Abstract
Modulation of NF-E2 related factor 2 (Nrf2) has been shown in several neurodegenerative disorders. The overexpression of Nrf2 has become a potential therapeutic avenue for various neurodegenerative disorders such as Parkinson, Amyotrophic lateral sclerosis, and Alzheimer’s disease. The expression of phase II detoxification enzymes is governed by the cis-acting regulatory element known as antioxidant response element (ARE). The transcription factor Nrf2 binds to ARE thereby transcribing multitude of antioxidant genes. Keap1, a culin 3-based E3 ligase that targets Nrf2 for degradation, sequesters Nrf2 in cytoplasm. Disruption of Keap1-Nrf2 interaction or genetic overexpression of Nrf2 can increase the endogenous antioxidant capacity of the brain thereby rendering protection against oxidative stress in neurodegenerative disorders. This review primarily focuses on targeted Nrf2 overexpression as a promising therapeutic strategy for the treatment of neurodegenerative disorders.
Introduction
Oxidative stress has been implicated in numerous disorders, including neurodegenerative disorders. The free radical-mediated oxidative stress is a widely accepted mechanism towards the formation of reactive oxygen species (ROS) (e.g., superoxide radical O2−. or hydrogen peroxide H2O2) and reactive nitrogen species (RNS) (e.g. nitric oxide radical; NO., dinitrogen tetroxide; N2O4 or peroxynitrite; ONOO−) that can damage biomolecules such as proteins and lipids [1]. The source of free radicals could be exogenous factors such as ionizing radiation, photochemical reactions, environmental toxins among others or endogenous biochemical and enzymatic processes. These reactive species are found intracellularly and extracellularly.
Various antioxidant systems are operational in the human body that inhibits the activity of ROS/RNS. Some examples of antioxidant systems are antioxidant enzymes (e.g., superoxide dismutase, glutathione peroxidase), antioxidant proteins (e.g., thioredoxin, peroxyredoxin) and antioxidant molecules (e.g., lipoic acid, GSH, vitamin E). During disease conditions, the level of ROS/RNS may increase, antioxidant capacity may decrease or both may occur at the same time [1]. This condition is often described as oxidative stress.
The brain is particularly vulnerable to free radical damage and oxidative stress because of high amounts of polyunsaturated fatty acids (PUFA) that are easily oxidizable by ROS, the presence of significant amount of trace metal ions such as iron (II) and copper (I) that can catalyze various redox reactions to produce ROS, the high consumption of oxygen compared to other organs, and the low antioxidant levels. These factors lead to increased lipid peroxidation, as well as protein, DNA and RNA oxidation causing neuronal dysfunction and/or death [2, 3]. Such changes have been mechanistically implicated in the pathogenesis and progression of neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson disease (PD), amyotrophic lateral sclerosis (ALS) and Huntington disease (HD) [4]. Hence, studies focusing on molecules that modulate the cells endogenous antioxidant capacity are of vital significance and can form new therapies targeting the treatment of the neurodegenerative disorders.
Nrf2-ARE Pathway
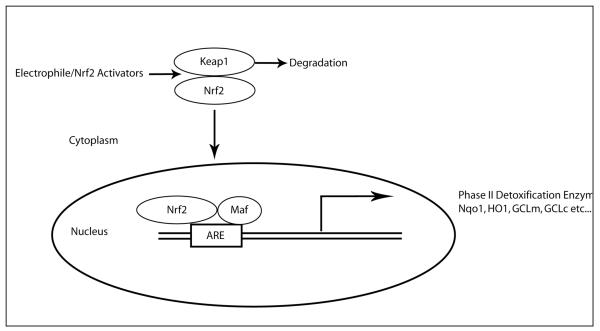
One such way the cell regulates its endogenous antioxidant capacity is through activation of the transcription factor NF-E2-related factor 2 (Nrf2). The antioxidant response element (ARE) is a cis-acting regulatory element that governs the expression of phase II detoxification enzymes. Nrf2, a member of Cap ‘n’ collar/basic-leucine zipper family transcription factor, regulates the ARE containing genes. Nrf2 has six erythroid-derived CNC homology protein (ECH) domains. The cytoplasmic protein Keap1 is associated with Nrf2 via a Neh2 domain [5]. Keap1 functions as adaptor for the culin 3-based E3 ligase. Under normal unstressed condition, Nrf2 is ubiquitinated and rapidly degraded (half life ~ 20 min) by ubiquitin-proteasome system [6-8]. Under conditions of oxidative stress by either reactive electrophiles, toxins or ARE inducers, the interaction between Nrf2 and Keap1 is disrupted and Nrf2 translocates to the nucleus. In the nucleus, it binds to small Maf proteins that increase the transcription rate of ARE-driven genes (Fig 1).
Figure 1.
Schematic representation of Keap1-Nrf2 interaction and activation. Under basal conditions Keap1 continuously degrades Nrf2 following ubiquitination. Electrophiles and oxidants directly modify reactive cysteine residues on Keap1 thereby disrupting Keap1-Nrf2 interaction. Nrf2 translocates to nucleus and binds to small Maf protein and transcribes ARE-driven genes.
