Abstract
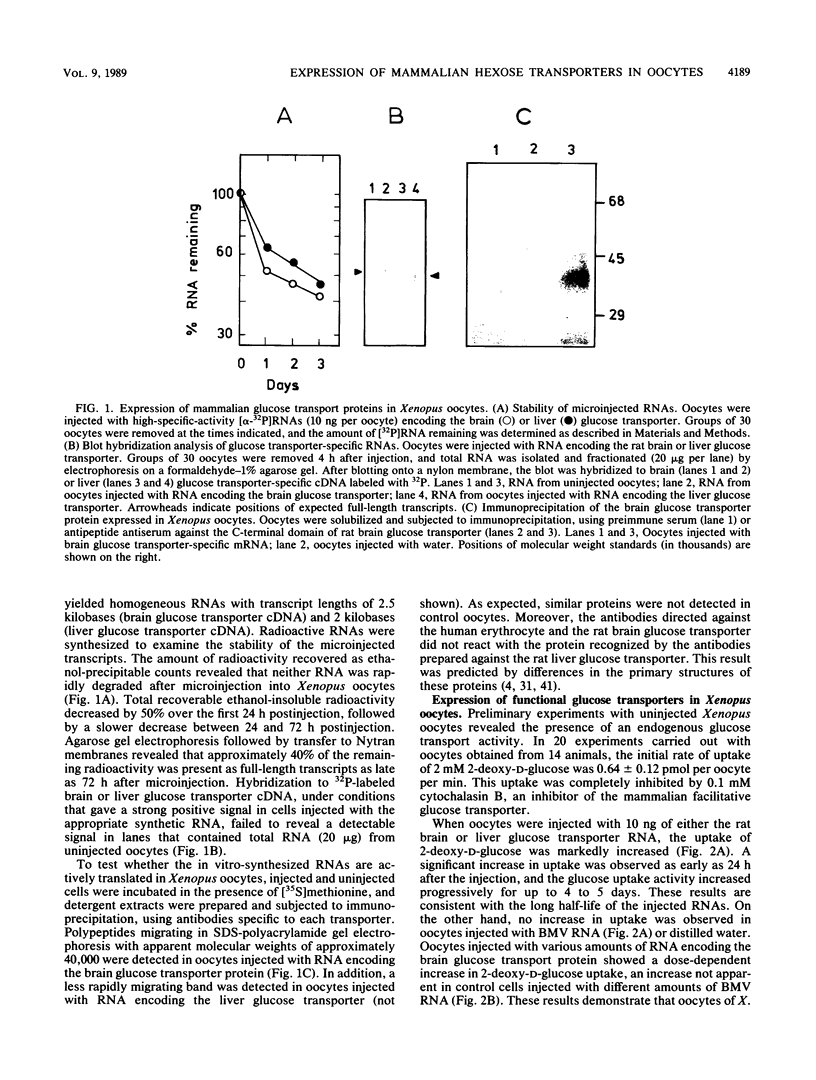
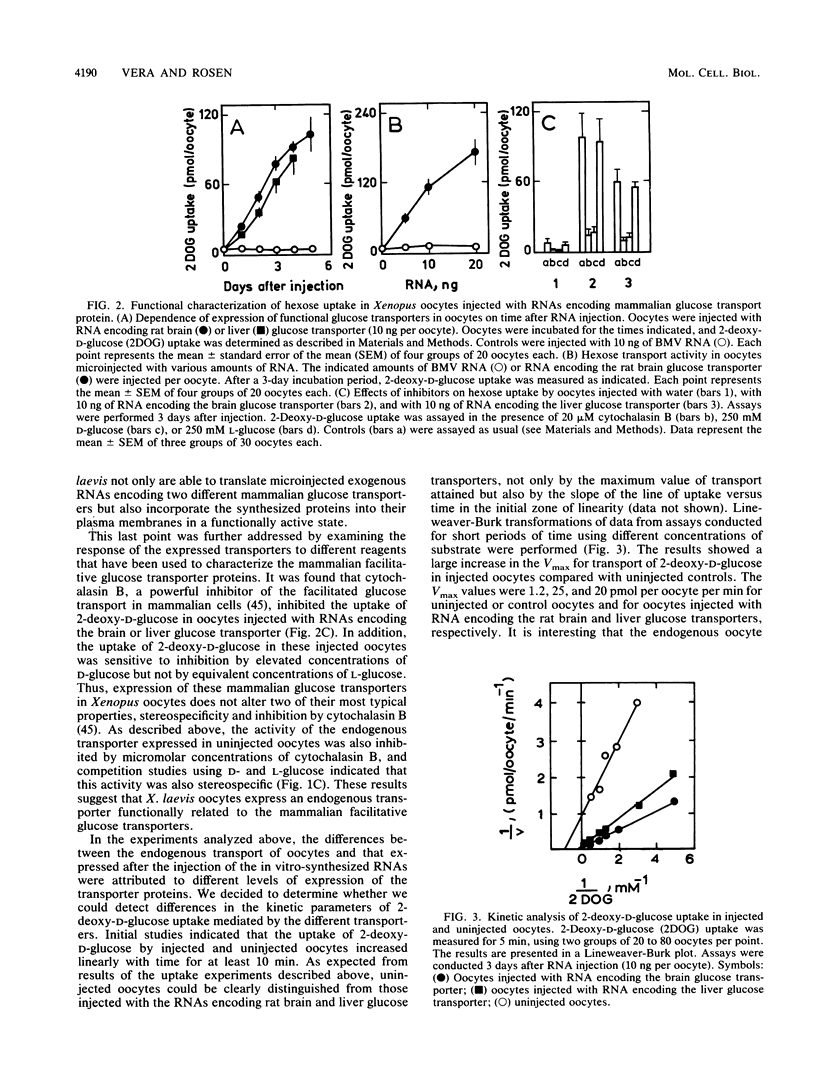
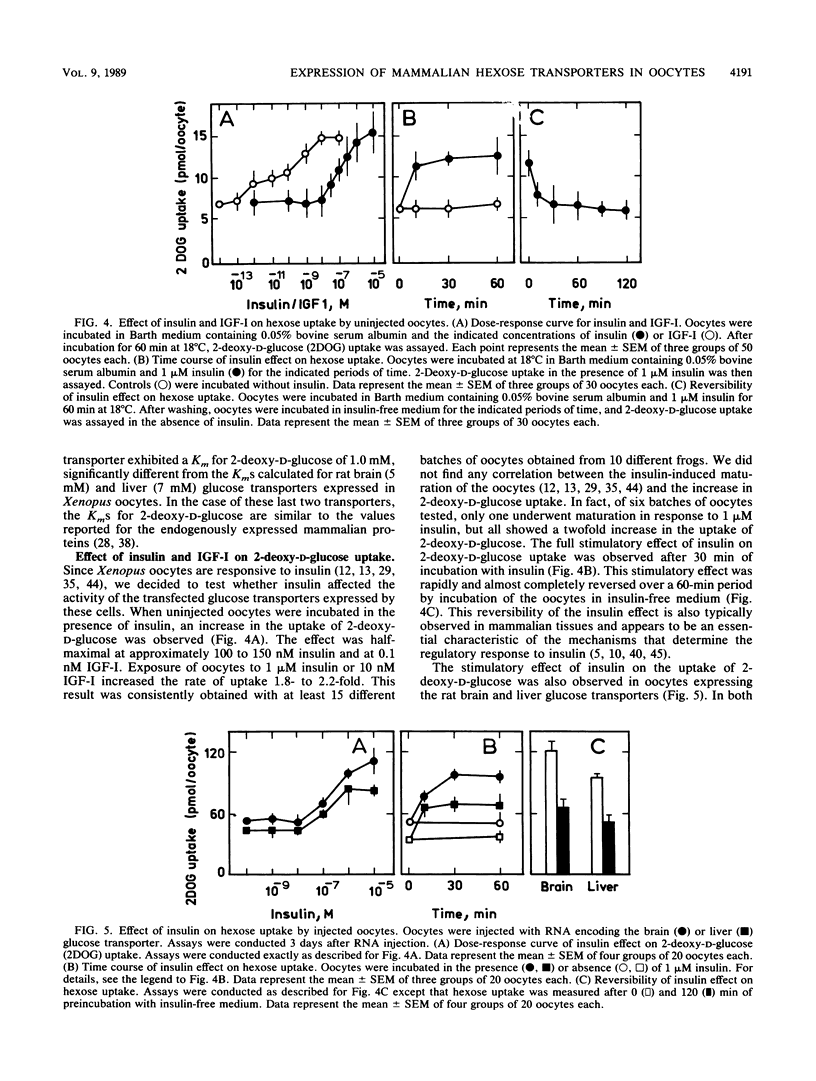
We report the functional expression of two different mammalian facilitative glucose transporters in Xenopus oocytes. The RNAs encoding the rat brain and liver glucose transporters were transcribed in vitro and microinjected into Xenopus oocytes. Microinjected cells showed a marked increase in 2-deoxy-D-glucose uptake as compared with controls injected with water. 2-Deoxy-D-glucose uptake increased during the 5 days after microinjection of the RNAs, and the microinjected RNAs were stable for at least 3 days. The expression of functional glucose transporters was dependent on the amount of RNA injected. The oocyte-expressed transporters could be immunoprecipitated with anti-brain and anti-liver glucose transporter-specific antibodies. Uninjected oocytes expressed an endogenous transporter that appeared to be stereospecific and inhibitable by cytochalasin B. This transporter was kinetically and immunologically distinguishable from both rat brain and liver glucose transporters. The uniqueness of this transporter was confirmed by Northern (RNA) blot analysis. The endogenous oocyte transporter was responsive to insulin and to insulinlike growth factor I. Most interestingly, both the rat brain and liver glucose transporters, which were not insulin sensitive in the tissues from which they were cloned, responded to insulin in the oocyte similarly to the endogenous oocyte transporter. These data suggest that the insulin responsiveness of a given glucose transporter depends on the type of cell in which the protein is expressed. The expression of hexose transporters in the microinjected oocytes may help to identify tissue-specific molecules involved in hormonal alterations in hexose transport activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Shibasaki Y., Ohno S., Taira H., Lin J. L., Kasuga M., Kanazawa Y., Akanuma Y., Takaku F., Oka Y. Rabbit brain glucose transporter responds to insulin when expressed in insulin-sensitive Chinese hamster ovary cells. J Biol Chem. 1989 Feb 25;264(6):3416–3420. [PubMed] [Google Scholar]
