Abstract
Background:
Endoscopic ultrasound (EUS) is a resource-intensive endoscopic procedure, but may result in high downstream health resource utilization and multispecialty impact. Our aim was to examine the downstream impact of EUS by specialty and by indication.
Methods:
A retrospective chart review was performed at an academic medical center for patients for whom EUS represented the first point of contact at the study institution within a 20-month period.
Results:
A total of 552 EUS procedures were reviewed and 208 represented the first point of contact. The most common principle indication involved the pancreas (n = 117, 56%). Downstream health utilization was calculated for an average of 313 days postprocedure (range: 35–632 days). Following unique referral for endoscopic ultrasound, 52% of the patients in the study were retained within the institution for further management and, of these, 34% had a major intervention in the form of surgery or chemoradiotherapy. Compared with other indications, patients presenting with a pancreatic mass were significantly more likely to remain in the study institution for further management (62% versus 39%, p = 0.005), were more likely to have a downstream surgery (29% versus 14%, p = 0.020) and were more likely to have downstream chemo-radiotherapy (11% versus 3%, p = 0.012).
Conclusions:
EUS represents a unique portal of entry into tertiary referral medical centers. First point of contact EUS referrals are associated with major downstream health resource utilization and significantly increased utilization for mass lesions of the pancreas.
Keywords: endoscopic ultrasound, fine-needle aspiration, health utilization, pancreatic mass
Introduction
Endoscopic ultrasound (EUS) is utilized for minimally invasive diagnostic and therapeutic interventions, frequently involving the diagnosis and staging of gastrointestinal (GI) malignancies. When employed to evaluate GI malignancy or for cancer staging, EUS may change clinical management in 31% of patients, resulting in less invasive and less risky management [Nickl et al. 1996].
Due to the anatomic location of the pancreas posterior to the stomach and to its easy accessibility by EUS, EUS has a prominent role in evaluating and managing lesions of the pancreas. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is the most sensitive and specific modality for the diagnosis of pancreatic cancers, with studies showing EUS-FNA sensitivity of 80–94%, specificity of 97–100% and accuracy of 84–92% [Wiersema et al. 1997; Gress et al. 1997; Williams et al. 1999; Harewood and Wiersema, 2002; Raut et al. 2003; Eloubeidi et al. 2003].
However, EUS equipment is expensive, the procedure duration is comparatively long, the training requirements are extensive, and reimbursement is not reflective of the discrepancy in procedure length and complexity. These factors create a disincentive to the incorporation of EUS into medical centers and practice groups. Nevertheless, EUS may have comparatively greater downstream health resource utilization than other interventional endoscopic procedures, including colonoscopy, endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic mucosal resection (EMR) [Harewood et al. 2009; Atkinson and Schmulewitz, 2009]. We sought to examine downstream health resource utilization for patients uniquely referred for EUS, and for whom EUS represented the first point of contact within the institution, to determine the relative impact by specialty and by procedural indication.
Methods
Study design and population
The study was a retrospective review of EUS procedures performed within a 20-month period at a tertiary referral, academic medical center. The Institutional Review Board of Emory University approved the study. All patients provided written informed consent for the clinical procedures. Downstream health utilization was calculated within the 20-month period for the patients for whom EUS represented the first point of contact. A downstream health service was included only if it was a direct result of findings at EUS and would not have occurred at the study institution in the absence of EUS.
An EUS procedure was considered the first point of contact if a patient was referred directly for an EUS procedure from outside the study institution. Patients were included if they had prior care at the study institution and this prior care was for an unrelated indication (e.g. bone fracture and orthopedic surgery 5 years prior to EUS). Patients were included if they received an imaging test at the study institution prior to the EUS if these patients had been referred from outside the study institution for EUS and cross sectional imaging was ordered by the consultants in preparation for the procedure. EUS procedures were included as first point of contact for inpatients if the transfer request was for endoscopic ultrasound or an EUS guided intervention (e.g. pseudocyst and pancreatitis). EUS procedures were not included if they resulted from internal referral within the study institution.
EUS procedures were included if they had been performed ≥1 month prior to the end of follow up to allow a minimum of 1 month for examination of intra-institution follow up to have occurred.
Downstream health utilization was examined for clinic visits including surgery, gastroenterology, hematology-oncology, radiation-oncology, nutrition, pain center, psychiatry, endocrine, rehabilitation and anticoagulation clinics. Surgeries included major operations such as pancreaticoduodenectomy (Whipple procedure) as well as minor operations such as chemotherapy port placement. Endoscopy procedures included EUS, ERCP, endoscopic gastroduodenoscopy (EGD) and colonoscopy. Imaging included computed tomography (CT), magnetic resonance imaging (MRI), octreotide scanning, positron emission tomography (PET), ultrasound and hepatobiliary iminodiacetic acid (HIDA) scan. Interventional radiology procedures included percutaneous transhepatic biliary catheter placement, gastrojejunostomy tube placement and peripherally inserted central catheter (PICC) line insertion.