In addition, Keap1 may also be involved in accompanying Nrf2 out of the nucleus by shuffling itself from the cytoplasm to the nucleus [9-11]. The actual mechanism of disruption of the Keap1-Nrf2 interaction remains elusive but it is reported that ARE inducers may directly modify cysteine thiols groups in Keap1 [12] that leads to the release of Nrf2, thereby increasing Nrf2 activity [13-16]. Furthermore, activation of several kinases may participate in this process through phosphorylation of Nrf2 at serine and threonine residues that could mediate/modify translocation of Nrf2 to nucleus [17]. Finally, the major Nrf2 activation pathway is believed to be that discussed above, however, other data suggests that there may be exceptions. Members of the fibroblast growth factor family have been shown to transcriptionally regulate Nrf2 [18, 19], thereby increasing both Nrf2 mRNA and protein levels that could contribute to activation of Nrf2 and induction of ARE-driven genes.
Nrf2-ARE driven genes
A multitude of genes involved in redox status, anti-inflammation and detoxification are transcribed by Nrf2-ARE pathway activation. These genes are known to be involved in cytoprotection from various oxidative insult and cellular injuries in numerous different tissues and organs including brain. Antioxidant enzyme systems regulated by Nrf2 include, but not limited to, redox regulation [superoxide dismutase (SOD), catalase (CAT), sulfaredoxin (Srx), thioredoxin (Trx), peroxiredoxin (Prdx) system], glutathione synthesis and metabolism [glutathione peroxidase (Gpx), glutathione reductase (GR), γ-glutamine cysteine ligase (GCL) and synthase (GCS)], quinone recycling [NAD(P)H quinone oxidoreducase (Nqo1)] and iron homeostasis [heme oxygenase 1 (HO-1), Ferritin]. Some antioxidant genes have more active roles than others in brain depending on the disease condition, cellular environment or cell type.
Nqo1 is an antioxidant enzyme involved in two-electron reduction of endogenous quinones utilizing NADH or NADPH as a reducing co-factor [20]. Additionally, Nqo1 is also involved in α-tocopherol (vitamin E) metabolism and regeneration [21]. Small molecules such as phenolic and polycyclic aromatic hydrocarbons are known to induce Noq1 activity [22-24]. Various small molecules such as tertiary butyl hydroquinone (tBHQ) and sulforaphane, antioxidants, and H2O2 are known to activate Nqo1 mediated via the ARE [25-27]. Nqo1 is highly expressed in the central nervous system and lung epithelium and tissues that require high antioxidant protection [28]. In brain, astrocytes are known to show high level of Nqo1 compared to other cell type under normal condition. Gliosis is a common pathological hallmark in neurodegenerative disorder such as AD and PD. These reactive astrocytes show increased levels of Nqo1 that potentially indicate the presence of oxidative stress [29, 30].
Intracellular peroxidases are cleared by a group of enzymes that are transcribed by Nrf2-ARE pathway. The peroxisomal CAT catalyzes the conversion of H2O2 to water and molecular oxygen. However, the specific activity of CAT is much lower in brain than peripheral tissue [31]. Gpx is another enzyme that metabolizes H2O2 and depends on reduced glutathione (GSH). The oxidized GSH (GSSG) is recycled to GSH by GR. GSH, a tripeptide γ-glutamyl-cysteinyl-glycine, is the most abundant low molecuar weight thiol expressed ubiquitously. It is widely recognized as an endogenous non-enzymatic antioxidant and an oxyradical scavenger, and is thereby critical to maintaining a reducing environment in the cell and protect against oxidative damage by ROS [32-36]. GSH has been implicated in a wide range of metabolic processes, including cell division, DNA repair, regulation of enzyme activity, activation of transcription factors, modulation of anion and cation homeostasis, and protection against oxidative damage [37].
The consecutive action of two cytosolic enzymes, GCL and GCS, catalyze the synthesis of GSH. The first step involves ligation of γ-glutamate to cysteine to form γ-glutamylcysteine; a reaction catalyzed by GCL. This step is the rate-limiting step in GSH production and GCL is feedback inhibited by GSH itself. GSH is present in 1-3mM concentration throughout the brain [38], acting as a high capacity detoxification agent. GSH maintains the cellular redox balance depending upon the pH of the cellular compartment and is involved in various biosynthetic processes as well [35]. The level of GSH is reduced in specific regions of the central nervous system in various neurodegenerative disorders with concomitant increase in GSSG levels allowing for increased oxidative stress-mediated neuronal cell dysfunction and/or loss in these disorders [38, 39]. The basal and inducible levels of GCL, GCS and GR are regulated by Nrf2-ARE pathway [40-43]. GSH, in conjugation with GR, GPx, glutathione-S-transferase (GST) and NADPH provide protection against various toxic electrophiles and hydrogen peroxide.
GSTs are key detoxification enzymes that catalyze the conjugation of various electrophiles, reactive alkenals, and numerous other xenobiotic to GSH. These GSH-S-conjugates are removed from cells by the multidrug resistant protein-1 (MRP-1), an ATP binding cassette (ABC) family protein [44, 45]. MRP-1 is an integral plasma membrane protein that exports glutathione-S conjugates out of the cell in an ATP-dependent manner [46, 47]. Studies have shown reduced GST activity in brain and ventricular fluids in AD [48]. Increased expression of GST leads to increased resistance towards oxidative stress in neuroblastoma cells and provides protection against HNE-mediated toxicity in neuronal cell culture [48]. Several members of the GST family and MRP1 expression levels are regulated by Nrf2 [49, 50].
