Abstract
Purpose
The aim of this study is to assess the effects of age on the short-term outcomes of a laparoscopic resection of colorectal cancer in elderly (≥75 years old), as compared with younger (<75 years old), patients.
Methods
A retrospective analysis of patients who underwent laparoscopic surgery for colorectal cancer between January 2007 and December 2009 was performed. There were two groups: age <75 years old (group A) and age ≥75 years old (group B). The perioperative outcomes between group A and group B were compared.
Results
The study included 824 patients in group A and 92 patients in group B. The body mass index (BMI) and the American Society of Anesthesiologists (ASA) score were significantly different between group B and group A (BMI: 22.5 vs. 23.5, P = 0.002; ASA score: 1.88 vs. 1.48, P = 0.001). Mean operating times were similar between the groups (325.4 minutes vs. 351.6 minutes, P = 0.07). We observed a higher overall complication rate in group B than in group A (12.0% vs. 6.2%, P = 0.047), but the number of severe complications of Accordion Severity Classification ≥3 (those that required an invasive procedure) was not significantly different between the two groups (6.5% vs. 3.4%, P = 0.142). There was no significant difference in the length of hospital stay (13.0 days vs. 12.0 days, P = 0.053).
Conclusion
Although the elderly patients had a significantly higher overall postoperative complication rate, no significant difference was seen in either the number of severe complications of Accordion Severity Classification ≥3 or in the length of hospital stay. A laparoscopic colorectal cancer resection in elderly patients, especially those aged 75 years or older, is safe and feasible.
Keywords: Colorectal cancer, Laparoscopic surgery, Elderly, Morbidity, Mortality
INTRODUCTION
Recently, the Korean Statistical Information Service has reported that by the year 2026, approximately 20.8 percent of the population in Korea will be more than 65 years old [1]. This increasing population is associated with a high incidence of surgically-resectable colorectal cancer. Eventually, every colorectal surgeon will have to face the dilemma of how best to perform colorectal resections in elderly patients.
In general, open colorectal surgery in the elderly, in comparison with younger patients, is known to be associated with increased morbidity and mortality and with a longer length of hospital stay [2-4]. However, due to the development of minimally invasive techniques, most colorectal procedures can be performed using a laparoscopic approach. Many randomized controlled studies, including the MRC CLASICC trial, the COST trial, and the COLOR trial, have reported that laparoscopic colorectal surgery had better features, including early recovery, shorter hospital stay, smaller incision and less pain, than open colorectal surgery [5-11]. These advantages are particularly important in elderly patients as they can help improve short-term outcomes and reduce morbidity and mortality. However, not many studies have addressed the issue of laparoscopic colorectal surgery in elderly patients. Thus, the aim of this study was to evaluate the effects of age on the short-term outcomes of laparoscopic resection of colorectal cancer in elderly (≥75 years old), as compared to younger (<75 years old), patients.
METHODS
All patients who underwent an elective laparoscopic colorectal cancer resection between January 2007 and December 2009 at our department were retrospectively reviewed. Data from our prospectively-collected computer database were extracted, and further clinical information was extracted from reviews of medical charts. Patients with recurrent colorectal cancer, those who required an emergency operation, or those who underwent surgery without resection were excluded. After exclusion, a total of 916 patients were analyzed. Patients were divided into two groups by their age: those younger than 75 years of age (group A, 824 patients) and those 75 years of age or older (group B, 92 patients).
Analyzed parameters included demographics, American Society of Anesthesiology (ASA) score, body mass index (BMI), comorbid conditions, preoperative carcinoembryonic antigen level, operative time, procedure performed, pathologic stage, length of hospital stay, postoperative complications, and mortality.
Operative time was defined as the time from the initial incision until the end of the operation with the dressing in place. Operative mortality was defined as death within 30 days of surgery. Postoperative morbidity was defined as complications that required additional treatment or that prolonged the hospital stay, and these complications were stratified by using the Accordion Severity Grading System as recommended by Strasberg et al. [12]. Only significant severe surgical complications (grade ≥3) requiring endoscopic or interventional procedures or surgical reoperation under general anesthesia were considered.
The IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. Descriptive data are reported as mean (±standard deviation) or the number of patients and percentage. The Student's t-test was used to compare normally-distributed variables. Comparisons between groups of discrete variables were made by using the chi-squared test or the Fisher's exact test when appropriate. Multivariate logistic regression models were generated to estimate the risk of postoperative surgical complications based on preoperative patient characteristics, comorbid conditions, and surgical approach. Comparisons between the groups with regard to recurrence and survival were done by using the log-rank test and were demonstrated by using Kaplan-Meier curves. The predictive values of different variables for overall survival (OS) rates were assessed using the univariate and multivariate Cox proportional-hazards regression models. All P-values < 0.05 were considered statistically significant.
