Abstract
Multiple sclerosis (MS)-related spasticity is associated with disability and impairment in quality of life. We report on a patient with secondary progressive MS and spastic tetraparesis (Expanded Disability Status Scale score 8.5). The right arm exhibited flexor spasticity resulting in functional disability despite multimodal symptomatic treatment. Intrathecal baclofen led to side effects despite decreasing efficacy. Low-dose nabiximols improved spasticity and function with recovery of daily-life activities and spasticity-related symptoms. Reduction of intrathecal baclofen ameliorated adverse drug reactions. Add-on cannabinoid therapy was effective in therapy-refractory spasticity with supra-additive effect in combining intrathecal baclofen and nabiximols, hypothetically explained by mutually complementing mechanisms of action.
Keywords: cannabinoids, CB1 receptor, multiple sclerosis, nabiximols, secondary progressive multiple sclerosis, spastic tetraparesis, symptomatic treatment
Introduction
Spasticity in multiple sclerosis (MS) is a common symptom contributing to disability [Thompson et al. 2010]. In addition to physiotherapy, guidelines recommend first-line antispastic treatment with oral baclofen or tizanidine [Gold et al. 2012; Thompson et al. 2005], although with low-level evidence due to limited quality and availability of data [Shakespeare et al. 2003]. For other agents (gabapentin, tolperisone) efficacy is even less certain or potential serious adverse reactions must be considered (dantrolene, benzodiazepines) [Gold et al. 2012]. More invasive and partly off-label treatment options are intrathecal baclofen (ITB), intrathecal triamcinolone acetonide (TCA) and intramuscular botulinum toxin.
In 2011, nabiximols, a phytocannabinoid formulation of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) was approved as add-on therapy for patients with MS and moderate to severe spasticity based on randomized, placebo-controlled, double-blind trials [Collin et al. 2007, 2010; Novotna et al. 2011], an open-label follow-up study [Wade et al. 2006] and a meta-analysis [Wade et al. 2010]. As the first trials had shown differential effects in the intention-to-treat and the per-protocol population [Collin et al. 2007, 2010], the latest study was designed as a two-phase trial in which the first phase separated responders from nonresponders [Novotna et al. 2011]. Only the responder group (42%) entered the second double-blind phase of the study. Mean improvement of spasticity in the first phase of the study was a change of 3.01 points in the Numeric Rating Scale (range 0–10), the second phase of the study revealed a significant difference between further active versus placebo treatment of 0.84 points.
We describe an MS case with tetraspasticity refractory to high-dose ITB and botulinum toxin, responding to low-dose nabiximols in a supra-additive fashion.
Method
We report a case involving a woman with secondary progressive MS and severe spastic tetraparesis. Informed consent was obtained for clinical data and videos.
Case report
The 54-year-old woman was diagnosed with relapsing–remitting MS according to Poser criteria in 1990. Previous immunotherapies included long-term oral and intravenous steroids, interferon β, oral immunosuppression, mitoxantrone and intravenous immunoglobulins. Nevertheless, she was diagnosed with secondary progressive MS at her first visit to our department in 2005. A predominantly left-sided spastic tetraparesis averted independent transfer. Still, she performed self-care functions using both arms [Expanded Disability Status Scale (EDSS) score 8.0]. Oral antispastic treatment led to sufficient symptom control (Table 1).
Table 1.
Symptomatic antispastic treatment of the patient: dates, medication and dosage.
| Date | Medication | Dose |
|---|---|---|
| May 2005 | Gabapentin | 1200 mg per day |
| Baclofen | 60 mg per day | |
| Tizanidine | 8 mg per day | |
| April 2008 | Gabapentin | 1200 mg per day |
| Baclofen | 100 mg per day | |
| Tizanidine | 4 mg per day | |
| Dantrolene | 75 mg per day | |
| May 2008 | Gabapentin | 1200 mg per day |
| ITB test doses | 25 µg bolus, 50 µg bolus, 75 µg bolus, 50 µg per day via catheter | |
| Botulinum toxin (intermittent) | Injection in the left upper extremity | |
| October 2008 | Gabapentin | 1200 mg per day |
| ITB via pump | Titration, see Figure 1 | |
| Tolperison | 450 mg per day (until June 2010) | |
| October 2010 | ITB via pump | Titration, see Figure 1 |
| Intrathecal TCA (intermittent) | 80 mg in 6–8-week intervals | |
| March 2011 | ITB via pump | Titration, see Figure 1 |
| Intrathecal TCA (intermittent) | 80 mg in 6–8-week intervals (until May 2011) | |
| Botulinum toxin (intermittent) | Injection in the right upper extremity | |
| August 2011 | ITB via pump | Titration, see Figure 1 |
| Start of cannabinoid treatment | 1 puff per day |
ITB, intrathecal baclofen; TCA, triamcinolone acetonide.