The chaperone protein HO-1, in concert with cytochrome p450, catalyzes degradation of heme to biliverdin that is subsequently converted to bilirubin. Both biliverdin and bilirubin have shown antioxidant and immunomodulatory properties [51, 52]. Increased expression of HO-1 has been reported in various neurodegenerative disorders such as AD, PD, ALS and multiple sclerosis [19, 53-55], which may be linked to an attempt by HO-1 to restore redox state or attenuate inflammation in these conditions [reviewed in, [56]]. Several line of evidence show that Nrf2-mediated regulation of HO-1 protect cells from toxic and oxidative injuries [57, 58].
Nrf2-mediated neuroprotection in neurodegenerative disorders
Aging is common risk factor in neurodegenerative disorders. Increased protein and lipid oxidation and decreased antioxidant defense has been implicated in neurodegenerative conditions. In consequence, a considerable importance has been given to Nrf2-ARE pathway as a potential therapeutic target towards prevention of these disorders [59-61].
In CNS, the Nrf2-ARE dependent gene expression is preferentially less activated in neurons compared to astrocytes [57, 62, 63]. Additionally, astrocytes have higher GSH content than neurons [64, 65]. Hence, neurons often depend on astrocytes for protection against oxidative stress. Several lines of evidence show that neurons are more resistant to the oxidative stress in presence of astrocytes [66-68]. Most of the studies are targeted at either astrocytic Nrf2 overexpression-mediated protection of neurons or modulating endogenous neuronal antioxidant capacity by small molecule Nrf2 activators or cell specific overexpression. In the following sections, we will review disease specific studies involving Nrf2 activation/overexpression as a therapeutic strategy to modulate the progression of major neurodegenerative disorders.
Alzheimer’s disease
AD is an age-associated progressive neurodegenerative disorder characterized by memory loss, cognitive dysfunction and is the most common form of dementia in the elderly population effecting more than 5 million Americans. Pathological hallmarks of AD includes brain atrophy due to neuronal and synapse loss, senile plaques predominantly consisting of fibrillar amyloid β-peptide and neurofibrillary tangles (NFT) made up of hyperphosphorylated tau, a cytoskeletal protein [69]. Some of the major risk factors for AD are unhealthy aging in sporadic AD cases, the presence of ApoE-4 alleles in both sporadic and familial AD [70] and genetic factors, such as mutation in amyloid precursor protein (APP) and presenilin-1 (PS1) in familial AD [71] among others. AD brain is characterized by mitochondrial dysfunction, reactive gliosis and oxidative damage to lipids and proteins [72-76].
Growing evidence demonstrates that the AD brain is under tremendous oxidative stress. A significantly increased HO-1 expression was reported in post-mortem AD temporal cortex and hippocampus compared to aged-matched control [73]. Additionally, an increased Nqo1 activity and expression was found in astrocytes and neurons of AD brain [30, 77] and Nrf2 was predominantly localized in cytoplasm in AD hippocampal neurons [78]. Furthermore, there is increased protein oxidation [reviewed in [79, 80]] and lipid peroxidation [81-83] in AD brain when compared to aged matched controls. Recent studies in aged APP/PS1 AD mouse models showed reduced Nrf2, Nqo1, GCL catalytic subunit (GCLC) and GCL modifier subunit (GCLM) mRNA and Nrf2 protein levels [84]. Additionally, in a triple transgenic AD mouse, the GSH/GSSG ratio was reported to be reduced [85].
Antioxidant therapy in human AD clinical trials has meet with limited to no success. A α-tocopherol (vitamin E) treatment in AD patients delayed progression compared to placebo treated individuals [86], suggesting an increase in antioxidant capacity can alter AD pathogenesis. However, the majority of antioxidant trials have not shown similar positive outcomes [86, 87]. These failures could be due to many factors including bioavailability, distribution, metabolism or blood-brain-barrier penetration. Alternatively, the clinical trial design is not necessarily optimized to actually determine effectiveness of such treatments. The trial length and stage of disease may be to short and to late, respectively, for antioxidant treatments to have a positive outcome leading to multiple failed trials. Manipulation of the cells own endogenous antioxidant pathways via Nrf2 activation/overexpression does show significant protection against Aβ-mediated toxicity, in vitro [84, 88]. In addition, stereotactic injection of lentiviral-Nrf2 into the hippocampus of the APP/PS1 AD mouse model improved spatial learning, but did not alter Aβ levels compared to wild type littermate controls [89]. Synthetic triterpenoid compounds have been shown to activate Nrf2-ARE pathway in various rodent models [90-94]. Recently in transgenic mice carrying two human APP mutations (Tg19959 mouse), synthetic triterpenoids attenuated inflammation and oxidative stress. Additionally, these mice also showed improved spatial memory retention and reduced Aβ plaque load [95]. These results and several other in vitro studies suggest that the Nrf2-ARE pathway is a viable drug target for the treatment of AD.
