Abstract
Self-conscious emotions such as embarrassment arise when one’s actions fail to meet salient social expectations and are accompanied by marked physiological and behavioral activation. We investigated the neural correlates of self-conscious emotional reactivity in 27 patients with behavioral variant frontotemporal dementia (bvFTD), a neurodegenerative disease that disrupts self-conscious emotion and targets brain regions critical for emotional functioning early in the disease course, and in 33 healthy older controls. Subjects participated in an embarrassing karaoke task in which they watched a video clip of themselves singing. They also watched a sad film clip; these data were used to control for non-self-conscious emotional reactivity in response to audiovisual stimuli. Using Freesurfer to quantify regional brain volumes from structural magnetic resonance imaging, right pregenual anterior cingulate cortex (pACC) gray matter volume was the only brain region that was a significant predictor of self-conscious emotion. Smaller pACC volume was associated with attenuated physiological and behavioral self-conscious emotional reactivity, and this relationship was not specific to diagnosis. We argue that these results reflect the significant role that right pACC plays in the visceromotor responding that accompanies self-conscious emotion and that neurodegeneration in this region may underlie the self-conscious emotional decline seen in bvFTD.
Keywords: emotion, cingulate, autonomic nervous system, behavior, neurodegenerative disease
It is not the simple act of reflecting on our own appearance, but the thinking what others think of us, which excites a blush.
(Charles Darwin)
INTRODUCTION
Self-conscious emotions such as embarrassment, shame, guilt and pride are social emotions in which the self stands at the forefront of awareness. Our use of the term ‘self’ in this context refers to one’s physical being, as well as the thoughts and feelings that constitute the subjective sense of that being (James, 1890). These emotions serve important interpersonal functions (Miller and Leary, 1992; Tangney, 1999; Lewis, 2000). Embarrassment, for example, typically arises when heightened attention is paid to the self after violation of a social rule (Keltner, 1995). Not only is embarrassment associated with autonomic nervous system responding including increases in heart rate, blood pressure, sweating (Keltner, 1995; Harris, 2001; Gerlach et al., 2003) and peripheral vasodilation (producing characteristic facial blushing), but it also has a characteristic behavioral display (e.g. smile control, gaze aversion and face touching; Shearn et al., 1990; Keltner, 1995). The physiological and behavioral changes that occur in embarrassment signal to others that one regrets the offending action (Miller, 2007) and may help motivate actions (e.g. apologizing) that redress social transgressions.
Self-conscious emotions are complex emotions that arise following measured comparisons of oneself against relevant social standards. As such, they likely rely upon a distributed neuroanatomical network that obtains and evaluates information about the social context and relays this information to structures involved in emotion generation. Patient and neuroimaging studies offer converging evidence that a network involving prefrontal cortex plays an important role in self-conscious emotion. While some studies of patients with orbitofrontal lesions secondary to traumatic brain injury suggest that this region may be important for the regulation, and not generation, of self-conscious emotion (Beer et al., 2003), others suggest that damage to this region results in dampened self-conscious emotional reactivity (Krajbich et al., 2009). Functional neuroimaging studies, which typically elicit self-conscious emotion by having subjects read embarrassing vignettes (Berthoz et al., 2002; Takahashi et al., 2004), view unflattering photographs of oneself (Morita et al., 2008) and recall guilt-inducing memories (Shin et al., 2000), have consistently found that self-conscious emotions (with perhaps the exception of pride; Takahashi et al., 2008) activate medial prefrontal cortex (Shin et al., 2000; Takahashi et al., 2004; Morita et al., 2008) and anterior insula (Shin et al., 2000; Morita et al., 2008). Activity in anterior cingulate cortex (ACC), a region within the medial prefrontal cortex that coordinates synchronized, multisystem emotional responses to salient environmental stimuli (Patterson et al., 2002; Critchley, 2005; Wager et al., 2009), has also been found during self-conscious emotional responding (Shin et al., 2000; Morita et al., 2008).
Pregenual ACC (pACC) is a ventral subregion of ACC (i.e. Brodmann areas 24a–c, 25, 32 and 33) that coordinates the autonomic, visceromotor and endocrine system activity that accompany emotion (Vogt et al., 1992; Devinsky et al., 1995; Bush et al., 2000; Ongur and Price, 2000; Saper, 2002; Kober et al., 2008). Through strong connections with orbitofrontal cortex, temporal pole and central pattern generators including the amygdala, periaqueductal gray and hypothalamus (Saper, 2002), pACC is well-situated for receiving afferent input (from prefrontal cortex and temporal pole, which are important for processing the social milieu) and for initiating subsequent emotional responding (via efferent connections with central pattern generators, which activate autonomic and behavioral responding). Thus, this region may be particularly important for self-conscious emotion because of its role in relaying complex social appraisals, which are intrinsic to emotions like embarrassment, to emotion generation structures.
Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disease that affects anterior insula and pACC early in the disease course (Schroeter et al., 2008; Seeley et al., 2008). Declines in social and emotional behavior (e.g. disinhibition, apathy and loss of empathy) are hallmark features of bvFTD that are sensitive to underlying pathology (Rascovsky et al., 2011). Patients with bvFTD have trouble recognizing situations that would typically elicit self-conscious emotion (Moll et al., 2011) and have diminished physiological and behavioral self-conscious emotional reactivity when measured in a laboratory setting (Sturm et al., 2006, 2008).
In the present study, we measured self-conscious emotional reactivity in patients with bvFTD and in healthy control subjects. We examined whether neurodegeneration of pACC underlies the diminished self-conscious emotion that we have found in bvFTD and whether variability in pACC volume in control subjects is associated with individual differences in self-conscious emotion. Subjects participated in an embarrassing karaoke task in which they watched video recordings of themselves singing while their peripheral physiological responding and facial behavior were monitored. To control for the effects of non-self-conscious emotional reactivity in response to viewing a dynamic, audiovisual stimulus, subjects also watched a sad film clip. Given its role in visceromotor emotional responding, we hypothesized that, independent of diagnosis, smaller pACC volume would predict lower physiological (i.e. autonomic and somatic) and behavioral (i.e. emotional facial expressions) self-conscious emotional reactivity.
