Cord blood (CB) has been used as an alternative source for unrelated stem cell transplantation (SCT) for patients with hematologic malignances, bone marrow failure syndromes, and immune deficiencies. Since the first patient (a child with Fanconi anemia) was successfully treated with CB transplantation (CBT) in 1988, over 20,000 patients worldwide have received unrelated CBT. In Korea, approximately 500 patients have received unrelated CBT since its first successful use in a patient with acute leukemia in 1998 [1]. Disadvantages of the treatment, such as limited cell doses, slow engraftment, and a high rate of graft failure, were major obstacles in the early CBT era, although several advantages over conventional bone marrow transplantation (BMT) and peripheral blood stem cell (PBSC) transplantation (PBSCT) have been reported. However, the findings from many clinical trials and primary research studies have led to substantial improvement in the availability and outcomes of CBT. Most recent studies of unrelated CBT have shown outcomes comparable to those of unrelated BMT or PBSCT [2, 3]. Therefore, CB is no longer just an alternative source for unrelated SCT; furthermore, unrelated donors for SCT have to be selected according to patient status because there are advantages and disadvantages associated with different stem cell sources.
Although the clinical availability of CB has been expanded, and because it is expected to be a good source for unrelated SCT, several problems need to be solved before the utilization of banked CB can be increased, and before it can become a good option for physicians who have to select optimal unrelated stem cell donors. Recently, the CB Committee of the Korean Society of Blood & Marrow Transplantation conducted an on-site questionnaire study targeting transplant physicians and nurses to determine the current problems associated with the low utilization rate of banked CB. On the basis of this ongoing study, I would like to present a proposal for improving the utilization rate of banked CB.
First, it is essential to manage the quality of banked CB and to gain transplant physicians' trust in its use. Although most CB banks have their own standard operating procedure based on international standards, all CB banks should undergo scheduled inspections from an international organization such as the Foundation for the Accreditation of Hematopoietic Cell Therapy or a national organization according to the relevant laws in each country. Several countries including Korea and Japan have enacted laws for CB management. In Korea, the establishment of the CB Act has been effective in establishing a public CB banking program and managing the quality of banked CB; as a result, one of the largest pre-existing CB banks was found not to comply with the regulations and was closed down. An objective inspection program is needed to ensure that the trust of transplant physicians' is gained for using foreign and domestic banked CB as an important source for life-saving treatments.
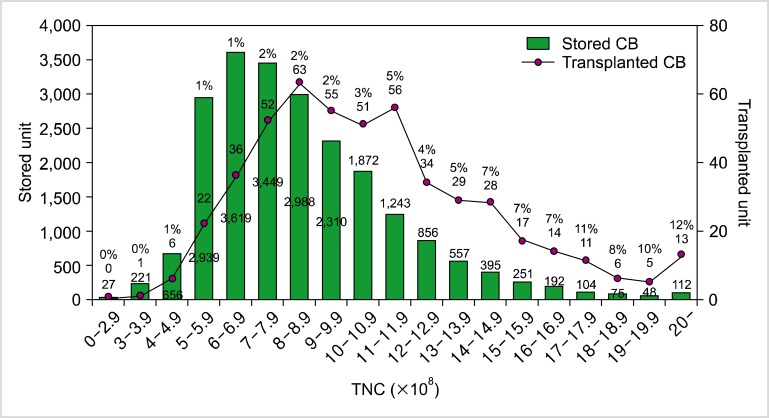
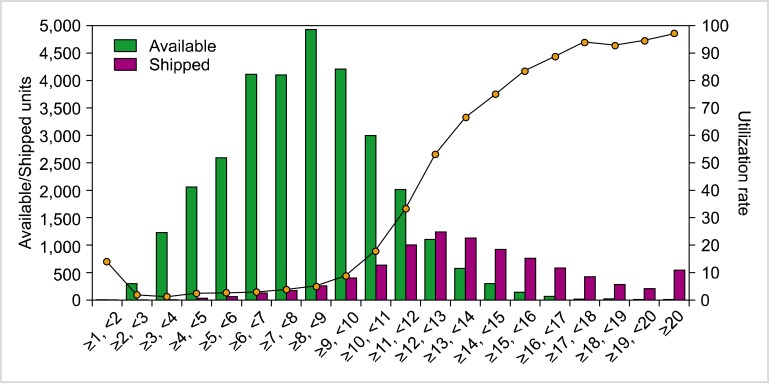
Second, the standards for banking CB have to be raised so that greater use can be made of banked CB. The approximate rates of the utilization of banked public CB are as follows: 3-4% (20,000/450,000-600,000) in the World Marrow Donor Association, 3% (4,800/122,000) in the National Marrow Donor Program (until 2010), 29% (8,948/30,779) in Japan (until 2012), and 1.3% (626/47,808) in Korea (until 2012). Although there are other factors that affect utilization rate, the cell dose of banked CB is an important consideration for transplant physicians. According to recent data from the Korean Network for Public Cord Blood Banks (KoreaCORD), only 1% of the transplanted CB units are derived from stored CB units with total nucleated cell (TNC) counts of 5-5.9×108; the proportion steadily increases up to 12% for stored CB units