Parkinson Disease
PD is a progressive neurodegenerative disorder characterized by motor symptoms such as tremor, bradykinesia, posture instability and rigidity and non-motor neuropsychiatric problems such as mood, cognition, behavior and sometimes dementia [reviewed in [96]]. The main pathological hallmark of PD is loss or dopaminergic neurons in substantia nigra pars compacta that project to striatum and lewy body inclusions primarily composed of abnormal accumulation of α-synuclein bound to ubiquitin [97] as well as increased gliosis characterized by both astrogliosis and microgliosis. Familial PD, although accounting for only 15% of total cases [98] involves mutations in specific genes that code for α-synuclein (SNCA), parkin (PRKN), leucinerich repeat kinase 2 (LRRK2 or dardarin), PTEN-induced putative kinase 1 (PINK1) and DJ1 [99]. Oxidative stress has been implicated in PD and this is consistent with the predominantly nuclear distribution of Nrf2 [78] along with mitochondrial dysfunction in the dopaminergic neuron [100-103]. In the postmortem PD brain, an increased HO-1 and Nqo1 expression was observed in reactive glial cells [29, 53].
Our laboratory and others have showed differential sensitivity of Nrf2 deficient mice towards 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a chemical model of PD [104, 105]. It has also been reported that there is a greater loss of dopamine transporter levels in striatum of Nrf2 knockout mice post MPTP administration compared to wildtype mice [106]. Moreover, Nrf2 activation protects dopaminergic neurons from 6-hydroxydopamine (dopamine analog) toxicity and MPP+ toxicity, in vitro [107, 108] and in vivo [107]. Additional in vivo evidence for Nrf2-mediated neuroprotection was demonstrated by transplantation of astrocytes overexpressing Nrf2 in striatum [107]. Mice receiving the Nrf2 overexpressing astrocytes were increasingly resistant to 6-hydroxydopamine-induced toxicity. Finally, in a separate study, our laboratory showed that transgenic mice with astrocyte-specific Nrf2 overexpression (GFAP-Nrf2) completely reversed dopaminergic neuronal loss in substantia niagra and loss of terminal in striatum [104]. These study strongly support the concept that modulation of the Nrf2-ARE pathway in astrocyte is sufficient to confer protection to neurons in mouse models of PD.
Huntington Disease
HD is a progressive autosomal dominant neurodegenerative disorder that is caused by a CAG repeats expansion in the HD gene resulting in an expansion of polyglutamines at the N-terminus of the huntingtin protein and accumulation of the mutant protein as cytoplasmic and nuclear aggregate inclusions [109]. HD is pathologically characterized by degeneration in neostriatal (caudate and putamen) and cerebral cortex that is believed to be the underlying contributor of motor impairment, cognitive decline and psychiatric symptoms that worsen as the disease progresses. Oxidative stress due to mitochondrial dysfunction has been implicated in human patients and HD animal models [110]. Defects in mitochondrial complex II, III and IV were observed in striatum of post mortem HD brain [111, 112]. 3-Nitropropionic acid (3NP) and malonate are mitochondrial complex II inhibitors that produce striatal medium spiny neuron degeneration, a characteristic feature observed in HD [113-116]. An increase in ROS production due to disruption of electron transport chain by these inhibitors is also observed. Our laboratory and others have demonstrated that mice lacking Nrf2 are more susceptible to mitochondrial complex II inhibitors compared to wild type mice and grafting of astrocytes overexpressing Nrf2 or chemical activation of Nrf2 protected from 3NP- and malonate-induced lesioning in striatum respectively [58, 117]. In a separate study, mice overexpressing Nrf2 in astrocytes (GFAP-Nrf2) were resistant to malonate-induced lesioning in vivo. In the same study, the mouse neuroprogenetor cells (NPCs) infected with either adeno-Nrf2 or adeno-GFP virus were grafted in straital region and were subjected to malonate-induced lesioning. The Nrf2 overexpressing NPCs showed significant protection against malonate toxicity [117]. Additionally, cystamine, an Nrf2-ARE activator, conferred protection in both in vitro and in vivo model of 3NP toxicity [118]. More recently, synthetic triterpenoids were shown to activate Nrf2 and rescued rodents against 3NP-mediated striatal lesioning. Synthetic triterpinoids also rendered protection against 3NP-mediated increased DNA and protein oxidation, lipid peroxidation, and disrupted glutathione homeostasis [93]. A transgenic mouse model of HD (N171-82Q mice) also showed improved motor function and improved longevity when synthetic triterpinoids were fed in diet. A reduction in oxidative stress marker and straital atrophy was also observed [119]. Most recently, dimethyl fumarate (DMF), an Nrf2-ARE activator, was given orally to the R6/2 and YAC 128 mouse model of HD. Mouse receiving DMF showed increased neuronal Nrf2 and a significant improvement in motor function and preservation of neurons in motor cortex and striatum [120]. These studies strongly suggest that small molecule intervention or ex-vivo manipulation of Nrf2-ARE pathway is a promising therapeutic strategy got treatment of HD.