Data collection
Data on patient demographics, dates of service utilization, indications, procedural details and endoscopist, as well as downstream health resource utilization were abstracted from the electronic medical record and were compiled to form the study database.
Statistical analysis
Downstream health utilization was examined using descriptive statistics. Patients referred for evaluation of pancreatic mass lesions were compared with other indications in terms of downstream surgery, downstream chemotherapy and downstream radiation therapy. Noncontinuous variables were compared using the Chi-square tests for contingency tables. A p value ≤ 0.05 was considered statistically significant.
Results
The mean patient age at time of EUS procedure was 59 years, with a range of 12 to 97 years. There were 108 females and 100 males in the subset uniquely referred for EUS. The average duration of procedure was 35 minutes [standard deviation (SD): 14 minutes, range: 6 to 76 minutes]. Downstream health utilization was calculated over an average of 313 days following the EUS procedure date (range: 35 to 632 days).
The majority of EUS procedures were for pancreatic indications, and pancreas-related indications constituted 56% of the total for first point of contact EUS (117/208). These included pancreatic mass (n = 45), pancreatic cyst (n = 41), evaluation of pancreatitis (n = 14), dilated bile duct and/or pancreatic duct (n = 12), evaluation/staging of pancreatic neuroendocrine tumor (n = 3), celiac plexus neurolysis (n = 1) and evaluation/staging of pancreatic cancer (n = 1).
A total of 552 EUS procedures were reviewed and 208 represented the first point of contact. Downstream health utilization for the 208 first point of contact EUS cases were represented by contacts across the institution across specialties. Total conglomerated utilization was represented by surgeries (55), clinic visits outside the department of gastroenterology (255), additional endoscopic procedures (73), interventional radiology procedures (31), and imaging tests (262) (Table 1).
Table 1.
First point of contact EUS procedures (n = 208) and total downstream health utilization by type of referral.
| Indication by organ of interest | n | Surgeries (total) | Clinic visits (total) | Chemotherapy and radiation treatments (total) | Endoscopy and interventional radiology procedures (total) | Imaging (total) |
|---|---|---|---|---|---|---|
| Pancreasa | 117 | 31 | 219 | 87 | 56 | 168 |
| Stomachb | 41 | 9 | 42 | 0 | 16 | 43 |
| Esophagusc | 24 | 6 | 10 | 1 | 16 | 15 |
| Duodenumd | 10 | 1 | 7 | 0 | 10 | 19 |
| Othere | 16 | 8 | 21 | 11 | 6 | 17 |
| GRAND TOTAL | 208 | 55 | 299 | 99 | 104 | 262 |
Includes indications: pancreatic mass, pancreatic cyst, evaluation of pancreatitis, dilated bile duct and/or pancreatic duct, evaluation/staging of neuroendocrine tumor of the pancreas, celiac plexus neurolysis and evaluation/staging of pancreatic cancer.
Includes indications: gastric mass and gastric ulcer.
Includes indications: esophageal mass, evaluation/staging of esophageal cancer, dysphagia, mass in the gastroesophageal junction, periesophageal mass and surveillance/restaging of dysplasia associated with Barrett’s esophagus.
Includes indications: duodenal mass, ampullary adenoma, and duodenal ulcer.
Includes indications: abdominal pain, abnormal imaging, abdominal mass, celiac lymphadenopathy, diarrhea, mediastinal mass and surveillance/restaging of cholangiocarcinoma.
Of 208 patients for whom EUS represented the first point of contact, 108 (52%) were retained at the study institution for health services following the EUS procedure and 36 (17%) stayed in the institution for one or more surgeries. Additionally, 84 patients (40%) had one or more clinic appointments; 72 patients (35%) had one or more imaging tests; 49 patients (24%) had one or more endoscopic or interventional radiology procedures; and 15 patients (7%) had clinic appointments with hematology-oncology, of which 9 (60%) ultimately received chemotherapy ± radiation treatment. The most frequent downstream clinic appointment was at the surgical clinic (47/208 patients, 23%).
