Abstract
Colorectal cancer (CRC) is posing an increasingly important burden on the health care system, with western countries seeing a growing incidence of the disease. Except for germline DNA mutations which have been attributed to less than 5% of patients, little is known about the main causes of CRC. However, environment factors such as food, lifestyle and medication are now suspected to have a major influence on inducing cancers. Today, exhaustive quantitative and qualitative evaluation of all environmental factors is not possible. Various environment-induced diseases have been characterized based on colon microflora, also called microbiota, analyses. Growing data have shown specific changes in microflora (i.e. dysbiosis) in the stools of patients with colon cancer or those adherent to the colonic mucosa. Thus, it appears that microbiota may be considered a platform offering host and environment interactions for studying CRCs. The hypothesis that colon cancer might be a bacteria-related disease is suggested and perspectives are discussed.
Keywords: bacteria, bacteria–host interaction, colon cancer, environment, genetic
Introduction
Colorectal cancer (CRC) is a common disease of high economic costs in developed countries. Its incidence has been steadily increasing over the last five decades and mortality rates due to this disease remain at very high levels. A good understanding of the factors promoting carcinogenesis in CRC should enable a better policy of prevention and a more efficient screening protocol. The majority of CRC occurrences are considered sporadic, meaning they are due to the environment rather than constitutional genetic alteration. Among environmental factors, the western lifestyle is considered to impact on CRC occurrence.
Microbes are major actors in biological environments. About 16% of cancers around the world have been estimated to be caused by microbes and several cancers in the liver and gastrointestinal tract are clearly identified as being microbe related [deMartel et al. 2012]. Among these, Helicobacter pylori has been considered by the World Health Organization Agency for Research on Cancer to be associated with gastric adenocarcinoma and lymphoma involving the mucosa-associated lymphoid tissue. Evidence on such an association has been obtained from epidemiological data, experimental results in animal models and interventional trials by eradication of the bacterium in humans. Our good understanding of the physiology and pathophysiology of the gastric function in relation to a single microorganism has made it possible to better understand human gastric carcinogenesis.
In contrast to H. Pylori, colon microflora is a very complex system hosting several billions of bacteria and multitudes of functions. Furthermore, due to the complexity of the current disease model used for colon carcinogenesis, it is not possible at present to make a conclusive link between carcinogenesis in the colon to a single bacterium or function. In this article we focus on data that support the hypothesis that ‘CRCs may be a bacteria-related disease’ and we elaborate on mechanisms that could be involved.
Epidemiological data
The incidence of CRCs is increasing in all western countries and in many developing countries, suggesting that the main cause might be related to the western lifestyle. Mortality due to CRC and its associated global cost to healthcare systems justify deployment of screening programmes based on biological testing prior to colonoscopy or direct access to colonoscopy in average-risk and higher-risk populations respectively [Sobhani et al. 2011a].
Age-related risk in the general population
About 3–6% of the population aged 50–75 years present with adenomatous polyps of varying sizes or cancer [Quintero et al. 2012]. Adenomatous polyps are precancerous lesions that undergo a generative process requiring interactions of various factors (mainly) from the environment. Therefore, time to detection is very important because it takes about 10–15 years for polyps to evolve into cancer if they are not removed. Thus tools for mass screening are considered taking into account patient age and mucosal polyp phenotypes [Bretthauer and Kalager, 2012].
Gene- and familial-related risks
Although no more than a dozen genes are involved in early colon carcinogenesis, less than 5% of CRC cases result from a constitutional mutation. In these cases, the relatives who carry the mutated gene remain at very high risk of developing cancer (Table 1). In this group, the risk of developing cancer varies from 35% to 100% depending on the mutated gene, age and location of the tumour or prenoplastic lesions. Even though the hereditary germline mutation constitutes the main risk factor, the actual development of cancer among carriers in these family syndromes likely depends on environmental factors. In addition, about 10–15% of patients with CRC or adenomas have other affected family members whose conditions do not fulfil the criteria for a constitutional gene-related carcinoma. A simple family history of CRC (defined as one or more close relatives with CRC in the absence of a known hereditary colon cancer) confers a two- to sixfold increase in CRC risk. A personal history of adenomatous polyps confers a 15–20% risk of subsequently developing polyps and increases the risk of CRC in relatives with diagnosed adenomas before the age of 60. The estimated relative risk (RR) for this opulation is 2.59 (95% confidence interval 1.46–4.58) [Winawer et al. 1996]. Interestingly, the RR of CRCs for spouses also appears to be higher than the RR in the general population [Quintero et al. 2012].
Table 1.
Risk of colorectal cancer in gene mutation carriers.
| Syndrome | Risk in mutation carriers |
|---|---|
| FAP | 90% by age 45 years |
| Attenuated FAP | 69% by age 80 years |
| Lynch syndrome | 40–80% by age 75 years |
| MYH*-associated polyposis | 35–53% |
| Peutz-Jeghers syndrome | 39% by age 70 years |
| Juvenile polyposis syndrome | 17–68% by age 60 years |
Although absolute risk is clearly higher than average risk in the general population, note the impact of age and range of risk depending on time to expose to the environment factors.
Guidelines of National Cancer Institute at the National Institutes of Health (http://www.cancer.gov/cancertopics/pdq/genetics/colorectal/HealthProfessional).
FAP, familial adenomatous polyposis. *MYH: MUTYH (mutY Homolog (E. coli) gene) is a human gene located on the short arm (p) of chromosome 1.
Putative roles of genetic and environment factors have been analyzed by two studies performed in twins and in spouses. After analysis of environmental factors, they explain the degree to which hereditary factors contribute to familial CRC [Lichtenstein et al. 2000; Hemminki and Chen, 2004] and support intrafamilial transmission of cancer. Briefly, familial clusters account for approximately 20% of all CRC cases in developed countries, while highly penetrant Mendelian CRC diseases contribute only up to 5% of familial cases. Overall, shared environmental factors may contribute to 90% or more of CRC cancers.
Environment
Although CRC remains one of the most common cancers, incidence rates vary up to 10-fold in both sexes worldwide. The highest rate is estimated in western Europe, Australia and New Zealand, and the lowest in Africa and India. Elevated intake of red meat and animal fat increases CRC while greater consumption of fibre reduces this risk [Greer and O’Keefe, 2011]. The link between western diet and elevated risk of colon cancer has been established for quite some time [O’Keefe, 2008]. The effect of diet on colonic mucosa can either maintain colonic health or promote chronic inflammation [Greer and O’Keefe, 2011]. The first interesting explanation of this observation was that dietary constituents might influence colon cancer prevalence and it was hypothesized that these might be involved in CRC carcinogenesis through the microbial flora of the gut. Microbiota changes (dysbiosis) have been documented in patients with colon cancer or adenomatous polyps compared with control groups [Greer and O’Keefe, 2011; McIllmurray and Langman, 1975; DeFillipo et al. 2010; Shen et al., 2010; Pagnini et al. 2011].