Amyotrophic Lateral Sclerosis
ALS is caused by the progressive degeneration of motor neurons in the spinal cord, brainstem, and motor cortex [121] characterized by progressive weakness in muscle, spasticity and muscle atrophy. Pathological contributors to ALS are oxidative damage, neuroinflammation, and mitochondrial dysfunction. Although the etiology of sporadic ALS remains unclear, approximately 5-10% of ALS is familial and about 20% of the familial ALS cases are associated a toxic gain-of-function mutation in Cu/Zn-superoxide dismutase (SOD1) [122]. The overexpression of mutant hSOD1 in rodent models has been shown to cause an ALS-like phenotype [123, 124]. A strong gliosis surrounding degenerating motor neurons and increase oxidative stress markers were observed in ALS patients and rodent models of ALS [125].
Oxidative stress has been implicated in ALS and most likely affects the course of the disease [126, 127]. Modulation in Nrf2-ARE pathway in response or as a consequence of oxidative stress has been well documented. The neurons from primary motor cortex and spinal cord from postmortem ALS tissue and primary embryonic motor neurons from ALS rat model showed reduced Nrf2 mRNA and protein expression [128, 129]. Our laboratory showed increase in ARE-driven gene in spinal cord and muscle of ALS mice crossed with ARE-hPAP (human placental alkaline phosphatase) reporter mice prior to disease onset [130]. Others also show a strong increase in HO-1 was found at onset of disease symptoms in an ALS rat model [19].
Our laboratory and others have demonstrated a toxic effects of astrocytes isolated from the hSOD1G93A rat [131] or mouse on co-cultured motor neurons [132, 133]. Studies have shown that activation or overexpression of Nrf2 in the hSOD1 mutant astrocytes completely reversed their toxicity toward motor neurons the co-culture system. These observations translated to the in vivo situation since crossing the GFAP-Nrf2 mice with multiple mouse models of ALS lead to a delay onset of disease and increased life span in the ALS mice [134]. These observations were first in vivo evidence that Nrf2 activation in astrocytes can be beneficial to protect neurons in chronic neurodegenerative models suggesting that Nrf2 activation should be a suitable therapeutic target in ALS. Recently, triterpenoids that are potent activators of Nrf2-ARE pathway showed significant attenuation in weight loss, enhanced motor function and extended life span in hSOD1G93A mice when treated at pre-symptomatic age. Furthermore, when treatment of these mice was initiated at symptom onset, there was also significant neuroprotection and slowed disease progression. These data provide further evidence that compounds activating the Nrf2-ARE pathway can be potential therapeutic agents in treating ALS [135].
Recent Patents on Nrf2 Modulation
The manipulation of the Nrf2-ARE pathway at the genetic level is being studied through the use of siRNA or antisense oligonucleotides against Keap1 to activate/overexpress Nrf2. Antisense drugs are being researched to study neurodegenerative disorders, cancer, metabolic disorders and disorders with inflammatory components among others. Antisense drug fomiversen, marketed as Vitravene, has been approved by the US food and drug administration (FDA) for treatment of cytomegalovirus retinitis. Since then numerous antisense therapies have been tested but have not produced significant clinical result. This hasn’t diminished the potential of gene therapies. Antisense oligonucleotide can bind to the target RNA and disrupt RNA splicing, transcription, translation and replication thereby modulating gene expression. Our laboratory recently showed that siRNA-mediated knockdown of Keap1 activated Nrf2-ARE pathway in mouse cortical astrocytes and provide partial protection against MPTP-mediated toxicity in mouse, in vivo [136]. The overexpression of target gene can also be achieved by viral-mediated gene transduction but it is too early to conclude on efficacy of viral-mediated gene therapy in human neurodegenerative disorder cases. Nrf2 modulation in various neurodegenerative disorders has been previously described in this review. Hence using the antisense oligonucleotide against Keap1, lenti-viral-mediated Nrf2 overexpression or siRNA against keap1-mediated overexpression of Nrf2 treatment can prove beneficial in neurodegenerative disorders. Among recent patents, Curna, Inc. filed patent for use of antisense for treatment of Nrf2-related disorders. The initial study published under international application for the patent cooperation treaty (PCT) showed that antisense CUR-0330 and CUR 0332 showed 2 to 3 fold increase in Nrf2 mRNA expression compared to control (PCT/US2010/027394). The invention is targeted at inhibition of natural antisense transcript to Nrf2 as a strategy towards modulation of Nrf2 expression in disease models.
The modulation of Nrf2 expression by using several other pharmacological interventions to inhibit Keap1 and Nrf2 interaction are under investigation. Table 1 lists some of the other patents on small molecule that interact at the specific region of Keap1 that binds to Nrf2. Disruption of these Keap1-Nrf2 interacting region activates Nrf2. A detailed description of chemicals and small molecules that are targeted towards disruption of Keap1-Nrf2 interaction towards increasing the biological activity of Nrf2 as a strategy towards protection against neurodegenerative disorder is discussed in following section.
Table 1.