RESULTS
Demographics and clinical characteristics
The demographics and the clinical characteristics of the patients in the two groups are summarized in Table 1. The median age was 61 years old in group A (range, 14 to 74 years) and 78 years old in group B (range, 75 to 93 years) (P < 0.001). As expected, group B presented with a significantly lower average BMI (22.5 vs. 23.5, P = 0.002), a higher average ASA score (1.88 vs. 1.48, P = 0.001) and more comorbid conditions. In particular, a higher proportion of Hypertension (65.2% vs. 35.7%), pulmonary disease (7.6% vs. 2.7%), and ASA scores II and III (80.4% vs. 46.1%) were observed in group B.
Table 1.
Demographics and clinical characteristics
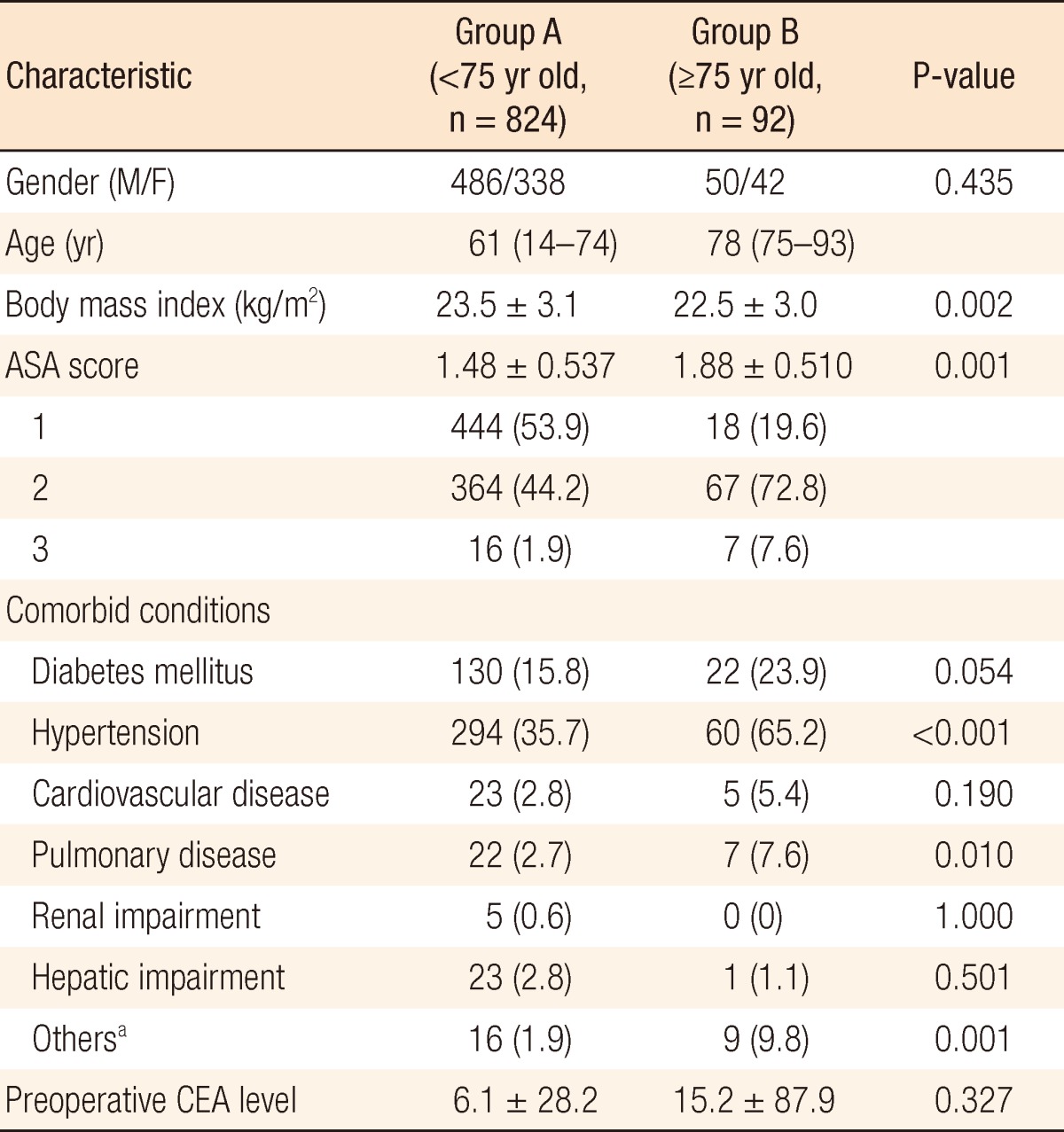
Values are presented as median (range), mean±standard deviation, or number (%).