Microbiota
The intestinal microbe population, known as ‘intestinal microbiota’ is heterogeneous and complex, and is composed of more than 1000 different bacterial species. Analysis of the microbiota makes it possible to analyze ‘factors from the environment’ (e.g. nutrients) and interactions with the host. This complex ecosystem contributes to the maturation of the immune system, provides a direct barrier against colonization of the intestine by pathogens [Gaboriau-Routhiau et al. 2011] and seems able to metabolize procarcinogens and carcinogens from the environment [Bordonaro et al. 2008]. Although more than 80% of intestinal bacteria cannot be cultured, identification of all bacteria has become possible by using high technology to perform whole DNA genome sequencing. This metagenomic approach has allowed characterization of healthy individuals’ microbiota [Qin et al. 2010], which is now considered as a reference. Several studies have indicated that the bacterial community in an individual host is relatively stable in the distal digestive tract throughout adult life, although there is much variation from person to person [Eckburg et al. 2005]. The ‘core microbiome’ hypothesis, which states that a similar set of microbial functions can be found at the gene level in all individuals, despite there being substantial variation at the species level, may be a possible explanation for this phenomenon. Nevertheless, it seems plausible to expect that certain individuals (as determined by genetic makeup and lifestyle) carry a higher proportion of bacterial drivers than others and are therefore predisposed to bacteria-related diseases such as obesity, asthma, autoimmunity, or inflammatory bowel diseases [Ley, 2010; Bach, 2002] and CRC. The main challenge is how to describe healthy conditions. In spite of large variations in healthy individuals’ microflora [Eckburg et al. 2005], it has been shown that intestinal microbiota variation is generally stratified, not continuous, and might respond differently to diet and drug intake based on healthy individuals’ microbiota analyses through different continents and countries [Arumugam et al. 2011]. Arumugam and colleagues identified three major enterotypes suggesting the impact of host–environment can be categorized through the microbiota. Further, data-driven marker genes or functional modules could be identified for host properties. For example, 12 genes significantly correlate with age and three functional modules with the body mass index, hinting at the diagnostic potential of microbial markers [Arumugam et al. 2011].
Bacteria and colorectal cancer links
The bacterial density in the large intestine (~1012 cells per ml) is much greater than that in the small intestine (~102 cells per ml), and this is paralleled by an approximately 12-fold increase in cancer risk for the large intestine compared with the small intestine. These two observations combined point towards the hypothesis that colon cancer may be induced by bacteria.
Historical data
Streptococcus bovis/gallolyticus was traditionally considered as a lower grade pathogen involved in endocarditis. Although McCoy and Mason suggested a relationship between colonic carcinoma and the presence of infectious endocarditis in 1951, it was only in 1974 that the association of S. bovis and colorectal neoplasia was recognized [McCoy and Mason, 1951].
In 1971, a study aimed to identify associations between the human microbiota composition and colorectal carcinogenesis without any specific hypothesis concerning a group of bacteria. However, the study had to be abandoned because of technical difficulties in recovering all the bacteria from the stools, including a large majority that were anaerobic. Later on, 13 bacterial species were shown to be significantly associated with a high risk of colon cancer and the western diet [Savage, 1977]. However, these results were somewhat unconvincing because of the small number of people investigated without performing any intestinal investigation (i.e. radiology or colonoscopy).
Clinical data
The phylogenetic core has been characterized in healthy people and therefore a comparison with other individual groups has become possible. Recently, we reported that the phylogenetic core of human microbiota was significantly different in the stools of patients with colon cancer compared with those of matched individuals with normal colonoscopy; this result has been confirmed by others [Marchesi et al. 2011]. Although, these studies did not reveal whether the phylogenetic core changes in the stools of patients with colon cancer were the cause or consequence of the disease, quantification of major bacterial dominant and subdominant groups allowed identification of the Bacteroides family as predominant in patients with colon cancer (Figure 1). Interestingly, Bacteroides fragilis, a common intestinal commensal species from this bacteria family, induces spontaneous colonic tumourigenesis in ApcMin/+ mice [Wu et al. 2009] compared with germ-free animals. More generally, in germ-free conditions lower numbers of tumours are observed than when the conditions involve a conventional microbiota, irrespective of whatever tumours are induced by chemical agents or due to germline mutation (Table 2). However, the possibility that intestinal microorganisms have a direct effect on the initiation and progression of sporadic CRC has been ignored. A growing amount of recent data support the hypothesis that CRC can be initiated by some bacteria called ‘drivers’ and promoted by others called ‘passengers’ [Tjalsma et al. 2012]. For a driver bacteria adherence to the mucosa is presumed while bacteria found in stools are not always detected among those attached to the epithelium and vice versa.
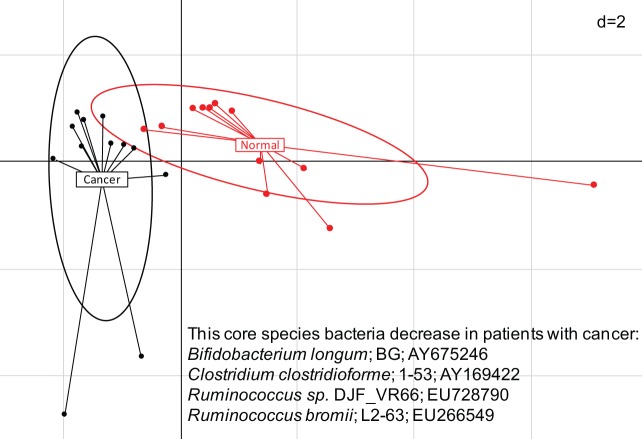
Figure 1.
Composition of bacteria in the stools of patients with colon cancer.
Fresh samples were collected prior to the colonoscopy, DNA was extracted and submitted to pyrosequencing analyses of the 16S rRNA V3–V4 region and comparisons of bacteria sequences were performed between individuals with normal colonoscopy and patients with colon cancer on the basis of bacterial species abundance. This region belongs to the phylogenetic core and differentiates patients with cancer from healthy individuals. Principal component analysis, based on the 16S rRNA gene sequence abundance of 10 discriminate phylogenetic core species, was carried out with six normal individuals (red points) and six patients with cancer (black points) with two replicates. Two first components (PC1 and PC2) were plotted and represented 57.95 % of the whole inertia. Individuals (represented by their sample id) were clustered and centre of gravity computed for each class. This is the first comparison of stool composition in healthy individuals and patients with colon cancer and shows significant dysbiosis (figure is due to Julien Tap).
Table 2.
