Abstract
Objective
To evaluate the frequency of glucocerebrosidase (GBA) mutations in cases and controls enrolled in the Genetic Epidemiology of Parkinson’s Disease (GEPD) study.
Methods
We sequenced all exons of the GBA gene in 278 Parkinson disease (PD) cases and 179 controls enrolled in GEPD, with a wide range of age at onset (AAO), and that included a subset of 178 Jewish cases and 85 Jewish controls. Cases and controls were recruited without knowledge of family history of PD, and cases were oversampled in the AAO < 50 years category.
Results
13.7% of PD cases (38/278) carried GBA mutations, compared with 4.5% of controls (8/179) (odds ratio [OR] 3.4, 95% CI 1.5 to 7.4). The frequency of GBA mutations was 22.2% in 90 cases with AAO ≤ 50 years, compared with 9.7% in 185 cases with AAO > 50 years (OR 2.7, 95% CI 1.3 to 5.3). Adjusting for age at the time of evaluation, sex, family history of PD, and Jewish ancestry, GBA carriers had a 1.7-year-earlier AAO of PD (95% CI 0.5 to 3.3, p < 0.04) than noncarriers. The average AAO of PD was 2.5 years earlier in carriers with an AAO ≤ 50 years compared with noncarriers (95% CI 0.6 to 4.5, p < 0.01) and this was not seen in the AAO > 50 years group. The frequency of GBA mutations was higher in a subset of 178 cases that reported four Jewish grandparents (16.9%) than in cases who did not report Jewish ancestry (8.0%) (p < 0.01). Nine different GBA mutations were identified in PD cases, including 84insGG, E326K, T369M, N370S, D409H, R496H, L444P, RecNciI, and a novel mutation, P175P.
Conclusions
This study suggests that the Glucocerebrosidase gene may be a susceptibility gene for Parkinson disease and that Glucocerebrosidase mutations may modify age at onset.
Gaucher disease (GD; MIM 230800), a lysosomal lipid storage disease, is one of the most common genetic diseases reported in the Ashkenazi Jewish population and is caused by mutations in the β-glucocerebrosidase (GBA) gene (reviewed in reference 1). In adult-onset “nonneuronopathic” GD (Type 1), a range of neurologic manifestations can occur, which can include parkinsonism.2–8 A family history of parkinsonism has been reported in patients with GD.7 An association of the GBA N370S mutation was recently reported in Ashkenazi Jews,9 and four studies report an increased frequency of different GBA mutations (84insGG, N370S, L444P, RecNciI, K198T, T369M, E326K, IVS1 + 1, V394L, and R496H) in Parkinson disease (PD) cases compared with controls,10–13 and in pathologically confirmed dementia with Lewy bodies.14 The GBA mutations, L444P and N370S, were found at a similar frequency in Norwegian PD cases (2.3%, 7/311) and controls (1.7%, 8/474), suggesting that these disease alleles are not risk factors for parkinsonism in this population.15 In the present study, we explored the contribution of the GBA locus to PD by sequencing the GBA gene in PD cases and controls enrolled in a study of the genetic epidemiology of PD16 (GEPD), that included a subset (cases n = 178, controls n = 85) who reported that all four grandparents were Jewish.
METHODS
Subjects
PD cases and controls were a subset of participants in the GEPD study.16 We included all 178 cases and 85 controls who reported that all four grandparents were Jewish. One hundred PD cases who reported that all four grandparents were not Jewish were frequency matched to PD cases of Jewish ancestry by age at onset (AAO) of PD and sex. Ninety-four randomly chosen controls who were previously sequenced for mutations in the parkin gene and did not report Jewish ancestry were also included.17 All cases were recruited from the Center for Parkinson’s Disease and Other Movement Disorders at Columbia University. All met research criteria for PD.18 The majority of controls were recruited by random digit dialing and were frequency matched by age, sex, ethnicity, and area code/exchange. The remaining controls were recruited from a 50% sample of Medicare recipients aged ≥ 65 years who resided in the Washington Heights community.16 All controls underwent the same evaluation as cases, which included a medical history, Unified Parkinson’s Disease Rating Scale (UPDRS), 19 and modified Mini-Mental State Examination (MMSE).20 Family history of PD and related disorders in first-degree relatives was obtained using a structured interview that has been shown to be reliable and valid.16,21 Information on Jewish ancestry in each of the grandparents was obtained during that interview. Information about Ashkenazi origin was not specifically obtained; however, approximately 90% of Jews in the United States are Ashkenazi.22
Molecular genetic analysis
Sequencing
PCR and amplification of the GBA gene was performed. The PCR and sequencing primers used for amplification of GBA have been described previously.23 Cycle sequencing in forward and reverse directions was performed on purified PCR products and run on an ABI 3700 genetic analyser (Applied Biosystems, Foster City, CA). Sequence chromatograms were viewed and genotypes determined using Sequencher (Genecodes).
