Abstract
Changes in cardiac gene expression contribute to the progression of heart failure by affecting cardiomyocyte growth, function, and survival. The Na+ -Ca2+ exchanger gene (Ncx1) is upregulated in hypertrophy and is often found elevated in end-stage heart failure. Studies have shown that the change in its expression contributes to contractile dysfunction. Several transcriptional pathways mediate Ncx1 expression in pathological cardiac remodeling. Both α-adrenergic receptor (α-AR) and β-adrenergic receptor (β-AR) signaling can play a role in the regulation of calcium homeostasis in the cardiomyocyte, but chronic activation in periods of cardiac stress contributes to heart failure by mechanisms which include Ncx1 upregulation. Our studies have even demonstrated that NCX1 can directly act as a regulator of “activity-dependent signal transduction” mediating changes in its own expression. Finally, we present evidence that histone deacetylases (HDACs) and histone acetyltransferases (HATs) act as master regulators of Ncx1 expression. We show that many of the transcription factors regulating Ncx1 expression are important in cardiac development and also in the regulation of many other genes in the so-called fetal gene program, which are activated by pathological stimuli. Importantly, studies have revealed that the transcriptional network regulating Ncx1 expression is also mediating many of the other changes in genetic remodeling contributing to the development of cardiac dysfunction and revealed potential therapeutic targets for the treatment of hypertrophy and failure.
Keywords: Na+ -Ca2+ exchanger, Transcriptional regulation, α-Adrenergic, β -Adrenergic, HDAC
11.1 Introduction
The Na+-Ca2+ exchanger (NCX1) is one of the essential regulators of Ca2+ homeostasis within cardiomyocytes and is an important regulator of contractility. NCX1 plays a critical role in maintaining the balance of Ca2+ flux across the sarcolemmal membrane in excitation-contraction coupling (Bers 2002). Cardiac muscle contracts in response to the rise in [Ca2+]i which is released from the sarcoplasmic reticulum (SR) and from in flux across the sarcolemma through voltage-sensitive channels. SR Ca2+ -ATPase (SERCA) recycles Ca2+ from the cytosol into the lumen of the SR, and NCX1 mediates the movement of [Ca2+]i across the sarcolemma to the extracellular space. The exchanger catalyzes the electrogenic exchange of Ca2+ and Na+ across the plasma membrane in either the Ca2+ -in flux or Ca2+-efflux mode. NCX1 transports approximately 28% of the cytosolic Ca2+ during a contraction-relaxation cycle in large animals and humans, with 70% being reaccumulated in the SR (via SERCA) (Bassani and Bers 1994; Bers and Bridge 1989; Bers et al. 1990). Alterations in any of the activities associated with this complex process cause a corresponding change in the amount of Ca2+ released from the SR and the resulting force of cardiac contraction.
Heart disease can arise from either congenital abnormalities or a combination of acquired longstanding disorders such as hypertension, injury resulting from myocardial infarction, or myocarditis due to an infectious agent. Pathological stimuli such as prolonged mechanical stress or abnormal neurohumoral activation result in an increase in ventricular wall stress, necessitating an increase in contractile Ca2+ to maintain cardiac output. These stimuli induce a phase of cardiac hypertrophy in which individual cardiomyocytes increase in size and assembly of sarcomere proteins as a mechanism of augmenting cardiac output. But persistent pathological stress on the heart leads to continued hypertrophic growth and remodeling characterized by interstitial fibrosis, reexpression of cardiac embryonic genes, and transition to heart failure, arrhythmia, and sudden death (Molkentin and Dorn 2001). The exchanger is regulated at the transcriptional level in animal models of cardiac hypertrophy (Kent et al. 1993; Menick et al. 1996), ischemia, and failure (Studer et al. 1997; Hobai and O’Rourke 2000; Pogwizd et al. 2001; Sipido et al. 2002; Ahmmed et al. 2000; Litwin and Bridge 1997). Importantly, both NCX1 mRNA and protein levels are significantly upregulated in human end-stage heart failure (Hasenfuss et al. 1994, 1996, 1997; Studer et al. 1994). The diastolic performance of failing human myocardium correlates inversely with protein levels of NCX1 (Hasenfuss et al. 1999), and upregulation of the NCX1 gene (Ncx1) alone contributes directly to impaired SR loading and contractile dysfunction (Schillinger et al. 2000; Ranu et al. 2002). Studer et al. have demonstrated that there is an increase of NCX1 and a decrease in SERCA mRNA and protein in patients with dilated cardiomyopathy and coronary artery disease (Studer et al. 1994).
The cardiomyocytes sense many of the pathological stimuli outlined above either by membrane-bound receptors which are targets of the hormones, cytokines, or by growth factors and initiate intracellular signaling cascade in response to their binding. These signal transduction pathways mediate the cardiac growth/disease response affecting nuclear factors and the regulation of gene expression. Most of the efforts of trying to suppress the pathological outcomes of hypertrophy and heart failure have focused on the signaling pathways that alter gene expression. The paracrine/autocrine actions of growth factors such as transforming growth factor-β (TGF β) and connective tissue growth factor (CTGF), cytokines such as TNF-α, as well as neurohormonal mediators such as norepinephrine, acetylcholine, phenylephrine, endothelin-1, and angiotensin II can all activate cardiac hypertrophy. Treatment of neonatal or adult cardiomyocytes with TGFβ, TNF-α, endothelin-1, or angiotensin II induces profound changes in cardiac gene expression including the reexpression of fetal isoforms of contractile proteins including β-myosin heavy chain, α-skeletal actin, and increased production of “stress markers” such as atrial natriuretic factor (ANF) and B-type natriuretic peptide (BNP) (Sadoshima and Izumo 1993a, b). Although endothelin-1 enhances Ncx1 expression in renal epithelial cells, angiotensin II upregulates Ncx1 in vascular smooth muscle cells (Kita et al. 2004) and TNF-α induces significant increases in Ncx1 expression in human airway smooth muscle; treatment with TGFβ, TNF-α, endothelin-1, or angiotensin II does not alter the expression of NCX1 in adult cardiocytes (Mani, S. and Menick D. R. unpublished). What controls NCX1 expression in the heart? Unraveling these pathways should give us a better understanding of this complex process at the molecular level and reveal novel therapeutic targets for the prevention of adverse cardiac remodeling, cardiac hypertrophy, ischemia-reperfusion injury, and heart failure. In this chapter, we introduce recent findings on the signal transduction pathways, signaling factors, and transcription factors, which regulate Ncx1 gene and contribute to the pathological process of heart failure.
