Abstract
Recent years have substantially broadened our view on the pathogenesis of multiple sclerosis (MS). While earlier concepts focused predominantly on T lymphocytes as the key cell type to mediate inflammatory damage within central nervous system (CNS) lesions, emerging evidence suggests that B lymphocytes may play a comparably important role both as precursors of antibody-secreting plasma cells and as antigen-presenting cells (APCs) for the activation of T cells. With greater appreciation of this pathogenic B-cell function in MS, B-cell-directed therapies, and in particular B-cell-depleting monoclonal antibodies targeting the CD20 molecule, have gained enormous interest over recent years. Clinical trials demonstrated that anti-CD20 treatment, which depletes immature and mature B cells but spares CD20 negative plasma cells, rapidly reduces formation of new inflammatory CNS lesions. While these findings clearly corroborate a pathogenic contribution of B cells, recent experimental but also clinical findings indicate that not all B cells contribute in an equally pathogenic manner and that certain subsets may in contrast mediate anti-inflammatory effects. In this review, we summarize current findings in support of pathogenic B-cell function in MS, including the encouraging clinical data which derived from anti-CD20 MS trials. Further, we review novel findings suggestive of regulatory properties of B-cell subsets which may be collaterally abolished by pan-CD20 depletion. In conclusion, we aim to provide an outlook on how this currently differentiating concept of pro- and anti-inflammatory B-cell function could be harnessed to further improve safety and effectiveness of B-cell-directed therapeutic approaches in MS.
Keywords: Multiple sclerosis, B cell, anti-CD20, experimental autoimmune encephalomyelitis, antibody, cellular subsets, intrathecal, immune phenotyping, therapy
Introduction
B cells may contribute to multiple sclerosis (MS) pathogenesis in more than one way; they are the source of differentiating plasma cells which secrete autoreactive antibodies possibly contributing to demyelination within the inflamed central nervous system (CNS). Earlier maturation stages as well as memory B cells may provide specifically recognized antigen to other antigen-presenting cells (APCs), or alternatively, directly present processed antigen in the context of constitutively expressed major histocompatibility complex (MHC) class II. Emerging evidence suggests that, in addition, B cells are potent regulators of ongoing immune processes capable of providing both pro- and anti-inflammatory cytokines. In the first part of this review, we describe these various B-cell functions in order to provide the basis to appreciate, but also critically evaluate current B-cell-directed therapeutic approaches.
In the second part, we recapitulate existing clinical data on the effectiveness of antibody-removing therapeutic plasma exchange (PE) and summarize the findings obtained from recent clinical MS trials testing anti-CD20 B-cell depletion. In addition, we provide experimental and clinical data suggesting that not all patients may equally benefit from B-cell-directed therapeutic approaches based on possible differences in MS pathogenesis, and that not all B cells expressing CD20 may be equally pathogenic.
Based on the emerging concept of pro- and anti-inflammatory B-cell functions in conjunction with the clinical data obtained to date, we lastly discuss how these findings could be possibly utilized to further ‘fine tune’ B-cell-directed therapeutic approaches in MS.
The role of B cells in multiple sclerosis
It is instrumental to recognize that the role of B cells in MS is diverse. While antigen-activated B cells differentiate into antibody-secreting plasma cells and serve as potent APCs, naïve B cells are substantially weaker APCs and may exert anti-inflammatory properties modulating effector function of other immune cells. Further, it is likely that subgroups of patients with MS differ in their extent of pathogenic B-cell involvement; while CNS lesions in the majority of individuals consistently contain B cells, plasma cells and antibodies, other, histologically distinct subtypes rather display a primary oligodendrocyte dystrophy without an apparent pathogenic contribution of B cells or B-cell-derived products [Lucchinetti et al. 2000]. Which respective role(s) B cells may play in a patient with MS could thus relate to the individual’s B-cell repertoire, activation and maturation status at a given time point as well as to the underlying pathogenic subtype.
B cells as precursors of antibody secreting plasma cells
Among possible pathogenic B-cell functions in MS, the role of antibodies produced by terminally differentiated plasma cells has been studied most extensively [Weber et al. 2011] (Figure 1). An oligoclonal antibody response generated by a limited repertoire of activated B cells remains a hallmark diagnostic finding in the cerebrospinal fluid (CSF) [Obermeier et al. 2008; von Budingen et al. 2010]. Since 1942, when these oligoclonal bands were fist described by Kabat and colleagues [Kabat et al. 1942], numerous investigations attempted to identify their target antigens. Some findings implicated that these antibodies may eventually recognize components of the myelin sheath; however no unequivocal evidence of such CNS reactivity has been established to date. While the occurrence of oligoclonal antibodies in the CSF is thus tightly associated with the diagnosis of MS, it is still unclear whether these immunoglobulins actively contribute to pathogenesis or progression of the disease.
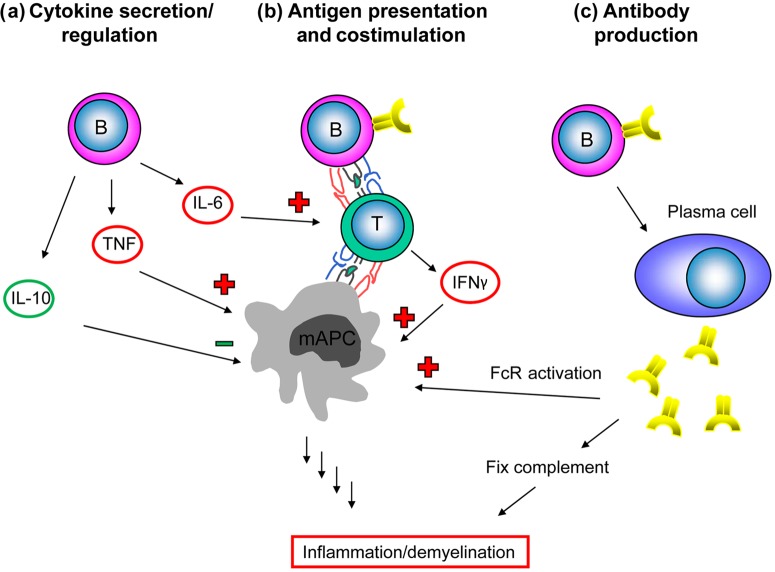
Figure 1.
The role of B cells in multiple sclerosis. B cells act as (a) potent producers of regulating cytokines, (b) antigen-presenting cells (APCs) for the activation of T cells and (c) precursors of antibody-secreting plasma cells. FcR, Fc receptor; IFNγ, interferon γ; IL-6/10, interleukin 6/10; mAPC, myeloid APC; TNF, tumor necrosis factor.