METHODS
Subjects
Sixty subjects (including 27 patients with bvFTD and 33 healthy controls) diagnosed according to standard research criteria (Neary et al., 1998) were included in the study. We included both healthy subjects and patients with a neurodegenerative disease to provide adequate variability in our anatomical and emotional measures and thereby increase the statistical power of our analyses. Table 1 presents the age and sex data for each group.
Table 1.
Characteristics of subjects classified by diagnostic group
| HC | bvFTD | Overall | |
|---|---|---|---|
| n | 33 | 27 | 60 |
| Age: M (s.d.) | 65.0 (10.5) | 60.4 (6.5) | 63.0 (9.1) |
| Sex: M:F | 16:17 | 20:7 | 36:24 |
| CDR: M (s.d.) | 0.0 (0.1) | 1.2 (0.5) | 0.6 (0.7) |
Notes: HC = healthy controls, bvFTD = behavioral variant frontotemporal dementia, CDR = Clinical Dementia Rating Scale. Means (M) and standard deviations (s.d.) listed for each group.
Clinical evaluation
Subjects were evaluated by a multidisciplinary team at the University of California, San Francisco Memory and Aging Center. Subjects underwent a clinical interview; neurological examination; laboratory evaluation; neuropsychological evaluation (Kramer et al., 2003) and a functional assessment of dementia severity, the Clinical Dementia Rating Scale (CDR; Morris, 1993). The total CDR score (scores range from 0 to 3, with higher scores indicating greater functional impairment) was computed for each subject (Morris, 1993). Table 1 presents CDR scores for each group.
Emotional evaluation
Procedure
Subjects were assessed at the University of California, Berkeley. Subjects signed consent forms and were then seated in a well-lit, 3 m × 6 m experiment room. All stimuli and instructions were presented on a 21-inch color television monitor at a distance of 1.75 m from the subject. Subjects participated in our standard day-long assessment of emotional functioning that assesses a number of aspects of emotional reactivity, regulation and empathy (Levenson et al., 2008). For this study, data were used from two tasks that tested emotional reactivity in response to dynamic visual stimuli.
Karaoke task
Self-conscious emotional reactivity was assessed with a karaoke singing task (Shearn et al., 1990; Sturm et al., 2008), which has been shown to elicit self-conscious emotions (e.g. embarrassment) reliably in older adults (Sturm et al., 2008). Subjects were asked to relax during a 60-s pretrial baseline during which an ‘X’ appeared on the television monitor. The song title (‘My Girl’) was then displayed on the monitor for 9 s followed by playing the song (the version by The Temptations was used) for 2 min and 33 s (lyrics appeared on the monitor and sound was presented over headphones). Subjects were asked to sing along with the song but were not told that they would later be viewing the tape of their performance. When the song ended, the experimenter returned to the room and removed the headphones. The only instruction given for the next task was to watch the television monitor. The videotape that had just been recorded was then played on the television monitor (subjects saw themselves sitting through the pretrial baseline and then heard and saw themselves singing without hearing The Temptations’ version in the background).
Sad film
Non-self-conscious emotional reactivity was assessed with a film-viewing task in which subjects watched a sad film clip. The film clip, which was excerpted from the film The Champ, depicts a young boy crying as he watches his father die. This film has been shown to elicit sadness reliably in older adults (Seider et al., 2011). Subjects were asked to relax during a 60-s pretrial baseline during which an ‘X’ appeared on the television monitor. The sad film averaged 2 min and 13 s.
Measures
Physiological reactivity
Physiological measures were monitored continuously using a Grass Model 7 polygraph, a computer with analog-to-digital capability and an online data acquisition and analysis software package written by Robert W. Levenson. The software computed second-by-second averages for the following measures: (i) heart rate (Beckman miniature electrodes with Redux paste were placed in a bipolar configuration on opposite sides of the subject’s chest; the inter-beat interval was calculated as the interval, in milliseconds, between successive R waves); (ii) finger pulse amplitude (a UFI photoplethysmograph recorded the amplitude of blood volume in the finger using a photocell taped to the distal phalanx of the index finger of the non-dominant hand); (iii) finger pulse transmission time (the time interval in milliseconds was measured between the R wave of the electrocardiogram (ECG) and the upstroke of the peripheral pulse at the finger site, recorded from the distal phalanx of the index finger of the non-dominant hand); (iv) ear pulse transmission time (a UFI photoplethysmograph attached to the right earlobe recorded the volume of blood in the ear, and the time interval in milliseconds was measured between the R wave of the ECG and the upstroke of peripheral pulse at the ear site); (v) systolic and (vi) diastolic blood pressure (a blood pressure cuff was placed on the middle phalanx of the middle finger of the non-dominant hand and continuously recorded the systolic and diastolic blood pressure using an Ohmeda Finapress 2300); (vii) skin conductance (a constant-voltage device was used to pass a small voltage between Beckman regular electrodes [using an electrolyte of sodium chloride in unibase] attached to the palmar surface of the middle phalanges of the ring and index fingers of the non-dominant hand); (viii) general somatic activity (an electromechanical transducer attached to the platform under the subject’s chair generated an electrical signal proportional to the amount of movement in any direction); (ix) respiration period (a pneumatic bellows was stretched around the thoracic region and the inter-cycle interval was measured in milliseconds between successive inspirations); (x) respiration depth (the point of the maximum inspiration minus the point of maximum expiration was determined from respiratory tracing) and (xi) finger temperature (a thermistor attached to the distal phalanx of the little finger of the non-dominant hand recorded temperature in degrees Fahrenheit). This array of measures was selected to sample from major autonomic (cardiac, vascular, electrodermal and respiratory) and somatic systems that are important for emotional responding. Table 2 shows mean physiological levels for each group.