11.2 α-Adrenergic Receptor-Stimulated Ncx1 Upregulation
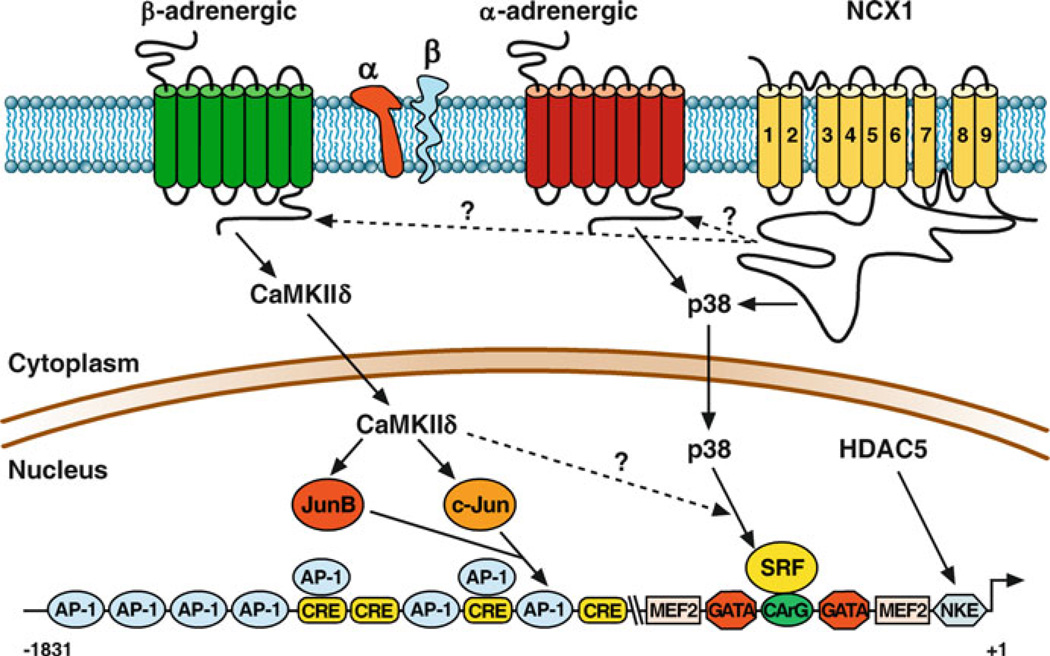
α- and β-adrenergic agonists play a major role in regulating cardiac metabolism and function. α-adrenergic stimulation stimulates chronotropic and inotropic effects on the heart and can activate many of the hypertrophic growth pathways in isolated adult and neonatal cardiomyocytes (Lee et al. 1988). The Ncx1 gene contains three promoters (H1, K1, and Br1) and multiple 5′-untranslated exons upstream from the coding region. As a result of alternative promoter usage and the resulting alternative splicing, there are multiple tissue-specific variants of NCX1 (Barnes et al. 1997; Lee et al. 1994; Kofuji et al. 1993; Quednau et al. 1997). In our initial characterization of the NCX1 cardiac promoter (Barnes et al. 1996, 1997), we demonstrated that a construct containing the first 250 bp of the 5′-flanking region, H1 exon, and 67 bp of the first intron is sufficient for cardiac-directed expression and α-adrenergic stimulation of the luciferase reporter gene. We have since shown that a construct containing only 184 bases of the 5′- flanking region, H1 exon, and 67 bp of the first intron is not only sufficient for cardiac-directed expression but also for α-adrenergic stimulation of the luciferase reporter gene (Xu et al. 2006). This is also in agreement with what has been reported for the rat Ncx1 minimal promoter (Nicholas et al. 1998). There are consensus sequences for a number of potential DNA-binding factors in the Ncx1 cardiac minimal promoter (Fig. 11.1). There are two potential binding sites for the GATA family of zinc- fingered transcription factors (A/T) GATA(A/G) and two CANNTG motifs (E-boxes) which are potential target sites for the basic helix-loop-helix (bHLH) family of transcription factors. This region also contains a single myocyte enhancer factor 2 (MEF-2) element, a CArG element, and a binding site for the cardiogenic homeodomain factor Nkx-2.5. It is of interest to note that the sequence of both GATA elements, CArG element, MEF element, and −153 E-Box are perfectly conserved in the feline and rat Ncx1 promoter (Nicholas et al. 1998). The mutational analysis revealed that both the CArG box at −80 and the GATA element at −50 were required for expression in rat neonatal cardiomyocytes but were not required for α-adrenergic induction (Cheng et al. 1999). In contrast to what we found in neonatal cardiomyocytes, the −80 CArG element mediates a part of the α-adrenergic-stimulated upregulation and is required for Ncx1 upregulation in response to p38 stimulation in isolated adult cardiomyocytes (Xu et al. 2005).
Figure 11.1.