ASA, American Society of Anesthesiologists; CEA, carcinoembryonic antigen; BPH, benign prostatic hyperplasia.
aGroup A: hyperlipidemia (4), BPH (8), hyperthyroidism (1), tuberculous spondylitis (1), and stroke (2); group B: stroke (2), iron deficiency anemia (1), hyperthyroidism (1), Parkinson's disease (2), and BPH (3).
The operative details are shown in Table 2. The difference in the mean operative times between the both groups was not statistically significant (group A, 351.6 minutes vs. group B, 324.4 minutes; P = 0.07). The numbers of lymph nodes harvested were 19.9 ± 14.0 in group A and 20.0 ± 13.6 in group B (P = 0.931). The proximal and the distal resection margins of the specimen were 10.8 cm and 7.0 cm in group A and 12.8 cm and 7.9 cm in group B. The proximal resection margin was significantly longer in group B than in group A (P = 0.04), and the distal margins were similar in both groups (P = 0.274). The performed procedures (P = 0.202) and the pathologic tumor-node-metastasis stage distributions (P = 0.152) were similar in both groups of patients.
Table 2.
Operative details
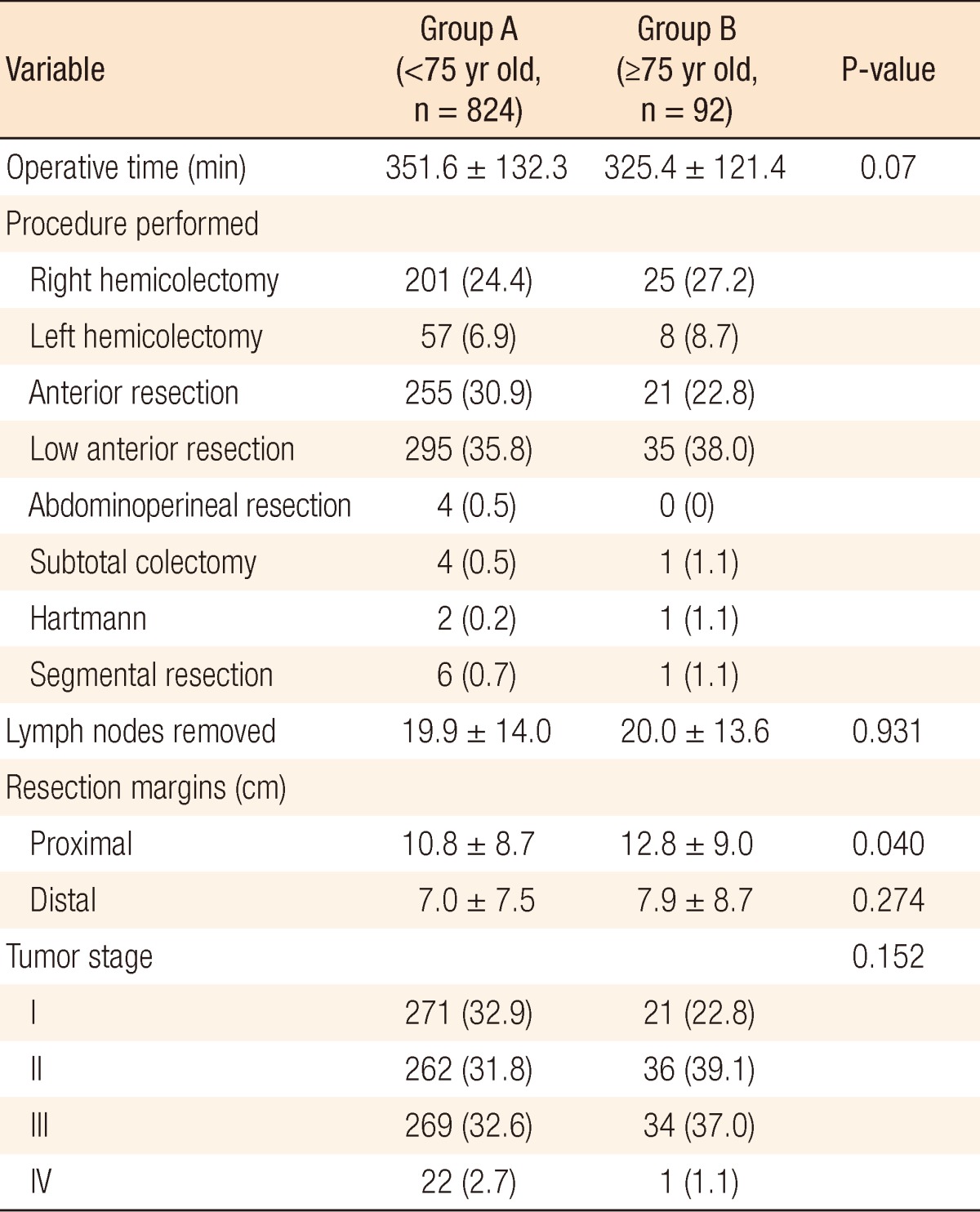
Values are presented as mean±standard deviation or number (%).