Recent patents in Nrf2-ARE pathway activators for treatment of central nervous system disorders.
| S.No | Patent | Application Number | Title | Applicant | PubDate |
|---|---|---|---|---|---|
| 1 | WO/2011/156889 | PCT/CA2011/000649 | NOVEL MODULATORS OF NRF2 AND USES THEREOF | TRT Pharma Inc. | 12/22/11 |
| 2 | WO/2010/107733 | PCT/US2010/027394 | TREATMENT OF NUCLEAR FACTOR (ERYTHROID- DERIVED 2)-LIKE 2 (NRF2) RELATED DISEASES BY INHIBITION OF NATURAL ANTISENSE TRANSCRIPT TO NRF2 |
OPKO CURNA, LLC. |
9/23/10 |
| 3 | WO/2010/036711 | PCT/US2009/058050 | METHODS OF MODULATING PROTEIN HOMEOSTASIS, METABOLIC SYNDROME, HEAVY METAL INTOXICATION AND NRF2 TRANSCRIPTION FACTORS |
Bach Pharma Inc. | 4/1/10 |
| 4 | WO/2009/036204 | PCT/US2008/076064 | PHASE II DETOXIFICATION AND ANTIOXIDANT ACTIVITY |
Joslin Diabetes center Inc. |
3/19/09 |
| 5 | WO/2008/136838 | PCT/US2007/071933 | NOVEL AMIDE DERIVATIVES OF CDDO AND METHODS OF USE THEREOF |
Trustees of Dartmouth College |
11/13/08 |
| 6 | WO/2008/108825 | PCT/US2007/021748 | NEUROPROTECTIVE COMPOSITIONS AND METHODS | Burnham Institute for medical research |
9/12/08 |
| 7 | WO/2007/008652 | PCT/US2006/026503 | METHODS AND COMPOSITIONS DIRECTED TO DJ-1 AS REGULATOR OF THE ANTI-OXIDANT TRANSCRIPTION FACTOR NRF2 |
the University of North Carolina at Chapel Hill |
1/18/07 |
| 8 | WO/2007/005879 | PCT/US2006/026056 | COMPOSITIONS AND METHODS FOR THE TREATMENT OR PREVENTION OF DISORDERS RELATING TO OXIDATIVE STRESS |
The Johns Hopkins University |
1/11/07 |
| 9 | WO/2008/097596 | PCT/US2008/001602 | NRF2 SCREENING ASSAYS AND RELATED METHODS AND COMPOSITIONS |
Biogen Idec Inc. | 8/14/08 |
Targeting the Keap1-Nrf2 Interaction
Keap1 has four discrete domains. The BTB (Broad complex, Tramtrack and Bric-à-Brac) domain important for stress sensing and homodimerization of Keap1 protein [137], the intervening region (IVR) domain, a double glycine repeat (DGR) and C-terminal Kelch domain contains six conserved Kelch repeat sequences that binds to the Neh2 domain of Nrf2 [138, 139]. Keap1 not only sequesters Nrf2 in cytoplasm but also plays a role in its subsequent ubiquitination and proteasomal degradation [6, 7, 140, 141]. Keap1 represses the Nrf2 activation, which is a master regulator of antioxidant genes, hence making it a potential drug target towards treatment of oxidative stress related disorders, including neurodegenerative disorders. Increasing Nrf2 expression or biological activity by small molecules such quinone based compounds, flavonoids, polyphenols, α, β-unsaturated esters, reactive electrophiles among others can be achieved in various cell types, however, they are limited by bioavailability and blood-brain-barrier permeability. Chemical modification of certain base molecules that can interrupt Keap1-Nrf2 interaction can achieve the desirable increased expression or biological activity of Nrf2 in brain.
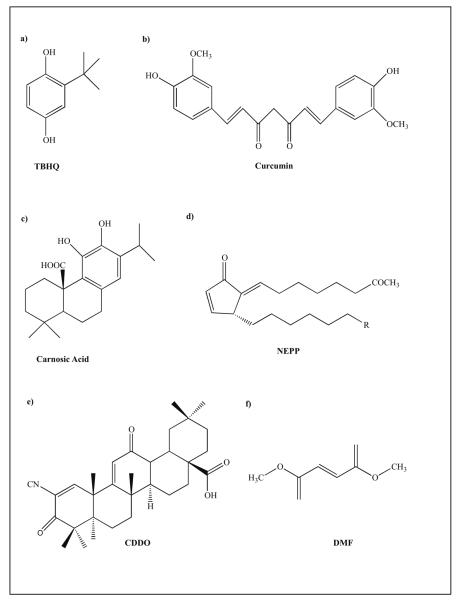
Among the 27 cysteine residues on Keap1, C273 and C288 in the linker region and C151 in the BTB region were identified as key sites that chemicals bind to leading to Nrf2 release and Nrf2 activation [13, 14, 16, 142, 143]. The reactive electrophiles potentially alkylate the cysteine residues thereby releasing and stabilizing Nrf2 [142, 144]. Electrophiles that can S-alkylate are classified as α, β-unconjugated enones such as curcumin and neurite-outgrowth promoting prostaglandin (NEPP) or polyphenols such as catechol-like carnosic acid, and resveratrol among others. Polyphenols, although not electrophilic, can cross the hydrophobic lipid membrane and get oxidized intracellularly to form quinone-like electrophiles. Hence, these catechol-like molecules can function as pro-drugs. Mechanisms of protection by these electrophiles differ and have been described previously [62, 145-147]. Carnosic acid, a catechol-like electrophile has been shown to protect neurons against glutamate-mediated toxicity in vitro and brain against ischemia reperfusion injury in vivo via s-alkylation of Keap1 and activation of Nrf2-ARE pathway [148]. Among other non-flavanoid polyphenols, Curcumin and resveratrol have shown to activate Nrf2 leading to subsequent protection of neural cells from oxidative insult and toxins in in vitro and in vivo models of AD and PD. Flavonoid polyphenols such as epigallocatechin 3-gallate (EGCG) and quercetine, and organo-sulphur based compounds such as sulforaphane are potent activator of Nrf2 that have been shown to be neuroprotective against oxidative stress in vitro. The chemical analogues of these base molecules (Fig 2) have been patented (PCT/US2007/021748, Lipton and Satoh 2007) and are under investigation.