- Axelrod J. D., Pilch P. F. Unique cytochalasin B binding characteristics of the hepatic glucose carrier. Biochemistry. 1983 Apr 26;22(9):2222–2227. doi: 10.1021/bi00278a025. [DOI] [PubMed] [Google Scholar]
- Baly D. L., Horuk R. Dissociation of insulin-stimulated glucose transport from the translocation of glucose carriers in rat adipose cells. J Biol Chem. 1987 Jan 5;262(1):21–24. [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok J., Gibbs E. M., Lienhard G. E., Slot J. W., Geuze H. J. Insulin-induced translocation of glucose transporters from post-Golgi compartments to the plasma membrane of 3T3-L1 adipocytes. J Cell Biol. 1988 Jan;106(1):69–76. doi: 10.1083/jcb.106.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Czech M. P. Reconstitution of D-glucose transport activity from cytoplasmic membranes. Evidence against recruitment of cytoplasmic membrane transporters into the plasma membrane as the sole action of insulin. J Biol Chem. 1980 Nov 10;255(21):10382–10386. [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Czech M. P. Characterization of (3H)cytochalasin B binding to the fat cell plasma membrane. J Biol Chem. 1976 May 25;251(10):2905–2910. [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- El-Etr M., Schorderet-Slatkine S., Baulieu E. E. Meiotic maturation in Xenopus laevis oocytes initiated by insulin. Science. 1979 Sep 28;205(4413):1397–1399. doi: 10.1126/science.472755. [DOI] [PubMed] [Google Scholar]
- El-Etr M., Schorderet-Slatkine S., Baulieu E. E. The Role of Zinc and Follicle Cells in Insulin-Initiated Meiotic Maturation of Xenopus laevis Oocytes. Science. 1980 Nov 21;210(4472):929–930. doi: 10.1126/science.210.4472.929. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorga F. R., Lienhard G. E. Equilibria and kinetics of ligand binding to the human erythrocyte glucose transporter. Evidence for an alternating conformation model for transport. Biochemistry. 1981 Sep 1;20(18):5108–5113. doi: 10.1021/bi00521a003. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Derechin V., James D. E., Tordjman K., Ahern S., Gibbs E. M., Lienhard G. E., Mueckler M. Insulin-stimulated translocation of the HepG2/erythrocyte-type glucose transporter expressed in 3T3-L1 adipocytes. J Biol Chem. 1989 Feb 5;264(4):2180–2184. [PubMed] [Google Scholar]