Table 2.
Groups means and standard deviations of the physiological and behavioral measures of self-conscious emotional reactivity
| HC | bvFTD | Overall | |
|---|---|---|---|
| Standardized physiological composite score* | 0.10 (0.58) | −0.14 (0.33) | −0.01 (0.50) |
| Non-standardized individual physiological measures | |||
| Cardiac inter-beat interval (ms)** | 848.5.0 (133.7) | 724.0 (116.8) | 792.5 (140.0) |
| Finger pulse amplitude (U) | 6.5 (5.9) | 5.1 (4.7) | 5.9 (5.4) |
| Finger pulse transit time (ms) | 266.6 (27.0) | 257.5 (29.6) | 262.6 (28.3) |
| Ear pulse transit time (ms) | 183.0 (31.9) | 179.0 (22.5) | 181.3 (28.1) |
| Systolic blood pressure (mmHg) | 148.8 (26.2) | 146.0 (18.1) | 147.4 (22.6) |
| Diastolic blood pressure (mmHg) | 81.9 (15.2) | 85.8 (11.4) | 83.7 (13.6) |
| Skin conductance (µMhos) | 2.5 (1.5) | 2.6 (2.4) | 2.6 (1.9) |
| General somatic activity (U) | 2.0 (2.1) | 1.5 (1.3) | 1.8 (1.8) |
| Respiration period (ms) | 3259.7 (465.2) | 3422.9 (1259.2) | 3333.6 (908.1) |
| Respiration depth (U) | 217.9 (105.3) | 232.0 (113.9) | 224.3 (108.4) |
| Finger temperature (degrees Fahrenheit) | 85.7 (5.5) | 87.4 (6.9) | 86.4 (6.2) |
| Behavioral reactivity | |||
| Self-conscious emotional behavior composite score** | 6.88 (8.6) | 1.92 (3.1) | 4.65 (7.1) |
Notes: For physiological reactivity, mean levels of individual channels (not corrected for baseline levels) and standard deviations (in parentheses) during the karaoke task. Higher values indicate greater physiological arousal for skin conductance, general somatic activity, systolic and diastolic blood pressure, respiration depth and finger temperature; lower values indicate greater physiological arousal for cardiac inter-beat interval, finger pulse amplitude, finger pulse transit time, ear pulse transit time, and respiration period. For behavioral reactivity, mean levels of self-conscious emotional behavior during the karaoke task. For abbreviations, see Table 1. *P < 0.10, **P < 0.05.
Emotional behavior
Subjects’ behavior was videotaped continuously using a remote-controlled, high-resolution video camera. Subjects’ facial behavior during the karaoke and sad film tasks was later coded by a team of trained coders. For the karaoke task, the first 20 s of subjects watching themselves singing, the period which is typically the most embarrassing, was coded. For the sad film, a highly intense 20-s period of the film was coded. For both tasks, coders used a modified version of the Emotional Expressive Behavior coding system (Gross and Levenson, 1993) to code each second for nine emotional behaviors (anger, disgust, happiness/amusement, contempt, sadness, embarrassment, fear, surprise and confusion) on an intensity scale ranging from 0 to 3. The code for embarrassment was based on (Keltner and Buswell’s 1997) description, which includes gaze aversion, smiling and laughter, smile suppression, blushing and face-touches. The code for happiness/amusement included smiling and laughing. Inter-coder reliability was high (intraclass correlation coefficient = 0.82). Table 2 shows mean levels for each group.
Data reduction
Physiological reactivity
Physiological reactivity scores were computed for the karaoke and sad film tasks. For the karaoke task, we subtracted the average level of each physiological measure while subjects watched themselves during the pre-singing baseline period (the first 50 s of the baseline) from the average level while they watched themselves singing (the first 20 s that they watched themselves singing). For the sad film, we subtracted the average level of each measure during the first 50 s of the baseline period from the average level during a 20-s period that included the most intense portion of the film.
To control for Type I error due to multiple dependant measures, we calculated a single composite measure of physiological reactivity for each task (e.g. Sturm et al., 2006, 2008). To calculate these scores, we computed standardized scores for each physiological reactivity score and reverse-scored as needed (i.e. cardiac inter-beat interval, finger pulse amplitude, finger pulse transmission time, ear pulse transmission time and respiration period) so that larger values reflected greater physiological arousal. The standardized scores were then averaged, which produced two composite physiological reactivity scores for each subject (one for the karaoke task and one for the sad film). Although correlations among measures of autonomic and somatic nervous system activity are typically low at rest, we have found much higher coherence among these measures when subjects are in the throes of emotion (Mauss et al., 2005; Sze et al., 2010). Consistent with this, in control subjects, the individual physiological measures exhibited an acceptable level of internal consistency during the karaoke task (Cronbach α = 0.76) and sad film (Cronbach α = 0.62).
Emotional behavior
For each emotional code, we summed the intensity scores for each occurrence during the karaoke task and sad film. We computed a behavioral composite score for self-conscious emotional behavior for each task (by averaging the embarrassment and happiness/amusement codes) and for negative emotional behavior (by averaging the anger, disgust, sadness, fear and surprise codes).
Brain imaging
Structural neuroimaging acquisition
All subjects underwent a structural magnetic resonance imaging (MRI) at the San Francisco Veterans Administration Hospital. MR images were acquired on a 1.5 T Magnetom VISION system (Siemens 75 Inc., Iselin, NJ, USA) using a standard quadrature head coil. A volumetric magnetization-prepared rapid gradient echo (MP RAGE) MRI (repetition time/echo time/inversion time 10/4/300 ms) sequence was used to obtain images of the entire brain-in a coronal orientation, with 1.0 mm2 in-plane and 1.5 mm through-plane resolution.
