Abstract
Although creativity has been called the most important of all human resources, its neural basis is still unclear. In the current study, we used fMRI to measure neural activity in participants solving a visuospatial creativity problem that involves divergent thinking and has been considered a canonical right hemisphere task. As hypothesized, both the visual creativity task and the control task as compared to rest activated a variety of areas including the posterior parietal cortex bilaterally and motor regions, which are known to be involved in visuospatial rotation of objects. However, directly comparing the two tasks indicated that the creative task more strongly activated left hemisphere regions including the posterior parietal cortex, the premotor cortex, dorsolateral prefrontal cortex (DLPFC) and the medial PFC. These results demonstrate that even in a task that is specialized to the right hemisphere, robust parallel activity in the left hemisphere supports creative processing. Furthermore, the results support the notion that higher motor planning may be a general component of creative improvisation and that such goal-directed planning of novel solutions may be organized top-down by the left DLPFC and by working memory processing in the medial prefrontal cortex.
Keywords: creativity, laterality, hemispheres, art, fMRI
INTRODUCTION
Creativity has been defined as a behavior or product that is both novel and useful (Sternberg and Lubart, 1996). Although creativity may be the most important of all human resources (Edward de Bono), its neural basis remains difficult to understand. The problem can be approached at the level of large-scale systems using brain imaging, but the studies carried out to date yield conflicting results. While some have claimed that creative problem solving is driven by processing in the right hemisphere (Finkelstein et al., 1991; Rotenberg, 1994; Miller et al., 1996, 1998, 2000; Murai et al., 1998), previous thinkers and recent studies indicate the importance of processing in both hemispheres (Bogen and Bogen, 1969; Kwong et al., 1992; Atchley et al., 1999; Aziz-Zadeh et al., 2009; Lindell, 2010) in supporting the creative process. However, the majority of these studies used language-related tasks, which largely force a lateralization of activity to the left hemisphere (e.g. word generation; anagrams). Conceivably, the finding of activity in both hemispheres during creative tasks might be due to the fact that the left hemisphere is necessary to complete the task and the right hemisphere is recruited to provide creative processing. It is thus reasonable to ask what happens when the task itself is presumed to recruit the right hemisphere. Is the right hemisphere sufficient for completing the creative task without further processing in the left hemisphere? Or will the equivalent left hemisphere regions be recruited for additional creative processing? The primary aim of the current study was to use functional magnetic resonance imaging (fMRI) to measure neural activity in subjects solving a visuospatial creativity problem that has been considered a canonical right hemisphere task.
Visual creativity, the production of novel and useful visual forms (Dake, 1991), is a primary component of fields such as painting, photography, sculpture and architecture. There have been only a handful of fMRI studies on visual creativity. A study on product design where novice and expert designers mentally imagined designing new pen prototypes found that in experts, there was increased dominance of right prefrontal regions over left prefrontal regions (Kowatari et al., 2009). Case studies on visual creativity have suggested decreased activity in fusiform gyrus when artists copy drawings of faces (Solso, 2001) and decreases in artistic abilities following stimulation of the left subthalamic nucleus (Drago et al., 2009). Furthermore, electroencephalography (EEG) studies of artistic creativity support a role of the right prefrontal cortex, showing greater synchrony in the right hemisphere during visual perception and visual memory, but only in artists (Bhattacharya and Petsche, 2002). Thus, it appears that each study yields different results depending on the task and the participant group, leaving the overall pattern of results difficult to interpret.
In trying to understand the creative process, some studies have focused on divergent thinking. Divergent thinking is defined as creative thinking that may follow many lines of thought and tends to generate new and original solutions (e.g., listing creative uses for a common object; www.merriam-webster.com; Campbell, 1960). This is in contrast to convergent thinking tasks for which there is one correct answer and which are most commonly used in intelligence tests (e.g., solving an anagram; Campbell, 1960). The common finding in the majority of fMRI studies on divergent thinking indicates the importance of the prefrontal cortex in both hemispheres, presumably for its role in working memory and executive attention [for a review, see (Dietrich and Kanso, 2010)].
With regard to visuospatial processing in general, previous data indicate involvement of the inferior parietal lobule, as well as higher motor regions [supplementary motor cortex (SMA), pre-SMA, premotor cortex, inferior frontal gyrus] in visuospatial processing. While lesion data indicate the importance of the right temporo-parietal cortex in visuospatial processing (Samuelsson et al., 1997; Swan, 2001), bilateral activation for components of visuospatial processing [e.g. mental rotation (Ng et al., 2001)] has also been shown. Thus, while the right hemisphere may be specialized for visuospatial processing, the left hemisphere also may be involved. Nevertheless, in line with the lesion data, it does appear that activity in the right inferior parietal cortex is a stronger rate-limiting step in visuospatial processing (Ng et al., 2001). In contrast, activity in the motor regions during visuospatial processing has commonly been found in the left hemisphere, and it has been speculated that participants use motor imagery of the dominant right hand to ‘manually’ rotate and manipulate the stimulus (Vingerhoets et al., 2002; Windischberger et al., 2003).