Agonist-specific regulation of Ncx1. β-AR agonist isoproterenol (1 µM) or dobutamine (1 µM) activates CaMKinase II which then activates JunB and c-Jun, which sequentially binds to AP-1 elements and activates Ncx1 expression. α-AR agonist phenylephrine (10 µM) activates SRF via MAP kinase-p38 activation. SRF binds to CArG elements and mediates Ncx1 upregulation. HDACs regulate Ncx1 via the NKE element by deacetylating Nkx2.5. Inhibition of NCX1 by its reverse mode inhibitor KB-R7943 activates Ncx1 upregulation via activating MAP kinase p38 pathway
11.3 β-Adrenergic Receptor-Stimulated Ncx1 Upregulation
β-adrenergic receptor activation is common during times of cardiac stress. Initially, this leads to increases in heart rate and contractility contributing to increased cardiac output. However, chronic β-AR stimulation leads to changes in cardiac gene expression and eventual heart failure. In congestive heart failure, the heart is under intense sympathetic stimulation with very high levels of circulating norepinephrine (Bristow 2000; Vatner et al. 2000). The changes in gene expression with chronic β-AR stimulation are the same as what is observed in heart failure (Lowes et al. 2002; Rothermel et al. 2001; Sucharov et al. 2006). Ncx1 is upregulated at both the transcriptional and protein levels with β-AR stimulation in neonatal rat cardiomyocytes and in the adult rat heart (Golden et al. 2000, 2001). β-AR-stimulated upregulation of Ncx1 is largely dependent on CaMKII activation in the adult heart (Mani et al. 2010). β-AR-stimulated changes in cardiomyocyte gene expression are classically mediated by the cAMP-responsive element-binding protein (CREB) and activating protein-1 (AP-1) transcription factors which bind respectively to CRE and AP-1 promoter elements which are present in the Ncx1 promoter (Fig. 11.1). Although CREB is phosphorylated by β-AR stimulation in adult cardiomyocytes, it does not mediate Ncx1 upregulation. Mutation of the Ncx1 promoter AP-1 elements demonstrates that although no specific AP-1 element is required, retention of a single AP-1 element is sufficient for the majority of the β-AR-stimulated upregulation (Mani et al. 2010). Chromatin immunoprecipitation (ChIP) analysis demonstrates that β-AR stimulation activates an ordered recruitment of JunB, between 30 and 60 min, which then is replaced by c-Jun (between 2 and 6 h) binding to either the −546 AP-1 or −581 AP-1 element of the endogenous Ncx1 promoter (Mani et al. 2010).
β-AR activation is one of the most important pathways regulating E-C coupling in the heart. β-AR stimulation results in increased amplitude and rate of cardiomyocyte [Ca2+]I at each beat resulting in increased contractility. Calcium-/calmodulin-dependent kinase II (CaMKII) is activated by β-AR stimulation in response to the increase in level and frequency of calcium transients (for review (Maier and Bers 2002)). There are four CaMKII isoforms, α, β, γ, and δ. CaMKIIδ is the predominant form in the heart, and the α- and β-isoforms are expressed only in nerve tissue (Tobimatsu and Fujisawa 1989). CaMKII phosphorylates several downstream targets in common with cAMP-activated PKA including the ryanodine receptor, phospholamban, and L-type Ca2+ channel complex and thus also plays an important role in regulating E-C coupling in the heart (Lindemann et al. 1983; Takasago, et al. 1989; Karczewski et al. 1997; Maier and Bers 2002). CaMKII has also been shown to phosphorylate Na+ channels, which may contribute to arrhythmogenesis in heart failure (Wagner et al. 2006). CaMKII is activated in hypertrophy and has been shown to induce dilated cardiomyopathy and heart failure (Zhang et al. 2003; Hoch et al. 1999). In addition to acutely modulating calcium in flux, SR calcium release and uptake, chronic activation of CaMKII results in the induction of the fetal gene program, which is expressed in cardiac hypertrophy and failure. Our findings that β-AR-stimulated Ncx1 upregulation is dependent on CaMKII further illuminate the important role CaMKII has in the chronic dysregulation of cardiac Ca2+ homeostasis and E-C coupling. Transgenic overexpression of the cytosolic splice variant, CaMKIIδc, induces severe heart failure associated with SR Ca2+ leak and reduced SR Ca2+ content (Zhang et al. 2003). Correspondingly, the upregulation of Ncx1 contributes directly to limiting SR loading, contractile dysfunction, and greater potential for delayed afterdepolarizations, which lead to ventricular tachycardia (Pogwizd et al. 1999, 2001; Schillinger et al. 2000; Ranu et al. 2002). Further, numerous studies of human and animal models of heart failure demonstrate that diastolic performance in the failing heart correlates inversely with protein levels of NCX1 (Hasenfuss et al. 1999; Pogwizd et al. 1999; Weisser-Thomas et al. 2005). Importantly, inhibition of CaMKII activity prevents cardiac arrhythmias and suppresses afterdepolarizations (Anderson 2004). Dysregulation of Ncx1 expression can be added to the list of downstream adverse effects of chronic β-AR stimulation events mediated by the activation of CaMKII.
11.4 Identification of Regulatory Elements Mediating In Vivo Cardiac-Specific Expression and Upregulation
From our in vitro studies, we proposed that Ncx1H1 promoter regulates expression in the heart, the K1 promoter regulates expression in the kidney, and the Br1 promoter regulates expression in the brain as well as low-level ubiquitous expression. In order to test whether the H1 promoter directed the correct spatiotemporal pattern of Ncx1 expression in the developing and adult heart, we engineered a transgenic mouse model with the −1,831 to 67 bp of intron 1 encompassing exon H1 for the feline Ncx1 gene. The full-length 1831Ncx1H1 promoter was expressed in a heart-restricted pattern both in early embryos (E7.75–E14) and in late embryos (post-E14) when Ncx1 is expressed in other tissues (Muller et al. 2002). Ncx1 -driven reporter gene expression was detected in the cardiogenic plate by E7.75–E8.0, before the first heartbeat. The spatiotemporal expression of the reporter is identical to that previously described for endogenous Ncx1 (Koushik et al. 1999). High levels of reporter gene expression were restricted to cardiomyocytes in both ventricles and atria in the adult heart. No reporter gene activity was detected in the kidney, liver, spleen, uterus, or skeletal muscle, but trace activity was detected in the brain. Importantly, there was a twofold upregulation of Ncx1H1 promoter activity in the left ventricle after 7 days of transverse aortic constricted induced pressure overload compared with both sham and control animals (Muller et al. 2002).