Further evidence for a role of antibodies in MS derives from histopathological studies in which B cells, as well as B cell-derived plasma cells and antibodies are found in a majority of inflammatory CNS plaques. Molecular analyses of B cells in brain lesions demonstrated an accumulation of clonotypic B cells, reflective of a restricted immune response. Investigated sequences further showed signs of hypermutations, indicating an ongoing and maturating B-cell response to a target antigen within the local compartment [Baranzini et al. 1999; Owens et al. 1998, 2001]. Following antigen recognition, B cells mature to plasma blasts and plasma cells, which produce large amounts of antibodies. As indicated above, histopathological studies revealed a distinct pattern of lesion pathology. Importantly, all lesions of an individual patient appear to belong to one of these subtypes [Lassmann et al. 2001; Lucchinetti et al. 2000]. The most frequently found pattern of lesion pathology is characterized by significant antibody deposits and complement activation, suggesting that the locally produced antibody response may indeed contribute to CNS demyelination [Breij et al. 2008].
The role of antibodies in development and progression of CNS autoimmune disease has been intensively investigated in the animal model of MS, experimental autoimmune encephalomyelitis (EAE), which enables to dissect the role of immune components in a mechanistic manner [Steinman and Zamvil, 2006]. Similar to most active CNS lesions in patients with MS, B cells, plasma cells and antibodies are found in areas of myelin breakdown in EAE [Merkler et al. 2006]. Due to the primarily demyelinating nature of inflammatory CNS lesions, antigens within the myelin sheath are the prevalent putative targets studied. Within various candidate myelin antigens, myelin oligodendrocyte glycoprotein (MOG) is the one that is investigated most due to its extracellular location on the outermost myelin lamellae [Lalive et al. 2011]. This location makes it an exposed target accessible to an initial autoimmune attack against the intact myelin sheath. Immunization with MOG intact protein induces fulminant EAE in rodents and nonhuman primates, which is associated with a robust antibody response against MOG. Mechanistically, it is clear that these antibodies alone are not capable of initiating EAE in otherwise naïve animals. However, certain studies suggest that they may facilitate CNS damage and promote progression of ongoing EAE [Benkhoucha et al. 2012]. More than 20 years ago, Schluesener and colleagues reported that intravenous administration of a monoclonal antibody against MOG enhanced CNS demyelination and induced fatal relapses in a rodent disease model [Schluesener et al. 1987]. Notwithstanding this seminal observation, it should be noted that the antibody was injected at a quantity that cannot be produced endogenously. Further evidence for a pathogenic role of antibodies in CNS autoimmune disease derives from experiments in B-cell-deficient mice. Whereas B cells or antibodies are not required when mice are immunized with the short encephalitogenic T-cell determinant peptide (p)35-55, B-cell-deficient mice were found to be resistant to EAE induced by active immunization with human recombinant MOG protein [Lyons et al. 1999]. Reconstitution with the antigen-specific antibody was sufficient to restore EAE susceptibility, suggesting a crucial role for antibody-mediated antigen recognition in this model [Lyons et al. 2002]. In order to study the role of MOG-specific antibodies which are endogenously produced, a transgenic mouse model was engineered to express the rearranged heavy chain of a pathogenic antibody recognizing a conformational determinant of MOG. Strikingly, these mice contained high titers of MOG antibodies, and developed severe EAE with greater inflammation and demyelination compared with wild-type mice [Litzenburger et al. 1998]. However, it is important to note that the MOG-recognizing antibody was not only secreted by B-cell-derived plasma cells, but also expressed as membrane-bound B-cell receptor (BCR). Accordingly, the observed enhancement of EAE severity and CNS demyelination in these studies may indicate a pathogenic role for plasma cell-secreted myelin-specific antibodies; alternatively, although not mutually exclusive, accelerated EAE severity may reflect a pathogenic role for myelin-specific B cells in processing and presentation of myelin antigen to encephalitogenic T cells.
B cells as antigen-presenting cells for activation of T cells
Besides differentiating into antibody-secreting plasma cells, B cells may contribute to the development and progression of CNS autoimmune disease as APCs for activation of T cells [Weber and Hemmer, 2010] (Figure 1). Encephalitogenic T helper 1 (Th1) and Th17 cells are thought to play a central role in the pathogenesis of CNS autoimmune disease. In general, activation of CD4+ T cells requires recognition of antigen in the context of MHC class II molecules, which are constitutively expressed on professional APCs, such as dendritic cells, monocytes/macrophages but also B cells. T-cell antigen recognition has to occur twice, once in the periphery, which enables activated T cells to infiltrate the CNS, and another time within the CNS, which triggers and directs encephalitogenic T-cell effector function. Several types of APCs may contribute to T-cell activation in CNS autoimmune disease. They can be generally divided into resident (CNS) and nonresident (bone-marrow-derived) APCs. Among resident APCs, astrocytes and parenchymal microglia could potentially participate [Fontana et al. 1984; Soos et al. 1998], whereas their in vivo contribution is still under debate [Stuve et al. 2002]. In our current understanding, nonresident, peripheral APCs in contrast have the more important role in initiation of CNS demyelinating disease. All subtypes of bone-marrow-derived APCs, B cells, macrophages and dendritic cells are found in EAE and active MS lesions. In EAE, it has been demonstrated that MHC class II restricted antigen presentation by dendritic cells is sufficient to induce CNS autoimmunity [Greter et al. 2005], suggesting that antigen presentation by other APCs, resident and nonresident, is not required. However, these data do not exclude that other APCs contribute to MHC II restricted activation of T cells or even be required in another setting. Whereas dendritic cells are the most potent APCs for presentation of peptide antigen, antigen-specific B cells are specialized to process and present protein antigen which they recognize through their BCR [Constant et al. 1995a, 1995b; Lanzavecchia, 1985; van der Veen et al. 1992]. Hereby, they are specialized to serve as efficient APCs for the activation of T cells, particularly when the amount of antigen is limited [Rivera et al. 2001]. A detailed study further elucidated the role of B cells as APCs and the reciprocal stimulation of B cells by activated T cells [Harp et al. 2008]; the authors elegantly demonstrated that activation of B cells by a combination of the T-cell products CD40L and interleukin (IL)-4, but not by unspecific stimuli such as a toll-like receptor ligand and IL-2-rendered B cells capable of activating T cells in a myelin-antigen-specific manner. These data indicate that T-cell-mediated activation of B cells is crucial for their capability to process and present antigen to T cells and that both populations thereby stimulate each other in a reciprocal manner.