Statistical analysis
Demographic and clinical characteristics of PD cases compared with controls and mutation carriers compared with noncarriers were analyzed using χ2 tests or Fisher exact tests for categorical data and Student’s t tests for continuous data. Logistic regression was used to calculate the log odds and 95% CIs for being a GBA mutation carrier in the entire sample, and stratified by Jewish and non-Jewish ancestry. Separate analyses were performed for carriers and noncarriers of the NS370S mutation. Multiple logistic regression was used to examine the effect of GBA mutation carrier status on AAO of PD adjusting for age at the time of the evaluation, sex, family history of PD, and Jewish ancestry. Separate analyses were performed for cases recruited with AAO ≤ 50 years and AAO > 50 years because the original sample was enriched for cases with AAO ≤ 50 years, and slightly different inclusion criteria were used.16 Empirical distributions of AAO in PD carriers and noncarriers were plotted to examine the difference in AAO.
RESULTS
Mutations identified in the GBA gene
GEPD
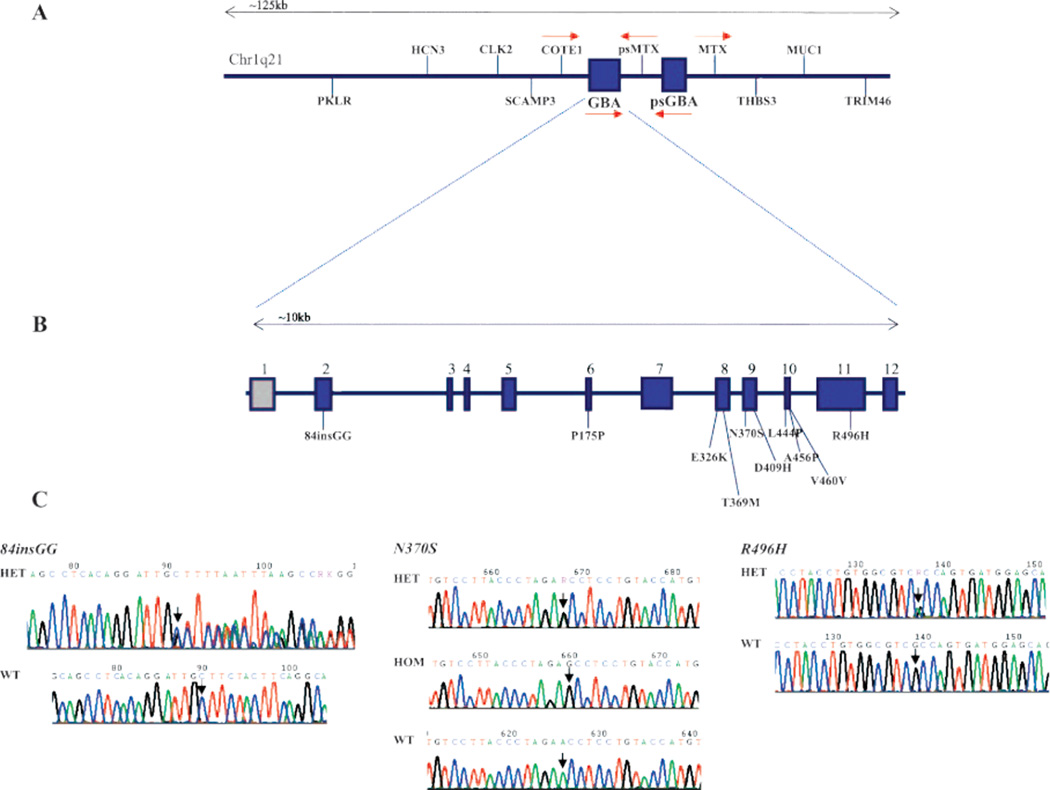
We sequenced all exons of the GBA gene in a total of 278 cases and 179 controls enrolled in GEPD. Overall, nine different mutations were identified in PD cases, including the frameshift mutation 84insGG; six missense mutations, N370S, D409H, R496H, L444P, E326K, and T369M; the recombinant mutation RecNciI (L444P + A456P + V460V); and the synonymous substitution P175P (table 1 and figure 1). Eight of the mutations, 84insGG, N370S, D409H, R496H, L444P, E326K, T369M, and RecNciI, have been reported previously. The synonymous substitution P175P is novel and was absent in 358 control chromosomes. We identified a total of 3 homozygous (Jewish), 34 heterozygous (Jewish and non-Jewish), and 1 compound heterozygous (non-Jewish) PD case that carried GBA mutations (table 1).
Table 1.