The minimal (184Ncx1) promoter drives reporter gene expression at levels three- to fourfold greater than the 1831Ncx1 promoter in both neonatal and adult cardiomyocytes (Xu et al. 2006) because of the deletion of putative repressor elements distal to the minimal 184-bp promoter construct (S. Mani, L. Xu, and D. R. Menick, unpublished data). As discussed above, the 1831Ncx1 promoter is upregulated in response to α-adrenergic and β-adrenergic stimulation in both adult and neonatal cardiomyocytes and responsive to pressure-overload hypertrophy in the adult heart. However, the 184Ncx1 promoter is upregulated in response to α-adrenergic stimulation in neonatal cardiomyocytes but is recalcitrant to α-adrenergic and β-adrenergic stimulation in adult cardiomyocytes.
To test whether the Ncx1 minimal promoter contains sufficient DNA regulatory elements to direct cardiac-specific expression, we established 184Ncx1 -β-galactosidase transgenic mouse lines. The data revealed that the 184Ncx1 minimal promoter retains the necessary enhancer elements to drive the correct spatiotemporal pattern of Ncx1 expression in development but not for upregulation in response to pressure overload. Our data show that at least a single distal AP-1 element is required for the majority of the β-AR-stimulated upregulation (Mani et al. 2010). This AP-1 element may also be required for upregulation of Ncx1 expression in response to pressure overload. Mutational analysis revealed that both the −80 CArG and the −50 GATA elements were required for expression in isolated adult cardiomyocytes (Cheng et al. 1999). ChIP assays in adult cardiocytes demonstrate that SRF and GATA4 are associated with the proximal region of the endogenous Ncx1 promoter. Transgenic lines were established for the 1831Ncx1 promoter-luciferase containing mutations in the 221280 CArG or −50 GATA element. No luciferase activity was detected during development, in the adult, or after pressure overload in any of the −80 CArG transgenic lines. Therefore, the –80 CArG element appears to be critical to Ncx1 cardiac expression and regulation (Xu et al. 2006). The Ncx1 −50 GATA mutant promoter was sufficient for driving the normal spatiotemporal pattern of Ncx1 expression in development and for upregulation in response to pressure overload, but importantly, expression was no longer cardiac restricted. Our work demonstrates that the −50 GATA element is critical for cardiac-restricted expression of Ncx1 (Xu et al. 2006).
11.5 NCX1 Acts as a Regulator of Activity-Dependent Transcription
Many studies have demonstrated that NCX1 inhibitors can act as positive inotropic drugs for the treatment of ischemia-reperfusion injury and congestive heart failure (Hobai et al. 2004; MacDonald and Howlett 2008; Ozdemir et al. 2008; O’Rourke 2008). All three benzyloxyphenyl NCX inhibitors, KB-R7943, SN-6, and SEA-0400, have been reported to confer some cardioprotective effects against ischemia-reperfusion injury and heart failure.
Although KB-R7943, SN-6, and SEA-0400 have been utilized in a variety of animal and cell models, most studies have focused only on the acute effects on INCX1 and Ca2+ homeostasis. The potential for modulation of NCX1 activity to correct the impaired contractile properties seen in diseased cardiomyocytes makes it an extremely attractive target for therapeutic intervention. However, these studies primarily focus on acute treatment with NCX1 inhibitors. Interestingly, cardiac NCX1 expression is increased at both the transcriptional and protein levels in response to chronic inhibition of NCX1 activity with KB-R7943. The level of upregulation is similar to what we have observed with pressure-overload hypertrophy (Muller et al. 2002). Ncx1 upregulation is mediated by p38, which is activated within 5 min of KB-R7943 treatment and persists for more than 72 h (Fig. 11.1). These studies impart compelling insight into the regulation of NCX1 function and expression. Importantly, treatment of adult cardiomyocytes with a second NCX1 inhibitor, SN-6 (Niu et al. 2007), also results in activation of p38.
11.6 Regulation of NCX1 Expression by HDACs and HATs
The reversible acetylation of histones plays a critical role in gene regulation as well as many other nuclear events. Protein acetylation is regulated by histone acetyltransferases (HATs), while protein deacetylation is regulated by histone deacetylases (HDACs). Many transcriptional activators are HATs or recruit HATs, allowing acetylation to be targeted to specific gene promoters. Conversely, HDACs are associated with transcriptional repressor complexes, which are also recruited to specific gene promoters. There is a rapidly growing list of nonhistone nuclear and cytosolic proteins that undergo reversible acetylation (Minucci and Pelicci 2006; Yang and Gregoire 2005). These findings have established that acetylation of nonhistone proteins plays multiple roles in the regulation of many cellular processes.