Despite extended oral treatment (Table 1), in 2008 the patient exhibited gradual worsening of spasticity, with severe spasticity in both legs and the left arm whereas the right arm retained some activities of daily living (EDSS 8.5).
Implantation of an electronically adjustable ITB pump (Medtronic SynchroMed II, model 8637-20, Medtronic, Inc., Minneapolis, MN, USA) resulted in a profound antispastic effect on the lower extremities. However, increasing flexor spasticity of the left arm did not respond to ITB but intermittent botulinum toxin resulted in better passive motility. The dominant right arm was not functionally affected.
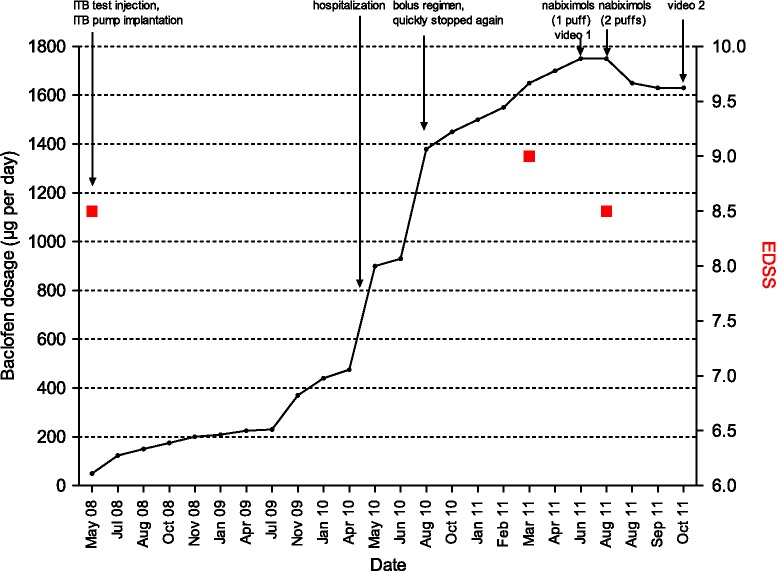
In 2010, ITB dosage was increased more than threefold (Figure 1). Malfunction of the pump/catheter dislocation was excluded via lower spinal magnetic resonance imaging (MRI) and radioscopy. Cerebral MRI showed marked cortical and subcortical brain atrophy and high T2-lesion load. Spinal MRI revealed substantial atrophy of the medulla oblongata and cervical spinal cord with diffuse T2-signal. Active, gadolinium-enhancing lesions were not present on cerebral and spinal T1-weighted MRI.
Figure 1.
Course of intrathecal baclofen (ITB) dosage and relevant clinical events over time. EDSS, Expanded Disability Status Scale.
On high-dose ITB (1450 µg per day) and intermittent intrathecal TCA administration (Table 1), EDSS and function of the right arm and hand remained stable.
In 2011, the patient’s symptoms worsened markedly with fluctuating flexor spasticity leading to loss of function of the right arm (EDSS 9.0, Figure 1). Neurological examination revealed severe tetraspasticity with fixed extensor position of the left foot, insuperable flexor malposition and contractures of the left arm and hand. The patient was only intermittently able to assist during transfer. She complained about pain and muscular cramps in the legs, neck and shoulders. Both intravenous and intrathecal steroid application were ineffective.
Botulinum toxin was injected in the right arm (dosage not known), resulting in a loss of function due to plegia of the right forearm and hand.
Increased ITB (Figure 1, 1750 µg per day) led to side effects with relevant impact on daily living (sleepiness), still without efficacy on flexor spasticity of the right arm.
In August 2011, the patient reported loss of function of the right hand as the most disabling symptom because this was previously the only function left. She could not conduct simple activities of daily living for herself.