Recent findings suggest that this interplay between activated B and T cells may progressively shift into the CNS throughout the chronic disease course of MS. This development appears to be promoted by B-cell-fostering chemokines such as CXCL13, BAFF and APRIL, which are paradoxically provided by the inflamed CNS itself [Mackay et al. 2003; Krumbholz et al. 2005; Kowarik et al. 2012]. Evidence for compartimentalized pathogenic B-cell function was primarily derived from the CNS histological analysis of patients at a later stage of MS. In many, but not all cases, organized B-cell structures which are found physiologically solely in secondary lymphoid organs could be identified within the CNS [Serafini et al. 2004]. Correlation with clinical parameters further suggested that these follicles, which are predominantly located within the meninges, could be associated with a more severe secondary-progressive disease course [Magliozzi et al. 2007]. Occurrence of such ectopic follicles is generally suggestive of B-cell replication and activation within the inflamed target organ. Accordingly, at a later disease stage, pathogenic B-cell properties may primarily relate to these meningeal B-cell follicles and rely gradually less on infiltration of peripherally activated B cells into the CNS. Such disease- and stage-specific compartmentalization of B-cell function not only further supports involvement of B cells and B-cell-derived products in development and progression of MS, but is even more so instrumental for the intent to target pathogenic B-cell function throughout the chronic course of MS therapeutically.
Cytokine-producing B-cell (subsets) with pro- and anti-inflammatory properties
Besides acting as potent APCs and a source for plasma cells producing CNS demyelinating antibodies, B cells may regulate inflammatory processes through provision of pro- but also anti-inflammatory cytokines (Figure 1). On the proinflammatory side, activated B cells produce substantial amounts of IL-6, which is in return crucial for development of encephalitogenic Th17 cells [Korn et al. 2008]. A recent report suggests that IL-6 production by B cells is a major pathogenicity factor for B cells and that, intriguingly, B cells from patients with MS contain a higher frequency of activated IL-6-producing subsets [Barr et al. 2012].
Not all B cells and B-cell subsets may, however, unequivocally contribute to CNS autoimmune disease in a pathogenic manner. As APCs, distinct B-cell subsets appear to preferentially facilitate development of pro- or anti-inflammatory T-cell subsets. Such functional dichotomy has been well established for other APCs, such as dendritic cells. In one classification, two subsets of B cells, peritoneal B1 and conventional follicular B2 cells, can be distinguished. They differ in respect to lineage, location, gene expression, antibody repertoire, proliferative response and immunoglobulin secretion. B1 cells are the predominant population of B cells in the peritoneal cavity, while they are rather rare in secondary lymphoid organs [Hardy, 2006]. B1 cells are thought to participate in autoimmunity, presumably through potent antigen presentation [Sato et al. 2004]. When used as APCs, B1 cells indeed governed generation of Th1 and Th17 cells whereas conventional B2 cells promoted development of induced regulatory T cells with suppressive capacity [Zhong et al. 2007]. These findings highlight that, similar to subsets of dendritic cells, B-cell subsets may be capable of fostering development of anti-inflammatory T cells, which may relate to their respective expression of costimulatory molecules and T-cell-polarizing cytokines.
Besides generation of T cells with anti-inflammatory effector function, B cells may further exert regulatory properties through provision of anti-inflammatory IL-10. Animal models of human autoimmunity suggest that, in particular, nonactivated, naïve B cells regulate autoimmune responses [Fillatreau et al. 2002] and control proinflammatory differentiation of other APCs [Moulin et al. 2000]. A recent report suggests that development of IL-10-producing B cells could be further fostered by an IL-21-dependent mechanism [Yoshizaki et al. 2012]. In EAE, B-cell-deficient mice failed to recover from acute EAE, suggesting that B cells may have the ability to support recovery and prevent progression to chronic or relapsing CNS autoimmune disease [Wolf et al. 1996]. In another study, mice that contained B cells unable to produce IL-10 did not recover from EAE either. When these mice were reconstituted with IL-10-competent B cells, they again recovered from EAE, demonstrating that B-cell-produced IL-10 plays a key role in recovery from an acute attack of CNS autoimmunity [Fillatreau et al. 2002]. Accumulating evidence suggests that equivalent regulatory B-cell properties exist in humans as well [Mauri and Blair, 2010]. In a recent report, Iwata and colleagues described a subset of IL-10-producing B cells in healthy individuals and patients with various autoimmune conditions. Among the later, this regulatory B-cell subset was also found in patients with MS. Frequency and IL-10 production of these B cells were comparable to healthy individuals [Iwata et al. 2011]. Functionally, these regulatory B cells inhibited tumour necrosis factor (TNF) release of monocytes isolated from the identical patient, further fueling the concept that regulatory B-cell subsets control proinflammatory activity of other APCs.
Collectively, these findings indicate that, besides serving as efficient APCs and a source of antibody-secreting plasma cells, B-cell subsets regulate autoimmune processes through fostering development of anti-inflammatory T cells and suppression of proinflammatory APC activity. This scenario epitomizes the complexity of B-cell function in CNS autoimmune disease and highlights the possibility that unselective B-cell-directed therapeutic approaches may collaterally eradicate pre-existing B-cell regulation.
B-cell-directed therapeutic approaches
Plasmapheresis
Historically, plasmapheresis was the first therapeutic strategy for treatment of MS directed specifically against B-cell components, specifically B-cell-derived antibodies. Mechanistically, it conceptualizes a possible pathogenic role of antibodies in MS and accordingly lowers their abundance. The first report of efficacy of therapeutic PE in MS goes back to 1980, when Dau and colleagues evaluated this therapeutic approach in patients with both acute and progressive forms of MS [Dau et al. 1980]. Since then, several studies investigated the usefulness of PE in progressive forms of MS, mostly as a reflection of the general lack of therapies for these patients. Unfortunately, none of these reports could substantiate a distinct benefit of PE in these conditions [Vamvakas et al. 1995]. In contrast, a clear clinical benefit could be established for early treatment of acute inflammatory CNS demyelinating disease. In the pivotal trial of Weinshenker and colleagues, 8 out of 19 patients undergoing an acute relapse substantially improved versus 1 out of 19 in the control group [Weinshenker et al. 1999]. Based primarily on these data, PE is considered a rescue therapy for steroid-refractory relapses [Seifert et al. 2012]. Interestingly, the relative clinical benefit in treatment of this condition seems to vary considerably between individual patients. A study comparing the CNS lesion subtype and responsiveness to PE treatment revealed that patients with the B-cell- and antibody-dominated lesion subtype II clinically improved, whereas patients with other subtypes failed to do so [Keegan et al. 2005]. While this important finding again supports the pathogenic role of antibodies in many patients with MS, it also underscores the importance of treating subtypes of patients with MS differently. In an approach to identify predictive parameters besides histology, a recent study suggests that clinical and radiographic features, such as a lower Expanded Disability Status Scale score and the presence of a ring-enhancing lesion on magnetic resonance imaging (MRI) scans, may predict the clinical response to PE [Magana et al. 2011].