Freesurfer volumes of interest
MR images were analyzed using Freesurfer, http://surfer.nmr.mgh.harvard.edu, (Dale et al., 1999; Fischl et al., 1999, 2001; Segonne et al., 2004), a surface-based structural MR image analysis tool that segments white matter and tessellates both gray and white matter surfaces. Non-brain tissue is removed using a hybrid watershed/surface deformation procedure (Segonne et al., 2004) and intensity normalization (Sled et al., 1998), followed by automated Talairach transformation and volumetric segmentation of cortical and subcortical gray and white matter, subcortical structures, basal ganglia and ventricles, used to calculate total intracranial volume (Fischl et al., 2002, 2004). The LONI Pipeline environment (http://pipeline.loni.ucla.edu) was used to distribute Freesurfer processing tasks to an offsite CPU cluster located at UCLA-LONI. Cortical regions of interest were defined as described by Desikan et al. (2006).
Because the neurodegenerative diseases in our sample may produce diffuse brain atrophy, we took a whole-brain approach and examined 79 cortical and subcortical regional volumes that were generated by Freesurfer. These included brainstem volumes and volumes in the right and left insula, pACC, anterior mid-cingulate cortex, superior frontal gyrus, rostral middle frontal gyrus, caudal middle frontal gyrus, pars opercularis, pars orbitalis, pars triangularis, lateral orbitofrontal cortex, medial orbitofrontal cortex, frontal pole, precentral gyrus, paracentral lobule, entorhinal cortex, parahippocampal gyrus, temporal pole, fusiform gyrus, superior temporal gyrus, middle temporal gyrus, inferior temporal gyrus, transverse temporal cortex, banks of the superior temporal sulcus, postcentral gyrus, superior parietal cortex, inferior parietal cortex, precuneus, lingual gyrus, pericalcarine cortex, cuneus, lateral occipital cortex, amygdala, hippocampus, accumbens area, cerebellar cortex, thalamus, caudate, putamen and pallidum.
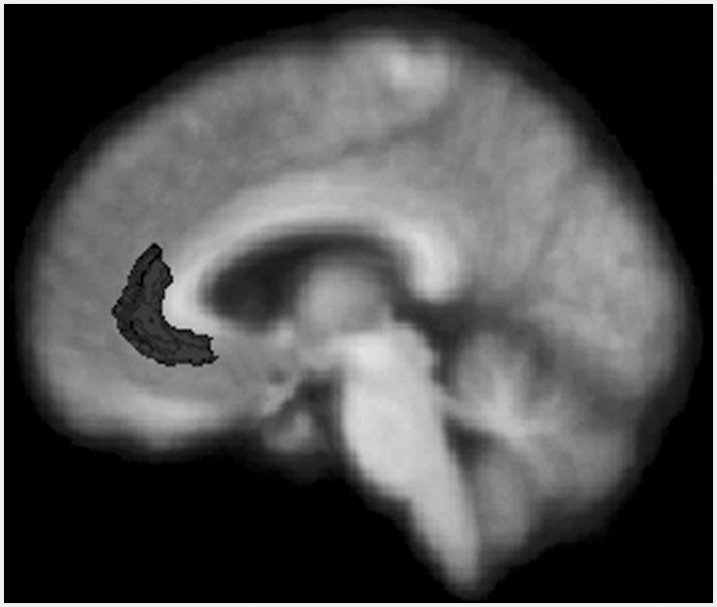
Manual data quality checks were completed for each MR image to determine whether Freesurfer had accurately segmented gray from white matter in the ACC (Figure 1). A total of four bvFTD patients were excluded from our original sample of 65 subjects due to poor image quality or problems with gray–white segmentation of the ACC.
Fig. 1.
pACC as delineated by Freesurfer. The figure is the composed of a standardized average MRI coregistered with average regions representing the pACC (dark gray).
Statistical analyses
Although we hypothesized that pACC volume would predict self-conscious emotional reactivity, we took a broad approach in order to determine whether any other brain regions were also associated. We ran two-tailed partial correlations (controlling for total intracranial volume) between self-conscious emotional reactivity (i.e. the physiological and behavioral composite scores) and 79 regional brain volumes generated by Freesurfer (specific regions are detailed above in the Brain Imaging section). We planned to include any regions for which rp values were greater than 0.20 [a small to medium effect size (Cohen, 1992), which is a permissive inclusion threshold], as additional candidate predictor variables in our multiple regression analyses.
Next, we ran multiple regression analyses in order to examine whether: (i) right or left pACC gray matter volume predicted self-conscious physiological or behavioral reactivity above and beyond multiple demographic and clinical variables and (ii) any other brain regions were also significant predictors of self-conscious emotion. This is a conservative test of our hypothesis that pACC is important for self-conscious emotion because this region must emerge as a significant predictor from a group of candidate predictor regions. In step one, we forced age, sex, CDR total (to control for disease severity), sad film reactivity (i.e. the physiological or negative emotional behavioral composite score, to control for effects of non-self-conscious emotional reactivity), study wave (to account for subtle differences in data processing of the sad film that occurred between subjects who were studied early or late in our data collection), diagnosis (parameterized 0 = bvFTD, 1 = controls; to rule out the possibility that significant findings held true only in one diagnostic group) and total intracranial volume (a total of gray matter, white matter, and cerebrospinal fluid volume, to control for individual differences in head size) into the model. In step two, we used a ‘forward’ entry model to let the statistical program determine which regions accounted for a significant amount of variance above and beyond the control variables; variables that did not account for significant variance were thus excluded from the model. In this second step, we included as predictor candidates the right or left pACC gray matter volume in addition to all of the other ipsalateral brain regions that passed the 0.2 inclusion threshold from our partial correlation analyses. We favored a ‘forward’ entry model instead of a ‘backward’ entry model because of sample size limitations. We also examined our models to insure that there was only weak multicollinearity among variables (VIF < 4).