GBA mutations identified in cases and controls and allele frequencies
| Mutation/SNP, cDNA Acc#, M16328 |
No. of cases |
Jewish ancestry |
Exon | Amino acid change |
Predicted effect on protein function |
Zygosity | Allele frequency, non-Jewish controls, n = 188 chr |
Allele frequency, Jewish controls, n = 170 chr |
|---|---|---|---|---|---|---|---|---|
| Nt.84insGG | 5 | Jewish | 2 | NA | Frameshift/Null | Het | 0 | 0 |
| Nt.1226 A>G | 23 | 21 Jewish + 2 non-Jewish | 9 | N370S | Missense/Mild | 21 Het, 2 Hom | 0 | 0.02 |
| Nt.1604G>A | 1 | Jewish | 11 | R496H | Missense/Mild | Het | 0 | 0 |
| Nt.1223C>T | 3 | 1 Jewish, 2 non-Jewish | 8 | T369M | Unknown/Modifier allele? | Het | 0.005 | 0.01 |
| Nt.1093 G>A | 1 | Jewish | 8 | E326K | Unknown/Modifier allele? | Het | 0 | 0.005 |
| Nt.1067 C>T | 1 | Jewish | 7 | P175P | Unknown | Hom | 0 | 0 |
| Nt.1448T>C + nt.1483G>C + nt.1497G>C | 1 | Non-Jewish | 10 | RecNciI (L444P + A456P + V460V) | Missense/Severe | Comp. het | 0 | 0 |
| Nt.1343 A>T | 1 | Non-Jewish | 9 | D409H | Missense/Severe | Het | 0 | 0 |
| Nt.1448T>C | 2 | Non-Jewish | 10 | L444P | Missense/Severe | Het | 0 | 0 |
SNP = single nucleotide polymorphism; cDNA = complementary DNA; NA = not applicable.
Figure 1.
Schematic of genes located at the chr1q21 locus that includes GBA (A); a schematic of the GBA gene showing the location of mutations identified in Parkinson disease (PD) cases (B); and sequence chromatograms showing the location of the N370S, 84insGG, and R496H mutations in PD cases (C)
All PD cases included in the current study have been analyzed for parkin mutations and genotyped for the LRRK2 mutations G2019S, L1114L, I1122V, R1441C, and Y1699C. None of the GBA mutation carriers had parkin mutations. However, one Jewish PD case heterozygous for N370S also carried the LRRK2 G2019S mutation.
Jewish PD cases and controls
16.9% (30/178) of Jewish PD cases carried GBA mutations, compared with 7.1% (6/85) of Jewish controls (odds ratio [OR] 2.7, 95% CI 1.1 to 6.7). Six different mutations were identified in Jewish PD cases, including the frameshift mutation 84insGG; four missense mutations, N370S, R496H, E326K, and T369M; and the synonymous substitution P175P (table 1 and figure 1). Five of the mutations, 84insGG, N370S, R496H, E326K, and T369M, have been reported previously and are common in the Jewish population.24 The synonymous substitution P175P is novel and was absent in 358 control chromosomes, which included 178 Jewish control chromosomes. We identified a total of 3 homozygous and 28 heterozygous Jewish PD cases that carried GBA mutations (table 1). The following mutations were also observed in controls: N370S, T369M, and E326K (table 1). The N370S allele frequency observed in Jewish controls is consistent with our previously published study, which included only Jewish PD cases and Jewish controls genotyped for the GBA N370S mutation from GEPD.12 An allele frequency of approximately 1% was observed for T369M in Jewish controls,2 and one Jewish control carried E326K.
Non-Jewish PD cases and controls
Eight percent (8/100) of non-Jewish PD cases carried GBA mutations, compared with 2.1% (2/94) of non-Jewish controls (OR 4.0, 95% CI 0.8 to 19.3). Four different missense mutations were identified in non-Jewish PD cases, including N370S, T369M, D409H, and L444P, and one non-Jewish PD case carried the RecNciI mutation (table 1 and figure 1). Four non-Jewish mutation carriers were heterozygous, and one carrier was compound heterozygous (table 1). One non-Jewish control carried the T369M mutation. None of the non-Jewish controls carried the N370S mutation or any of the other mutations identified in non-Jewish PD cases (table 1).
Frequency and clinical characteristics of mutation carriers
GEPD
The demographic characteristics of sequenced cases and controls are shown in table 2. Compared with GEPD subjects who were not sequenced, cases and controls were older, were more likely to be white, and had more years of education (p < 0.01; data not shown). Sequenced cases also had a later AAO compared with all cases in GEPD (p < 0.01; data not shown). Controls were more likely to be non-white compared with cases (p < 0.01) because of the inclusion of a group of non-Jewish controls.17 There were more Jewish cases than Jewish controls in the GEPD study, as reflected in this analysis (p < 0.01; table 2). Thirty-eight PD cases (13.7%) and 8 controls (4.5%) carried GBA mutations, including 30 Jewish PD cases and 6 Jewish controls. Seventy percent of the Jewish carriers had the N370S mutation, compared with 25% of non-Jewish carriers.
Table 2.