Anti-CD20-mediated B-cell depletion
Based on the capability of B cells to serve as potent APCs for activation of encephalitogenic T cells but also to develop into plasma cells secreting pathogenic antibodies, substantial interest has developed for testing B-cell depletion as a therapeutic strategy in MS. The target molecule of B-cell depleting antibodies is CD20, a surface molecule that well characterizes the B-cell lineage. CD20 is expressed throughout B-cell maturation starting from pre B cells up to memory B cells and is only lost upon their terminal differentiation into plasma cells. Rituximab and its further humanized successor ocrelizumab are monoclonal antibodies against CD20 which efficiently deplete circulating immature and mature B cells, but spare CD20-negative plasma cells. Effector mechanisms of anti-CD20 antibodies are complement dependent or cellular cytotoxicity as well as induction of B-cell apoptosis. Originally, rituximab was generated for the treatment of non-Hodgkin’s B-cell lymphoma and was approved for this indication in 1997. In 2006, anti-CD20 rituximab was also approved for the treatment of rheumatoid arthritis refractory to TNFα blocking agents.
Recent clinical trials testing rituximab and its successor ocrelizumab in the treatment of MS generated encouraging results; in the initial double-blind, placebo-controlled phase II trial rituximab-mediated depletion of B cells led to a rapid decline in newly developing gadolinium-enhancing inflammatory CNS lesions in patients with relapsing remitting MS (RRMS) [Hauser et al. 2008]. This 48-week trial included 104 patients, 69 of whom received rituximab (a single course administered intravenously 1000 mg on days 1 and 15), while 35 patients received placebo. While this phase II trial was not primarily designed to generate data on clinical effectiveness, rituximab treatment was also associated with a significant reduction in the number of relapses. Another double-blind, placebo-controlled phase II/III trial tested rituximab in patients with primary progressive MS. A total of 439 patients were enrolled who received rituximab (two infusions of 1000 mg each, 2 weeks apart) or placebo every 24 weeks through week 96. While the primary endpoint, time to confirmed disease progression, was not reached, B-cell depletion significantly reduced lesion formation in a subgroup of younger patients with active CNS inflammation [Hawker et al. 2009]. Most recently, data on the further humanized anti-CD20 antibody ocrelizumab in the treatment of RRMS was reported. This 24-week, placebo-controlled and active comparator phase II study included 220 patients who were randomized to one of four arms: 600 mg ocrelizumab, 2000 mg ocrelizumab (two infusions on days 1 and 15), placebo or 30 μg interferon β1a intramuscularly every week as an open-label arm. Similar to the initial trial using rituximab, ocrelizumab significantly reduced the development of newly developing gadolinium-enhancing CNS lesions as well as the calculated annualized relapse rate compared with placebo and the active interferon β1a arm [Kappos et al. 2011].
Both clinical trials using rituximab or ocrelizumab in RRMS showed a rapid effect on lesion development. This immediate and robust effect suggests that abolishment of cellular B-cell functions like antigen presentation or cytokine production rather than a secondary decline in titers of potentially pathogenic antibodies may account for the clinical effectiveness of anti-CD20 in MS. Indeed, contrary to the initial concept, anti-CD20 treatment was not even associated with a significant reduction in immunoglobulin in these MS trials [Hauser et al. 2008]. To date, several preclinical and clinical immunologic studies aimed to address the mechanism of anti-CD20 in the treatment of CNS autoimmune disease. In EAE induced by recombinant MOG protein, the clinical benefit of anti-CD20 was associated with a reduced frequency of Th1 and Th17 cells both in peripheral immune compartments as well as in the CNS [Weber et al. 2010]. In the same study it could be demonstrated that B cells, when activated in an antigen-specific manner, can serve as potent APCs, suggesting in conjunction that anti-CD20 may abolish clinically relevant B-cell APC function. These preclinical findings were paralleled and confirmed by human studies. In patients with RRMS, anti-CD20 B-cell depletion similarly diminished proliferation and proinflammatory differentiation of peripheral T cells [Bar-Or et al. 2010], again pointing towards abrogation of APC function as the primary mechanism. Interestingly, rituximab treatment led to a reduction of B cells, but also of T cells within the CSF of patients with RRMS, supporting the same hypothesis [Cross et al. 2006].
In a very recent study, Barr and colleagues suggested that therapeutic B-cell depletion may ameliorate EAE by ablation of pathogenic IL-6-secreting B cells [Barr et al. 2012]. Interestingly, the same group showed in this study, but also in past studies [Duddy et al. 2007], that in patients with MS, B cells are chronically activated and matured in a proinflammatory manner. Among other parameters, the B cells of patients with MS produced elevated levels of IL-6. In this context, another promising therapeutic approach for MS and related demyelinating disorders could be the selective inhibition of the IL-6 receptor using tocilizumab, a novel anti-inflammatory agent for rheumatoid arthritis. Intriguingly, tocilizumab was shown to impair somatic hypermutation of pre-switch memory B cells [Muhammad et al. 2011] and recent case reports suggest that it may also be effective in inflammatory CNS demyelinating disorders [Araki et al. 2012]. One mechanism for clinical benefit could refer to the fact that B-cell-derived IL-6 in conjunction with transforming growth factor β can promote preferential differentiation of naïve T cells into Th17 effector cells. Taken together, these observations support the assumption that antigen presentation and production of proinflammatory cytokines, such as IL-6, are key pathogenic features of B cells which are controlled by anti-CD20 treatment.
In light of the broad depletion of B cells at various maturation stages, the number of serious side effects associated with anti-CD20 treatment is surprisingly low. Of course, anaphylactic reactions to the antibody or its components are potentially severe or even fatal adverse events. The further humanized anti-CD20 antibody ocrelizumab was generated to minimize the likelihood of such an event even further in the future. Immunologically, the approach of B-cell depletion as a therapeutic strategy naturally contains the risk of immunosuppression, while the frequency of reported infectious events throughout the relatively short MS studies reported to date is low. Few reports indicate that anti-CD20 treatment may be associated with an increased risk of developing progressive multifocal leukoencephalopathy (PML). Although no such case has been described in the above-mentioned clinical trials or in an off-label setting in patients with MS, cases of PML have occurred in patients treated with rituximab for other indications. Carson and colleagues recapitulated 57 HIV-negative patients (52 patients with lymphoproliferative disorders, two patients with systemic lupus erythematosus, one patient with rheumatoid arthritis, one patient with an idiopathic autoimmune pancytopenia, one patient with immune thrombocytopenia) who had developed PML after treatment with rituximab in combination with other immunosuppressive agents [Carson et al. 2009]. Recently, another study reported four patients with PML in an estimated cohort of 129,000 patients treated with rituximab for rheumatoid arthritis, suggesting an increased risk of one case in 25,000 treated individuals with rheumatoid arthritis [Clifford et al. 2011]. However, whether development of PML truly refers to anti-CD20 treatment apart from the underlying condition and cotreatment, and whether accordingly the risk of PML is increased in patients with MS treated with anti-CD20 remains to be determined.