Finally, to determine whether any potential findings between self-conscious emotional reactivity and right pACC held in the bvFTD and healthy control groups independently, we repeated the multiple regression analyses in each diagnostic group separately.
RESULTS
Physiological reactivity
Right hemisphere
Partial correlation analyses found that physiological reactivity during the karaoke task correlated with right pACC gray matter volume, rp(57) = 0.35, P = 0.01. Gray matter volume in right pars triangularis, rp(57) = 0.22, P = 0.10; right lateral orbitofrontal cortex, rp(57) = 0.23, P = 0.08; right precentral gyrus, rp(57) = 0.20, P = .13; right temporal pole, rp(57) = 0.22, P = 0.10; right lateral occipital cortex, rp(57) = 0.21 P = 0.12; right caudate, rp(57) = 0.20, P = 0.13; right putamen, rp(57) = 0.23, P = 0.08, also emerged as associated regions (i.e. rp values greater than 0.2). Table 3 shows volumes for each group.
Table 3.
Group means and s.d.’s for regional brain volumes
| HC | bvFTD | Overall | |
|---|---|---|---|
| Right hemisphere | |||
| pACCa,b | 1722.9 (329.8) | 1499.7 (354.2) | 1622.5 (356.1) |
| Anterior mid-cingulate cortexb | 2000.5 (374.2) | 1873.0 (355.5) | 1943.1 (368.4) |
| Pars triangularisa | 3880.9 (1018.8) | 2971.0 (828.0) | 3471.5 (1036.1) |
| Pars orbitalis | 2673.4 (493.9) | 2011.0 (597.3) | 2375.3 (632.4) |
| Medial orbitofrontal cortex | 4926.0 (670.6) | 3594.2 (925.4) | 4326.7 (1033.3) |
| Lateral orbitofrontal cortexa,b | 7527.0 (860.6) | 5743.4 (1198.2) | 6724.4 (1354.7) |
| Superior frontal gyrus | 21 221.8 (2963.5) | 16 278.1 (3805.0) | 18 997.1 (4158.7) |
| Precentral gyrusa | 12 560.3 (1601.8) | 11 145.4 (2701.3) | 11 923.6 (2260.8) |
| Superior temporal gyrusb | 11 339.6 (1277.0) | 9530.7 (2090.9) | 10 525.6 (1906.5) |
| Middle temporal gyrusb | 12 110.9 (1648.2) | 8582.7 (2861.4) | 10 523.2 (2866.1) |
| Entorhinal cortex | 1689.3 (439.1) | 1190.0 (461.7) | 1464.6 (511.1) |
| Parahippocampal gyrus | 1863.0 (245.8) | 1610.7 (376.6) | 1749.5 (333.6) |
| Temporal polea,b | 2304.1 (458.2) | 1529.4 (680.7) | 1955.5 (684.9) |
| Fusiform gyrus | 8965.6 (1490.7) | 7272.1 (1336.3) | 8203.5 (1647.5) |
| Inferior parietal cortexb | 14 265.8 (2385.4) | 12 193.4 (2670.9) | 13 333.2 (2703.9) |
| Superior parietal cortexb | 12 798.8 (1896.9) | 11 219.7 (2510.4) | 12 088.2 (2314.4) |
| Lateral occipital cortexa | 12 804.5 (1527.3) | 12 927.6 (2543.8) | 12 859.9 (2029.9) |
| Caudatea,b | 3517.7 (445.4) | 2740.7 (735.8) | 3168.1 (526.1) |
| Putamena,b | 4805.5 (662.7) | 3735.0 (836.5) | 4323.8 (913.8) |
| Pallidumb | 1505.1 (374.3) | 1285.7 (356.2) | 1406.4 (379.5) |
| Amygdalab | 1717.2 (246.1) | 1322.5 (454.8) | 1539.6 (404.0) |
| Hippocampusb | 4026.0 (412.3) | 3057.8 (834.5) | 3590.3 (796.9) |
| Accumbens areab | 517.8 (86.9) | 338.3 (109.6) | 437.1 (132.3) |
| Left hemisphere | |||
| pACC | 2310.0 (449.4) | 1963.3 (571.9) | 2154.0 (532.9) |
| Anterior mid-cingulate cortex | 1797.8 (374.9) | 1626.1 (474.9) | 1720.5 (427.8) |
| Pars triangularis | 3721.9 (705.4) | 3274.1 (771.0) | 3520.4 (763.1) |
| Pars orbitalisa | 2065.7 (423.3) | 1686.7 (379.7) | 1895.1 (443.7) |
| Medial orbitofrontal cortexa,b | 4326.3 (574.8) | 3609.8 (715.7) | 4003.9 (730.8) |
| Lateral orbitofrontal cortexa,b | 7522.6 (973.4) | 5963.4 (1450.1) | 6821.0 (1432.6) |
| Superior frontal gyrusa,b | 22 607.6 (3512.0) | 18 403.2 (3566.2) | 20 715.6 (4091.8) |
| Precentral gyrus | 12 342.2 (1811.7) | 10 889.9 (2500.1) | 11 688.67 (2250.7) |
| Superior temporal gyrusb | 12 658.9 (1799.2) | 10 599.4 (2057.2) | 11 732.1 (2165.2) |
| Middle temporal gyrus | 11 052.7 (1544.6) | 9348.2 (2341.1) | 10 285.7 (2107.3) |
| Entorhinal cortexb | 1833.4 (301.8) | 1344.8 (438.8) | 1613.5 (440.8) |
| Parahippocampal gyrusb | 2088.2 (294.7) | 1868.0 (500.9) | 1989.2 (412.2) |
| Temporal polea,b | 2404.2 (372.9) | 1889.9 (610.5) | 2172.7 (553.4) |
| Fusiform gyrusb | 9179.8 (1381.1) | 8086.3 (1803.7) | 8687.7 (1664.1) |
| Inferior parietal cortex | 11 615.5 (1949.0) | 10 828.2 (2327.4) | 11 261.2 (2145.5) |
| Superior parietal cortex | 12 917.6 (1914.2) | 12 802.3 (2036.4) | 12 865.7 (1954.0) |
| Lateral occipital cortex | 12 939.7 (1816.4) | 13 719.3 (2129.6) | 13 290.5 (1985.2) |
| Caudateb | 3538.3 (428.0) | 3216.0 (586.0) | 3393.3 (526.1) |
| Putamena | 5010.2 (596.5) | 4166.7 (881.6) | 4630.6 (845.3) |
| Palliduma,b | 1573.1 (298.8) | 1411.7 (317.1) | 1500.5 (315.1) |
| Amygdalab | 1690.5 (260.7) | 1339.5 (380.8) | 1532.6 (363.0) |
| Hippocampusb | 3795.2 (374.3) | 3086.7 (714.7) | 3476.4 (653.8) |
| Accumbens area | 482.8 (114.5) | 398.5 (160.4) | 444.9 (142.2) |
Notes: Mean volumes (in cubic millimeters) and s.d.’s (in parentheses) for brain regions that were associated with aphysiological or bbehavioral reactivity during the karaoke task in the partial correlation analyses (rp > 0.20). For abbreviations, see Table 1.