In the current study, we investigated the neural basis of visual creativity by comparing brain activity when participants complete two different tasks: a creative, divergent thinking task where they must mentally manipulate three shapes (e.g. ‘C’, ‘0’, ‘8’) to create a recognizable object (e.g. a smiley face) vs a mental rotation, convergent thinking task where participants had to rotate three parts to create a recognizable shape (e.g. rectangle; Figure 1 and Supplementary Data). The creative task has been used in previous behavioral studies investigating visual creativity (Finke and Slayton, 1988), while the control task was created and piloted for the current study (see ‘Experimental Methods’ section). We chose these tasks because both involve visuospatial processing as well as a naming component. Thus the main difference between the two tasks is visual creative processing, while basic visuospatial processing and verbal naming should be subtracted out in the comparison as they are common to the two tasks. Furthermore, the creative task requires novel responses (divergent thinking), whereas the control task requires a single correct answer (convergent thinking). As we hypothesized that creativity utilizes both hemispheres as opposed to the hemisphere dominant for the task (right parietal regions), we predicted that the visual creativity task would show more bilateral activity in parietal regions known to be active for visuospatial processes as compared to the control task. Additionally, as the creative task may involve stronger motor imagery of the dominant hand, we expected left motor regions to be more active during the creative task. Furthermore, as divergent thinking tasks have been reported to activate the prefrontal cortex due to the importance of working memory and cognitive planning in this task (for a review see Dietrich and Kanso, 2010), we also expected activity in this region during divergent creative processing.
Fig. 1.
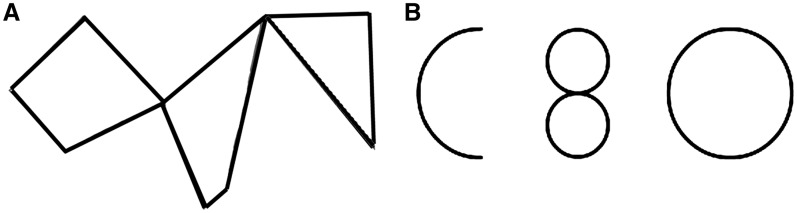
Example of control stimuli (A) and creative stimuli (B). The answer for (A) is rectangle, and for (B), sample answers include a smiley face, Homer Simpson or a man in a Volkswagen bug.
EXPERIMENTAL METHODS
Participants
Thirteen normal healthy adult participants (7 females, 6 males; mean age 23.15, s.d. = 3.36 years), over 18 years of age, were enrolled in the study. All participants were recruited by posted advertisement from a pool of architects and architect students, as pretesting showed that this population performs well on both the creative visual task and the control mental rotation task. Equal ability on these two tasks was important for the current study, as we did not want to confound the tasks by difficulty for the participant. Furthermore, all subjects were pretested and only subjects who performed well on both tasks (>75% accuracy on the control task, ability to complete the creative task in the allotted time and reported the two tasks to be equally difficult) participated in the fMRI study. At the time of the study, all participants were architecture students and some had limited internship experience; four had prior work experience in architecture or were currently working part time as architects.
In addition, because we were interested in laterality, all participants were right-handed, as measured by a modified Edinburgh Handedness Inventory (Oldfield, 1971). All participants had normal or corrected-to-normal vision, had no neurological or psychiatric history as measured by a questionnaire and passed an MRI safety screening questionnaire. Written informed consent was obtained from all participants before inclusion in the study. This study was approved by the University of Southern California Institutional Review Board and was performed in accordance with the 1964 Declaration of Helsinki.
Stimuli
Participants completed two different tasks, a visual creativity task and a control task. The creative task involved presenting participants with three distinct shapes (e.g. a circle, an ‘8’ and a ‘C’), and then asking them to assemble the shapes into a namable composite image (e.g. smiling face; Figure 1). The control task involved presenting participants with an ordinary shape (square, triangle and rectangle) that had been trisected into three pieces which were rotated apart from each other, with the three pieces hinging around shared vertices. Participants were asked to mentally rotate the constituent pieces around these hinge points to reconstruct and name the original shape (see Supplementary Data for list of all stimuli). There were a total of 20 creative stimuli and 20 control stimuli, all in black and white, at a size of 680 × 400 pixels (W × H). Each stimulus was shown once.
The stimuli were piloted on a separate group of 20 participants to assess equal task difficulty and length of time to complete the task between creative and control stimuli. Both types of stimuli took approximately the same amount of time to complete (control: 16 s; creative: 17 s; P = 0.2116), indicating that creative and control stimuli tasks did not differ by completion time. In addition, participants did not report one group being more difficult than the other.