Demographic characteristics of sequenced cases and controls
| Total, n = 457 |
Cases, n = 278 |
Controls, n = 179 |
Significance, cases vs controls |
|
|---|---|---|---|---|
| % Male (n) | 60.2 (275) | 62.6 (174) | 56.4 (101) | 0.20 |
| Age (SD), y | 65.3 (11.3) | 65.6 (11.3) | 64.9 (11.3) | 0.57 |
| Race | <0.01 | |||
| % White (n) | 98.0 (448) | 100.0 (278) | 95.0 (170) | |
| % African American (n) | 0.7 (3) | 0 | 1.7 (3) | |
| % Hispanic (n) | 0.9 (4) | 0 | 2.2 (4) | |
| % Other (n) | 0.4 (2) | 0 | 1.1 (2) | |
| Years of education (SD) | 15.9 (3.0) | 16.0 (3.0) | 15.8 (2.9) | 0.64 |
| % Jewish (n) | 57.6 (263) | 64.0 (178) | 47.5 (85) | <0.01 |
| % with mutation (n) | 10.1 (46) | 13.7 (38) | 4.5 (8) | <0.01 |
| % with family history of PD (n) | 13.8 (63/456) | 18.8 (52) | 6.2 (11) | <0.01 |
PD = Parkinson disease.
The odds of being a carrier of a GBA mutation based on specific demographic and clinical characteristics are shown in table 3. Overall, PD cases were 3.4 times as likely as controls (95% CI 1.5 to 7.4) to carry a GBA mutation. Individuals of Jewish ancestry were significantly more likely to carry a mutation than non-Jews (in both cases and controls), and this was also true for Jewish PD cases compared with non Jewish PD cases (OR 2.3, 95% CI 1.02 to 5.30, p = 0.045). Of note, family history of PD in a first-degree relative did not influence the log odds of carrying a mutation in the entire group or when stratified by Jewish ancestry. We also analyzed carriers and noncarriers of N370S mutations separately. PD cases were 5.6 times (95% CI 1.3 to 24.3) as likely as controls to carry N370S mutations, and early-onset cases were 3.4 times as likely (95% CI 1.2 to 7.5) as late-onset cases to carry this specific mutation. Family history of PD in a first-degree relative had no influence on the presence of the N370S mutation.
Table 3.
Proportions of cases and controls with the mutation and odds ratios
| Total | No. with mutation |
% | Odds ratio (95% CI) | |
|---|---|---|---|---|
| All subjects | ||||
| Controls | 179 | 8 | 4.5 | |
| All PD cases | 278 | 38 | 13.7 | 3.4 (1.5–7.4) |
| Late-onset PD cases | 185 | 18 | 9.7 | |
| Early-onset PD cases* | 90 | 20 | 22.2 | 2.7 (1.3–5.3) |
| Cases without a family history of PD | 226 | 32 | 14.2 | |
| Cases with a family history of PD | 52 | 6 | 11.5 | 0.8 (0.3–2.0) |
| Non-Jewish | 194 | 10 | 5.2 | |
| Jewish | 263 | 36 | 13.7 | 2.9 (1.4–6.0) |
| Non-Jewish cases | 100 | 8 | 8.0 | |
| Jewish cases | 178 | 30 | 16.9 | 2.3 (1.0–5.3) |
| Jewish subjects | ||||
| Controls | 85 | 6 | 7.1 | |
| All PD cases | 178 | 30 | 16.9 | 2.7 (1.1–6.7) |
| Late-onset PD cases | 124 | 16 | 12.9 | |
| Early-onset PD cases* | 53 | 14 | 26.4 | 2.4 (1.1–5.4) |
| Cases without a family history of PD | 154 | 25 | 16.2 | |
| Cases with a family history of PD | 24 | 5 | 20.8 | 1.4 (0.5–4.0) |
| Non-Jewish subjects | ||||
| Controls | 94 | 2 | 2.1 | |
| All PD cases | 100 | 8 | 8.0 | 4.0 (0.8–19.3) |
| Late-onset PD cases | 61 | 2 | 3.3 | |
| Early-onset PD cases* | 37 | 6 | 16.2 | 5.7 (1.1–30.0) |
| Cases without a family history of PD | 72 | 7 | 9.7 | |
| Cases with a family history of PD | 28 | 1 | 3.6 | 0.3 (0.04–2.9) |
Early-onset Parkinson disease (PD) defined as age at onset ≤ 50 years.
We also compared the clinical characteristics of PD cases who carried GBA mutations with PD cases who did not carry mutations. Mutation carriers report an earlier AAO and current age at evaluation compared with noncarriers (p < 0.01). Carriers did not differ from noncarriers in the presence of the cardinal features of PD, UPDRS Part III score, modified MMSE score, or Hoehn and Yahr score. However, carriers were more likely to report tremor as a first symptom compared with noncarriers (p < 0.01). They also were more likely to report the presence of dyskinesias; however, the dose of levodopa was significantly higher among carriers (687.0 mg, SD 396.5 mg) than among noncarriers (493.4 mg, SD 343.5 mg, n = 155, p = 0.02). The demographic and clinical characteristics of Jewish mutation carriers did not differ from those of non-Jewish mutation carriers (table E-1 on the Neurology® Web site at www.neurology.org).