In the multiple regression analysis, right pACC was the only brain region (standardized β = 0.32, P < 0.05, R2 change = 0.08, N = 60) that was a significant predictor of physiological reactivity during the karaoke task above and beyond other predictor variables. Since no other brain regions (i.e. pars triangularis, lateral orbitofrontal cortex, precentral gyrus, temporal pole, lateral occipital cortex, caudate, putamen) explained a significant portion of the variance above and beyond the contribution of right pACC, they were not included in the final model. No other clinical or demographic variables were significant predictors. Overall, the final model (which included age, sex, CDR total, sad film reactivity, study wave, diagnosis, total intracranial volume and right pACC volume) accounted for 6.6% (adjusted R2) of the total variance in physiological reactivity during the karaoke task.
Left hemisphere
Partial correlation analyses revealed that physiological reactivity during the karaoke task was associated with left superior frontal gyrus, rp(57) = 0.25, P = 0.06; left pars orbitalis, rp(57) = 0.22, P = 0.10; left lateral orbitofrontal cortex, rp(57) = 0.24, P = 0.07; left medial orbitofrontal cortex, rp(57) = 0.25, P = 0.05; left temporal pole, rp(57) = 0.23, P = 0.08; left putamen, rp(57) = 0.24, P = 0.07 and left pallidum, rp(57) = 0.20, P = 0.13, and not left pACC, rp(57) = 0.06, P = 0.65. In the multiple regression analysis, there were no significant clinical or demographic predictors. Neither left pACC gray matter volume (P = 0.71) nor any other brain regions were significant predictors of physiological reactivity during the karaoke task. The final model included age, sex, CDR total, sad film reactivity, study wave, diagnosis and total intracranial volume but no brain regions (P = 0.48, R2 change = 0.11, N = 60).
Emotional behavior
Right hemisphere
Partial correlation analyses revealed associations between emotional behavior during the karaoke task and right pACC gray matter volume, rp(58) = 0.50, P = 0.001. Right anterior mid-cingulate cortex, rp(57) = 0.29, P = 0.03; right lateral orbitofrontal cortex, rp(57) = 0.31, P = 0.02; right temporal pole, rp(57) = 0.28, P = 0.04; right superior temporal gyrus, rp(57) = 0.31, P = 0.02; right middle temporal gyrus, rp(57) = 0.40, P = 0.002; right superior parietal cortex, rp(57) = 0.30, P = 0.02; right inferior parietal cortex, rp(57) = 0.30, P = 0.02; right amygdala, rp(57) = 0.20, P = 0.13; right hippocampus, rp(57) = 0.27, P = 0.04; right accumbens area, rp(57) = 0.32, P = 0.01; right caudate, rp(57) = 0.31, P = 0.02; right putamen, rp(57) = 0.23 P = 0.08 and right pallidum, rp(57) = 0.36, P = 0.01, also emerged as associated regions.
The multiple regression analysis revealed that right pACC gray matter volume was the only significant predictor of emotional behavior during the karaoke task above and beyond other variables (standardized β = 0.35, P < 0.01, R2 change = 0.09, N = 60). Since no other brain regions (i.e. anterior mid-cingulate cortex, lateral orbitofrontal cortex, right temporal pole, superior temporal gyrus, middle temporal gyrus, superior parietal cortex, inferior parietal cortex, amygdala, hippocampus, accumbens area, caudate, putamen, pallidum) explained a significant portion of the variance above and beyond the contribution of right pACC, they were not included in the final model. Total intracranial volume (standardized β = −0.33, P < 0.05) was the only other significant predictor of self-conscious emotional behavior. Overall, this final model (which included age, sex, CDR total, sad film reactivity, study wave, diagnosis, total intracranial volume and right pACC volume) accounted for 33.4% (adjusted R2) of the total variance in emotional behavior during the karaoke task.
Left hemisphere
Partial correlation analyses revealed that left superior frontal gyrus, rp(57) = 0.25, P = 0.06; left lateral orbitofrontal cortex, rp(57) = 0.24, P = 0.07; left medial orbitofrontal cortex, rp(57) = 0.25, P = 0.06; left entorhinal cortex, rp(57) = 0.29, P = 0.03; left parahippocampal gyrus, rp(57) = 0.31, P = 0.02; left temporal pole, rp(57) = 0.22, P = 0.10; left fusiform gyrus, rp(57) = 0.22, P = 0.10; left superior temporal gyrus, rp(57) = 0.27, P = 0.04; left amygdala, rp(57) = 0.21, P = 0.12; left hippocampus, rp(57) = 0.25, P = 0.06; left caudate, rp(57) = 0.32, P = 0.01 and left pallidum, rp(57) = 0.26, P = 0.05, were associated with emotional behavior during the karaoke task. Left pACC was not associated with self-conscious emotional behavior, rp(57) = 0.11, P = 0.43. In the multiple regression analysis, there were no significant clinical or demographic predictors. Neither left pACC gray matter volume (P = 0.95) nor any other brain regions were significant predictors of emotional behavior during the karaoke task. The final model included age, sex, CDR total, sad film reactivity, study wave, diagnosis and total intracranial volume but no brain regions (P < 0.01, R2 change = 0.33, N = 60).