Task
Creative and control trials were randomly intermixed across four different runs, with five of each condition per run. Each stimulus was seen only once during the entire scanning session. Participants were instructed to complete the creative task as quickly and creatively as possible and the control task as quickly and accurately as possible and were given practice trials outside the scanner. Inside the scanner, participants were given two button boxes to hold and asked to press two buttons, one with each hand, as soon as they completed the task. Bimanual responses were used to avoid a possible confounding effect of lateralization due to using one hand only. The onset of the first response was then used to determine the length of that trial for later analysis. Following task completion, there was a jittered rest with fixation cross, after which they were shown the prompt ‘What is your answer?’ Participants were instructed to speak their answer (the name of the object that they mentally produced either in the creative or control task) into the MRI-compatible microphone that was placed by their mouths and their response was digitally recorded. These responses were later transcribed by a research assistant.
General procedure and design
The images were presented through a projector onto a rear-projection screen attached to the head coil and located above the participant’s head. The experiment utilized a single event-related design in which all conditions (creative, control, rest/fixation cross, waiting and answer) were evenly distributed across four runs. Trial order included either a creative or control task, followed by a fixation cross presented for a jittered interstimulus interval (2–6 s), an answer period (6 s) and a fixed rest (15 s). Each of the functional runs lasted 466 s (233 TRs) and included four creative trials, four control trials and eight rests, answer periods and waiting periods. Creative and control stimuli (30 s each) were counterbalanced and trial order was randomized across participants.
fMRI image acquisition and analysis
Scanning was performed on a Siemens 3-T Trio scanner with a standard head coil. Thirty-seven axial slices of functional images covering the whole brain were acquired using a gradient-echo echo-planar pulse sequence (64 × 64 × 37 matrix with a spatial resolution of 3.5 × 3.5 ×3.5 mm, repitition time (TR) = 2000 ms, echo time (TE) = 30 ms, field of view (FOV) = 224 mm, flip angle = 90°). Anatomical images were obtained using a MPRAGE sequence (208 coronal slices, 256 ×256 ×208 matrix with a spatial resolution of 1 × 1 × 1 mm, TR = 1950 ms, TE = 2.56 ms, FOV = 256 mm; flip angle = 90°).
fMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The following prestatistics processing were applied to individual participants: motion correction using MCFLIRT (Jenkinson et al., 2002), slice-timing correction using Fourier space time series phase-shifting; nonbrain removal using FSL’s Brain Extraction Tool (BET) (Smith, 2002), spatial smoothing using a Gaussian kernel of full with at half maximum (FWHM) 5 mm, grand-mean intensity normalization of the entire 4D data set by a single multiplicative factor and highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 65.0 s) (Jenkinson et al., 2002; Smith, 2002). For each participant, a time-series statistical analysis was carried out using FILM GLM with local autocorrelation correction (Woolrich et al., 2001). Z (Gaussianized T/F) statistic images were then thresholded at P = 0.001 (uncorrected), and registered to a high-resolution standard space image [2 × 2 × 2 mm3 Montreal Neurological Institute (MNI) space] using FLIRT (FSL’s Linear Image Registration Tool) (Jenkinson and Smith, 2001; Jenkinson et al., 2002).
A group-level analysis was carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) Stage 1, which employed a mixed-effects model that includes both fixed effects and random effects from cross-session/participant variance (Beckmann et al., 2003; Woolrich et al., 2004; Woolrich, 2008). Z (Gaussianised T/F) statistic images at this level were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05 (Worsley et al., 2001).
RESULTS
Behavioral results
For the control task, the accuracy was 93% with an average time of task completion at 12.63 s, with a standard deviation of 9.8 s. For the creative task, participants were able to come up with a correct answer in the time allotted in all trials. The average time for completing the creative task was 20 s with a standard deviation of 9.11 s. The two tasks did not significantly differ in reported difficulty. As we were interested in comparing the cognitive process used in each task rather than the accuracy of the end solution, all trials were included in our subsequent analyses. The mean percentage of creative responses that were of faces/people was 14.1% ± 13%. The mean percentage of creative responses that were of a biological category (people + animals) was 25% ± 15%. Thus, the responses in the creative task were heterogeneous and were not major from any given category (biological, faces, animals, etc.).
Creative vs rest
To find brain regions that were active during the creative task, we compared activity in this task to rest. Significantly, active regions included the left inferior frontal gyrus (pars opercularis, pars orbitalis), left superior frontal gyrus (SMA/pre-SMA complex) (pre-SMA), left precentral gyrus (premotor cortex), as well as bilaterally, the lateral prefrontal cortex, inferior parietal lobule, the superior parietal lobule, the precuneus, the middle occipital gyrus, the anterior cingulate sulcus, the striatum, the insula and the cerebellum.