Putative role of gene and environment in various population-related risks in animal models.
| Host characteristics | Conventional | SPE | Germ free | Mechanisms | Reference |
|---|---|---|---|---|---|
| APC Min/+ | Reference (100%) | Dec. ≈–20% | Dec. ≈–50% | Bacteria-induced proliferation and possible gene mutation accumulation | Arthur and Jobin, 2011; Zhu et al. 2011; Wu et al. 2009 |
| IL10+/+ | Reference (20%) | Inc. 20–40% | n.t. | Inflammation enhancement | Arthur et al. 2012; Arthur and Jobin, 2011; Zhu et al. 2011 |
| + AOM | |||||
| IL10–/– | Inc. (62%) | ||||
| + AOM | |||||
| IL10–/–/ MyoD88–/– | Reference (100%) | Dec. | Dec. | Failure of myeloid cell action | Arthur et al. 2012; |
| Toll-like receptor–/– | Role of adherent bacteria and receptors | Arthur et al. 2012; Arthur and Jobin, 2011; Zhu et al. 2011; Vijay-Kumar et al. 2010 | |||
| Tlr4–/– | Reference (100%) | 0 | 0 | ||
| Toll-like receptor+/+ | Inc. (≈200%) | Inc. (≈130%) | Inc. (≈110%) | ||
| + AOM | |||||
| + DSS | |||||
| NLR nuclear nod like receptor | Bacterial invasion activates microbial sensors, initiate a protective response to clear invading bacteria and restore homeostasis | Arthur et al. 2012; Zhu et al. 2011 | |||
| PYCARD (inflamasome adaptor protein)–/– versus WT | Inc. +++ | Inc.+ | n.t. | ||
| NLRP3–/– versus WT | Inc.++ | n.t. | n.t. | ||
| NLR4–/– | Inc.++ | n.t. | n.t. | ||
| Caspase–/– versus WT | Inc.+++ | n.t. | n.t. | ||
| Nod sensors | Reference | Increased numbers of commensal bacteria | Arthur et al. 2012; Zhu et al. 2011 | ||
| Nod2–/– versus WT | Inc.++ | Inc.+ | No change | Impaired antimicrobial peptide secretion | |
| Nod1–/– versus WT | Inc. ++ (inflammation and tumours) | Inc.+ | No change | ||
| NLRP3 | |||||
| IL6–/–, Stat3–/– | Reference | Dec. | n.t. | n.t. | Arthur et al. 2012 |
| versus | |||||
| WT | |||||
| IL6+/+ | Reference | Dec. | Dec. | IL-6/STAT3 signaling axis modulates homeostasis via proliferation and antiapoptotic effects | Arthur et al. 2012 |
| IL6–/– and Stat3–/– | Dec. | No change | No change | ||
| +DDS or +AOM |
Germline mutated animal models develop inflammation or cancer spontaneously or under chemical (azoxymethane, DSS). In the majority of these, bacteria (conventional microbiota or specific pathogens) accelerate the carcinogenesis. The receptors for bacteria or bacterial production initiate signaling cascades that activate numerous downstream effector systems, including mitogen-activated protein kinases, nuclear factor κB, and interferon regulatory factors that then modulate apoptosis, proliferation, cell migration, and inflammation. Various members of the TLR family (TLR2, TLR4, TLR5, and TLR9) and their adaptor protein MyD88 are involved in innate immune signaling pathways associated with the inflammation and tumours.
Some increases have been semi-quantitatively evaluated and indicated as + to +++.
AOM, azoxymethane; APC, adenomatous polyposis coli; Dec., decrease; DSS, Dextran Sodium Sulfate induces colitis in rodents; IL, interleukin; Inc., increase; NLR, nod like receptor; NLRP3, intra cellular Nod-like receptor; NLRP3-/-, homozygote mutated mouse model; Nods, Nucleotide Orgomerization Domain receptors; n.t., not tested; Stat3, signal transducer and activator of transcription 3; TLR, toll-like receptor; WT, wild type.
Mucosa-adherent bacteria
Epithelium-adherent bacteria appear to be different from stool microbiota in terms of abundance and heterogeneity. Fusobacterium nucleatum is not a dominant species in the stools and has been detected from tumour biopsies in patients with colon cancer by two independent groups (Figures 2 and 3). These groups have suggested that this bacterium might be the cause of CRC occurrence [Castellarin et al. 2012; Kostic et al. 2012] while it has not been prevalent in stools in other studies [Marchesi et al. 2011; Sobhani et al. 2011b]. F. nucleatum is commonly found in the dental plaque of many primates, including humans, and is frequently associated with gum disease. It is a key component of periodontal plaque due to its abundance and its ability to coaggregate with other species in the oral cavity. This bacterium shows the ability to associate with viruses, which adhere to host tissue cells and modulate the host’s immune response [Bolstad et al. 1996]. However, a causative role of Fusobacterium has not been demonstrated and so this bacterium could be a cofactor of an as yet unidentified cause. Therefore, studies on large series of patients examining the abundance and heterogeneity of bacteria in normal tissue and cancer tissue of the same individuals are needed.
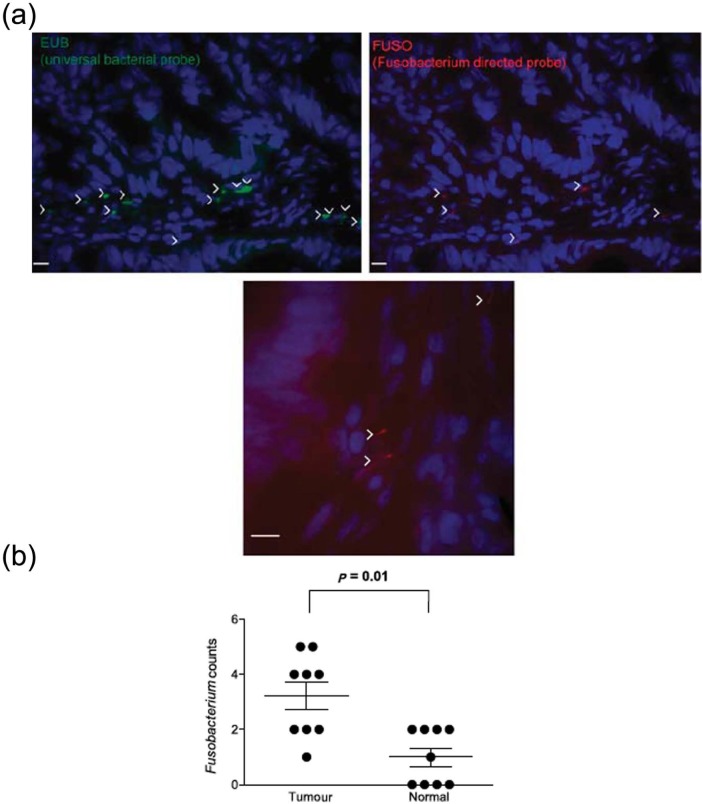
Figure 2.
Fusobacterium in colorectal tumours [Kostic et al. 2012].
(a) Fluorescence in situ hybridization (FISH) using an Oregon-Green 488-conjugated ‘universal bacterial’ 16S rDNA-directed oligonucleotide probe (EUB338, green) (top left), and Cy3-conjugated Fusobacterium (FUSO, red) (top right and bottom centre) 16S rDNA-direct oligonucleotide probe demonstrates the presence of bacteria and Fusobacterium in the colonic mucosa of colorectal tumour samples. Representative images are shown with a 10 μm scale bar in the lower corner of each panel; white arrowheads mark bacteria. Epithelial cell nuclei were stained with 4’,6-diamidino-2-phenylindole. (b) Shows whether Fusobacterium was enriched in tumour versus normal pairs. Each dot represents data from either a tumour or a normal sample from nine tumour/normal paired cases. The mean, standard error of the mean and p values (calculated by a Wilcoxon matched-pair signed rank test) are shown.
Figure 3.