Follow-up regression analyses in the bvFTD and healthy control groups separately
Patients with bvFTD
For physiological reactivity during the karaoke task, right pACC gray matter volume continued to be the only brain region that was a significant predictor above and beyond other variables (standardized β = 0.42, P < 0.05, R2 change = 0.13, N = 27). Physiological reactivity during the sad film was also a significant predictor in this model (standardized β = −0.58, P < 0.05). For emotional behavior during the karaoke task, right pACC gray matter volume continued to be the only brain region that was a significant predictor (standardized β = 0.52, P < 0.05, R2 change = 0.16, N = 27). CDR (standardized β = −0.47, P < 0.05) and study wave (standardized β = 0.60, P < 0.05) were also significant predictors in this model.
Healthy controls
For physiological reactivity during the karaoke task, neither right pACC gray matter volume (standardized β = 0.35, P < 0.09, R2 change = 0.09, N = 33) nor any other variables or brain regions were significant predictors in the model. For emotional behavior during the karaoke task, right pACC gray matter volume continued to be the only brain region that was a significant predictor above and beyond other variables (standardized β = 0.41, P < 0.05, R2 change = 0.14, N = 33). No other variables were significant predictors in this model.
DISCUSSION
Self-conscious emotions are complex emotions that arise when the self is compared with social standards (Lewis, 1995; Miller and Leary, 1992). The majority of prior research on the neuroanatomy of self-conscious emotion has been based on either functional neuroimaging studies (Shin et al., 2000; Berthoz et al., 2002; Takahashi et al., 2004; Morita et al., 2008) or studies of patients with acquired brain lesions (Beer et al., 2003). In the present study, we related regional brain volumes to direct measurements of physiological and behavioral responding that occurred during an embarrassing karaoke task. Our sample included both patients with bvFTD, a neurodegenerative disease that targets pACC and anterior insula early in the disease course (Schroeter et al., 2008; Seeley et al., 2008), and healthy control subjects. We included both of these groups to provide us with sufficient variability in emotional reactivity and regional brain volumes to detect meaningful brain–emotion relationships. We found that, across the groups, smaller right pACC gray matter volume predicted lower physiological and behavioral self-conscious emotional reactivity. This relationship held above and beyond multiple other variables (i.e. age, sex, CDR total, diagnosis, study wave, sad film reactivity and total intracranial volume). Since the findings held while accounting for the control task, this suggests that the relationship cannot be explained by an association between right pACC and more generalized emotional reactivity.
Our results suggest that right pACC plays an important role in both the physiological and behavioral aspects of self-conscious emotion. Finding that diagnosis was not a significant predictor in the regression models provides some indication that the relationship between right pACC volume and self-conscious emotional reactivity is not limited to either group. Additional support for this conclusion derives from the follow-up analyses of the bvFTD and control groups separately, which revealed that the findings for emotional behavior were consistent with those found in the total sample: smaller right pACC volume predicted lower levels of self-conscious emotional behavior. For physiological reactivity, smaller right pACC volume predicted lower physiological reactivity in the bvFTD group only. In the healthy controls, the pattern of results was the same; however, it fell to trend levels, likely reflecting the lower anatomical and physiological variability in the normal group and lower power in these follow-up analyses.
Although the present study highlights the particular importance of pACC in self-conscious emotion, it is apparent that this region works in concert with other cortical and subcortical structures that support emotion (Devinsky et al., 1995; Bush et al., 2000; Kober et al., 2008). The pACC, via its strong neuroanatomical connections with both prefrontal regions and central pattern generators (Vogt et al., 1992; Devinsky et al., 1995; Bush et al., 2000; Saper, 2002), is well-configured to play a particularly important role in self-conscious emotion by helping relay social-cognitive information to emotion generation systems. In our partial correlation analyses, we found evidence that cortical (e.g. anterior mid-cingulate cortex, lateral orbitofrontal cortex, superior temporal gyrus, middle temporal gyrus, temporal pole) and subcortical (e.g. amygdala, pallidum, caudate, accumbens, hippocampus, putamen) structures that are important for emotion in general may also be important for self-conscious emotion. Although regression models that included pACC and other relevant brain regions did not reveal significant contributions of these additional brain regions above and beyond that of right pACC, it is still likely that these regions play a role in self-conscious emotional reactivity.
Limitations
There are limitations inherent in the present study. First, results indicate that the pACC plays an important role in a specific self-conscious emotion (i.e. embarrassment). It remains to be determined whether pACC volume influences other self-conscious emotions (e.g. pride, guilt) and other emotions that are thought to activate pACC [e.g. disgust, fear, anger (Phan et al., 2002; Murphy et al., 2003; Kober et al., 2008)]. Second, our regional brain volumes were based on structural MRI. Given that some regional volumes encompass multiple subregions (e.g. the insula volumes includes anterior, middle and posterior insula regions and the pACC region could be further subdivided into pregenual and subgenual subregions), it is possible that these subregions have different levels of involvement with self-conscious emotion. Future work that utilizes imaging techniques that allow for more detailed whole-brain analysis (e.g. voxel-based morphometry) may enable us to elucidate with more specificity the subregions that are involved in different types of emotion.