Control vs rest
To find brain regions that were active during the control task, we compared activity in this task to rest. Significantly, active regions included the right middle frontal gyrus and inferior frontal gyrus (pars opercularis), and bilaterally the inferior parietal lobule, the fusiform gyrus, the middle occipital gyrus, insula and the cerebellum.
Creative vs control
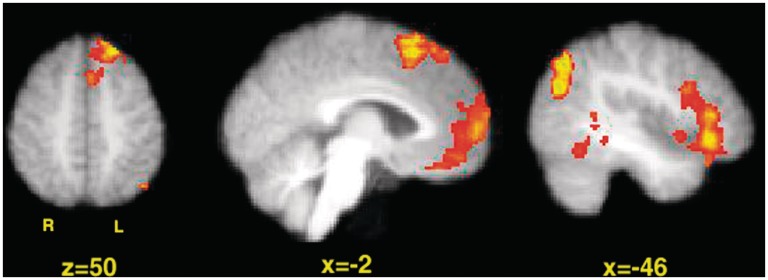
To find brain regions that were more active specifically during visual creative processing, we compared activity in the creative task to activity in the control task. Significant regions of activation were found in the medial prefrontal cortex, the left superior frontal gyrus [with peaks in the SMA, pars orbitalis and dorsolateral prefrontal cortex (DLPFC)], the premotor cortex, the left lateral occipital cortex, the left inferior parietal lobule and the left posterior middle temporal gyrus (Figure 2 and Table 1).
Fig. 2.
Activation pattern for the contrast ‘Creative > Control’. Left: SMA and left superior frontal gyrus are active. Middle: SMA and mPFC are active. Right: left IFG, left parietal cortex and left middle temporal gyrus are active. Images were thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05.
Table 1.
Activation peaks for the contrasts ‘Creative > Control’ and ‘Control > Creative’
| Z | x | y | z | Anatomical region | L/R | BA | Cluster no. | No. of voxels |
|---|---|---|---|---|---|---|---|---|
| Creative > control | ||||||||
| 4.1 | −2 | 20 | 56 | Superior frontal gyrus/SMA | L | 6 | 3 | 3590 |
| 3.7 | −8 | 66 | 24 | Superior Frontal Gyrus/mPFC | L | 8 | 3 | |
| 3.6 | −20 | 38 | 52 | Superior Frontal Gyrus/DLPFC | L | 8 | 3 | |
| 3.5 | −24 | 46 | 22 | Superior frontal gyrus | L | 8 | 3 | |
| 3.1 | −42 | 12 | 26 | Premotor cortex | L | 6 | 2 | 2182 |
| 3.5 | −38 | 28 | −20 | Pars orbitalis | L | 47 | 2 | |
| 3.5 | −46 | 26 | −6 | Pars orbitalis | L | 47 | 2 | |
| 4.4 | −50 | −74 | 26 | Lateral occipital gyrus | L | 39 | 1 | 2122 |
| 4.3 | −44 | −68 | 42 | Posterior parietal cortex | L | 39 | 1 | |
| 3.8 | −56 | −46 | 2 | Posterior middle temporal gyrus | L | 22 | 1 | |
| Control > creative | ||||||||
| 4.7 | 34 | −36 | 48 | Posterior parietal cortex | R | 40 | 2 | 7786 |
| 4.4 | 54 | −20 | 42 | Postcentral gyrus | R | 3 | 2 | |
| 3.6 | −40 | −40 | 52 | Postcentral gyrus | L | 2 | 2 | |
| 4.2 | 24 | −50 | 46 | Precuneus | R | 7 | 1 | 1362 |
| 4.1 | 14 | −88 | 16 | Inferior occipital gyrus | R | 18 | 1 |
Included in the table are the zstat for the peak, the MNI coordinates, anatomical region, hemisphere (L/R), Brodmann area (BA), cluster number and number of voxels significantly active per cluster.
Control vs creative
Brain regions that were more active during the control task as compared to the creative task included the bilateral postcentral gyrus, the right inferior parietal lobule, the right precuneus and right inferior occipital gyrus (Table 1).
DISCUSSION
As hypothesized, both the visual creativity task and the control task as compared to rest activated a variety of areas including the posterior parietal cortex bilaterally and motor regions, which are known to be involved in visuospatial rotation of objects (Milivojevic et al., 2009). Interestingly, however, directly comparing the two tasks indicated that the creative task more strongly activated left hemisphere regions, including the posterior parietal cortex, the superior frontal gyrus, the premotor cortex, the inferior frontal gyrus, the DLPFC and the medial prefrontal cortex (mPFC), while the control task more strongly activated the right postcentral gyrus, right posterior parietal cortex and visual processing regions. Thus, while both tasks involve bilateral processing, the creative task more strongly recruits the left hemisphere, while the control visuospatial task more strongly recruits the right hemisphere.