In order to better understand the beneficial but also potentially harmful consequences of treatment interfering with B cells in MS, the ‘atacicept experience’ should be kept in mind. Here, depletion of the two B-cell-fostering chemokines BAFF and APRIL led to a counterintuitive worsening of MS and clinical trials had to be stopped [Hartung and Kieseier, 2010]. One plausible explanation for this unexpected outcome could be that the treatment primarily affected CD27-negative naïve B cells while having only little effect on memory B cells [Genovese et al. 2011; Tak et al. 2008]. Along the same lines, CD20 is expressed on a wide range of B-cell maturation stages, and accordingly, besides depletion of pathogenic B cells, pan-CD20 bears the potential to collaterally abolish pre-existing B-cell regulation of CNS autoimmune disease. In this regard, a recent study demonstrated that anti-CD20 treatment of patients with MS or other neuroinflammatory diseases is associated with elevated activation and increased proinflammatory cytokine production by circulating myeloid APCs [Lehmann-Horn et al. 2011]. In EAE, such APCs lacking in vivo B-cell regulation displayed enhanced T-cell-polarizing properties with a preferential development of proinflammatory Th1 and Th17 cells [Weber et al. 2010]. Collectively, these findings show that not all patients may equally benefit from the current approach of B-cell depletion targeting CD20. Some patients with minor pathogenic B-cell involvement and a preferential naïve B-cell phenotype could theoretically even deteriorate as single reports indicate for other inflammatory conditions [Benedetti et al. 2007; Broglio and Lauria, 2005; El Fassi et al. 2008; Renaud et al. 2003]. Accordingly, development of criteria determining pathogenic B-cell involvement in an individual patient prior to treatment initiation as well as pioneering more selective depletion strategies targeting solely B cells with pathogenic effector function may be a therapeutically desirable goal.
Future strategies
Recent years have greatly improved our understanding of how B cells participate in MS pathogenesis. The clinical trials testing therapeutic B-cell depletion exceeded the initial expectations and established that B cells are key players in progression of CNS autoimmune disease. It is thus clear that modulation of pathogenic B-cell function is an extraordinarily attractive therapeutic goal in MS. However, it is likely that individual patients differ in regard to pathogenic B-cell involvement. Further, apparently not all CD20-positive B-cell subsets play a pathogenic role and, ideally, B-cell subsets with regulatory properties would be spared by an improved therapeutic approach. Notwithstanding the exciting data that anti-CD20 brought into the world of MS in recent years, we accordingly aim to discuss in the following section how effectiveness and safety of this promising approach could be further improved in the future.
Intravitally identify patients with pathogenic B-cell involvement
As described in detail above, MS pathogenesis is likely more heterogeneous than previously thought. This emerging concept is primarily fueled by histologic analysis of CNS lesions, which revealed that CNS lesions within one patient are similarly composed whereas four general subtypes of lesion can be distinguished among different patients [Lucchinetti et al. 2000]. The probably most frequent B-cell- and antibody-dominated subtype positively responds to therapeutic PE, while the subtypes I, III and IV failed to do so [Keegan et al. 2005]. Accordingly, individual patients may also benefit from therapeutic B-cell depletion to a different extent. In order to anticipate the potential gain for an individual patient it would therefore be instrumental to assess pathogenic B-cell involvement prior to therapy initiation. In this regard, the recent observations of Bar-Or and colleagues in Montreal could be extraordinarily helpful; they found that B cells from patients with MS on average produce more proinflammatory TNF, lympotoxin, IL-6 and less regulatory IL-10 [Barr et al. 2012; Duddy et al. 2007]. Functionally, these B cells fostered development of encephalitogenic Th17 cells. Furthermore, the ratio between naïve and memory B cells appeared to be shifted towards a dominant proinflammatory memory phenotype, indicating that B cells were continuously activated. If future investigations may further consolidate these and possible further markers of pathogenic B-cell function in MS, such B-cell phenotyping prior to therapy initiation could be tremendously helpful.
Targeting pathogenic B-cell function within the central nervous system
Another promising approach to efficiently eradicate pathological B-cell function while preserving regulatory B-cell subsets may be to therapeutically target B cells within the CNS. Several findings support the concept of a CNS compartmentalized immune activity in MS; intrathecally located B cells produce oligoclonal bands in the CSF. Especially in the secondary progressive course of the disease clonally expanded populations of B cells in the CSF are detected [Colombo et al. 2000]. Further, in cases of secondary progressive MS, Magliozzi and colleagues found lymphoid follicle-like structures in the meningeal compartment that show germinal center formation [Magliozzi et al. 2007]. With regard to histological and clinical parameters these follicle-like structures contain proliferating B cells, are often adjacent to large subpial cortical lesions and are associated with disease progression. In secondary progressive MS, fewer activated blood-borne effector cells penetrate the blood–brain barrier and inflammation seems to be trapped within the CNS compartment [Bradl and Lassmann, 2009]. As a result, contrast enhancement in MRI scans declines, while diffusely spread CNS inflammation continues. Taken together, throughout the chronic course of MS, CNS inflammation may become gradually independent of recruitment of peripheral immune cells and, accordingly, an effective therapy may require local targeting of immune processes.
Anti-CD20 antibodies are well established in the therapy of systemic but also CNS lymphoma. However, intravenously applied anti-CD20 may allow only low antibody distribution to the cerebral compartment. While perivascular CNS B cells may be depleted by anti-CD20 treatment [Martin Mdel et al. 2009], only about 0.1% of the serum concentrations are reached within the CSF. Studies in cynomolgus monkeys provided pharmacokinetic data of intraventricularly administered rituximab, demonstrating high CSF concentrations, although with a short half life of approximately 5 h [Rubenstein et al. 2003]. A phase I study of intraventricular administration of rituximab in patients with CNS lymphoma established an antibody distribution along the craniospinal axis. Similar to the findings in monkeys, the CSF concentration rapidly declined after intrathecal injection whereas serum concentrations continuously increased [Rubenstein et al. 2007]. Intrathecal monotherapy led to a reduction of lymphoma activity as well as to remarkable clinical benefit in some patients. This study as well as several case reports revealed that intrathecal rituximab up to a dose of 25 mg was well tolerated and did not show severe adverse events [Antonini et al. 2007; Hong et al. 2009; Schulz et al. 2004].