Figure 2.
Example of some of the base compounds that activate Nrf2-ARE Pathway.
Another class of Nrf2 activators is triterpenoid-based compounds (Fig 2). Synthetic triterpenoids are derived from 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO). CDDO and it’s derivatives have been tested for their antioxidant and anti-inflammatory properties and are known to activate the Nrf2-ARE pathway in in vitro and in vivo in animal models of various disorders [90-94]. As mentioned earlier, in the neurodegenerative disorder paradigm, CDDO-methyl amine (CDDO-MA), an analogue of CDDO, showed improved memory and reduced Aβ plaques and oxidized proteins in AD transgenic mouse model [95], as well as showed neuroprotective properties against acute and chronic toxicity models of PD and HD and in transgenic mouse model of HD [93, 119], identifying these compounds as potential therapeutics for neurodegenerative disorders. The synthetic CDDO based analogues have been patented (PCT/US2007/071933) for testing against inflammatory and oxidative stress-mediated neurological disorders.
The ester of fumaric acid is a class of electrophiles that is known to be neuroprotective in mouse of models of multiple sclerosis (MS) and HD by activating the Nrf2-ARE pathway [120, 149-152]. In the experimental autoimmune encephalomyelitis (EAE) mouse model of MS, reduced oxidative stress and subsequent preservation of nerve fiber myelenation was observed post DMF administration and the protection was lost in Nrf2 knockout mouse [150]. The Nrf2-dependent genes were upregulated and motor function was improved in EAE mice upon DMF treatment. As discussed earlier, DMF also preserved neurons from motor cortex and striatum, reduced behavioral deficits, and improved life span in transgenic HD mice [120]. DMF increased Nqo1 level and was shown to protect rat cortical neurons against peroxide-mediated toxicity in vitro. Furthermore, oral administration of DMF or its metabolite mono-methyl fumarate (MMF) to mice was shown to increase Nrf2 levels [152]. These studies confirmed that Nrf2 activation by DMF and its metabolites could protect the CNS from oxidative insult in mouse models of neurodegeneration.
Conclusion
In past decade, Nrf2-ARE pathway activation has shown promising results for the treatment of many disorders including neurodegenerative disease. Several of these Nrf2 activators or their brain accessible synthetically modified compounds have passed phase II and III clinical trials. BG-12, an oral formulation of DMF (Biogen Idec, Inc.) is in phase III clinical trials for the treatment of MS. Bardoxolone methyl, an oral formulation of CDDO-MA (Reata Pharmaceuticals, Inc.) is currently in phase III clinical trials for chronic kidney disease in type II diabetes mellitus patients, but there are no existing clinical trails in pipeline for neurodegenerative disorders. EGCG, resveratrol and curcumin are in various phases of clinical trial for treatment and efficacy in neurodegenerative disorders such as AD, PD and ALS. The knowledge gained from these studies will further help in identifying clinically relevant approaches for activation of Nrf2 in CNS and potentially lead to finding treatments for these devastating neurological disorders.
Acknowledgments
This work was supported by grants ES08089 and ES10042 from the National Institute of Environmental Health Sciences (JAJ).