Jewish mutation carriers
Jewish PD cases with AAO ≤ 50 years were significantly more likely than those with AAO > 50 years to carry GBA mutations (OR 2.4, 95% CI 1.1 to 5.4). Family history of PD in a first-degree relative did not influence the log odds of carrying a GBA mutation in Jewish cases (OR 1.4, 95% CI 0.5 to 4.0).
Non-Jewish mutation carriers
GBA mutations were not associated with PD among non-Jewish PD cases compared with controls (OR 4.0, 95% CI 0.8 to 19.3). When non-Jewish PD cases were stratified by AAO, a total of 16.2% of PD cases had early-onset PD (AAO ≤ 50 years), compared with 3.3% of PD cases with late-onset PD (AAO > 50 years). As observed in PD cases with Jewish ancestry, non-Jewish PD cases with AAO ≤ 50 years were significantly more likely than those with AAO > 50 years to carry GBA mutations (OR 5.7, 95% CI 1.1 to 30.0). Family history of PD in a first-degree relative did not influence the log odds of carrying a GBA mutation in non-Jewish cases (OR 0.3, 95% CI 0.04 to 2.9).
GBA mutations modify age at onset of PD
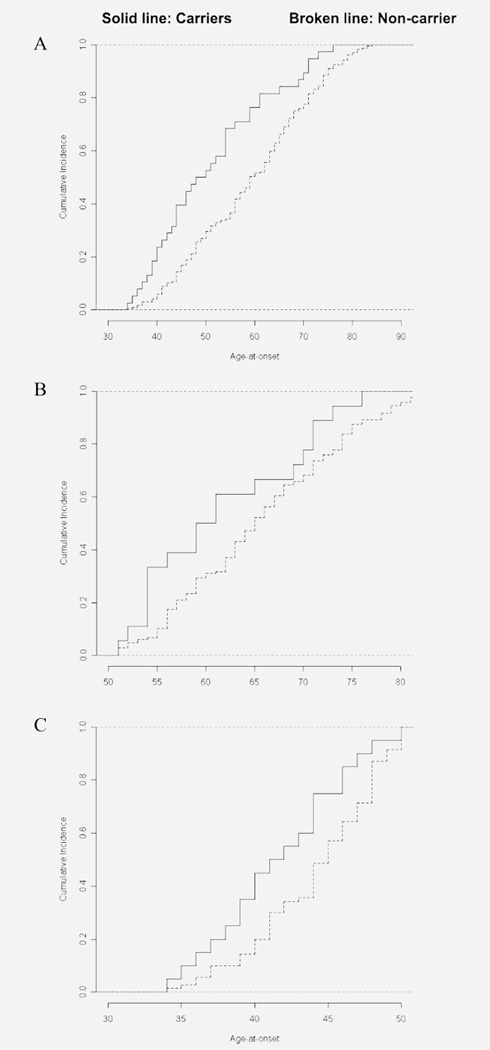
We constructed a model to predict AAO of PD, adjusting for the independent effects of age at evaluation, sex, whether the individual had an AAO ≤ 50 or > 50 years (since we oversampled for AAO ≤ 50 years), and whether the individual carried a GBA mutation. Carriers of GBA mutations had an AAO 1.7 years earlier than noncarriers (95% CI 0.04 to 3.3, p < 0.04). Among cases with AAO ≤ 50 years, the average AAO of PD was 2.5 years younger in GBA carriers than in noncarriers (95% CI 0.6 to 4.5 years, p < 0.01). However, AAO did not differ between carriers and noncarriers among cases with AAO > 50 years (95% CI −0.1 to 8.0 years, p = 0.06). When restricted to carriers and noncarriers of N370S, using the same model, carriers had a 2.0-year-earlier onset (95% CI 0.03 to 4.09 years, p < 0.04), and this difference was also confined to those who had an AAO < 50 years (3.0 years, 95% CI 0.45 to 5.70 years, p < 0.02). The cumulative incidence of PD in carriers and noncarriers is displayed graphically in the entire sample and in early-onset (≤50 years) and late-onset (>50 years) cases in figure 2. Significant differences in AAO are seen for the entire sample (figure 2A) and in the early-onset group (figure 2C).
Figure 2.
Cumulative incidence rates of Parkinson disease in carriers and noncarriers
(A) Entire sample, (B) late onset, (C) early onset.