Morbidity and mortality
Patients had similar postoperative courses in terms of time to ambulation, advancement of diet, and discharge. Details of the postoperative outcomes are summarized in Table 3. The median lengths of hospital stay were 12 days (interquartile range [IQR], 5 to 79) for group A and 13 days (IQR, 7 to 53) for group B, but this difference was not statistically significant (P = 0.053). Regarding the overall postoperative complications, there were more complications in group B (12.0%) than in group A (6.2%), and there was a statistically significant difference in the percentage of complications (P = 0.047). However, when the severity of the surgical complications was considered, for severe complications with an Accordion Severity Classification grade of more than 3, i.e., those requiring an endoscopic or interventional procedure without general anesthesia (grade 3), those requiring surgical reoperation under general anesthesia (grade 4), and those involving organ system failure (grade 5), there was no statistically significant difference between the two groups (6.5% vs. 3.4%, P = 0.142).
Table 3.
Postoperative outcomes
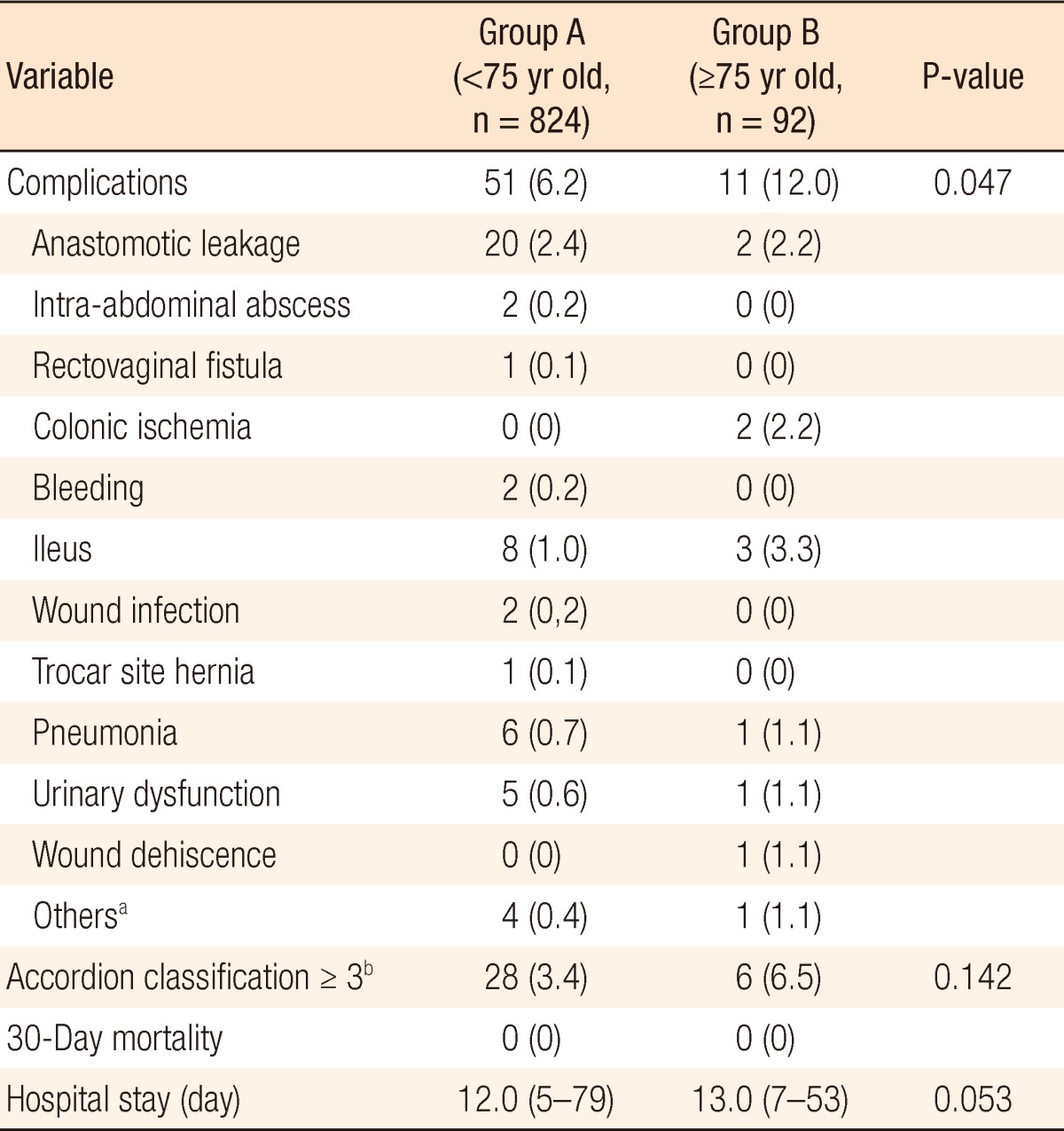
Values are presented as number (%) or median (interquartile range).