Interestingly, α-adrenergic, β-adrenergic, and pressure-overload-stimulated Ncx1 endogenous and reporter gene expression is inhibited in a dose-dependent manner by the class I/IIb HDAC inhibitor trichostatin A (TSA). In addition, only overexpression of the class IIb HDAC, HDAC5, resulted in significant upregulation of both the promoter-luciferase activity as well as the endogenous Ncx1 gene. Although HDACs are classically regarded as transcriptional repressors, there is mounting evidence that HDACs can serve to activate some genes, often through the direct deacetylation of transcription factors (Zupkovitz et al. 2006; Qiu et al. 2006; Nusinzon and Horvath 2006). Our co-immunoprecipitation data show that HDAC5 is in complex with HDAC1 and HDAC2, and ChIP demonstrates that HDAC1, HDAC2, and HDAC5 are recruited to the Ncx1 promoter by the Nkx2.5 transcription factor (Fig. 11.1). Overexpression of HDAC5 appears to decrease the level of HDAC1 and HDAC 2, whereas TSA treatment increases the level HDAC1 and HDAC 2 bound to the Ncx1 promoter. This suggests that association of HDAC5 with HDAC1 facilitates the deacetylation of a transcription factor associated with the Ncx1 promoter resulting in their dissociation from the promoter, but inhibition of HDAC activity prevents this. Our results demonstrate that Nkx2.5 is acetylated in adult cardiomyocytes and that TSA treatment dramatically increases this acetylation. We demonstrate that when Nkx2.5 is acetylated, it is found associated with HDAC5, whereas deacetylated Nkx2.5 is in complex with the histone acetyltransferase p300. Importantly, TSA treatment prevents p300 from being recruited to the endogenous Ncx1 promoter resulting in the repression of Ncx1 expression.
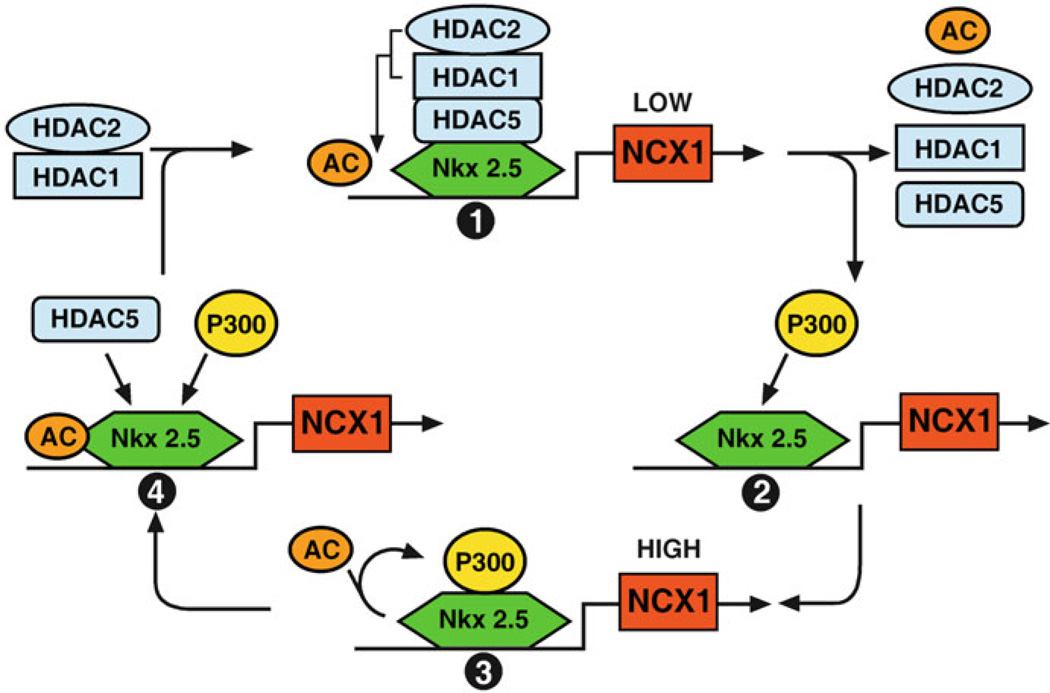
Based on our finding, we speculate that the Ncx1 promoter cycles through at least four kinetic steps of transcriptional competency, which we are currently experimentally testing (Fig. 11.2). The first step is a low transcriptional activity state (1) where acetylated Nkx2.5 recruits HDAC5 to the promoter. HDAC5 then complexes with HDAC1 and HDAC2, which mediates the deacetylation of Nkx2.5. Nkx2.5 deacetylation triggers the shift to the second kinetic step, the “transition state.” Importantly, TSA inhibits the transfer from the low-activity state (1) to the transition state (2). TSA treatment should trap all the cardiomyocyte Ncx1 promoters in the low transcriptional activity state (1). In the transition state, the HDAC 1/5 complex dissociates from the promoter and p300 in complex with coactivators is recruited to the promoter. The promoter is now in the high transcriptional activity state (3). We speculate that p300 bridges the transcription factors and coactivators with the preinitiation complex, stabilizing it at the initiation site and helps promote Pol II phosphorylation. As we have previously demonstrated, the makeup of the coactivators and transcription factors recruited to the Ncx1 promoter would depend on which signaling pathways were activated. Therefore, the combinatorial recruitment of coactivators would determine the extent and duration of Ncx1 transcriptional activation. We predict that the acetylation of Nkx2.5 by p300 results in its dissociation from the promoter and the subsequent recruitment of HDAC5 (transition state 4). This would cycle the promoter back to its low-activity state (1). Our model does not propose that HDAC5 is itself a coactivator but that HDAC5 is required for the recruitment of coactivators to the Ncx1 promoter in response to hypertrophic stimuli. The HDAC5-dependent recruitment of coactivators to the promoter in state (3) of our model determines the transcriptional productivity and duration of the high transcriptional state. This cycling between low transcriptional activity and high transcriptional activity states allows the cell to continuously regulate transcription in response to physiological and pathophysiological stimuli through restricting the duration of the high-activity state (3). Importantly, HDAC and HAT activity appears to act as a dominant regulator over many if not all the pathways that mediate NCX1 expression. Inhibition of HDAC activity trumps both α-adrenergic and β-adrenergic and more importantly prevents Ncx1 upregulation in the pressure-overloaded ventricle. This is clearly one of the reasons that HDAC inhibitor treatment has been found to be efficacious in several preclinical models of cardiac hypertrophy and failure.