Patients referred for evaluation of mass lesions of the pancreas were compared with patients referred for other indications. Of the patients with a pancreatic mass indication, 62% (28/45) were retained in the study institution for further management compared with 39% (63/163) of the patients with a nonpancreatic mass indication (p = 0.005). Patients with a pancreatic mass indication were significantly more likely to remain in the study institution for surgery, with 29% (13/45) having at least one downstream surgery compared with 14% (23/168) of patients with a nonpancreatic mass indication (p = 0.020). Patients with a pancreatic mass indication were also significantly more likely to remain within the study institution for chemotherapy ± radiation treatment, with 11% receiving chemoradiotherapy compared with 3% (4/163) of patients with a nonpancreatic mass indication (p = 0.012) (Figure 1).
Figure 1.
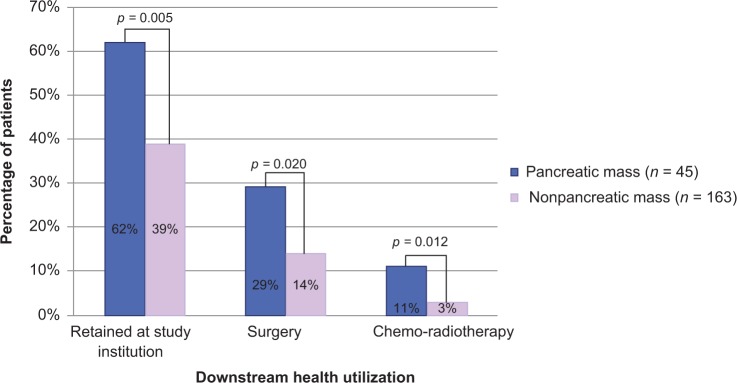
Proportion of patients retained in the institution, receiving downstream surgery, and receiving downstream chemo-radiotherapy stratified by procedural indication.
Conclusion
EUS is associated with major downstream health resource utilization and significant impact across subspecialties outside of and within the Department of Medicine. Following unique referral for EUS, more than half of the patients in the study were retained within the study institution for further management and, of these, 34% had a major intervention in the form of surgery or chemoradiotherapy. The majority of referrals (56%) were for pancreatic indications, consistent with the central role played by EUS in the evaluation of pancreatic lesions. Patients referred for a mass lesion of the pancreas were significantly more likely to remain at the study institution for further management, to receive downstream surgery and to receive chemoradiotherapy.
While surgical services, imaging modalities and oncology consult services may be available in the community, EUS is often underrepresented due to high upfront equipment and set-up costs, lengthy training requirements and comparatively lower reimbursement based on procedure duration. Thus, EUS represents a unique portal of entry into tertiary referral centers. In this study, approximately a third of all EUS referrals (208/552 patients, 38%) were unique and represented the first point of contact within the institution. Many of these patients will remain within the institution, over half in this study, emphasizing the importance of EUS as a key differentiator for referrals for diagnosis, staging and therapeutics. A previous study at our institution demonstrated an accuracy of 91% with EUS-FNA for tissue diagnosis, emphasizing the high fidelity of the technique despite a difficult tertiary referral based cohort [Sodikoff et al. 2012].
EUS is probably the best modality for the evaluation of pancreatic lesions and abnormalities. The anatomical location of the pancreas immediately posterior to the stomach makes it difficult to access percutaneously but straightforward for an EUS approach. Correspondingly, EUS-guided fine needle aspiration has been shown to have the greatest sensitivity and specificity for the evaluation of pancreatic mass lesions [Wiersema et al. 1997; Gress et al. 1997; Williams et al. 1999; Harewood and Wiersema, 2002; Raut et al. 2003; Eloubeidi et al. 2003]. The key role played by EUS in evaluating lesions of the pancreas was characterized in this study. Over half of the EUS referrals were for pancreatic indications, and patients presenting for evaluation of pancreatic mass lesions had significantly greater downstream utilization of surgical services, oncology and infusion, and radiation therapy. This demonstrates the critical role played by EUS in centers specializing in the evaluation and treatment of pancreaticobiliary disease.
Many patients referred for EUS have additional imaging studies performed in the study institution. MRI is utilized in most cases of pancreatic mass lesions for distant staging and for examination of vasculature that could define a patient as locally advanced (e.g. involvement of the superior mesenteric artery (SMA)). Whole body PET/CT and EUS are used in the study institution protocol for the staging of esophageal malignancies. Additional imaging studies are frequently performed by oncology or surgery for the follow up of patients undergoing chemoradiotherapy or postresection to assess for remission or recurrence.
There are a number of limitations in this study. The study was retrospective and relied on the electronic medical record to capture encounter data following the initial referral. The study sought to examine patients who were uniquely referred for EUS and did not evaluate or characterize the sizeable cohort of patients for whom EUS did not represent the first point of contact. The impact in this cohort was not examined. The duration of time for characterization of downstream health utilization following EUS was also variable, with some cases receiving 35 days of follow up and other cases receiving over 600 days of follow up. This may have underestimated the downstream impact in cases with less follow-up time.