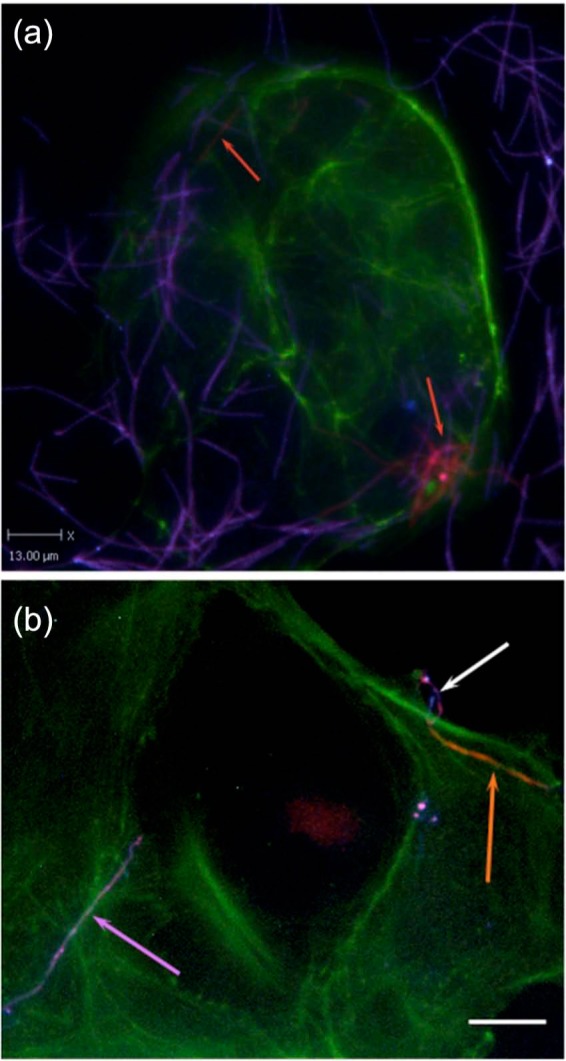
Fusobacterium invading colon cancer Caco-2 cells, in vitro [Castellarin et al. 2012].
Representative differentially stained immunofluorescence image showing strain CC53 invading Caco-2 cells. (a) The differential staining method allows for delineation between bacteria that have penetrated the host cells (labelled for actin in green) to reside within them (orange, also indicated with orange arrows), and bacteria present on the outside of the cell (purple). CC53 shows a very long, fine, thread-like cell morphology. (b) Detail of CC53 invasion. Top right: a representative CC53 cell in the process of invading the Caco-2 host cell [image is differentially stained as for (a)]. The long, thread-like cells appear to penetrate the host cell pole first. Orange arrow: the CC53 cell that is internalized. White arrow: the external portion of the same bacterial cell that has looped around on itself, demonstrating apparent flexibility. Purple arrow: a single CC53 cell that has not invaded the host cells, for contrast. The scale bar is 15 μm. In the immunofluorescence micrograph, CC53 shows a very long flexible cell morphology. Green: actin (Caco-2 cells); orange: invasive and internalized bacteria; purple: bacteria external to the cell.
Streptococcus gallyliticus and Fusobacterium are probably passenger species because in precancerous adenomatous polyps, analysis of the 16S rRNA genes from adherent bacteria has not shown these two species as being significant according to phylogenetic and taxonomic analyses [Shen et al. 2010; Pagnini et al. 2011]. Overall, Firmicutes (62%), Bacteroidetes (26%) and Proteobacteria (11%) are the most dominant phyla in bacteria adherent to these precancerous lesions. Although, significant differences in bacterial composition between cases and controls have been identified, surprisingly, in one of these studies [Pagnini et al. 2011], biopsies from adenomatous polyps showed 20-fold relative reduction of mucosa-adherent bacteria compared with normal tissue. The authors suggested that adenoma mucosa might exert increased antibacterial activity compared with normal mucosa due to α-defensin production, which was found to be significantly increased in adenomas. However, to date, no experimental data are available to support this hypothesis.
Mechanisms involved
In the original model of colon carcinogenesis, it has been proposed that only tubular and tubulovillous adenomas had the potential to progress to invasive adenocarcinoma (Figure 4) [Fearon and Vogelstein, 1990]. Serrated polyps including sessile serrated adenomas (SSAs) and traditional serrated adenomas (TSAs) are now recognized as having the potential for malignant transformation as well [Goldstein, 2006; Noffsinger, 2009]. A subset of hyperplastic polyps that are not usually recognized as precancerous lesions may progress to serrated neoplasms (SSAs or TSAs) and a fraction of these SSAs progress to cancer [Baker et al. 2004]. In these histological lesions various alterations in tumour genes are detected, such as adenomatous polyposis coli (APC) and β-catenin (CTNNB1) genes deleted in CRC and P53 genes, oncogenes such as Kirsten rat sarcoma (K-ras) and myelocytomatosis, and abnormalities such as chromosome instability, microsatellite instability and aberrant DNA methylation at CpG islands [Noffsinger, 2009]. These gene alterations can be induced by chemical or environment factors. The occurrence of tumours is due to DNA alterations in the stem cells at the base of the villus crypt. These changes appear to be permanent and prone to the accumulation of additional mutations [Baker, 2009].
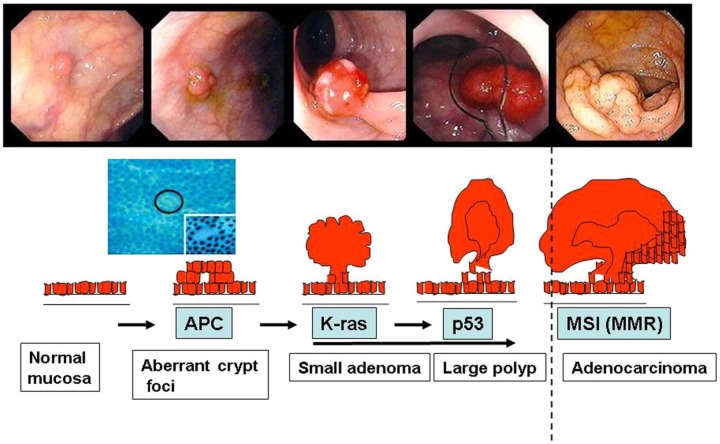
Figure 4.
Colorectal carcinogenesis from normal to invasive cancer.
The slides on the top are from colonoscopy after cleaning preparation. The genes involved in each step are also indicated. High magnification shows aberrant crypt foci is needed to detect precancerous lesions in macroscopic normal mucosa. APC, adenomatous polyposis coli; K-ras, Kirsten rat sarcoma; MMR, mismatch repair; MSI, microsatellite instability.
Stem cell as pivotal target from normal to neoplastic lesion
The stem cells and their descendant proliferating crypt precursors [Reya and Clevers, 2005] with a large number of differentiated cells (enterocytes, enteroendocrine cells and goblet cells) occupy the crypt. They self renew to regenerate the epithelium after injury while progenitor cells arrest their cell cycle and differentiate when they reach the tip of the crypt and when the number of cells produced by the crypt compartment is compensated by apoptosis at the tip of the crypt [Clevers, 2006; van de Wetering et al. 2002; Vogelmann and Amieva, 2007]. Thus, it is presumed that the first carcinogenic event occurs in stem cells or progenitor cells and CRC is considered as the consequence of a succession of steps with identifiable specific gene mutations in cells [Fearon and Vogelstein, 1990]. Here, the adherent bacteria could be considered as a crucial link with the environment, offering interactions with stem cells to yield focal lesions. In this regard the mucus layer [Coic et al. 2012] and crypt-specific core microbiota [Pédron et al. 2012] are considered as facilitators. In the two main hereditary syndromes involving no lethal germline mutations (APC, mismatch repair system) the delay for achieving critical accumulation of mutations in the stem cells up to the development of cancer is significantly shorter (20–45 years age) than in sporadic cancers (65 years age).