Figure 11.2.
Regulation of Ncx1 gene by HDAC’s. Model for the role of acetylation in Ncx1 transcriptional regulation. We speculate that the Ncx1 promoter cycles through at least four kinetic steps. Step 1: Acetylated Nkx2.5 recruits HDAC5, HDAC1, and HDAC2 complex to the promoter. The presence of the HDAC complex allows for low levels of Ncx1 expression. Step 2: When the HDAC complex deacetylates Nkx2.5, HDAC5, HDAC1, and HDAC2 dissociate from the promoter and the HAT, p300, is recruited to the promoter. Step 3: p300 and its associated coactivators stabilize the Poll II complex to allow a high level of transcription of the Ncx1 gene. Step 4: When p300 acetylates Nkx2.5, it dissociates from the promoter and HDAC5, HDAC1, and HDAC2 are recruited to the promoter. Importantly, TSA treatment traps the Ncx1 promoters in the low transcriptional state. HDAC5 is not itself a coactivator, but deacetylation of Nkx2.5 is required for the recruitment of activators to the Ncx1 promoter in response to hypertrophic stimuli
11.7 Conclusions
The process of pathological cardiac hypertrophy involves the change in expression of many genes activated by multiple receptors triggering intracellular signaling cascades that activate transcription factors, enhancers, and repressors. Work over the past 15 years has made major advances in the identification of the molecular regulators involved in Ncx1 expression in the normal and hypertrophic heart. The MAP kinase, p38, is required for both α-adrenergic-stimulated and NCX1 activity-dependent upregulation. p38 appears to mediate Ncx1 regulation through SRF activation which binds to the −80 CArG site of the Ncx1 promoter. Although the −80 CArG element is required for upregulation, the 184Ncx1 minimal promoter is recalcitrant to α-adrenergic-stimulated and NCX1 activity-dependent transcription. Further, the 184Ncx1 minimal promoter does not contain the necessary enhancer elements to drive upregulation in response to pressure overload. Therefore, one or more distal elements, possibly one or more of the AP1 elements, required for β-adrenergic-stimulated upregulation, are requisite with the −80 CArG for hypertrophic upregulation.
HDACs appear to act as master regulators of Ncx1 expression in the adult heart. Inhibition of class I and class IIb HDACs prevents Ncx1 upregulation by α-, β-adrenergic stimulation, activity-dependent transcriptional activation and, most importantly, pressure overload. Notably, HDAC inhibition has been shown to attenuate pathological cardiac remodeling in several preclinical models of cardiac hypertrophy and failure. In addition to blocking ncx1 upregulation, it prevents the dysregulation of many other cardiac genes and highlights the potential for using HDAC inhibitors in the treatment of heart failure (McKinsey 2012).
Acknowledgements
This work was supported by NIH R01HL095696 (DRM) and NIH T32HL07260 (MSL, OC, and DK).
References
- Ahmmed GU, Dong PH, Song G, Ball NA, Xu Y, Walsh RA, Chiamvimonvat N. Changes in Ca2+ cycling proteins underlie cardiac action potential prolongation in a pressure-overloaded guinea pig model with cardiac hypertrophy and failure. Circ. Res. 2000;86:558–570. doi: 10.1161/01.res.86.5.558. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc. Med. 2004;14:152–161. doi: 10.1016/j.tcm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Barnes KV, Dawson MM, Menick DR. Initial characterization of the feline sodium-calcium exchanger gene. Ann. N. Y. Acad. Sci. 1996;779:121–125. doi: 10.1111/j.1749-6632.1996.tb44778.x. [DOI] [PubMed] [Google Scholar]
- Barnes KV, Cheng G, Dawson MM, Menick DR. Cloning of cardiac, kidney, and brain promoters of the feline ncx1 gene. J. Biol. Chem. 1997;272:11510–11517. [PubMed] [Google Scholar]
- Bassani RA, Bers DM. Na-Ca exchange is required for rest-decay but not for rest-potentiation of twitches in rabbit and rat ventricular myocytes. J. Mol. Cell. Cardiol. 1994;26:1335–1347. doi: 10.1006/jmcc.1994.1152. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM, Bridge JH. Relaxation of rabbit ventricular muscle by Na-Ca exchange and sarcoplasmic reticulum calcium pump. Ryanodine and voltage sensitivity. Circ. Res. 1989;65:334–342. doi: 10.1161/01.res.65.2.334. [DOI] [PubMed] [Google Scholar]
- Bers DM, Lederer WJ, Berlin JR. Intracellular Ca transients in rat cardiac myocytes: role of Na-Ca exchange in excitation-contraction coupling. Am. J. Physiol. 1990;258 :C944–C954. doi: 10.1152/ajpcell.1990.258.5.C944. [DOI] [PubMed] [Google Scholar]
- Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- Cheng G, Hagen TP, Dawson ML, Barnes KV, Menick DR. The role of GATA, CArG, E-box, and a novel element in the regulation of cardiac expression of the Na+ -Ca2+ exchanger gene. J. Biol. Chem. 1999;274:12819–12826. doi: 10.1074/jbc.274.18.12819. [DOI] [PubMed] [Google Scholar]
- Golden KL, Fan QI, Chen B, Ren J, O’Connor J, Marsh JD. Adrenergic stimulation regulates Na+ /Ca2+ exchanger expression in rat cardiac myocytes. J. Mol. Cell. Cardiol. 2000;32:611–620. doi: 10.1006/jmcc.2000.1104. [DOI] [PubMed] [Google Scholar]
- Golden KL, Ren J, O’Connor J, Dean A, DiCarlo SE, Marsh JD. In vivo regulation of Na/Ca exchanger expression by adrenergic effectors. Am. J. Physiol. Heart Circ. Physiol. 2001;280 :H1376–H1382. doi: 10.1152/ajpheart.2001.280.3.H1376. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+ -ATPase in failing and nonfailing human myocardium. Circ. Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Preuss M, Lehnart S, Prestle J, Meyer M, Just H. Relationship between diastolic function and protein levels of sodium-calcium-exchanger in end-stage failing human hearts. Circulation. 1996;94:I-443. [Google Scholar]
- Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H. Calcium handling proteins in the failing human heart. Basic Res. Cardiol. 1997;92:87–93. doi: 10.1007/BF00794072. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+ -Ca2+ -exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- Hobai IA, O’Rourke B. Enhanced Ca2+ -activated Na+ -Ca2+ exchange activity in canine pacing-induced heart failure. Circ. Res. 2000;87:690–698. doi: 10.1161/01.res.87.8.690. [DOI] [PubMed] [Google Scholar]
- Hobai IA, Maack C, O’Rourke B. Partial inhibition of sodium/calcium exchange restores cellular calcium handling in canine heart failure. Circ. Res. 2004;95:292–299. doi: 10.1161/01.RES.0000136817.28691.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+ /calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ. Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- Karczewski P, Kuschel M, Baltas LG, Bartel S, Krause EG. Site-specific phosphorylation of a phospholamban peptide by cyclic nucleotide- and Ca 2+ /calmodulin-dependent protein kinases of cardiac sarcoplasmic reticulum. Basic Res. Cardiol. 1997;92(Suppl 1):37–43. doi: 10.1007/BF00794066. [DOI] [PubMed] [Google Scholar]
- Kent RL, Rozich JD, McCollam PL, McDermott DE, Thacker UF, Menick DR, McDermott PJ, Cooper G., IV Rapid expression of the Na+ -Ca2+ exchanger in response to cardiac pressure overload. Am. J. Physiol. 1993;265:H1024–H1029. doi: 10.1152/ajpheart.1993.265.3.H1024. [DOI] [PubMed] [Google Scholar]
- Kita S, Katsuragi T, Iwamoto T. Endothelin-1 enhances the activity of Na+ /Ca2+ exchanger type 1 in renal epithelial cells. J. Cardiovasc. Pharmacol. 2004;44(Suppl 1):S239–S243. doi: 10.1097/01.fjc.0000166262.19852.b5. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Lederer WJ, Schulze DH. Na/Ca exchanger isoforms expressed in kidney. Am. J. Physiol. 1993;265:F598–F603. doi: 10.1152/ajprenal.1993.265.4.F598. [DOI] [PubMed] [Google Scholar]
- Koushik SV, Bundy J, Conway SJ. Sodium-calcium exchanger is initially expressed in a heart-restricted pattern within the early mouse embryo. Mech. Dev. 1999;88:119–122. doi: 10.1016/s0925-4773(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Lee HR, Henderson SA, Reynolds R, Dunnmon P, Yuan D, Chien KR. Alpha 1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells. Effects on myosin light chain-2 gene expression. J. Biol. Chem. 1988;263:7352–7358. [PubMed] [Google Scholar]
- Lee SL, Yu AS, Lytton J. Tissue-specific expression of Na+ -Ca2+ exchanger isoforms. J. Biol. Chem. 1994;269:14849–14852. [PubMed] [Google Scholar]
- Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. Beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J. Biol. Chem. 1983;258:464–471. [PubMed] [Google Scholar]
- Litwin SE, Bridge JH. Enhanced Na+ -Ca2+ exchange in the infarcted heart. Implications for excitation-contraction coupling. Circ. Res. 1997;81:1083–1093. doi: 10.1161/01.res.81.6.1083. [DOI] [PubMed] [Google Scholar]
- Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, Quaife RA, Bristow MR. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N. Engl. J. Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- MacDonald AC, Howlett SE. Differential effects of the sodium calcium exchange inhibitor, KB-R7943, on ischemia and reperfusion injury in isolated guinea pig ventricular myocytes. Eur. J. Pharmacol. 2008;580:214–223. doi: 10.1016/j.ejphar.2007.10.055. [DOI] [PubMed] [Google Scholar]
- Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. J. Mol. Cell. Cardiol. 2002;34:919–939. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- Mani SK, Egan EA, Addy BK, Grimm M, Kasiganesan H, Thiyagarajan T, Renaud L, Brown JH, Kern CB, Menick DR. Beta-adrenergic receptor stimulated Ncx1 upregulation is mediated via a CaMKII/AP-1 signaling pathway in adult cardiomyocytes. J. Mol. Cell. Cardiol. 2010;48:342–351. doi: 10.1016/j.yjmcc.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu. Rev. Pharmacol. Toxicol. 2012;10:303–319. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- Menick DR, Barnes KV, Thacker UF, Dawson MM, McDermott DE, Rozich JD, Kent RL, Cooper G., IV The exchanger and cardiac hypertrophy. Ann. N. Y. Acad. Sci. 1996;779:489–501. doi: 10.1111/j.1749-6632.1996.tb44823.x. [DOI] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- Muller JG, Isomatsu Y, Koushik SV, O’Quinn M, Xu L, Kappler CS, Hapke E, Zile MR, Conway SJ, Menick DR. Cardiac-specific expression and hypertrophic upregulation of the feline Na+ -Ca2+ exchanger gene H1-promoter in a transgenic mouse model. Circ. Res. 2002;90:158–164. doi: 10.1161/hh0202.103231. [DOI] [PubMed] [Google Scholar]
- Nicholas SB, Yang W, Lee SL, Zhu H, Philipson KD, Lytton J. Alternative promoters and cardiac muscle cell-specific expression of the Na+/Ca2+ exchanger gene. Am. J. Physiol. 1998;274:H217–H232. doi: 10.1152/ajpheart.1998.274.1.H217. [DOI] [PubMed] [Google Scholar]