Such compartment-specific depletion of CNS B cells may provide an innovative approach in therapy of secondary progressive MS. Ideally, pathogenic B-cell function would be specifically targeted in the inflamed compartment whereas the peripheral immune system including regulatory B-cell subsets could be spared. It remains to be determined to which degree peripheral B cells are affected by the observed rise of anti-CD20 concentration in the serum following intrathecal administration. However, simultaneous depletion of central and peripheral compartments could even be desirable in light of new findings suggesting a bilateral exchange of B cells across the blood–brain barrier throughout the chronic course of MS [von Budingen et al. 2012]. A clinical study combining intrathecal and systemic B-cell depletion in treatment of secondary progressive MS started in September 2010 [ClinicalTrials.gov identifier: NCT01212094].
Identify and therapeutically target pathogenic B-cell subsets
As outlined above, the role of B cells in MS is likely to be more heterogeneous than previously anticipated. While accumulating evidence suggests that antigen-activated B cells substantially contribute to the development and progression of MS, the role of naïve B cells is more likely to be regulatory in nature. In this regard, it has been demonstrated that activated and memory B cells in patients with MS produce proinflammatory cytokines and foster development of Th17 cells, while unactivated, naïve B cells predominantly release regulatory IL-10. Reflecting the same dichotomy, anti-CD20 treatment was found to exacerbate EAE in a setting in which B cells were not activated, whereas depletion of activated B cells was associated with clinical benefit [Weber et al. 2010]. In the treatment of patients with MS and neuromyelitis optica (NMO), anti-CD20 B-cell depletion was further associated with a proinflammatory differentiation of monocytes, most likely due to collateral abrogation of IL-10-mediated B-cell regulation [Lehmann-Horn et al. 2011]. Jointly, these data suggest that the pool of CD20-positive B cells contains both subsets with pathogenic and regulatory properties. Accordingly, in order to enhance the safety and effectiveness of B-cell depletion as a therapeutic approach in MS, it may be instrumental to identify and dissect B-cell subpopulations with pathogenic function from entities with regulatory properties, which may ultimately allow selectively abrogating pathogenic B-cell function utilizing a more specific target.
Conclusion
The past few years substantially increased our understanding that B cells contribute to MS pathogenesis in many more ways than as precursors of terminally differentiated, antibody-secreting plasma cells. Pathogenic properties of earlier B-cell maturation stages primarily relate to the ability to specifically capture antigen with high affinity via the B-cell receptor. Bound antigen can then either be processed and presented in the context of constitutively expressed MHC II or transferred and provided to other APCs. In addition, antigen-activated B cells can act as potent producers of proinflammatory cytokines promoting ongoing inflammation as well as de novo development of encephalitogenic T cells. Appreciation of these relatively novel insights was substantially accelerated by the pivotal clinical trials depleting CD20-positive B cells in the treatment of MS. The clinical effectiveness of anti-CD20 was shown to relate primarily to abrogation of APC function and inflammatory cytokine secretion of B cells. Notwithstanding these encouraging and enlightening results, recent experimental and clinical data suggest that not all B cells may contribute pathogenically, and that some B-cell subsets, such as naïve B cells, may in contrast downregulate ongoing inflammation in a therapeutically desirable manner. These findings raise the possibility that, based on the predominant B-cell phenotype, individual patients may differentially benefit from anti-CD20 therapy. Further, these observations suggest that selective targeting of pathogenic B-cell function while sparing regulatory B-cell properties could be advantageous. In conclusion, while B cells turned out to be an extraordinarily attractive target in MS, we should be eager to harness the rapidly evolving concept of B-cell subsets with distinct functions to guide the development and use of B cell-directed therapeutic strategies.
Footnotes
Funding: M.S.W. is supported by the Else Kröner Fresenius Stiftung (A69/2010), TEVA, the Deutsche Forschungsgemeinschaft (DFG; WE 3547/4-1), the US National Multiple Sclerosis Society (NMSS; PP 1660) and the ProFutura program of the University of Göttingen.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Klaus Lehmann-Horn, Department of Neurology, Technische Universität München, Munich, Germany.
Helena C. Kronsbein, Department of Neurology, Technische Universität München, Munich, Germany
Martin S. Weber, Department of Neuropathology and Department of Neurology, University Medical Center, Georg August University, Robert-Koch-Str. 40, 37099 Göttingen, Germany
References
- Antonini G., Cox M., Montefusco E., Ferrari A., Conte E., Morino S., et al. (2007) Intrathecal anti-CD20 antibody: an effective and safe treatment for leptomeningeal lymphoma. J Neurooncol 81: 197–199 [DOI] [PubMed] [Google Scholar]
- Araki M., Aranami T., Matsuoka T., Nakamura M., Miyake S., Yamamura T. (2012) Clinical improvement in a patient with neuromyelitis optica following therapy with the anti-IL-6 receptor monoclonal antibody tocilizumab. Mod Rheumatol 11 July (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Fawaz L., Fan B., Darlington P., Rieger A., Ghorayeb C., et al. (2010) Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 67: 452–461 [DOI] [PubMed] [Google Scholar]
- Baranzini S., Jeong M., Butunoi C., Murray R., Bernard C., Oksenberg J. (1999) B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol 163: 5133–5144 [PubMed] [Google Scholar]
- Barr T., Shen P., Brown S., Lampropoulou V., Roch T., Lawrie S., et al. (2012) B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209: 1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti L., Franciotta D., Vigo T., Grandis M., Fiorina E., Ghiglione E., et al. (2007) Relapses after treatment with rituximab in a patient with multiple sclerosis and anti myelin-associated glycoprotein polyneuropathy. Arch Neurol 64: 1531–1533 [DOI] [PubMed] [Google Scholar]
- Benkhoucha M., Molnarfi N., Santiago-Raber M. L., Weber M. S., Merkler D., Collin M., et al. (2012) IgG glycan hydrolysis by EndoS inhibits experimental autoimmune encephalomyelitis. J Neuroinflammation 9: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M., Lassmann H. (2009) Progressive multiple sclerosis. Semin Immunopathol 31: 455–465 [DOI] [PubMed] [Google Scholar]