aOne small bowel injury, one uncontrolled blood pressure, two chyle drainage, one gastric ulcer bleeding. bAccordion classification ≥3 mean severe complication, requires an invasive procedure.
Results from the multivariate logistic regression revealed that age more than 75 years (odds ratio [OR], 2.71; P = 0.006) and an operation time longer than 360 minutes (OR, 2.47; P = 0.001) were associated with a significant increased risk of overall complications and that an operation time longer than 360 minutes (OR, 3.51; P = 0.001) and diabetes mellitus (OR, 2.97; P = 0.025) were associated with a significant increased risk of severe complications with an Accordion Severity Classification grade of more than 3. Age was not found to be associated with an increased risk of severe complications (Table 4).
Table 4.
Multivariate logistic regression analysis for overall complications and severe complications
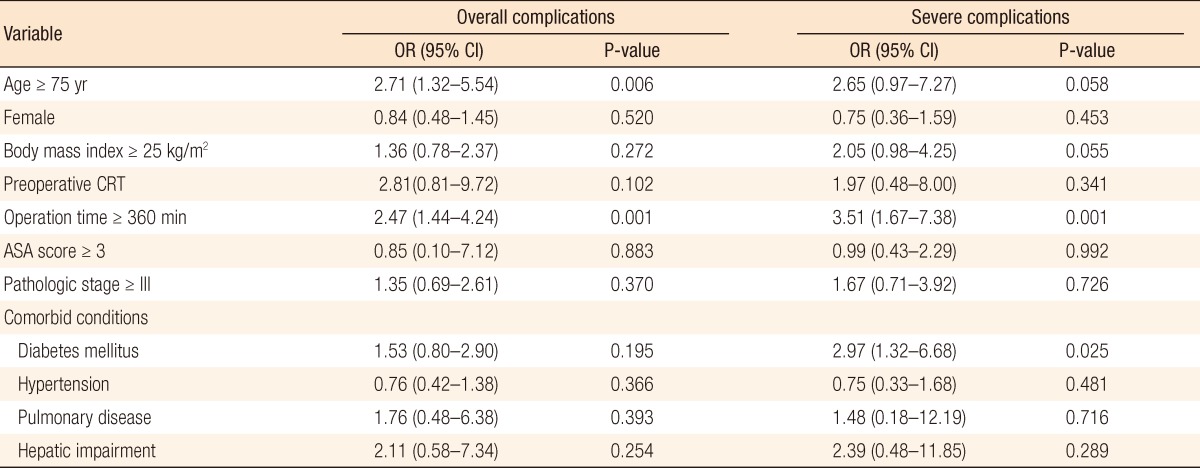
OR, odds ratio; CI, confidence interval; CRT, chemoradiotherapy; ASA, American Society of Anesthesiologists.
Oncological outcomes
The median follow-up time from surgery was 33 months (range, 1 to 65 months). At three years, overall local recurrence was 4.7% (group A, 4.3% vs. group B, 8.0%; P = 0.084), distant metastasis was noted in 12.5% (group A, 12.6% vs. group B, 16.0%; P = 0.264) of the patients, disease-free survival (DFS) was 85.5% (group A, 85.8% vs. group B, 79.4%; P = 0.11), and OS was 90.3% (group A, 91.2% vs. group B, 82.4%; P < 0.001). The local recurrence, distant metastasis and DFS rates, but not the OS rate, were not significantly different between the groups (Table 5).
Table 5.