Nabiximols was added (one puff per day owing to high-dose ITB side effects). Initial dizziness resolved 3 weeks later maintaining the initial dosage. We observed a gain of function in the right forearm and hand (Video 1). The patient was able to move her hand and forearm for the first time since the beginning of 2011. Daily ITB was reduced by 6.9 % (Figure 1); nabiximols was increased (two puffs per day).
After 6 weeks the patient had further improved and gained strength of the right arm. She was able to drink, comb her hair and write (Video 2). Pain and paroxysmal cramps decreased; no further adverse drug reactions occurred. ITB-related side effects resolved after dose reduction. EDSS stabilized at 8.5 (Figure 1).
Discussion
Progressive loss of efficacy of antispastic drugs even with high-dose ITB treatment is commonly encountered. Exceedingly high ITB dosage without therapeutic efficacy may point to paradoxical treatment unresponsiveness [Cooper and Ridley, 2006]. Baclofen reduces γ aminobutyric acid B (GABA-B) receptor density in animal models [Kroin et al. 1993]. In addition, desensitization mediated by GABA-B receptor agonists has been postulated as a molecular mechanism for development of tolerance [Wetherington and Lambert, 2002].
Since our patient remained stable after the addition of nabiximols and symptoms were well controlled, we did not further reduce ITB dosage. Drastic reduction of dosage has been described to improve spasticity in rare cases of paradoxical ITB treatment unresponsiveness [Cooper and Ridley, 2006].
Not unexpectedly, treatment with other substances with mechanisms of action similar to baclofen via GABA-ergic pathways (gabapentin, benzodiazepines) was without benefit [Kroin et al. 1993].
The gradual loss of antispastic effect of intrathecal TCA in our patient may be associated with marked upper spinal cord atrophy, which has been described as a response predictor for repetitive intrathecal TCA treatment [Lukas et al. 2009].
A hypothetical basis for the supra-additive therapeutic effect of nabiximols in our patient may be the modulation of the endocannabinoid system as a different molecular target and complementary site of action.
Phytocannabinoids bind presynaptic cannabinoid receptors 1 (CB1-R), predominantly present in the central nervous system on both GABA- and glutamate-ergic synapses, and therefore mimic the negative feedback mechanism of endocannabinoids such as anandamide or 2-arachidonoylglycerol [Pertwee, 2006].
In addition to CB1-R activation, the endocannabinoid system is involved in immunoregulatory processes via cannabinoid receptor 2 (CB2-R) with implications for neuroinflammatory diseases [Basu and Dittel, 2011].
THC and CBD have different affinities to CB1-R with differential effects [Pertwee, 2008; Russo and Guy, 2006]. Whereas the pivotal clinical trials have shown the efficacy of add-on nabiximols therapy with mean dosages of 7–15 puffs per days [Collin et al. 2007; Novotna et al. 2011; Wade et al. 2006, 2010], we observed supra-additive efficacy of only one to two puffs of nabiximols in our patient on high-dose ITB comedication.
Despite profound adverse drug reactions of high-dose ITB in this patient, at least with low dosage side effects of the THC/CBD treatment were mild and transient. However, long-term safety and side effects need to be further investigated [Leussink et al. 2012]. In particular, the psychoactive effects and the influence of cannabinoids on neurocognitive function are still controversially discussed [Aragona et al. 2009; Papathanasopoulos et al. 2008; Robson, 2011]. Although we did not observe short-term effects in our patient, possible cognitive decline needs to be evaluated in a long-term perspective.
Add-on nabiximols therapy may improve spasti-city not sufficiently controlled by GABA-ergic medication including ITB. Presumably owing to different mechanisms of action, low dosages of nabiximols may be sufficient in these cases.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: A. Stroet has received personal compensation for activities with Novartis, Sanofi and Almirall Hermal GmbH. N. Trampe has nothing to disclose. A. Chan has received personal compensation for activities with Almirall Hermal GmbH, Bayer Schering, Biogen Idec, Merck Serono, Novartis and Teva Neuroscience, research support from Bayer Schering, Biogen Idec, Merck Serono and Novartis and research grants from the German Ministry for Education and Research [BMBF, ‘German Competence Network Multiple Sclerosis’ (KKNMS), CONTROL MS, 01GI0914].
Contributor Information
Anke Stroet, Department of Neurology, St Josef-Hospital, Ruhr-University Bochum, Gudrunstr. 56, D-44791 Bochum, Germany.