with TNC counts of 20×108 or more. In addition, although around half (45.7%) of stored CB units have TNC counts of 5-7.9×108, only 22.0% of all transplanted CB was derived from these stored units. In spite of the young age and low body weights of most CBT recipients in Korea, 78% of CB units selected for transplantation had TNC counts exceeding 8×108; even in cases of double CBT, 74.4% of the transplanted CB units had TNC counts of at least 8×108 (Fig. 1). On the basis of these data, we have decided to increase the minimum CB TNC count necessary to be banked for public purposes from 5×108 to 8×108 [4]. The utilization rates of banked CB in Japan are comparable to our data (Fig. 2).
Fig. 1.
The number of stored and transplanted CB units in Korea-CORD according to TNC count.
Fig. 2.
The number of available and shipped CB units along with the utilization rate according to TNC count (kindly provided by Takanashi at Tokyo Cord Blood Bank).
Third, the releasing cost is an important consideration in CB utilization. It would be difficult to estimate a reasonable releasing cost on the basis of the costs of processing and storing CB, which depend on the funding source for public CB banking programs. In a comparison of releasing costs for CB, BM, and PBSC in the United States, the costs for CB (21,000-45,000 USD) were comparable to those for unrelated BM (33,840 USD) and PBSC (30,885 USD). The releasing cost for CB in Taiwan (300,000 NTD) was greater than that for BM (120,000 NTD) and PBSC (120,000 NTD). On the contrary, CB releasing is free of charge in Japan, where it is covered by governmental funding. In Korea, although the releasing cost for CB (8,000,000 KW for 1 unit, 12,000,000 KW for 2 units) has been adjusted to that for unrelated BM or PBSC donation, it feels expensive for patients, even for recipients of 2 units. Because Korea enacted the CB Act in 2010 for national funding as well as strict quality control of CB banks, and because a national inventory of public CB units is supported by the government budget [5], the releasing cost for CB needs to be decreased or eliminated as in Japan. In our questionnaire study, most physicians (92.5%) reported difficulties in using CB for unrelated SCT associated with high cost, even when they identified transplantable CB units; these physicians expressed a desire for the releasing costs for CB to be decreased.
In conclusion, a strict and objective inspection program to maintain the quality of banked CB and the storage of high cell doses of CB must be in place before the utilization rate of banked CB can be improved. Furthermore, a reduction in the releasing cost for CB units would improve the use of banked CB.
ACKNOWLEDGMENTS
This study was supported by a grant of Korea Healthcare Technology R&D Project (A101712), Ministry for Health & Welfare, Republic of Korea. This study was supported by a grant of the Research Project, Seoul Metropolitan Public Cord Blood Bank (2012-001).
References
- 1.Park M, Lee SH, Lee YH, et al. Pre-engraftment syndrome after unrelated cord blood transplantation: a predictor of engraftment and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.01.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 3.Hwang WY, Samuel M, Tan D, Koh LP, Lim W, Linn YC. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13:444–453. doi: 10.1016/j.bbmt.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Lee YH, Kwon YH, Hwang K, et al. Analysis of stored and transplanted cord blood units from KoreaCORD: reappraisal of banking guidelines and selection strategy. Transfusion. 2013;53:123–127. doi: 10.1111/j.1537-2995.2012.03712.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee YH. The prospect of the government management for cord blood in Korea -at the time of enactment of the 『Cord blood management and research act』. Korean J Hematol. 2010;45:1–2. doi: 10.5045/kjh.2010.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]