Lesion data indicate that the right hemisphere is specialized for visuospatial processing; patients with visuospatial neglect more often have damage to the right parietal regions, indicating that the right parietal lobe may specialize in visuospatial abilities (Samuelsson et al., 1997; Swan, 2001). Furthermore, an fMRI study investigating the quantitative relationships between regional activation and behavioral performance measures on a visuospatial task found significant behavioral–physiological association more strongly in right parietal regions, though both hemispheres were involved (Ng et al., 2001). Our data correspond with this trend, demonstrating stronger right parietal activation for the control visuospatial task compared to the creative task.
While both tasks showed bilateral processing as compared to rest, our canonical right hemisphere task, however, additionally recruited the corresponding left hemisphere parietal regions during creative processing as compared to during control processing. This increased activity in the left posterior parietal cortex during the creative task may indicate that creative processing relies not only on the right hemisphere processing that is common to both tasks, but also additional left hemisphere processing. This finding is consistent with the notion that verbal creative processing is associated with stronger bilateral processing than noncreative processing (Bogen and Bogen, 1969; Aziz-Zadeh et al., 2009), suggesting that bilateral recruitment of regions may be a general characteristic of creative processing, regardless of the modality (verbal or visual). To our knowledge, this is the first report that a visuospatial task shows robust left hemisphere activity. This result of greater interhemispheric processing during creative processing is consistent with a previous study of white matter tractography using diffusion tensor imaging. In that study, it was suggested that individuals who scored higher on a measure of creativity showed greater fiber density in or near a number of regions including the body of the corpus callosum, the bilateral prefrontal cortices and the right inferior parietal lobule (Takeuchi et al., 2010). Thus creative processing, whether verbal or visual, may rely more on processing in both hemispheres.
In addition, we found that the creative task more strongly recruited left motor regions including the left SMA, inferior frontal gyrus and premotor cortex. This is consistent with previous studies on mental rotation where it has been speculated that activity in higher motor regions may correspond to participants engaged in motor imagery of the dominant right hand to rotate the stimuli. Previous data indicate that activity in the SMA is greater as motor planning increases (Winstein et al., 1997). Activity in these higher motor regions may be indicative of more complicated manipulations of the stimuli, which the creative task may engage. While in the control task objects may be manipulated along a specific axis, in the creative task they may be manipulated in multiple directions and dimensions. Indeed, manipulating information in numerous dimensions may be a component of the creative process in general. This is consistent with literature demonstrating that musical improvisation also engages the supplementary and premotor cortices more than an equivalent control task, and the authors also discuss the possibility of increased motor and temporal planning as a necessary component of creative musical improvisation (Brown et al., 2006; Bengtsson et al., 2007; Berkowitz and Ansari, 2008; Limb and Braun, 2008). Verbal tasks involving creative generation (a divergent thinking task) have also found significant activity in the inferior frontal gyrus, premotor cortex and SMA (Jung-Beeman et al., 2004; Brown et al., 2006; Mashal et al., 2007; Aziz-Zadeh et al., 2009; Fink et al., 2009). Taken together, the current study corresponds with previous studies in suggesting that creative generation may utilize higher planning areas in the brain, including the SMA, premotor cortex and inferior frontal gyrus. Here, we extend this finding beyond the musical and language domains into visual creativity as well.
We also find that the creative task as compared to the control task involves activity in the left DLPFC. In the current study, response times and participant reports indicate that there is no difference in task difficulty between the creative task and the control task, and both tasks require equal working memory loads. Thus, we do not expect this activation to reflect higher task demands or working memory alone. Instead, it may be attributed to the type of processing the creative task demands: open ended, divergent processing rather than deciding between one of several possible shapes (convergent thinking). We note that our creative task is a divergent thinking task and the control task is a convergent thinking task; thus it is not possible for us to distinguish between creative problem solving and divergent thinking in the current study. However, as creative problem solving is defined as a subtype of divergent thinking (www.merriam-webster.com), this may be a common difficulty in similar studies on creativity.
The DLPFC activity during the creative task may also be attributed to its role in top-down organization of the creative process. This is in line with previous studies indicating that the DLPFC is involved in planning and performing novel or complicated behavioral sequences, both motoric as well as cognitive sequences, such as language and thought [for a review, see (Fuster, 2001)]. Previous studies also show activity in the DLPFC during goal-related visual search (Pollmann and von Cramon, 2000), effortful problem solving and focused attention (Hampshire and Owen, 2006; Osaka et al., 2007; Israel et al., 2010). The DLPFC has also been found to be active during free selection in cognitive tasks involving high cognitive manipulation (D’Esposito et al., 1998). Furthermore, transcranial magnetic stimulation (TMS) experiments show that disruption of activity in the left DLPFC is correlated with responses that are more stereotypical in tasks involving pseudorandom generation of numbers (Jahanshahi et al., 1998) or letters (Jahanshahi and Dirnberger, 1999). In addition, repetitive TMS over the left DLPFC leads to longer reaction times during analogic reasoning, a finding which was not true for the right DLPFC (Boroojerdi et al., 2001). These functions of the DLPFC (planning novel and complex cognition, guiding search, focused attention, working memory, assisting to produce novel rather than stereotypical responses) may all be essential to visuospatial creativity.