DISCUSSION
This is the first study to sequence the GBA gene in both PD cases and matched controls. We have demonstrated that mutations in the GBA gene are associated with PD, are more frequent in Jewish PD cases than non-Jewish PD cases, and are more frequent in cases with AAO ≤ 50 years compared with AAO > 50 years in both Jewish and non-Jewish cases. Two other studies have also observed GBA mutations in early-onset PD cases.10,13 We also showed that among cases with AAO ≤ 50 years, the presence of a GBA mutation decreased AAO by almost 2 years compared with noncarriers. We did not demonstrate familial aggregation in Jewish or non-Jewish cases, suggesting that the mutations, the majority of which were heterozygous, have reduced penetrance in first-degree relatives. Reduced penetrance has also been observed for other PD susceptibility genes, particularly when heterozygous mutations are observed in PD cases. For example, in a case– control study of the parkin gene, family history of PD in a first-degree relative did not differ significantly between mutation carriers and noncarriers (p = 0.35).17 To date, there have been numerous reports of heterozygous mutations in PD cases in familial PD genes, including Parkin,17,25–36 DJ-1,31,37 and PINK1.38–40 It is still unclear whether a single heterozygous mutation in these genes is the causative factor or merely a risk allele. However, some studies indicate that heterozygous parkin mutation carriers may have an increased susceptibility to PD.41–45 The low penetrance observed for several PD susceptibility genes suggests that disease expression may be modified by additional genetic and environmental factors which may account for an “observed” decreased family history in mutation carriers.
In this study, the frequency of GBA mutations in cases with PD was 13.7% and was higher in PD cases with Jewish ancestry (16.9%) than in non-Jews (8%). Only one previously published study has sequenced the GBA gene in PD cases, and a carrier frequency of 21% (12/57) was observed.10 All other studies have assessed the frequency of “common” GBA mutations, and mutation frequencies ranging from 2.3% (7/311) in a Norwegian population15 to 5.7% (5/88)11 in a North American population and 31.3% (31/99)9 in an Ashkenazi Jewish population from Israel have been reported. Our results confirm that GBA mutations are risk factors in both sporadic and familial PD and modify AAO.
The frameshift mutation 84insGG; the missense mutations N370S, L444P, R496H, and D409H; and the recombinant mutation RecNciI (L444P + A456P + V460V) are predicted to result in decreased catalytic activity or result in conformational changes in the β-glucocerebrosidase protein and have been previously reported in PD cases. We identified one novel mutation, P175P, in the homozygous state. Four PD cases carried either T369M3 or E326K.1 We consider these variants to be polymorphisms because we also identified them in controls at a similar frequency. This is consistent with a previous publication that analyzed the GBA gene in PD cases.10 In GD, both T369M and E326K have been described as “mild” mutations or modifier alleles and are always observed with pathogenic mutations on the same allele. In our study, we did not observe a “second” mutation that co-occurred with either T369M or E326K, suggesting that they are unlikely to be pathogenic. However, the E326K variant has been observed together with a novel mutation T267I in a PD case who presented initially with parkinsonism and had a neuropathologic diagnosis of dementia with Lewy bodies (DLB).14
Although further studies will be required to determine the pathogenic role of different GBA variants identified in PD cases, two studies suggest that the N370S and L444P variants are pathogenic in the heterozygous state.10,46 In PD subjects heterozygous for the N370S variant, GBA enzyme activity in brain tissue is reduced (50% to 84%) compared with controls.10 PET studies in two GBA mutation carriers, a father (heterozygous for L444P) and his son (compound heterozygous for L444P and F213I), both of whom exhibited a parkinsonism phenotype, demonstrated that there is presynaptic dopaminergic neuronal dysfunction of the type normally seen in PD patients.46 A striatal dopaminergic deficit has also been observed in PD patients heterozygous for a parkin mutation.43
The identification of mutations in the GBA gene in PD and DLB cases and the associated neuropathologic findings suggest that the GBA gene may by a susceptibility gene for synucleopathies.
Supplementary Material
Acknowledgments
Supported by NIH NS50487 (L.N.C.) and NS36630 and RR00645 (K.M.) and the Parkinson’s Disease foundation (L.N.C. and K.M.).
GLOSSARY
- AAO
age at onset
- cDNA
complementary DNA
- GBA
glucocerebrosidase
- GD
Gaucher disease
- GEPD
Genetic Epidemiology of Parkinson’s Disease
- MMSE
Mini-Mental State Examination
- NA
not applicable