Kaplan-Meier estimates for oncologic outcomes
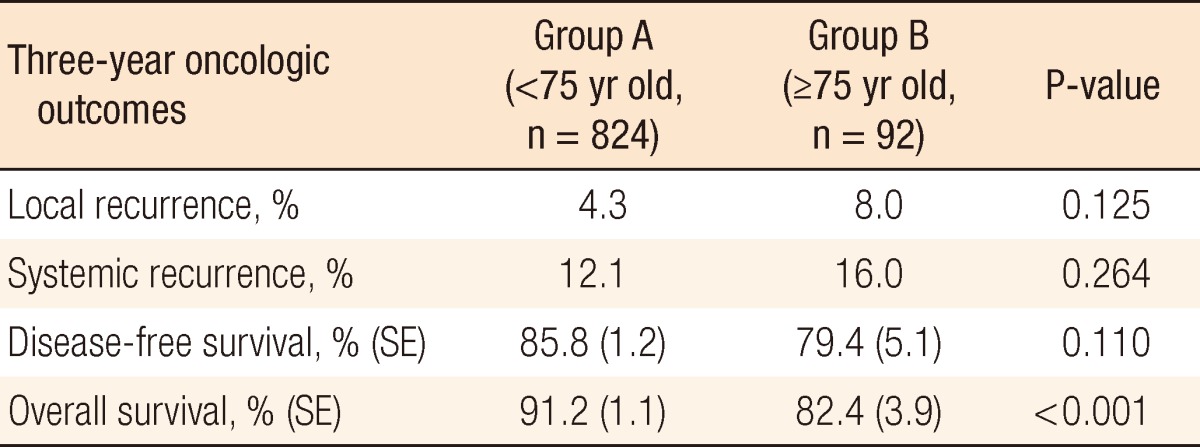
SE, standard error.
Variables identified as significant predictors of overall mortality are listed in Table 6. In the final multivariate Cox proportional hazard analysis, age (P = 0.002; hazard ratio [HR], 2.29), ASA score more than 3 (P = 0.004; HR, 3.89), pathologic stage more than III, i.e., Hypertension (P = 0.019; HR, 1.67), and hepatic impairment (P = 0.002; HR, 3.74) remained significant unfavorable prognostic factors of overall mortality in our study group.
Table 6.
Univariate and multivariate Cox proportional hazard analysis for overall mortality in colorectal cancer patients
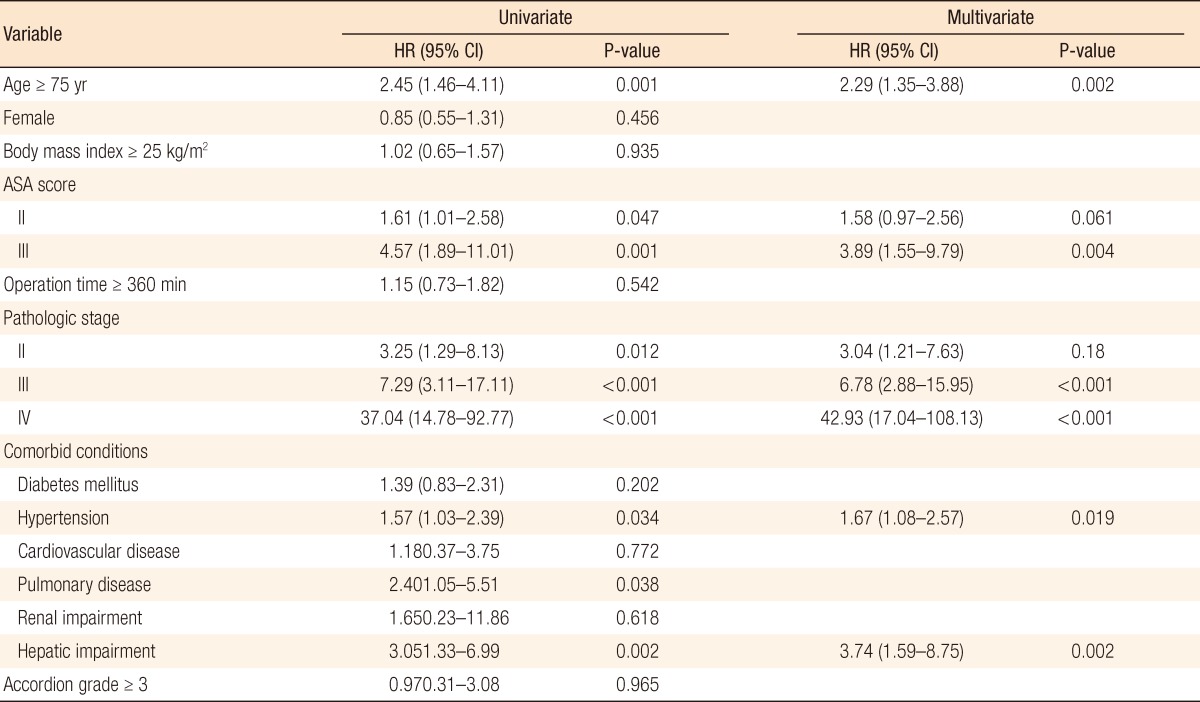
HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiologists.