The mPFC was also found to be more active in the creativity task. The peak of this activity was in the left hemisphere, though activity spreads bilaterally. Previous studies also indicate that the mPFC is a common part of the creativity network. Spontaneous counterfactual thinking, which is thought to be important for creativity (Kray et al., 2006), was found to be impaired in patients with PFC lesions (Gomez Beldarrain et al., 2005). Divergent thinking tasks commonly show activity in the mPFC, and this has been in part attributed to the high demand of working memory in these tasks [for a review, see (Dietrich and Kanso, 2010)]. An fMRI study on semantic divergence and creative story generation found activity in the right PFC and attributed it to increased monitoring as well as higher cognitive control for stringent monitoring for creative solutions (Howard-Jones et al., 2005). Furthermore, right prefrontal activation has been observed for other creative problem solving tasks, such as processing unusual semantic relationships (Schmidt et al., 2007). Additionally, a negative correlation has been found between producing original ideas and lesions in the right mPFC (Shamay-Tsoory et al., 2011). Thus, the current study extends previous findings that the mPFC is important to creative problem solving not only in language-related tasks and divergent thinking, but also in visual creativity.
Finally, we found increased activity in regions commonly associated with language processing (middle temporal gyrus, pars orbitalis) during the creativity task. While both tasks require a naming component, naming the produced creative figure may require more language processing than naming a shape. Activity in language regions may be attributed to the possibility that naming in the control task may have been simpler than in the creative task.
These results cannot be explained on the basis of difficulty. Pilot testing revealed that the two conditions did not significantly differ either in time required to complete the task or in reported difficulty. During scanning, there were also no significant differences in reported difficulty between the two tasks, though on average the creative task took longer to complete than the control task. However, we note that the analysis conducted here utilizes the time specific to each condition, so that the mental process for each condition is divided over the time reported to complete the task. In this way, each condition is represented per unit time; hence, the difference in overall time between the two conditions should not affect the current analysis. Future studies are necessary to see whether these findings are consistent with other types of visual creativity and visual divergent thinking tasks, as well as generalizable outside of our limited sample population of architects.
In summary, our results demonstrate that even in a task that is specialized to the right hemisphere (visuospatial processing), robust parallel activity in the left hemisphere supports creative processing. This novel finding suggests that creative processing recruits both hemispheres, including the one that is less dominant for that task. In particular, while in this study we find that visual creativity more strongly recruits left hemisphere activation despite being a right hemisphere task, previous reports show that creative tasks involving language also commonly find activity in the hemisphere nondominant for the task (right hemisphere activity, when it is a left hemisphere task). Furthermore, our results support the notion that higher motor planning may be a general component of creative improvisation (visual, verbal or auditory) and that such goal-directed planning of novel solutions may be organized top-down by the left DLPFC and by working memory processing in the mPFC. Thus, it may be that this pattern of activation is not only important for visual creativity but creativity in various domains.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
Authors thank Henryk Bukowski, Daniel Epstein, Mark Lay, Alicia Johnson and Antonio Damasio for their assistance with this study. This study was supported by the Brain and Creativity Institute, the Division of Occupational Science and Occupational Therapy, the National Science Foundation Graduate Research Fellowship, and the USC Provost PhD Fellowship.