The mosaic gene mutation is an exciting candidate mechanism to explain how accumulation of lethal gene mutations (i.e. K-ras, β−catenin) necessary at the very early colon carcinogenic steps in lesions such as aberrant crypt foci (ACFs) or adenomas could be observed. For instance, a constitutional lethal gene mutation (c.49G→A, p.Glu17Lys) in the oncogene AKT1 has recently been reported in tissues of patients with Proteus syndrome (characterized by the overgrowth of skin). By using a custom restriction enzyme test on DNA from patients with the Proteus syndrome, the authors have shown that the syndrome is caused by this lethal gene mutation through somatic mosaicism because skin tissues harbour admixtures of mutant alleles that range from 1% to approximately 50% [Lindhurst et al. 2011].
Focal and diffuse alteration in the intestinal mucosa
Stool versus adherent bacteria composition may impact diffuse and focal injuries respectively. In the mid 1970s and early 1980s, Deschner identified focal histologic lesions in the colonic mucosa in experimental animals as the earliest morphologic alteration preceding tumour development [Deschner, 1974]. Later this phenomenon was shown to be associated with a diffuse hyperproliferation in the intestinal mucosa [Lipkin, 1988]. The pitfalls in the use of colonic mucosal cell proliferation in assessing the potential risk of developing a colonic neoplasia [Whiteley and Klurfeld, 2000] and neoplastic paradigm (e.g. ACF) based on focal areas along the colon have been reviewed [Kinzler and Vogelstein, 1996]. Consequently quantitative changes in cell proliferation, associated with a loss of the epithelium’s ability to inhibit altered (gene mutated) progenitor cells in differentiation, increase the risk of neoplastic development [Wilcox et al. 1992; Zheng et al. 1999]. In this scheme, the human microbiota plays an important role, about which we have already learned a great deal through animal models. The transplant of human microbiota in the intestine of conventional animals stimulates cell proliferation in the intestine compared with germ-free animals (Table 2). The latter observation as well as antibiotic therapy accompanied with weight loss and mucosal atrophy are discussed by Reikvam and colleagues [Reikvam et al. 2011]. It is likely that this effect is direct and in need of bacterial attachment to the epithelial cells. However, an indirect long-term effect through cytokine production exerting a stimulatory effect has not been excluded.
Recognition of microbial species by the host
Humans, mice and other eukaryotes are equipped with an elegant repertoire of receptors, with each receptor recognizing specific conserved microbial patterns, such as components of bacterial cell walls or nucleic acids. These microbial sensors include several RNA helicases, lectin receptors and toll-like receptors [Vijay-Kumar et al. 2010]. These receptors are also expressed on intestinal epithelial cells including endocrine cells, as well as on various mucosal immune cells. They initiate signalling cascades involving mitogen-activated protein kinases, nuclear factor κB and interferon regulatory factors that in turn modulate apoptosis, proliferation and cell migration directly or via cytokines or hormones through a paracrine pathway. These mechanisms are in agreement with several clinical trials that have shown the importance of hyperproliferation in the normal mucosa of individuals at high risk of CRC [Mills et al. 2001; Ponz et al. 1988]. The substantial role of hyperproliferation induced by microbiota as driving CRC has been best demonstrated in gene-mutated animals. For instance, the ApcMin/+ mouse (i.e. with multiple intestinal neoplasia alleles of the APC gene), a model for human familial adenomatous polyposis, develops tens to hundreds of intestinal adenomas when raised in conventional or specific pathogen-free (SPF) conditions. However, mice raised in germ-free conditions exhibit a reduction of around 50% in intestinal tumours [Zhu et al. 2011]. The susceptibility of animals to developing cancer also depends on whether bacteria can induce mucosal inflammation (Table 2). More recently, a microbial composition has been found to influence the development of colitis-associated CRC. Similarly, in the newly developed AOM/IL10−/− model in which intestinal inflammation occurs spontaneously from the lack of immunosuppressive interleukin-10 (IL10), tumuorigenesis has been initiated with the colon- specific carcinogen azoxymethane (AOM); the mice have developed extensive intestinal inflammation and colonic adenomas under conventional or SPF conditions [Arthur et al. 2012]. However, mice monoassociated with Bacteroides vulgates exhibit fewer tumours, and more remarkably, those raised in germ-free conditions are found to be devoid of intestinal inflammation and tumours.
Bacteria-induced DNA alteration
Whether some bacteria can induce DNA damage is not so far documented. It has been suggested that enteropathogenic Escherichia coli possesses the ability to downregulate DNA mismatch repair proteins and, therefore, it could promote colonic tumuorigenesis by directly favouring DNA damage [Cuevas-Ramos et al. 2010]. Enterococcus faecalis induces aneuploidy in colonic epithelial cells and aggressive colitis in monoassociated IL10−/− mice. Furthermore, inhibitors of reactive oxygen and nitrogen species (RONS) prevent E. faecalis-induced aneuploidy [Arthur et al. 2012]. Thus the unique ability of this bacterium to induce RONS can lead to chromosomal instability in a susceptible host. Consequently, one needs to distinguish between indigenous intestinal bacteria able to drive the epithelial DNA damage and thereby contributing to the initiation of CRC, and intestinal niche alterations that favour the proliferation or inhibition of tumour growth. In contrast to driver mutations in the genomes of cancerous cells, bacterial drivers may disappear from cancerous tissue as they are outcompeted by passenger bacteria with a growth advantage in the tumour microenvironment. Species such as Bacteroides, Shigella, Citrobacter and Salmonella or E. coli may be considered as bacteria drivers since they are more abundant in the early stages of CRC, including adenomas, and disappear from cancerous tissue as the disease progresses. Moreover, Fusobacterium spp., Streptococcus gallolyticus subsp. gallolyticus, Clostridium septicum and Coriobacteriaceae (Slackia and Collinsella spp.), the genus Roseburia and the genus Faecalibacterium may be considered as passenger bacteria [Tjalsma et al. 2012].