CONCLUSION
The results of the present study indicate that right pACC plays an important role in physiological and behavioral aspects of self-conscious emotional reactivity. These findings have implications for basic affective neuroscience and speak to the neuroanatomical basis of self-conscious emotion dysfunction in bvFTD. In patients and healthy controls, variation in right pACC gray matter volume is associated with individual differences in behavioral (for both groups) and physiological (significantly for patients) reactivity when exposed to an embarrassing situation. In bvFTD, pACC is an early target for neurodegeneration, and loss in this region may help to explain patients’ attenuated self-conscious emotion.
Conflict of Interest
None declared.
Acknowledgments
Authors thank Victor Laluz and William Irwin for their help with the MRI data processing. Authors also thank Alan Bostrom for his statistical consultation. This work was supported by grants from the National Institute on Aging AG017766, AG019724; National Institute of Mental Health MH020006; the State of California Alzheimer’s Disease Research Center of California 03-75271; and the Hellman Family Center.
REFERENCES
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJR, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Darwin C. The Expression of the Emotions in Man and Animals (with introduction, afterword, and commentaries by P. Ekman) New York: Oxford University Press; 1872/1998. (Original work published 1872) [Google Scholar]
- Desikan RS, Segonne F, Fischl B, et al. An automated labelling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gerlach AL, Wilhelm FH, Roth WT. Embarrassment and social phobia: the role of parasympathetic activation. Journal of Anxiety Disorders. 2003;17:197–210. doi: 10.1016/s0887-6185(02)00197-4. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–86. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Harris CR. Cardiovascular responses of embarrassment and effects of emotional suppression in a social setting. Journal of Personality and Social Psychology. 2001;81:886–97. doi: 10.1037//0022-3514.81.5.886. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Vol. 1. Oxford: Holt; 1890. [Google Scholar]
- Keltner D. Signs of appeasement: evidence for the distinct displays of embarrassment, amusement, and shame. Journal of Personality and Social Psychology. 1995;68:441–54. [Google Scholar]
- Keltner D, Buswell BN. Embarrassment: Its distinct form and appeasement functions. Psychological Bulletin. 1997;122(3):250–270. doi: 10.1037/0033-2909.122.3.250. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. Journal of Neuroscience. 2009;29:2188–92. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cognitive and Behavioral Neurology. 2003;16:211–18. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ascher E, Goodkind M, McCarthy M, Sturm V, Werner K. Laboratory testing of emotion and frontal cortex. In: Goldenberg G, Miller BL, editors. Handbook of Clinical Neurology. Vol. 88 (3rd series): Neuropsychology and behavioral neurology. Edinburgh: Elsevier; 2008. pp. 489–98. [DOI] [PubMed] [Google Scholar]
- Lewis M. Embarrassment: the emotion of self exposure and evaluation. In: Tangney JP, Fischer KW, editors. Self-Conscious Emotions: The Psychology of Shame, Guilt, Embarrassment, and Pride. New York: Guilford Press; 1995. pp. 198–218. [Google Scholar]
- Lewis M. Self-conscious emotions: embarrassment, pride, shame, and guilt. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. New York: The Guilford Press; 2000. pp. 623–36. [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–90. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Miller RS. Is embarrassment a blessing or a curse? In: Tracy JL, Robins RW, Tangney JP, editors. The Self-Conscious Emotions: Theory and Research. New York: Guilford Press; 2007. pp. 245–62. [Google Scholar]
- Miller RS, Leary MR. Emotion and Social behavior. M Cliark. New York: Sage; 1992. Social sources and interactive functions of embarrassment: The case of embarrassment. [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, et al. Impairment of prosocial sentiments is associated with frontopolar and septal damage in frontotemporal dementia. NeuroImage. 2011;54:1735–42. doi: 10.1016/j.neuroimage.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Itakura S, Saito DN, et al. The role of the right prefrontal cortex in self-evaluation of the face: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:342–55. doi: 10.1162/jocn.2008.20024. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–14. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cerebral Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Patterson JC, Ungerleider LG, Bandettini PA. Task-independent functional brain activity correlation with skin conductance changes: an fMRI study. Neuroimage. 2002;17:1797–806. doi: 10.1006/nimg.2002.1306. [DOI] [PubMed] [Google Scholar]
- Phan LP, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25:433–69. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, von Cramon DY. Neural networks in frontotemporal dementia—a meta-analysis. Neurobiology of Aging. 2008;29:418–26. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of Neurology. 2008;65:249–55. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seider BH, Shiota MN, Whalen P, Levenson RW. Greater sadness reactivity in late life. Social, Cognitive and Affective Neuroscience. 2011;6:186–94. doi: 10.1093/scan/nsq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearn D, Bergman E, Hill K, Abel A, Hinds L. Facial coloration and temperature responses in blushing. Psychophysiology. 1990;27:687–93. doi: 10.1111/j.1469-8986.1990.tb03194.x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biological Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, Levenson RW. Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion. 2008;8:861–9. doi: 10.1037/a0013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Levenson RW, Rosen HJ, Allison SC, Miller BL. Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain. 2006;129:2508–16. doi: 10.1093/brain/awl145. [DOI] [PubMed] [Google Scholar]
- Sze JA, Gyurak A, Yuan JW, Levenson RW. Coherence between emotional experience and physiology: does body awareness training have an impact? Emotion. 2010;10:803–14. doi: 10.1037/a0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Matsuura M, Koeda M, et al. Brain activations during judgments of positive self-conscious emotion and positive basic emotion: pride and joy. Cerebral Cortex. 2008;18:898–903. doi: 10.1093/cercor/bhm120. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004;23:967–74. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Tangney JP. The self-conscious emotions: shame, guilt, embarrassment, and pride. In: Dalgleish T, Power MJ, editors. Handbook of Cognition and Emotion. New York: John Wiley & Sons; 1999. pp. 541–68. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in the cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat. I. Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009;47:821–35. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]