- Breij E., Brink B., Veerhuis R., van den Berg C., Vloet R., Yan R., et al. (2008) Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol 63: 16–25 [DOI] [PubMed] [Google Scholar]
- Broglio L., Lauria G. (2005) Worsening after rituximab treatment in anti-mag neuropathy. Muscle Nerve 32: 378–379 [DOI] [PubMed] [Google Scholar]
- Carson K., Evens A., Richey E., Habermann T., Focosi D., Seymour J., et al. (2009) Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 113: 4834–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford D., Ances B., Costello C., Rosen-Schmidt S., Andersson M., Parks D., et al. (2011) Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol 68: 1156–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M., Dono M., Gazzola P., Roncella S., Valetto A., Chiorazzi N., et al. (2000) Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol 164: 2782–2789 [DOI] [PubMed] [Google Scholar]
- Constant S., Sant’Angelo D., Pasqualini T., Taylor T., Levin D., Flavell R., et al. (1995a) Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J Immunol 154: 4915–4923 [PubMed] [Google Scholar]
- Constant S., Schweitzer N., West J., Ranney P., Bottomly K. (1995b) B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol 155: 3734–3741 [PubMed] [Google Scholar]
- Cross A., Stark J., Lauber J., Ramsbottom M., Lyons J. (2006) Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 180: 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dau P., Petajan J., Johnson K., Panitch H., Bornstein M. (1980) Plasmapheresis in multiple sclerosis: preliminary findings. Neurology 30: 1023–1028 [DOI] [PubMed] [Google Scholar]
- Duddy M., Niino M., Adatia F., Hebert S., Freedman M., Atkins H., et al. (2007) Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 178: 6092–6099 [DOI] [PubMed] [Google Scholar]
- El Fassi D., Nielsen C., Kjeldsen J., Clemmensen O., Hegedus L. (2008) Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease. Gut 57: 714–715 [DOI] [PubMed] [Google Scholar]
- Fillatreau S., Sweenie C., McGeachy M., Gray D., Anderton S. (2002) B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3: 944–950 [DOI] [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. (1984) Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 307: 273–276 [DOI] [PubMed] [Google Scholar]
- Genovese M., Kinnman N., de La Bourdonnaye G., Pena Rossi C., Tak P. (2011) Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum 63: 1793–1803 [DOI] [PubMed] [Google Scholar]
- Greter M., Heppner F., Lemos M., Odermatt B., Goebels N., Laufer T., et al. (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11: 328–334 [DOI] [PubMed] [Google Scholar]
- Hardy R. (2006) B-1 B cell development. J Immunol 177: 2749–2754 [DOI] [PubMed] [Google Scholar]
- Harp C., Lovett-Racke A., Racke M., Frohman E., Monson N. (2008) Impact of myelin-specific antigen presenting B cells on T cell activation in multiple sclerosis. Clin Immunol 128: 382–91 [DOI] [PubMed] [Google Scholar]
- Hartung H., Kieseier B. (2010) Atacicept: targeting B cells in multiple sclerosis. Ther Adv Neurol Dis 3: 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S., Waubant E., Arnold D., Vollmer T., Antel J., Fox R., et al. (2008) B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med 358: 676–688 [DOI] [PubMed] [Google Scholar]
- Hawker K., O’Connor P., Freedman M., Calabresi P., Antel J., Simon J., et al. (2009) Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 66: 460–471 [DOI] [PubMed] [Google Scholar]
- Hong S., Kim J., Chang J., Kim K., Kim S., Lee H., et al. (2009) A successful treatment of relapsed primary CNS lymphoma patient with intraventricular rituximab followed by high-dose chemotherapy with autologous stem cell rescue. Yonsei Med J 50: 280–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y., Matsushita T., Horikawa M., Dilillo D., Yanaba K., Venturi G., et al. (2011) Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117: 530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E., Moore D., Landow H. (1942) An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to the serum proteins. J Clin Invest 21: 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L., Li D., Calabresi P., O’Connor P., Bar-Or A., Barkhof F., et al. (2011) Ocrelizumab in relapsing–remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 378: 1779–87 [DOI] [PubMed] [Google Scholar]
- Keegan M., Konig F., McClelland R., Bruck W., Morales Y., Bitsch A., et al. (2005) Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet 366: 579–582 [DOI] [PubMed] [Google Scholar]
- Korn T., Mitsdoerffer M., Croxford A., Awasthi A., Dardalhon V., Galileos G., et al. (2008) IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 105: 18460–18465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarik M. C., Cepok S., Sellner J., Grummel V., Weber M. S., Korn T., et al. (2012). CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation 9: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M., Theil D., Derfuss T., Rosenwald A., Schrader F., Monoranu C., et al. (2005) BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med 201: 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalive P., Molnarfi N., Benkhoucha M., Weber M., Santiago-Raber M. (2011) Antibody response in MOG(35-55) induced EAE. J Neuroimmunol 240–241: 28–33 [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. (1985) Antigen-specific interaction between T and B cells. Nature 314: 537–539 [DOI] [PubMed] [Google Scholar]
- Lassmann H., Bruck W., Lucchinetti C. (2001) Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med 7: 115–121 [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn K., Schleich E., Hertzenberg D., Hapfelmeier A., Kumpfel T., von Bubnoff N., et al. (2011) Anti-CD20 B-cell depletion enhances monocyte reactivity in neuroimmunological disorders. J Neuroinflammation 8: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzenburger T., Fassler R., Bauer J., Lassmann H., Linington C., Wekerle H., et al. (1998) B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J Exp Med 188: 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C., Bruck W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47: 707–717 [DOI] [PubMed] [Google Scholar]
- Lyons J., Ramsbottom M., Cross A. (2002) Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur J Immunol 32: 1905–1913 [DOI] [PubMed] [Google Scholar]
- Lyons J., San M., Happ M., Cross A. (1999) B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol 29: 3432–3439 [DOI] [PubMed] [Google Scholar]
- Mackay F., Schneider P., Rennert P., Browning J. (2003) BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol 21: 231–264 [DOI] [PubMed] [Google Scholar]
- Magana S., Keegan B., Weinshenker B., Erickson B., Pittock S., Lennon V., et al. (2011) Beneficial plasma exchange response in central nervous system inflammatory demyelination. Arch Neurol 68: 870–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R., Howell O., Vora A., Serafini B., Nicholas R., Puopolo M., et al. (2007) Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130: 1089–1104 [DOI] [PubMed] [Google Scholar]