Indirect bacteria effects, energy balance and metabolism
An alternative pathway by which microbiota may drive carcinogenesis effects would be through hormone and metabolic changes [Ley, 2010]. A standard low-fat diet changing to a high-fat diet within weeks has been shown to be associated with a shift in the balance of the two dominant phyla, Bacteroidetes and Firmicutes, due to the expansion in one group of Firmicutes, the Mollicutes. This trait can be transferred by microbiota transplant into lean recipients. Similar changes in the proportion of Firmicutes and Bacteroidetes have been identified in overweight and obese humans, in genetically obese mice and in obesity-resistant mice fed a high-fat diet [Ley, 2010; Turnbaugh et al. 2006]. These highlight the critical role of specific members of the commensal microbiota in the development of metabolic- and metabolic-associated CRC. For instance hyperleptinaemia or hyperinsulinaemia are shown to increase the number of preneoplastic lesions in Apc–/– mice treated with exogenous leptin or in db/db rats treated with AOM [Hirose et al. 2004; Aparicio et al. 2005] respectively. In the first model, APC gene alteration is constitutional and affects all cells, including stem cells within crypts and submitted to pharmacologically obtained hyperleptinaemia. In the second model, AOM induces somatic gene alteration (i.e. β−catenin) including stem cells and endogenous hyperleptinaemia resulting from alteration of leptin receptor. In both models, leptin plays the role of epithelial cell stimulation. The serum leptin level is elevated in obese individuals. Men of all ages and postmenopausal women are at increased risk for CRC if they are obese [Frezza et al. 2006]. Visceral associated fat, the stigma of various hormone disorders, is considered the source of presumptive metabolic risk factors for colon cancer in mice. Mice with visceral associated fat develop hyperinsulinaemia and preneoplastic colonic mucosal changes more often than mice without visceral abdominal obesity. Insulin-like growth factors are mitogens and regulate energy-dependent growth processes, cell proliferation and inhibit apoptosis [Calle, 2007]. Findings on insulin resistance and colonic adenomas and cancers are reported by us and others [Sobhani et al. 1993, 2010; Frezza et al. 2006]. These results are consistent with those from prospective and interventional studies performed in obese individuals that have shown higher colonic cell proliferation and elevated numbers of ACFs in the colonic mucosa [Calle, 2007; Sainsbury et al. 2008].
Bacterial enzyme activity
The production of bioactive carcinogenic compounds as a result of environmental factors (diet, chemical agents) may be obtained through enzyme activities (β-glucuronidase, β-glucosidase, azoreductase and nitroreductase). For instance, AOM is first hydrolyzed in the liver to methylazoxymethanol and conjugated with glucuronic acid before transport to the intestine through bile secretion. Similarly, bacterial β-glucuronidase spontaneously yields the highly reactive methyl carbonium ion, a carcinogenic form from AOM, while inhibition of β-glucuronidase significantly reduces the ability of AOM to induce tumours in rats [Arthur and Jobin, 2011].
Chronic inflammation
Many cancers arise from sites of infection, chronic irritation and inflammation. The strongest association of chronic inflammation with malignant diseases is found in inflammatory bowel diseases of the colon [Balkwill and Mantovani, 2010] with a lifetime incidence of 10% [Wang and DuBois, 2010]. Thus, patients with chronic inflammatory bowel disease (Crohn’s disease, ulcerative colitis) are considered at high risk of CRC occurrence. These patients should undergo colonoscopy every 2–3 years, even when in clinical remission. In addition, a special zoom endoscopy or colouration process should be used to enhance the sensitivity of colonoscopy for detecting precancerous lesions such as ACF or high-grade dysplasia. Although the microbiota influences the development of colitis-associated CRC, the extent of host microbial recognition in this process is still unclear. A recent study performed in animals clearly showed that colitis can promote tumourigenesis by altering microbial composition and inducing the expansion of microorganisms with genotoxic capabilities [Arthur et al. 2012]. This involves two different groups of bacteria, those inducing chronic inflammation and those favouring carcinogenesis. Monocolonization with the commensal E. coli NC101 promoted invasive carcinoma in AOM-treated IL10–/– mice. Deletion of the polyketide synthase genotoxic island from E. coli NC101 decreases tumour multiplicity and invasion in AOM/IL10–/– mice, without altering intestinal inflammation. The role of bacteria stimulating proinflammatory mediators is therefore important. Wu and colleagues showed that the intestinal flora may promote colon tumour formation in APC knockout mice via immunologic mechanisms [Wu et al. 2009]. They were able to demonstrate that Bacteroides enterotoxigenic fragilis, a very common human commensal bacterium, induces cancer by a T helper 17 (TH17)-dependent pathway mechanism. They observed more ACFs and cancers in animals treated with this bacterium compared with controls. This effect could be suppressed by using anti-IL17 antibody. It is likely that the relationship between activation of regulatory T cells, overexpression of TH17 cells in the colonic mucosa and overexpression of the adherent Bacteroides group in the mucosa impacts the development of colon cancer. These observations are consistent with inflammatory and immune cell infiltration in the homologous normal mucosa of patients with cancer in our human colonoscopy series compared with normal mucosa in individuals with normal colonoscopy [Sobhani et al. 2011b]. However, whether this dysbiosis directly causes colon cancer or is a result of confounding factors from the environment remains to be investigated. In an experimental study performed on mice (personal unpublished data) we observed that the fresh stools of patients with colon cancer exert a higher proliferative effect on the intestinal mucosa in germ-free animals in which ACFs are also enhanced compared with the control germ-free mice after the transfer of fresh stools from gender- and age-matched normal individuals. Quantitative polymerase chain reaction measuring all bacteria and main groups did not reveal significant differences between recipient mice during the experimental period, suggesting that these main groups of bacteria are not likely to be the cause of the observed intestinal mucosa changes. However, 16S rRNA gene pyrosequencing results showed significant differences between the stools of patients with colon cancer and those of individuals with normal colon at baseline. Thus, we suggested the possible involvement of other bacteria groups or species [Sobhani et al. 2011c].
Anticancer bacterial effect
Exclusion of opportunistic pathogens by commensal bacteria may represent a natural defence against gastrointestinal diseases, including CRC. Probiotic bacteria (Lactobacillus spp. and Bifidobacterium spp.) exert anticarcinogenic effects, in part by inactivating microbial enzymes. For example, probiotic lactic acid bacteria including Lactobacillus casei and Lactobacillus acidophilus can decrease the activity of β-glucuronidase, azoreductase and nitroreductase. Bifidobacterium longum reduces AOM-induced aberrant crypt formation, which correlates with a decrease in AOM-activating β-glucuronidase activity. Lactobacillus spp. and Bifidobacterium spp. can inhibit DNA damage and tumourigenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine, 1,2-dime thylhydrazine [Arthur and Jobin, 2011]. In general, microbial fermentation of dietary fibres leads to short-chain fatty acid acetate, propionate and butyrate production. These are absorbed by colonocytes and used as a primary source of energy. They provide protection during the early stages of tumourigenesis [Scheppach, 1994].
The use of probiotics to prevent mild inflammation in the colonic mucosa via bacteria change would be the main promising therapeutic in the future.
Hypotheses and perspectives
We propose that the composition of the microbiota can shape a healthy immune response or predispose an individual to disease. Many factors can contribute to dysbiosis, including lifestyle, medical practices and likely host genetic phenotype. An individual with mutations in genes involved in immune regulatory mechanisms or proinflammatory pathways could lead to unrestrained inflammation in the intestine. It is possible that inflammation alone influences the composition of the microbiota, skewing it in favour of pathobionts. Alternatively, a host could ‘select’ or exclude the colonization of particular organisms.
This selection can be either active (as would be the case of an organism recognizing a particular receptor on the host) or passive (when the host environment is more conducive to fostering the growth of select organisms). In some conditions, selection of pathobionts by the host could tip the balance in favour of inflammation. Diet, toxic components and stress also have the potential to induce direct somatic gene alteration in the colonocytes or alternatively influence the microbiota. The overall results would be the failure to inhibit epithelium renewal in a mucosa with stem cells in which crucial genes are altered.