DISCUSSION
Until now, elderly patients have been shown to have worse clinical outcomes compared to younger patients. In particular, elderly patients with comorbid conditions often have difficulty with anesthesia and recovery. For this reason, patients and their physicians are reluctant to treat colorectal cancer surgically and tend to choose conservative or palliative management. However, minimally invasive surgery has become more prevalent in the field of colorectal surgery because of the advent of laparoscopic surgery and other advances in surgical techniques. Patients treated with these techniques have faster recovery because of smaller incisions, decreased pain, and less blood loss [13-15]. Especially, at Seoul St. Mary's Hospital, Korea, a 102-year-old elderly patient recently underwent laparoscopic colorectal cancer surgery successfully.
Several investigators [14,16-25] have reported in the literature that laparoscopic surgery in elderly patients is less morbid than previous techniques and should be considered more readily for the treatment of colorectal cancer. Yamamoto et al. [26] compared their results in 17 octogenarian patients who underwent laparoscopic colonic surgery for a carcinoma with the results in 34 matched patients, aged 60 or less. Although the ASA status was significantly higher in the octogenarian group (P = 0.001), the authors did not observe any significant difference in terms of the incidence of complications or the length of hospitalization.
Schwandner et al. [27] divided all patients into age-related groups: patients 50 years of age or younger (n = 65), patients ranging from 51 to 70 years of age (n = 138), and patients older than 70 years (n = 95). There were no statistically significant differences among the three groups relative to major complications, minor complications, or total laparotomy rate. However, the duration of operation and the postoperative hospitalization were significantly prolonged in patients older than 70 years.
Nevertheless, controversy as continued because other authors have reported a somewhat higher rate of complications in elderly patients [28-31]. Part of this uncertainty is due to the use of different cutoff definitions for the elderly. Furthermore, with increasing age, whether differences in terms of feasibility, operative time, and postoperative stay really exist between young and elderly patients undergoing a laparoscopic resection for colorectal cancer seems less clear. In the present study, by considering the normal life expectancy, we defined 75 years of age as the threshold between young and elderly patients [1].
Although many methods can be used to classify postoperative complications, we classified complications according to the Accordion Severity Classification of postoperative complications [12], paying particular attention to those classified as grade 3 or higher requiring invasive treatment. As a result, overall complications were more frequent in elderly patients (12.0% vs. 6.2%, P = 0.047). However, when we compared severe complications, there was no significant differences between the two groups (6.5% vs. 3.4%, P = 0.142). These differences are thought to be intrinsic to the nature of the patient, including high comorbidity and ASA score, rather than to be a feature of the laparoscopic procedure itself.
The total operation time in elderly patients tended to be shorter (325.4 minutes vs. 351.6 minutes; P = 0.07). We can postulate that laparoscopic surgery in elderly patients is performed more easily because of their lower average BMI. In addition, in this study, although more overall complications occurred in elderly patients, there were no significant differences either in the numbers of severe complications that required intervention or reoperation or in the lengths of hospital stay between the two groups.
The present study was subject to several limitations. Firsh, this is not a randomized controlled study; a prospective randomized controlled study is needed to demonstrate that laparoscopic surgery in elderly patients is truly a feasible procedure for colorectal cancer. Also, as this study was a single center study, generalizing the results to other patients is potentially limited. In conclusion, laparoscopic surgery for colorectal cancer can be performed as safely in elderly patients as in younger patients and should be considered as the first option in elderly patients undergoing a colorectal resection.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Korean Statistical Information Service [Internet] Daejeon: Statistics Korea; c2010. [cited 2012 Jun 15]. Statistics Korea. Available form: http://kosis.kr. [Google Scholar]
- 2.Alves A, Panis Y, Mathieu P, Mantion G, Kwiatkowski F, Slim K, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140:278–283. doi: 10.1001/archsurg.140.3.278. [DOI] [PubMed] [Google Scholar]
- 3.Fielding LP, Phillips RK, Hittinger R. Factors influencing mortality after curative resection for large bowel cancer in elderly patients. Lancet. 1989;1:595–597. doi: 10.1016/s0140-6736(89)91618-8. [DOI] [PubMed] [Google Scholar]