- Niu CF, Watanabe Y, Ono K, Iwamoto T, Yamashita K, Satoh H, Urushida T, Hayashi H, Kimura J. Characterization of SN-6, a novel Na+/Ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur. J. Pharmacol. 2007;573:161–169. doi: 10.1016/j.ejphar.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Nusinzon I, Horvath CM. Positive and negative regulation of the innate antiviral response and beta interferon gene expression by deacetylation. Mol. Cell. Biol. 2006;26:3106–3113. doi: 10.1128/MCB.26.8.3106-3113.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B. The ins and outs of calcium in heart failure. Circ. Res. 2008;102:1301–1303. doi: 10.1161/CIRCRESAHA.108.178095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir S, Bito V, Holemans P, Vinet L, Mercadier JJ, Varro A, Sipido KR. Pharmacological inhibition of Na/Ca exchange results in increased cellular Ca2+ load attributable to the predominance of forward mode block. Circ. Res. 2008;102:1398–1405. doi: 10.1161/CIRCRESAHA.108.173922. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ. Res. 1999;85:1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ. Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhao Y, Becker M, John S, Parekh BS, Huang S, Hendarwanto A, Martinez ED, Chen Y, Lu H, Adkins NL, Stavreva DA, Wiench M, Georgel PT, Schiltz RL, Hager GL. HDAC1 acetylation is linked to progressive modulation of steroid receptor-induced gene transcription. Mol. Cell. 2006;22:669–679. doi: 10.1016/j.molcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Quednau BD, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+ /Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. 1997;272:C1250–C1261. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- Ranu HK, Terracciano CM, Davia K, Bernobich E, Chaudhri B, Robinson SE, Bin Kang Z, Hajjar RJ, MacLeod KT, Harding SE. Effects of Na+ /Ca2+ - exchanger overexpression on excitation-contraction coupling in adult rabbit ventricular myocytes. J. Mol. Cell. Cardiol. 2002;34:389–400. doi: 10.1006/jmcc.2001.1521. [DOI] [PubMed] [Google Scholar]
- Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS. Myocyteenriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3328–3333. doi: 10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 1993a;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Signal transduction pathways of angiotensin II-induced c-fos gene expression in cardiac myocytes in vitro. Roles of phospholipidderived second messengers. Circ. Res. 1993b;73:424–438. doi: 10.1161/01.res.73.3.424. [DOI] [PubMed] [Google Scholar]
- Schillinger W, Janssen PM, Emami S, Henderson SA, Ross RS, Teucher N, Zeitz O, Philipson KD, Prestle J, Hasenfuss G. Impaired contractile performance of cultured rabbit ventricular myocytes after adenoviral gene transfer of Na+ -Ca2+ exchanger. Circ. Res. 2000;87:581–587. doi: 10.1161/01.res.87.7.581. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Volders PG, Vos MA, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc. Res. 2002;53:782–805. doi: 10.1016/s0008-6363(01)00470-9. [DOI] [PubMed] [Google Scholar]
- Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J, Drexler H. Gene expression of the cardiac Na+ -Ca2+ exchanger in end-stage human heart failure. Circ. Res. 1994;75:443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- Studer R, Reinecke H, Vetter R, Holtz J, Drexler H. Expression and function of the cardiac Na+ /Ca2+ exchanger in postnatal development of the rat, in experimental- induced cardiac hypertrophy and in the failing human heart. Basic Res. Cardiol. 1997;92:53–58. doi: 10.1007/BF00794068. [DOI] [PubMed] [Google Scholar]
- Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A beta1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1299–H1308. doi: 10.1152/ajpheart.00017.2006. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Shigekawa M. Phosphorylation of the cardiac ryanodine receptor by cAMP-dependent protein kinase. J. Biochem. 1989;106:872–877. doi: 10.1093/oxfordjournals.jbchem.a122945. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J. Biol. Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- Vatner SF, Vatner DE, Homcy CJ. Beta-adrenergic receptor signaling: an acute compensatory adjustment-inappropriate for the chronic stress of heart failure? Insights from Gsalpha overexpression and other genetically engineered animal models. Circ. Res. 2000;86:502–506. doi: 10.1161/01.res.86.5.502. [DOI] [PubMed] [Google Scholar]
- Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisser-Thomas J, Kubo H, Hefner CA, Gaughan JP, McGowan BS, Ross R, Meyer M, Dillmann W, Houser SR. The Na+ /Ca2+ exchanger/SR Ca2+ ATPase transport capacity regulates the contractility of normal and hypertrophied feline ventricular myocytes. J. Card. Fail. 2005;11:380–387. doi: 10.1016/j.cardfail.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Xu L, Kappler CS, Menick DR. The role of p38 in the regulation of Na+ -Ca2+ exchanger expression in adult cardiomyocytes. J. Mol. Cell. Cardiol. 2005;38:735–743. doi: 10.1016/j.yjmcc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Xu L, Renaud L, Muller JG, Baicu CF, Bonnema DD, Zhou H, Kappler CS, Kubalak SW, Zile MR, Conway SJ, Menick DR. Regulation of Ncx1 expression. Identification of regulatory elements mediating cardiac-specific expression and up-regulation. J. Biol. Chem. 2006;281:34430–34440. doi: 10.1074/jbc.M607446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Gregoire S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol. Cell. Biol. 2005;25:2873–2884. doi: 10.1128/MCB.25.8.2873-2884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ. Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- Zupkovitz G, Tischler J, Posch M, Sadzak I, Ramsauer K, Egger G, Grausenburger R, Schweifer N, Chiocca S, Decker T, Seiser C. Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol. Cell. Biol. 2006;26:7913–7928. doi: 10.1128/MCB.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]