Several questions remain to be addressed. First, the composition of microbiota should be fully analyzed using metagenomic sequencing on all bacteria genes to identity microorganisms promoting health or disease. These studies are currently in progress in Europe and in the USA. Potential dysbiotic microbiota could be found in patients with CRC and tested in experimental models. ‘Does the shift in the microbiota directly alter the course of disease?’ is a very important question and animal studies are required to address this. Is the information yielded by the metagenomic approach sufficient to initiate functional experiments in which a cause/effect relationship could be established using animal models? What would be the outputs of functional studies using dysbiotic microbiota obtained from germ-free animals and various disease states – inflammation, CRC? Although identification of microbial consortia associated with particular pathological conditions represents an important milestone, this critical step is not sufficient to fully understand the role of the microbiota in health and disease. Thus, it is crucial to work on the possibility and feasibility of manipulating the human microbiota and its metabolic capacity as an innovative approach to treating and preventing CRC.
Conclusion
The putative role of gene alterations and environmental factors in CRC genesis remains to be elucidated. More data are now available on the involvement of nutriment, hormone and metabolic disorders favouring CRC genesis. Colon microbiota should be considered as a novel window for analyzing exhaustive factors from the environment. Up to now, there has been no real evidence that bacteria might directly induce mutation in colonocytes. However, changes in energy uptake, metabolic disorders, and stimulation of epithelial cells and reduction of protective bacteria are possible ways through which carcinogenesis might be facilitated in humans.
Acknowledgments
We than Dr Farzam Ranjbaran for his kind contribution to the manuscript lecture and corrections and Julien Tap for his kind contribution to the figure construction.
Footnotes
Funding: Supported by ACD (association Charles Debrey).
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Iradj Sobhani, APHP-UPEC Paris12, 51 Av Mal deLattre de Tassigny, Créteil 94010, France.
Aurelien Amiot, Gastroenterology Unit, Albert Chenevier-Henri Mondor Hospital AP-HP, UPEC, Université Paris 12, Paris, France.
Yann Le Baleur, Gastroenterology Unit, Albert Chenevier-Henri Mondor Hospital AP-HP, UPEC, Université Paris 12, Paris, France.
Michael Levy, Gastroenterology Unit, Albert Chenevier-Henri Mondor Hospital AP-HP, UPEC, Université Paris 12, Paris, France.
Marie-Luce Auriault, Department of Pathology, Albert Chenevier-Henri Mondor Hospital AP-HP, UPEC, Université Paris 12, Paris, France.
Jeanne Tran Van Nhieu, Department of Pathology, Albert Chenevier-Henri Mondor Hospital AP-HP, UPEC, Université Paris 12, Paris, France.
Jean Charles Delchier, Gastroenterology Unit, Albert Chenevier-Henri Mondor Hospital AP-HP, UPEC, Université Paris 12, Paris, France.
References
- Aparicio T., Kermorgant S., Darmoul D., Guilmeau S., Hormi K., Mahieu-Caputo D., et al. (2005) Leptin and Ob-Rb receptor isoform in the human digestive tract during fetal development. J Clin Endocrinol Metab 90: 6177–6184 [DOI] [PubMed] [Google Scholar]
- Arthur J., Jobin C. (2011) The struggle within: microbial influences on colorectal cancer. Inflamm Bowel Dis 17: 396–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J., Perez-Chanona E., Muhlbauer M., Tomkovich S., Uronis J., Fan T., et al. (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338: 120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M., Raes J., Pelletier E., Le P., Yamada T., Mende D., et al. (2011) Enterotypes of the human gut microbiome. Nature 473: 174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J. (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347: 911–920 [DOI] [PubMed] [Google Scholar]
- Baker K., Zhang Y., Jin C., Jass J. (2004) Proximal versus distal hyperplastic polyps of the colorectum: different lesions or a biological spectrum? J Clin Pathol 57: 1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. (2009) Stem cells: fast and furious. Nature 458: 962–965 [DOI] [PubMed] [Google Scholar]
- Balkwill F., Mantovani A. (2010) Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther 87: 401–406 [DOI] [PubMed] [Google Scholar]
- Bolstad A., Jensen H., Bakken V. (1996) Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev 9: 55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordonaro M., Lazarova D., Sartorelli A. (2008) Butyrate and Wnt signaling: a possible solution to the puzzle of dietary fiber and colon cancer risk? Cell Cycle 7: 1178–1183 [DOI] [PubMed] [Google Scholar]
- Bretthauer M., Kalager M. (2012) Colonoscopy as a triage screening test. N Engl J Med 366: 759–760 [DOI] [PubMed] [Google Scholar]
- Calle E. (2007) Obesity and cancer. BMJ 335: 1107–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarin M., Warren R., Freeman J., Dreolini L., Krzywinski M., Strauss J., et al. (2012) Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22: 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Coic Y., Baleux F., Poyraz O., Thibeaux R., Labruyere E., Chretien F., et al. (2012) Design of a specific colonic mucus marker using a human commensal bacterium cell surface domain. J Biol Chem 287: 15916–15922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Ramos G., Petit C., Marcq I., Boury M., Oswald E., Nougayrede J. (2010) Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A 107: 11537–11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFillipo C., Cavalieri D., Di P., Ramazzotti M., Poullet J., Massart S., et al. (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107: 14691–14696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deMartel C., Ferlay J., Franceschi S., Vignat J., Bray F., Forman D., et al. (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13: 607–615 [DOI] [PubMed] [Google Scholar]
- Deschner E. (1974) Experimentally induced cancer of the colon. Cancer 34(Suppl. S3): 824–828 [DOI] [PubMed] [Google Scholar]
- Eckburg P., Bik E., Bernstein C., Purdom E., Dethlefsen L., Sargent M., et al. (2005) Diversity of the human intestinal microbial flora. Science 308: 1635–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E., Vogelstein B. (1990) A genetic model for colorectal tumorigenesis. Cell 61: 759–767 [DOI] [PubMed] [Google Scholar]
- Frezza E., Wachtel M., Chiriva-Internati M. (2006) Influence of obesity on the risk of developing colon cancer. Gut 55: 285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V., Lecuyer E., Cerf-Bensussan N. (2011) Role of microbiota in postnatal maturation of intestinal T-cell responses. Curr Opin Gastroenterol 27: 502–508 [DOI] [PubMed] [Google Scholar]
- Goldstein N. (2006) Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification. Am J Clin Pathol 125: 146–153 [PubMed] [Google Scholar]
- Greer J., O’Keefe S. (2011) Microbial induction of immunity, inflammation, and cancer. Front Physiol 1: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki K., Chen B. (2004) Familial risk for colorectal cancers are mainly due to heritable causes. Cancer Epidemiol Biomarkers Prev 13: 1253–1256 [PubMed] [Google Scholar]
- Hirose Y., Hata K., Kuno T., Yoshida K., Sakata K., Yamada Y., et al. (2004) Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis 25: 821–825 [DOI] [PubMed] [Google Scholar]
- Kinzler K., Vogelstein B. (1996) Lessons from hereditary colorectal cancer. Cell 87: 159–170 [DOI] [PubMed] [Google Scholar]