- Haspel H. C., Birnbaum M. J., Wilk E. W., Rosen O. M. Biosynthetic precursors and in vitro translation products of the glucose transporter of human hepatocarcinoma cells, human fibroblasts, and murine preadipocytes. J Biol Chem. 1985 Jun 25;260(12):7219–7225. [PubMed] [Google Scholar]
- Haspel H. C., Rosenfeld M. G., Rosen O. M. Characterization of antisera to a synthetic carboxyl-terminal peptide of the glucose transporter protein. J Biol Chem. 1988 Jan 5;263(1):398–403. [PubMed] [Google Scholar]
- Helgerson A. L., Carruthers A. Equilibrium ligand binding to the human erythrocyte sugar transporter. Evidence for two sugar-binding sites per carrier. J Biol Chem. 1987 Apr 25;262(12):5464–5475. [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Janicot M., Lane M. D. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2642–2646. doi: 10.1073/pnas.86.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C. Y., Rampal A. L. Cytochalasin B binding sites and glucose transport carrier in human erythrocyte ghosts. J Biol Chem. 1977 Aug 10;252(15):5456–5463. [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madar Z., Felig P. 3-O-methyl-D-glucose uptake in isolated rat hepatocytes. Effects of dexamethasone. Mol Pharmacol. 1983 Jan;23(1):141–145. [PubMed] [Google Scholar]
- Maller J. L., Koontz J. W. A study of the induction of cell division in amphibian oocytes by insulin. Dev Biol. 1981 Jul 30;85(2):309–316. doi: 10.1016/0012-1606(81)90262-1. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Shapiro D. J. Preparation of capped RNA transcripts using T7 RNA polymerase. Nucleic Acids Res. 1986 Jul 25;14(14):5936–5936. doi: 10.1093/nar/14.14.