- 4.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 6.Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–1192. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 7.Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Jr, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655–662. doi: 10.1097/SLA.0b013e318155a762. [DOI] [PubMed] [Google Scholar]
- 8.Liang JT, Huang KC, Lai HS, Lee PH, Jeng YM. Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann Surg Oncol. 2007;14:109–117. doi: 10.1245/s10434-006-9135-4. [DOI] [PubMed] [Google Scholar]
- 9.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 10.Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 11.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 12.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 13.Schwenk W, Bohm B, Muller JM. Postoperative pain and fatigue after laparoscopic or conventional colorectal resections: a prospective randomized trial. Surg Endosc. 1998;12:1131–1136. doi: 10.1007/s004649900799. [DOI] [PubMed] [Google Scholar]
- 14.Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 15.Delaney CP, Kiran RP, Senagore AJ, Brady K, Fazio VW. Case-matched comparison of clinical and financial outcome after laparoscopic or open colorectal surgery. Ann Surg. 2003;238:67–72. doi: 10.1097/01.sla.0000074967.53451.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stocchi L, Nelson H, Young-Fadok TM, Larson DR, Ilstrup DM. Safety and advantages of laparoscopic vs. open colectomy in the elderly: matched-control study. Dis Colon Rectum. 2000;43:326–332. doi: 10.1007/BF02258297. [DOI] [PubMed] [Google Scholar]
- 17.Law WL, Chu KW, Tung PH. Laparoscopic colorectal resection: a safe option for elderly patients. J Am Coll Surg. 2002;195:768–773. doi: 10.1016/s1072-7515(02)01483-7. [DOI] [PubMed] [Google Scholar]
- 18.Degiuli M, Mineccia M, Bertone A, Arrigoni A, Pennazio M, Spandre M, et al. Outcome of laparoscopic colorectal resection. Surg Endosc. 2004;18:427–432. doi: 10.1007/s00464-002-9267-y. [DOI] [PubMed] [Google Scholar]
- 19.Chautard J, Alves A, Zalinski S, Bretagnol F, Valleur P, Panis Y. Laparoscopic colorectal surgery in elderly patients: a matched case-control study in 178 patients. J Am Coll Surg. 2008;206:255–260. doi: 10.1016/j.jamcollsurg.2007.06.316. [DOI] [PubMed] [Google Scholar]
- 20.Frasson M, Braga M, Vignali A, Zuliani W, Di Carlo V. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum. 2008;51:296–300. doi: 10.1007/s10350-007-9124-0. [DOI] [PubMed] [Google Scholar]
- 21.Person B, Cera SM, Sands DR, Weiss EG, Vernava AM, Nogueras JJ, et al. Do elderly patients benefit from laparoscopic colorectal surgery? Surg Endosc. 2008;22:401–405. doi: 10.1007/s00464-007-9412-8. [DOI] [PubMed] [Google Scholar]
- 22.Fiscon V, Portale G, Frigo F, Migliorini G. Laparoscopic resection of colorectal cancer: matched comparison in elderly and younger patients. Tech Coloproctol. 2010;14:323–327. doi: 10.1007/s10151-010-0635-7. [DOI] [PubMed] [Google Scholar]
- 23.Roscio F, Bertoglio C, De Luca A, Frigerio A, Galli F, Scandroglio I. Outcomes of laparoscopic surgery for colorectal cancer in elderly patients. JSLS. 2011;15:315–321. doi: 10.4293/108680811X13125733357070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan KY, Konishi F, Kawamura YJ, Maeda T, Sasaki J, Tsujinaka S, et al. Laparoscopic colorectal surgery in elderly patients: a case-control study of 15 years of experience. Am J Surg. 2011;201:531–536. doi: 10.1016/j.amjsurg.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Law WL, Poon JT, Fan JK, Lo OS. Survival following laparoscopic versus open resection for colorectal cancer. Int J Colorectal Dis. 2012;27:1077–1085. doi: 10.1007/s00384-012-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Watanabe M, Hasegawa H, Baba H, Kitajima M. Short-term surgical outcomes of laparoscopic colonic surgery in octogenarians: a matched case-control study. Surg Laparosc Endosc Percutan Tech. 2003;13:95–100. doi: 10.1097/00129689-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Schwandner O, Schiedeck TH, Bruch HP. Advanced age: indication or contraindication for laparoscopic colorectal surgery? Dis Colon Rectum. 1999;42:356–362. doi: 10.1007/BF02236353. [DOI] [PubMed] [Google Scholar]
- 28.Bufalari A, Ferri M, Cao P, Cirocchi R, Bisacci R, Moggi L. Surgical care in octogenarians. Br J Surg. 1996;83:1783–1787. doi: 10.1002/bjs.1800831239. [DOI] [PubMed] [Google Scholar]
- 29.Isbister WH. Colorectal surgery in the elderly: an audit of surgery in octogenarians. Aust N Z J Surg. 1997;67:557–561. doi: 10.1111/j.1445-2197.1997.tb02038.x. [DOI] [PubMed] [Google Scholar]
- 30.Payne JE, Chapuis PH, Pheils MT. Surgery for large bowel cancer in people aged 75 years and older. Dis Colon Rectum. 1986;29:733–737. doi: 10.1007/BF02555321. [DOI] [PubMed] [Google Scholar]
- 31.Spivak H, Maele DV, Friedman I, Nussbaum M. Colorectal surgery in octogenarians. J Am Coll Surg. 1996;183:46–50. [PubMed] [Google Scholar]