- DLB
dementia with Lewy bodies
- OR
odds ratio
- PD
Parkinson disease
- SNP
single nucleotide polymorphism
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
Supplemental data at www.neurology.org
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Neudorfer O, Giladi N, Elstein D, et al. Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM. 1996;89:691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 3.Tayebi N, Callahan M, Madike V, et al. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab. 2001;73:313–321. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- 4.Guimaraes J, Amaral O, Sa Miranda MC. Adult-onset neuronopathic form of Gaucher’s disease: a case report. Parkinsonism Relat Disord. 2003;9:261–264. doi: 10.1016/s1353-8020(02)00096-2. [DOI] [PubMed] [Google Scholar]
- 5.Cormand B, Grinberg D, Gort L, et al. Molecular analysis and clinical findings in the Spanish Gaucher disease population: putative haplotype of the N370S ancestral chromosome. Hum Mutat. 1998;11:295–305. doi: 10.1002/(SICI)1098-1004(1998)11:4<295::AID-HUMU7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Machaczka M, Rucinska M, Skotnicki AB, Jurczak W. Parkinson’s syndrome preceding clinical manifestation of Gaucher’s disease. Am J Hematol. 1999;61:216–217. doi: 10.1002/(sici)1096-8652(199907)61:3<216::aid-ajh12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Tayebi N, Walker J, Stubblefield B, et al. Gaucher disease with parkinsonian manifestations: does glucocere-brosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab. 2003;79:104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 8.Varkonyi J, Rosenbaum H, Baumann N, et al. Gaucher disease associated with parkinsonism: four further case reports. Am J Med Genet. 2003;116A:348–351. doi: 10.1002/ajmg.a.10028. [DOI] [PubMed] [Google Scholar]
- 9.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocere-brosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–1977. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- 10.Lwin A, Orvisky E, Goker-Alpan O, et al. Glucocere-brosidase mutations in subjects with parkinsonism. Mol Genet Metab. 2004;81:70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Sato C, Morgan AJ, Lang AE, et al. Analysis of the glucocerebrosidase gene in Parkinson’s disease. Mov Disord. 2005;20:367–370. doi: 10.1002/mds.20319. [DOI] [PubMed] [Google Scholar]
- 12.Clark LN, Nicolai A, Afridi S, et al. Pilot association study of the beta-glucocerebrosidase N370S allele and Parkinson’s disease in subjects of Jewish ethnicity. Mov Disord. 2005;20:100–103. doi: 10.1002/mds.20320. [DOI] [PubMed] [Google Scholar]
- 13.Eblan MJ, Nguyen J, Ziegler SG, et al. Glucocerebrosidase mutations are also found in subjects with early-onset parkinsonism from Venezuela. Mov Disord. 2006;21:282–283. doi: 10.1002/mds.20766. [DOI] [PubMed] [Google Scholar]
- 14.Goker-Alpan O, Giasson BI, Eblan MJ, et al. Glucocere-brosidase mutations are an important risk factor for Lewy body disorders. Neurology. 2006;67:908–910. doi: 10.1212/01.wnl.0000230215.41296.18. [DOI] [PubMed] [Google Scholar]
- 15.Toft M, Pielsticker L, Ross OA, et al. Glucocere-brosidase gene mutations and Parkinson disease in the Norwegian population. Neurology. 2006;66:415–417. doi: 10.1212/01.wnl.0000196492.80676.7c. [DOI] [PubMed] [Google Scholar]
- 16.Marder K, Levy G, Louis ED, et al. Familial aggregation of early- and late-onset Parkinson’s disease. Ann Neurol. 2003;54:507–513. doi: 10.1002/ana.10711. [DOI] [PubMed] [Google Scholar]
- 17.Clark LN, Afridi S, Karlins E, et al. Case-control study of the parkin gene in early-onset Parkinson disease. Arch Neurol. 2006;63:548–552. doi: 10.1001/archneur.63.4.548. [DOI] [PubMed] [Google Scholar]
- 18.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psych. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahn S, Marsden CD, Calne D. Recent developments in Parkinson’s disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. [Google Scholar]
- 20.Stern Y, Sano M, Paulson JRM. Modified mini-mental state examination: validity and reliability. Neurology. 1987;37(suppl 1):179. [Google Scholar]
- 21.Marder K, Levy G, Louis ED, et al. Accuracy of family history data on Parkinson’s disease. Neurology. 2003;61:18–23. doi: 10.1212/01.wnl.0000074784.35961.c0. [DOI] [PubMed] [Google Scholar]
- 22.Ostrer H. A genetic profile of contemporary Jewish populations. Nat Rev Genet. 2001;2:891–898. doi: 10.1038/35098506. [DOI] [PubMed] [Google Scholar]
- 23.Stone DL, Tayebi N, Orvisky E, et al. Glucocere-brosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15:181–188. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E, Nguyen NJ, Henneberger MW, et al. Gaucher disease: gene frequencies in the Ashkenazi Jewish population. Am J Hum Genet. 1993;52:85–88. [PMC free article] [PubMed] [Google Scholar]