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Czech M. P. Photoaffinity labeling of insulin-sensitive hexose transporters in intact rat adipocytes. Direct evidence that latent transporters become exposed to the extracellular space in response to insulin. J Biol Chem. 1984 Jul 10;259(13):8125–8133. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. In vivo regulation of cyclic AMP phosphodiesterase in Xenopus oocytes. Stimulation by insulin and insulin-like growth factor 1. J Biol Chem. 1987 Aug 5;262(22):10644–10650. [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem. 1981 Jun 25;256(12):6368–6373. [PubMed] [Google Scholar]
- Sarkar H. K., Thorens B., Lodish H. F., Kaback H. R. Expression of the human erythrocyte glucose transporter in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5463–5467. doi: 10.1073/pnas.85.15.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. F. Characterization of cytochalasin B photoincorporation into human erythrocyte D-glucose transporter and F-actin. Biochemistry. 1983 May 24;22(11):2750–2756. doi: 10.1021/bi00280a024. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. Reassessment of the translocation hypothesis by kinetic studies on hexose transport in isolated rat adipocytes. J Biol Chem. 1988 Sep 5;263(25):12247–12252. [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Toyoda N., Flanagan J. E., Kono T. Reassessment of insulin effects on the Vmax and Km values of hexose transport in isolated rat epididymal adipocytes. J Biol Chem. 1987 Feb 25;262(6):2737–2745. [PubMed] [Google Scholar]
- Wall D. A., Patel S. Isolation of plasma membrane complexes from Xenopus oocytes. J Membr Biol. 1989 Feb;107(2):189–201. doi: 10.1007/BF01871724. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Misulovin Z. The role of zinc and follicle cells in insulin-initiated meiotic maturation of Xenopus laevis oocytes. Science. 1980 Nov 21;210(4472):928–930. doi: 10.1126/science.7001631. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- Williams T. F., Exton J. H., Park C. R., Regen D. M. Stereospecific transport of glucose in the perfused rat liver. Am J Physiol. 1968 Nov;215(5):1200–1209. doi: 10.1152/ajplegacy.1968.215.5.1200. [DOI] [PubMed] [Google Scholar]