Despite high upfront cost, extended training requirements and disparate reimbursement, EUS has a profound downstream institutional impact on medical and surgical subspecialties. As downstream utilization does not fall exclusively within gastroenterology or within the Department of Medicine, a broad, institutional perspective is required in evaluating the impact of the procedure. The importance of prompt downstream therapeutic and palliative care has been demonstrated in cases of pancreatic cancer where coordination of surgical, medical-oncology, radiation-oncology, radiology, interventional-radiology and gastroenterology services are key factors in patient outcome [Yamamoto et al. 2008; Gardner et al. 2010]. Such a multidisciplinary approach, as illustrated in our study, has implications for accountable care organizations that aim to provide coordinated care within an integrated delivery system [Enthoven and Tollen, 2005]. EUS is a critical component for a pancreaticobiliary center, and represents a major and probably differentiating portal of entry into the system. The aggregate impact of an upfront investment in EUS services could be further characterized in a prospective trial with an institution-level perspective.
Acknowledgments
This article was previously presented on 2 November 2011 at the annual meeting of the American College of Gastroenterology in Washington, DC.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Jamie B. Sodikoff, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA
Sagar S. Garud, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA
Steven A. Keilin, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA
Sheila J. Bharmal, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA
Melinda M. Lewis, Department of Pathology, Emory University School of Medicine, Atlanta, GA, USA
Qiang Cai, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA.
Field F. Willingham, Division of Digestive Diseases, Department of Medicine, Emory University, 1365 Clifton Road, NW, Building B, STE 1200, Atlanta, GA 30322, USA
References
- Atkinson M., Schmulewitz N. (2009) Downstream hospital charges generated from endoscopic ultrasound procedures are greater than those from colonoscopies. Clin Gastroenterol Hepatol 7: 862–867 [DOI] [PubMed] [Google Scholar]
- Eloubeidi M., Chen V., Eltoum I., Jhala D., Chhieng D., Jhala N., et al. (2003) Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol 98: 2663–2668 [DOI] [PubMed] [Google Scholar]
- Enthoven A., Tollen L. (2005) Competition in health care: it takes systems to pursue quality and efficiency. Health Aff (Millwood). Jul-Dec; Suppl Web Exclusives:W5-420–33 [DOI] [PubMed] [Google Scholar]
- Gardner T., Barth R., Zaki B., Boulay B., Mcgowan M., Sutton J., et al. (2010) Effect of initiating a multidisciplinary care clinic on access and time to treatment in patients with pancreatic adenocarcinoma. J Oncol Pract 6: 288–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gress F., Hawes R., Savides T., Ikenberry S., Lehman G. (1997) Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc 45: 243–250 [DOI] [PubMed] [Google Scholar]
- Harewood G., Stemmer W., Roth J., Waxman I. (2009) Resource-intensive endoscopy: revenue source or cash drain? Gastrointest Endosc 70: 272–277 [DOI] [PubMed] [Google Scholar]
- Harewood G., Wiersema M. (2002) Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol 97: 1386–1391 [DOI] [PubMed] [Google Scholar]
- Nickl N., Bhutani M., Catalano M., Hoffman B., Hawes R., Chak A., et al. (1996) Clinical implications of endoscopic ultrasound: the American Endosonography Club Study. Gastrointest Endosc 44: 371–377 [DOI] [PubMed] [Google Scholar]
- Raut C., Grau A., Staerkel G., Kaw M., Tamm E., Wolff R., et al. (2003) Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg 7: 118–126; discussion 127–128. [DOI] [PubMed] [Google Scholar]
- Sodikoff J., Johnson H., Lewis M., Garud S., Bharmal S., Keilin S., et al. (2012) Increased diagnostic yield of endoscopic ultrasound-guided fine needle aspirates with flow cytometry and immunohistochemistry. Diagn Cytopathol. [DOI] [PubMed] [Google Scholar]
- Wiersema M., Vilmann P., Giovannini M., Chang K., Wiersema L. (1997) Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 112: 1087–1095 [DOI] [PubMed] [Google Scholar]
- Williams D., Sahai A., Aabakken L., Penman I., van Velse A., Webb J., et al. (1999) Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut 44: 720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Imagawa D., Katz M. (2008) Multidisciplinary management of resectable adenocarcinoma of the pancreatic head. Expert Rev Anticancer Ther 8: 1611–1621 [DOI] [PubMed] [Google Scholar]