- Kostic A., Gevers D., Pedamallu C., Michaud M., Duke F., Earl A., et al. (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22: 292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R. (2010) Obesity and the human microbiome. Curr Opin Gastroenterol 26: 5–11 [DOI] [PubMed] [Google Scholar]
- Lichtenstein P., Holm N., Verkasalo P., Iliadou A., Kaprio J., Koskenvuo M., et al. (2000) Environmental and heritable factors in the causation of cancer – analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343: 78–85 [DOI] [PubMed] [Google Scholar]
- Lindhurst M., Sapp J., Teer J., Johnston J., Finn E., Peters K., et al. (2011) A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med 365: 611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin M. (1988) Biomarkers of increased susceptibility to gastrointestinal cancer: new application to studies of cancer prevention in human subjects. Cancer Res 48: 235–245 [PubMed] [Google Scholar]
- Marchesi J., Dutilh B., Hall N., Peters W., Roelofs R., Boleij A., et al. (2011) Towards the human colorectal cancer microbiome. PLoS One 6: e20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy W., Mason J., III (1951) Enterococcal endocarditis associated with carcinoma of the sigmoid; report of a case. J Med Assoc State Ala 21: 162–166 [PubMed] [Google Scholar]
- McIllmurray M., Langman M. (1975) Large bowel cancer: causation and management. Gut 16: 815–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S., Mathers J., Chapman P., Burn J., Gunn A. (2001) Colonic crypt cell proliferation state assessed by whole crypt microdissection in sporadic neoplasia and familial adenomatous polyposis. Gut 48: 41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noffsinger A. (2009) Serrated polyps and colorectal cancer: new pathway to malignancy. Annu Rev Pathol 4: 343–364 [DOI] [PubMed] [Google Scholar]
- O’Keefe S. (2008) Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol 24: 51–58 [DOI] [PubMed] [Google Scholar]
- Pagnini C., Corleto V., Mangoni M., Pilozzi E., Torre M., Marchese R., et al. (2011) Alteration of local microflora and alpha-defensins hyper-production in colonic adenoma mucosa. J Clin Gastroenterol 45: 602–610 [DOI] [PubMed] [Google Scholar]
- Pédron T., Mulet C., Dauga C., Frangeul L., Chervaux C., Grompone G., et al. (2012) A crypt-specific core microbiota resides in the mouse colon. MBio 3: e00116–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ponz L., Roncucci L., Di D., Tassi L., Smerieri O., Amorico M., et al. (1988) Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res 48: 4121–4126 [PubMed] [Google Scholar]
- Qin J., Li R., Raes J., Arumugam M., Burgdorf K., Manichanh C., et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464: 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero E., Castells A., Bujanda L., Cubiella J., Salas D., Lanas A., et al. (2012) Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 366: 697–706 [DOI] [PubMed] [Google Scholar]
- Reikvam D., Erofeev A., Sandvik A., Grcic V., Jahnsen F., Gaustad P., et al. (2011) Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One 6: e17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T., Clevers H. (2005) Wnt signalling in stem cells and cancer. Nature 434: 843–850 [DOI] [PubMed] [Google Scholar]
- Sainsbury A., Goodlad R., Perry S., Pollard S., Robins G., Hull M. (2008) Increased colorectal epithelial cell proliferation and crypt fission associated with obesity and roux-en-Y gastric bypass. Cancer Epidemiol Biomarkers Prev 17: 1401–1410 [DOI] [PubMed] [Google Scholar]
- Savage D. (1977) Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31: 107–133 [DOI] [PubMed] [Google Scholar]
- Scheppach W. (1994) Effects of short chain fatty acids on gut morphology and function. Gut 35: S35–S38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Rawls J., Randall T., Burcal L., Mpande C., Jenkins N., et al. (2010) Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes 1: 138–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani I., Alzahouri K., Ghout I., Charles D., Durand-Zaleski I. (2011a) Cost-effectiveness of mass screening for colorectal cancer: choice of fecal occult blood test and screening strategy. Dis Colon Rectum 54: 876–886 [DOI] [PubMed] [Google Scholar]
- Sobhani I., Jarrousse V., Guilemeau S., Blugeon S., Auriault M., et al. (2011c). Colon cancer patients’ fresh stools induced intestinal precancerous lesion in germ-free mice. Gut 60(Suppl. 3): A121409795 [Google Scholar]
- Sobhani I., LeGouvello S. (2009) Critical role for CD8+FoxP3+ regulatory T cells in colon cancer immune response in humans. Gut 58: 743–744 [DOI] [PubMed] [Google Scholar]
- Sobhani I., Lehy T., Laurent-Puig P., Cadiot G., Ruszniewski P., Mignon M. (1993) Chronic endogenous hypergastrinemia in humans: evidence for a mitogenic effect on the colonic mucosa. Gastroenterology 105: 22–30 [DOI] [PubMed] [Google Scholar]
- Sobhani I., Tap J., Roudot-Thoraval F., Roperch J., Letulle S., Langella P., et al. (2011b) Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One 6: e16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani I., Zeitoun J., Cahnteloup E., Charachon A., Levy M., Delchier J. (2010) Obesity and cancer: yin/yan effects of nutrition. Open Obesity J 2: 38–42 [Google Scholar]
- Tjalsma H., Boleij A., Marchesi J., Dutilh B. (2012) A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol 10: 575–582 [DOI] [PubMed] [Google Scholar]
- Turnbaugh P., Ley R., Mahowald M., Magrini V., Mardis E., Gordon J. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031 [DOI] [PubMed] [Google Scholar]
- Van de Wetering M., Sancho E., Verweij C., de Lau W., Oving I., Hurlstone A., et al. (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241–250 [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M., Aitken J., Carvalho F., Cullender T., Mwangi S., Srinivasan S., et al. (2010) Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann R., Amieva M. (2007) The role of bacterial pathogens in cancer. Curr Opin Microbiol 10: 76–81 [DOI] [PubMed] [Google Scholar]
- Wang D., DuBois R. (2010) Therapeutic potential of peroxisome proliferator-activated receptors in chronic inflammation and colorectal cancer. Gastroenterol Clin North Am 39: 697–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley L., Klurfeld D. (2000) Are dietary fiber-induced alterations in colonic epithelial cell proliferation predictive of fiber’s effect on colon cancer? Nutr Cancer 36: 131–149 [DOI] [PubMed] [Google Scholar]
- Wilcox D., Higgins J., Bertram T. (1992) Colonic epithelial cell proliferation in a rat model of nongenotoxin-induced colonic neoplasia. Lab Invest 67: 405–411 [PubMed] [Google Scholar]
- Winawer S., Zauber A., Gerdes H., O’Brien M., Gottlieb L., Sternberg S., et al. (1996) Risk of colorectal cancer in the families of patients with adenomatous polyps. National Polyp Study Workgroup. N Engl J Med 334: 82–87 [DOI] [PubMed] [Google Scholar]
- Wu S., Rhee K., Albesiano E., Rabizadeh S., Wu X., Yen H., et al. (2009) A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15: 1016–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Kramer P., Lubet R., Steele V., Kelloff G., Pereira M. (1999) Effect of retinoids on AOM-induced colon cancer in rats: modulation of cell proliferation, apoptosis and aberrant crypt foci. Carcinogenesis 20: 255–260 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Michelle L., Jobin C., Young H. (2011) Gut microbiota and probiotics in colon tumorigenesis. Cancer Lett 309: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]