- Martin Mdel P., Cravens P., Winger R., Kieseier B., Cepok S., Eagar T., et al. (2009) Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol 66: 1016–1020 [DOI] [PubMed] [Google Scholar]
- Mauri C., Blair P. (2010) Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol 6: 636–643 [DOI] [PubMed] [Google Scholar]
- Merkler D., Schmelting B., Czeh B., Fuchs E., Stadelmann C., Bruck W. (2006) Myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis in the common marmoset reflects the immunopathology of pattern II multiple sclerosis lesions. Mult Scler 12: 369–374 [DOI] [PubMed] [Google Scholar]
- Moulin V., Andris F., Thielemans K., Maliszewski C., Urbain J., Moser M. (2000) B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med 192: 475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad K., Roll P., Seibold T., Kleinert S., Einsele H., Dorner T., et al. (2011) Impact of IL-6 receptor inhibition on human memory B cells in vivo: impaired somatic hypermutation in preswitch memory B cells and modulation of mutational targeting in memory B cells. Ann Rheum Dis 70: 1507–1510 [DOI] [PubMed] [Google Scholar]
- Obermeier B., Mentele R., Malotka J., Kellermann J., Kumpfel T., Wekerle H., et al. (2008) Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med 14: 688–693 [DOI] [PubMed] [Google Scholar]
- Owens G., Burgoon M., Anthony J., Kleinschmidt-DeMasters B., Gilden D. (2001) The immunoglobulin G heavy chain repertoire in multiple sclerosis plaques is distinct from the heavy chain repertoire in peripheral blood lymphocytes. Clin Immunol 98: 258–263 [DOI] [PubMed] [Google Scholar]
- Owens G., Kraus H., Burgoon M., Smith-Jensen T., Devlin M., Gilden D. (1998) Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann Neurol 43: 236–243 [DOI] [PubMed] [Google Scholar]
- Renaud S., Gregor M., Fuhr P., Lorenz D., Deuschl G., Gratwohl A., et al. (2003) Rituximab in the treatment of polyneuropathy associated with anti-MAG antibodies. Muscle Nerve 27: 611–615 [DOI] [PubMed] [Google Scholar]
- Rivera A., Chen C., Ron N., Dougherty J., Ron Y. (2001) Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol 13: 1583–1593 [DOI] [PubMed] [Google Scholar]
- Rubenstein J., Combs D., Rosenberg J., Levy A., McDermott M., Damon L., et al. (2003) Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood 101: 466–468 [DOI] [PubMed] [Google Scholar]
- Rubenstein J., Fridlyand J., Abrey L., Shen A., Karch J., Wang E., et al. (2007) Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 25: 1350–1356 [DOI] [PubMed] [Google Scholar]
- Sato T., Ishikawa S., Akadegawa K., Ito T., Yurino H., Kitabatake M., et al. (2004) Aberrant B1 cell migration into the thymus results in activation of CD4 T cells through its potent antigen-presenting activity in the development of murine lupus. Eur J Immunol 34: 3346–3358 [DOI] [PubMed] [Google Scholar]
- Schluesener H., Sobel R., Linington C., Weiner H. (1987) A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol 139: 4016–4021 [PubMed] [Google Scholar]
- Schulz H., Pels H., Schmidt-Wolf I., Zeelen U., Germing U., Engert A. (2004) Intraventricular treatment of relapsed central nervous system lymphoma with the anti-CD20 antibody rituximab. Haematologica 89: 753–754 [PubMed] [Google Scholar]
- Seifert C. L., Wegner C., Sprenger T., Weber M. S., Brück W., Hemmer B., et al. (2012). Favourable response to plasma exchange in tumefactive CNS demyelination with delayed B-cell response. Multiple Sclerosis 18: 1045–1049 [DOI] [PubMed] [Google Scholar]
- Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F. (2004) Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos J., Morrow J., Ashley T., Szente B., Bikoff E., Zamvil S. (1998) Astrocytes express elements of the class II endocytic pathway and process central nervous system autoantigen for presentation to encephalitogenic T cells. J Immunol 161: 5959–5966 [PubMed] [Google Scholar]
- Steinman L., Zamvil S. (2006) How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol 60: 12–21 [DOI] [PubMed] [Google Scholar]
- Stuve O., Youssef S., Slavin A., King C., Patarroyo J., Hirschberg D., et al. (2002) The role of the MHC class II transactivator in class ii expression and antigen presentation by astrocytes and in susceptibility to central nervous system autoimmune disease. J Immunol 169: 6720–6732 [DOI] [PubMed] [Google Scholar]
- Tak P., Thurlings R., Rossier C., Nestorov I., Dimic A., Mircetic V., et al. (2008) Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum 58: 61–72 [DOI] [PubMed] [Google Scholar]
- Vamvakas E., Pineda A., Weinshenker B. (1995) Meta-analysis of clinical studies of the efficacy of plasma exchange in the treatment of chronic progressive multiple sclerosis. J Clin Apher 10: 163–170 [DOI] [PubMed] [Google Scholar]
- van der Veen R., Trotter J., Kapp J. (1992) Immune processing of proteolipid protein by subsets of antigen-presenting spleen cells. J Neuroimmunol 38: 139–146 [DOI] [PubMed] [Google Scholar]
- von Budingen H., Gulati M., Kuenzle S., Fischer K., Rupprecht T., Goebels N. (2010) Clonally expanded plasma cells in the cerebrospinal fluid of patients with central nervous system autoimmune demyelination produce ‘oligoclonal bands’. J Neuroimmunol 218: 134–139 [DOI] [PubMed] [Google Scholar]
- von Budingen H., Kuo T., Sirota M., van Belle C., Apeltsin L., Glanville J., et al. (2012) B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest 122: 4533–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Hemmer B. (2010) Cooperation of B cells and T cells in the pathogenesis of multiple sclerosis. Results Probl Cell Differ 51: 115–126 [DOI] [PubMed] [Google Scholar]
- Weber M., Hemmer B., Cepok S. (2011) The role of antibodies in multiple sclerosis. Biochim Biophys Acta 1812: 239–245 [DOI] [PubMed] [Google Scholar]
- Weber M., Prod’homme T., Patarroyo J., Molnarfi N., Karnezis T., Lehmann-Horn K., et al. (2010) B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol 68: 369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker B., O’Brien P., Petterson T., Noseworthy J., Lucchinetti C., Dodick D., et al. (1999) A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol 46: 878–886 [DOI] [PubMed] [Google Scholar]
- Wolf S., Dittel B., Hardardottir F., Janeway C., Jr (1996) Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med 184: 2271–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki A., Miyagaki T., DiLillo D., Matsushita T., Horikawa M., Kountikov E., et al. (2012) Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491: 264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Gao W., Degauque N., Bai C., Lu Y., Kenny J., et al. (2007) Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol 37: 2400–2404 [DOI] [PubMed] [Google Scholar]