REFERENCES
- Atchley RA, Keeney M, Burgess C. Cerebral hemispheric mechanisms linking ambiguous word meaning retrieval and creativity. Brain and Cognition. 1999;40:479–99. doi: 10.1006/brcg.1999.1080. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Kaplan JT, Iacoboni M. “Aha!”: the neural correlates of verbal insight solutions. Human Brain Mapping. 2009;30:908–16. doi: 10.1002/hbm.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Csikszentmihalyi M, Ullen F. Cortical regions involved in the generation of musical structures during improvisation in pianists. Journal of Cognitive Neuroscience. 2007;19:830–42. doi: 10.1162/jocn.2007.19.5.830. [DOI] [PubMed] [Google Scholar]
- Berkowitz AL, Ansari D. Generation of novel motor sequences: the neural correlates of musical improvisation. Neuroimage. 2008;41:535–43. doi: 10.1016/j.neuroimage.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Petsche H. Shadows of artistry: cortical synchrony during perception and imagery of visual art. Brain Research Cognitive Brain Research. 2002;13:179–86. doi: 10.1016/s0926-6410(01)00110-0. [DOI] [PubMed] [Google Scholar]
- Bogen JE, Bogen GM. The other side of the brain. III. The corpus callosum and creativity. Bulletin of the Los Angeles Neurological Society. 1969;34:191–220. [PubMed] [Google Scholar]
- Boroojerdi B, Phipps M, Kopylev L, Wharton CM, Cohen LG, Grafman J. Enhancing analogic reasoning with rTMS over the left prefrontal cortex. Neurology. 2001;56:526–8. doi: 10.1212/wnl.56.4.526. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Parsons LM. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. The European Journal of Neuroscience. 2006;23:2791–803. doi: 10.1111/j.1460-9568.2006.04785.x. [DOI] [PubMed] [Google Scholar]
- Campbell DT. Blind variation and selective retention in creative thought as in other knowledge processes. Psychological Review. 1960;67:380–400. doi: 10.1037/h0040373. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Ballard D, Aguirre GK, Zarahn E. Human prefrontal cortex is not specific for working memory: a functional MRI study. Neuroimage. 1998;8:274–82. doi: 10.1006/nimg.1998.0364. [DOI] [PubMed] [Google Scholar]
- Dake DM. The visual definition of visual creativity. Journal of Visual Literacy. 1991;1:99–118. [Google Scholar]
- Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin. 2010;136:822–48. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- Drago V, Foster PS, Okun MS, et al. Artistic creativity and DBS: a case report. Journal of the Neurological Sciences. 2009;276:138–142. doi: 10.1016/j.jns.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Fink A, Graif B, Neubauer AC. Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. Neuroimage. 2009;46:854–62. doi: 10.1016/j.neuroimage.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Finke RA, Slayton K. Explorations of creative visual synthesis in mental imagery. Memory & Cognition. 1988;16:252–7. doi: 10.3758/bf03197758. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Vardi J, Hod I. Impulsive artistic creativity as a presentation of transient cognitive alterations. Behavioral Medicine. 1991;17:91–4. doi: 10.1080/08964289.1991.9935164. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex—an update: time is of the essence. Neuron. 2001;30:319–33. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gomez Beldarrain M, Garcia-Monco JC, Astigarraga E, Gonzalez A, Grafman J. Only spontaneous counterfactual thinking is impaired in patients with prefrontal cortex lesions. Brain Research Cognitive Brain Research. 2005;24:723–6. doi: 10.1016/j.cogbrainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cerebral Cortex. 2006;16:1679–89. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Howard-Jones PA, Blakemore SJ, Samuel EA, Summers IR, Claxton G. Semantic divergence and creative story generation: an fMRI investigation. Brain Research Cognitive Brain Research. 2005;25:240–50. doi: 10.1016/j.cogbrainres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Israel SL, Seibert TM, Black ML, Brewer JB. Going their separate ways: dissociation of hippocampal and dorsolateral prefrontal activation during episodic retrieval and post-retrieval processing. Journal of Cognitive Neuroscience. 2010;22:513–25. doi: 10.1162/jocn.2009.21198. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Dirnberger G. The left dorsolateral prefrontal cortex and random generation of responses: studies with transcranial magnetic stimulation. Neuropsychologia. 1999;37:181–90. doi: 10.1016/s0028-3932(98)00092-x. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Profice P, Brown RG, Ridding MC, Dirnberger G, Rothwell JC. The effects of transcranial magnetic stimulation over the dorsolateral prefrontal cortex on suppression of habitual counting during random number generation. Brain: A Journal of Neurology. 1998;121(Pt 8):1533–44. doi: 10.1093/brain/121.8.1533. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, et al. Neural activity when people solve verbal problems with insight. PLoS Biology. 2004;2:E97. doi: 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatari Y, Lee SH, Yamamura H, et al. Neural networks involved in artistic creativity. Human Brain Mapping. 2009;30:1678–90. doi: 10.1002/hbm.