Nadine Trampe, Department of Neurology, St Josef-Hospital, Ruhr-University, Bochum, Germany.
Andrew Chan, Department of Neurology, St Josef-Hospital, Ruhr-University, Bochum, Germany.
References
- Aragona M., Onesti E., Tomassini V., Conte A., Gupta S., Gilio F., et al. (2009) Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clin Neuropharmacol 32: 41–47 [DOI] [PubMed] [Google Scholar]
- Basu S., Dittel B. (2011) Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res 51: 26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C., Davies P., Mutiboko I., Ratcliffe S. (2007) Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol 14: 290–296 [DOI] [PubMed] [Google Scholar]
- Collin C., Ehler E., Waberzinek G., Alsindi Z., Davies P., Powell K., et al. (2010) A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res 32: 451–459 [DOI] [PubMed] [Google Scholar]
- Cooper J., Ridley B. (2006) Response of intrathecal baclofen resistance to dose reduction. Neurology 67: 1495–1496 [DOI] [PubMed] [Google Scholar]
- Gold R., Hanschke S., Hemmer B., Wiendl H.(2012) DGN/KKNMS guideline for diagnosis and therapy of multiple sclerosis, online version. http://www.kompetenznetz-multiplesklerose.de/images/stories/PDF_Dateien/Leitlinie/dgn-kknms_ms-ll_20120809_frei.pdf
- Kroin J., Bianchi G., Penn R. (1993) Intrathecal baclofen down-regulates GABAB receptors in the rat substantia gelatinosa. J Neurosurg 79: 544–549 [DOI] [PubMed] [Google Scholar]
- Leussink V., Husseini L., Warnke C., Broussalis E., Hartung H., Kieseier B. (2012) Symptomatic therapy in multiple sclerosis: the role of cannabinoids in treating spasticity. Ther Adv Neurol Dis 5: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas C., Bellenberg B., Hahn H., Rexilius J., Drescher R., Hellwig K., et al. (2009) Benefit of repetitive intrathecal triamcinolone acetonide therapy in predominantly spinal multiple sclerosis: prediction by upper spinal cord atrophy. Ther Adv Neurol Dis 2: 42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotna A., Mares J., Ratcliffe S., Novakova I., Vachova M., Zapletalova O., et al. (2011) A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols*(Sativex(R)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 18: 1122–1131 [DOI] [PubMed] [Google Scholar]
- Papathanasopoulos P., Messinis L., Lyros E., Kastellakis A., Panagis G. (2008) Multiple sclerosis, cannabinoids, and cognition. J Neuropsychiat Clin Neurosci 20: 36–51 [DOI] [PubMed] [Google Scholar]
- Pertwee R. (2006) The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes 30(Suppl. 1): S13–S18 [DOI] [PubMed] [Google Scholar]
- Pertwee R. (2008) The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153: 199–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P. (2011) Abuse potential and psychoactive effects of delta-9-tetrahydrocannabinol and cannabidiol oromucosal spray (Sativex), a new cannabinoid medicine. Exp Opin Drug Saf 10: 675–685 [DOI] [PubMed] [Google Scholar]
- Russo E., Guy G. (2006) A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypoth 66: 234–246 [DOI] [PubMed] [Google Scholar]
- Shakespeare D., Boggild M., Young C. (2003) Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev 4: CD001332. [DOI] [PubMed] [Google Scholar]
- Thompson A., Jarrett L., Lockley L., Marsden J., Stevenson V. (2005) Clinical management of spasticity. J Neurol Neurosurg Psychiatry 76: 459–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A., Toosy A., Ciccarelli O. (2010) Pharmacological management of symptoms in multiple sclerosis: current approaches and future directions. Lancet Neurol 9: 1182–1199 [DOI] [PubMed] [Google Scholar]
- Wade D., Collin C., Stott C., Duncombe P. (2010) Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler 16: 707–714 [DOI] [PubMed] [Google Scholar]
- Wade D., Makela P., House H., Bateman C., Robson P. (2006) Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler 12: 639–645 [DOI] [PubMed] [Google Scholar]
- Wetherington J., Lambert N. (2002) GABA(B) receptor activation desensitizes postsynaptic GABA(B) and A(1) adenosine responses in rat hippocampal neurones. J Physiol 544: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]