- 25.Abbas N, Lucking CB, Ricard S, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson’s Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson’s Disease. Hum Mol Genet. 1999;8:567–574. doi: 10.1093/hmg/8.4.567. [DOI] [PubMed] [Google Scholar]
- 26.Hedrich K, Marder K, Harris J, et al. Evaluation of 50 probands with early-onset Parkinson’s disease for Parkin mutations. Neurology. 2002;58:1239–1246. doi: 10.1212/wnl.58.8.1239. [DOI] [PubMed] [Google Scholar]
- 27.West A, Periquet M, Lincoln S, et al. Complex relationship between Parkin mutations and Parkinson disease. Am J Med Genet. 2002;114:584–591. doi: 10.1002/ajmg.10525. [DOI] [PubMed] [Google Scholar]
- 28.Foroud T, Uniacke SK, Liu L, et al. Heterozygosity for a mutation in the parkin gene leads to later onset Parkinson disease. Neurology. 2003;60:796–801. doi: 10.1212/01.wnl.0000049470.00180.07. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira SA, Scott WK, Martin ER, et al. Parkin mutations and susceptibility alleles in late-onset Parkinson’s disease. Ann Neurol. 2003;53:624–629. doi: 10.1002/ana.10524. [DOI] [PubMed] [Google Scholar]
- 30.Lincoln SJ, Maraganore DM, Lesnick TG, et al. Parkin variants in North American Parkinson’s disease: cases and controls. Mov Disord. 2003;18:1306–1311. doi: 10.1002/mds.10601. [DOI] [PubMed] [Google Scholar]
- 31.Hedrich K, Djarmati A, Schafer N, et al. DJ-1 (PARK7) mutations are less frequent than Parkin (PARK2) mutations in early-onset Parkinson disease. Neurology. 2004;62:389–394. doi: 10.1212/01.wnl.0000113022.51739.88. [DOI] [PubMed] [Google Scholar]
- 32.Poorkaj P, Nutt JG, James D, et al. Parkin mutation analysis in clinic patients with early-onset Parkinson’s disease. Am J Med Genet. 2004;129A:44–50. doi: 10.1002/ajmg.a.30157. [DOI] [PubMed] [Google Scholar]
- 33.Bertoli-Avella AM, Giroud-Benitez JL, Akyol A, et al. Novel parkin mutations detected in patients with early-onset Parkinson’s disease. Mov Disord. 2005;20:424–431. doi: 10.1002/mds.20343. [DOI] [PubMed] [Google Scholar]
- 34.Khan NL, Horta W, Eunson L, et al. Parkin disease in a Brazilian kindred: manifesting heterozygotes and clinical follow-up over 10 years. Mov Disord. 2005;20:479–484. doi: 10.1002/mds.20335. [DOI] [PubMed] [Google Scholar]
- 35.Wu RM, Bounds R, Lincoln S, et al. Parkin mutations and early-onset parkinsonism in a Taiwanese cohort. Arch Neurol. 2005;62:82–87. doi: 10.1001/archneur.62.1.82. [DOI] [PubMed] [Google Scholar]
- 36.Sun M, Latourelle JC, Wooten GF, et al. Influence of heterozygosity for parkin mutation on onset age in familial Parkinson disease: the GenePD study. Arch Neurol. 2006;63:826–832. doi: 10.1001/archneur.63.6.826. [DOI] [PubMed] [Google Scholar]
- 37.Clark LN, Afridi S, Mejia-Santana H, et al. Analysis of an early-onset Parkinson’s disease cohort for DJ-1 mutations. Mov Disord. 2004;19:796–800. doi: 10.1002/mds.20131. [DOI] [PubMed] [Google Scholar]
- 38.Abou-Sleiman PM, Muqit MM, McDonald NQ, et al. A heterozygous effect for PINK1 mutations in Parkinson’s disease? Ann Neurol. 2006;60:414–419. doi: 10.1002/ana.20960. [DOI] [PubMed] [Google Scholar]
- 39.Djarmati A, Hedrich K, Svetel M, et al. Heterozygous PINK1 mutations: a susceptibility factor for Parkinson disease? Mov Disord. 2006;21:1526–1530. doi: 10.1002/mds.20977. [DOI] [PubMed] [Google Scholar]
- 40.Toft M, Myhre R, Pielsticker L, et al. PINK1 mutation heterozygosity and the risk of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:82–84. doi: 10.1136/jnnp.2006.097840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedrich K, Eskelson C, Wilmot B, et al. Distribution, type, and origin of Parkin mutations: review and case studies. Mov Disord. 2004;19:1146–1157. doi: 10.1002/mds.20234. [DOI] [PubMed] [Google Scholar]
- 42.Hilker R, Klein C, Ghaemi M, et al. Positron emission tomographic analysis of the nigrostriatal dopaminergic system in familial parkinsonism associated with mutations in the parkin gene. Ann Neurol. 2001;49:367–376. [PubMed] [Google Scholar]
- 43.Hilker R, Klein C, Hedrich K, et al. The striatal dopaminergic deficit is dependent on the number of mutant alleles in a family with mutations in the parkin gene: evidence for enzymatic parkin function in humans. Neurosci Lett. 2002;323:50–54. doi: 10.1016/s0304-3940(01)02529-0. [DOI] [PubMed] [Google Scholar]
- 44.Lohmann E, Periquet M, Bonifati V, et al. How much phenotypic variation can be attributed to parkin genotype? Ann Neurol. 2003;54:176–185. doi: 10.1002/ana.10613. [DOI] [PubMed] [Google Scholar]
- 45.Munhoz RP, Sa DS, Rogaeva E, et al. Clinical findings in a large family with a parkin ex3delta40 mutation. Arch Neurol. 2004;61:701–704. doi: 10.1001/archneur.61.5.701. [DOI] [PubMed] [Google Scholar]
- 46.Kono S, Shirakawa K, Ouchi Y, et al. Dopaminergic neuronal dysfunction associated with parkinsonism in both a Gaucher disease patient and a carrier. J Neurol Sci. 2007;252:181–184. doi: 10.1016/j.jns.2006.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.