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray LJ, Galinsky AD, Wong EM. Thinking within the box: the relational processing style elicited by counterfactual mind-sets. Journal of Personality and Social Psychology. 2006;91:33–48. doi: 10.1037/0022-3514.91.1.33. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences USA. 1992;89:5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb CJ, Braun AR. Neural substrates of spontaneous musical performance: an FMRI study of jazz improvisation. PLoS ONE. 2008;3:e1679. doi: 10.1371/journal.pone.0001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell AK. Lateral thinkers are not so laterally minded: hemispheric asymmetry, interaction, and creativity. Laterality. 2011;16(4):479–98. doi: 10.1080/1357650X.2010.497813. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, Jung-Beeman M. An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain and Language. 2007;100:115–26. doi: 10.1016/j.bandl.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Milivojevic B, Hamm JP, Corballis MC. Functional neuroanatomy of mental rotation. Journal of Cognitive Neuroscience. 2009;21:945–59. doi: 10.1162/jocn.2009.21085. [DOI] [PubMed] [Google Scholar]
- Miller BL, Boone K, Cummings JL, Read SL, Mishkin F. Functional correlates of musical and visual ability in frontotemporal dementia. British Journal of Psychiatry. 2000;176:458–63. doi: 10.1192/bjp.176.5.458. [DOI] [PubMed] [Google Scholar]
- Miller BL, Cummings J, Mishkin F, et al. Emergence of artistic talent in frontotemporal dementia. Neurology. 1998;51:978–82. doi: 10.1212/wnl.51.4.978. [DOI] [PubMed] [Google Scholar]
- Miller BL, Ponton M, Benson DF, Cummings JL, Mena I. Enhanced artistic creativity with temporal lobe degeneration. Lancet. 1996;348:1744–5. doi: 10.1016/s0140-6736(05)65881-3. [DOI] [PubMed] [Google Scholar]
- Murai T, Hanakawa T, Sengoku A, et al. Temporal lobe epilepsy in a genius of natural history: MRI volumetric study of postmortem brain. Neurology. 1998;50:1373–6. doi: 10.1212/wnl.50.5.1373. [DOI] [PubMed] [Google Scholar]
- Ng VW, Bullmore ET, de Zubicaray GI, Cooper A, Suckling J, Williams SC. Identifying rate-limiting nodes in large-scale cortical networks for visuospatial processing: an illustration using fMRI. Journal of Cognitive Neuroscience. 2001;13:537–45. doi: 10.1162/08989290152001943. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The Assessment and Analysis of Handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osaka M, Komori M, Morishita M, Osaka N. Neural bases of focusing attention in working memory: an fMRI study based on group differences. Cognitive, Affective & Behavioral Neuroscience. 2007;7:130–9. doi: 10.3758/cabn.7.2.130. [DOI] [PubMed] [Google Scholar]
- Pollmann S, von Cramon DY. Object working memory and visuospatial processing: functional neuroanatomy analyzed by event-related fMRI. Experimental Brain Research Experimentelle Hirnforschung Experimentation Cerebrale. 2000;133:12–22. doi: 10.1007/s002210000396. [DOI] [PubMed] [Google Scholar]
- Rotenberg VS. An integrative psychophysiological approach to brain hemisphere functions in schizophrenia. Neuroscience & Biobehavioral Reviews. 1994;18:487–95. doi: 10.1016/0149-7634(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Samuelsson H, Jensen C, Ekholm S, Naver H, Blomstrand C. Anatomical and neurological correlates of acute and chronic visuospatial neglect following right hemisphere stroke. Cortex. 1997;33:271–85. doi: 10.1016/s0010-9452(08)70004-2. [DOI] [PubMed] [Google Scholar]
- Schmidt GL, DeBuse CJ, Seger CA. Right hemisphere metaphor processing? Characterizing the lateralization of semantic processes. Brain and Language. 2007;100:127–41. doi: 10.1016/j.bandl.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Adler N, Aharon-Peretz J, Perry D, Mayseless N. The origins of originality: the neural bases of creative thinking and originality. Neuropsychologia. 2011;49:178–85. doi: 10.1016/j.neuropsychologia.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solso R. Brain activities in a skilled vs a novice artist: an fMRI study. Leonardo. 2001;34:31–4. [Google Scholar]
- Sternberg RJ, Lubart TJ. Investigating in creativity. American Psychologist. 1996;7:677–88. [Google Scholar]
- Swan L. Unilateral spatial neglect. Physical Therapy. 2001;81:1572–80. doi: 10.1093/ptj/81.9.1572. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, et al. White matter structures associated with creativity: evidence from diffusion tensor imaging. Neuroimage. 2010;51:11–18. doi: 10.1016/j.neuroimage.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, de Lange FP, Vandemaele P, Deblaere K, Achten E. Motor imagery in mental rotation: an fMRI study. Neuroimage. 2002;17:1623–33. doi: 10.1006/nimg.2002.1290. [DOI] [PubMed] [Google Scholar]
- Windischberger C, Lamm C, Bauer H, Moser E. Human motor cortex activity during mental rotation. Neuroimage. 2003;20:225–32. doi: 10.1016/s1053-8119(03)00235-0. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. Journal of Neurophysiology. 1997;77:1581–94. doi: 10.1152/jn.1997.77.3.1581. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41:286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. 2001. Statistical analysis of activation images. Functional MRI: An Introduction to Methods. , eds Jezzard P, Matthews PM, Smith SM (Oxford, NY), pp. 251–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.