Reference
- [1].Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- [2].Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience. 2001;103:373–83. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- [3].Lovell MA, Gabbita SP, Markesbery WR. Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF. J Neurochem. 1999;72:771–6. doi: 10.1046/j.1471-4159.1999.0720771.x. [DOI] [PubMed] [Google Scholar]
- [4].Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–58. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- [5].Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cullinan SB, Gordan JD, Jin J, Harper JW, Diehl JA. The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol Cell Biol. 2004;24:8477–86. doi: 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–9. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem. 2006;281:24756–68. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- [9].Nguyen T, Sherratt PJ, Nioi P, Yang CS, Pickett CB. Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem. 2005;280:32485–92. doi: 10.1074/jbc.M503074200. [DOI] [PubMed] [Google Scholar]
- [10].Sun Z, Zhang S, Chan JY, Zhang DD. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol. 2007;27:6334–49. doi: 10.1128/MCB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Velichkova M, Hasson T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol Cell Biol. 2005;25:4501–13. doi: 10.1128/MCB.25.11.4501-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–13. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Levonen AL, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J. 2004;378:373–82. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A. 2004;101:2040–5. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28:2758–70. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–51. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–40. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- [18].Braun S, Hanselmann C, Gassmann MG, auf dem Keller U, Born-Berclaz C, Chan K, Kan YW, Werner S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22:5492–505. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vargas MR, Pehar M, Cassina P, Martinez-Palma L, Thompson JA, Beckman JS, Barbeito L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J Biol Chem. 2005;280:25571–9. doi: 10.1074/jbc.M501920200. [DOI] [PubMed] [Google Scholar]
- [20].Iyanagi T, Yamazaki I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase) Biochim Biophys Acta. 1970;216:282–94. doi: 10.1016/0005-2728(70)90220-3. [DOI] [PubMed] [Google Scholar]
- [21].Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D. The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol. 1997;52:300–5. doi: 10.1124/mol.52.2.300. [DOI] [PubMed] [Google Scholar]
- [22].De Long MJ, Prochaska HJ, Talalay P. Induction of NAD(P)H:quinone reductase in murine hepatoma cells by phenolic antioxidants, azo dyes, and other chemoprotectors: a model system for the study of anticarcinogens. Proc Natl Acad Sci U S A. 1986;83:787–91. doi: 10.1073/pnas.83.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Prochaska HJ, Talalay P, Sies H. Direct protective effect of NAD(P)H:quinone reductase against menadione-induced chemiluminescence of postmitochondrial fractions of mouse liver. J Biol Chem. 1987;262:1931–4. [PubMed] [Google Scholar]
- [24].Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988;85:8261–5. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jaiswal AK. Antioxidant response element. Biochem Pharmacol. 1994;48:439–44. doi: 10.1016/0006-2952(94)90272-0. [DOI] [PubMed] [Google Scholar]
- [26].Li Y, Jaiswal AK. Human antioxidant-response-element-mediated regulation of type 1 NAD(P)H:quinone oxidoreductase gene expression. Effect of sulfhydryl modifying agents. Eur J Biochem. 1994;226:31–9. doi: 10.1111/j.1432-1033.1994.tb20023.x. [DOI] [PubMed] [Google Scholar]
- [27].Prestera T, Holtzclaw WD, Zhang Y, Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1993;90:2965–9. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Siegel D, Ross D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med. 2000;29:246–53. doi: 10.1016/s0891-5849(00)00310-5. [DOI] [PubMed] [Google Scholar]
- [29].van Muiswinkel FL, de Vos RA, Bol JG, Andringa G, Jansen Steur EN, Ross D, Siegel D, Drukarch B. Expression of NAD(P)H:quinone oxidoreductase in the normal and Parkinsonian substantia nigra. Neurobiol Aging. 2004;25:1253–62. doi: 10.1016/j.neurobiolaging.2003.12.010. [DOI] [PubMed] [Google Scholar]
- [30].Wang Y, Santa-Cruz K, DeCarli C, Johnson JA. NAD(P)H:quinone oxidoreductase activity is increased in hippocampal pyramidal neurons of patients with Aalzheimer’s disease. Neurobiol Aging. 2000;21:525–31. doi: 10.1016/s0197-4580(00)00114-7. [DOI] [PubMed] [Google Scholar]
- [31].Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279:32804–12. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- [32].Meister A. Mitochondrial changes associated with glutathione deficiency. Biochim Biophys Acta. 1995;1271:35–42. doi: 10.1016/0925-4439(95)00007-q. [DOI] [PubMed] [Google Scholar]
- [33].Darley-Usmar V, Halliwell B. Blood radicals: reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm Res. 1996;13:649–62. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- [34].Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–34. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- [35].Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267:4904–11. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- [36].Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–21. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- [37].Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- [38].Cooper J. Glutathione in the brain: disorders of glutathione metabolism. In: Barchi R, Kunk L, editors. The Molecular and Genetic Basis of Neurological Disease. Vol. 35. Butterworth-Heinemann; Boston: 1997. [Google Scholar]
- [39].Benzi G, Moretti A. Age- and peroxidative stress-related modifications of the cerebral enzymatic activities linked to mitochondria and the glutathione system. Free Radic Biol Med. 1995;19:77–101. doi: 10.1016/0891-5849(94)00244-e. [DOI] [PubMed] [Google Scholar]
- [40].Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys Acta. 2000;1517:19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- [41].Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med. 2009;46:443–53. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moinova HR, Mulcahy RT. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun. 1999;261:661–8. doi: 10.1006/bbrc.1999.1109. [DOI] [PubMed] [Google Scholar]
- [43].Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–36. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- [44].Sultana R, Butterfield DA. Oxidatively modified GST and MRP1 in Alzheimer’s disease brain: Implication for accumulation of reactive lipid peroxidation products. Neurochem Res. 2004;29:2215–2220. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- [45].Renes J, de Vries EE, Hooiveld GJ, Krikken I, Jansen PL, Muller M. Multidrug resistance protein MRP1 protects against the toxicity of the major lipid peroxidation product 4-hydroxynonenal. Biochem J. 2000;350(Pt 2):555–61. [PMC free article] [PubMed] [Google Scholar]
- [46].Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, Keppler D. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129:349–60. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [47].Conseil G, Deeley RG, Cole SP. Polymorphisms of MRP1 (ABCC1) and related ATP-dependent drug transporters. Pharmacogenet Genomics. 2005;15:523–33. doi: 10.1097/01.fpc.0000167333.38528.ec. [DOI] [PubMed] [Google Scholar]
- [48].Lovell MA, Xie C, Markesbery WR. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology. 1998;51:1562–6. doi: 10.1212/wnl.51.6.1562. [DOI] [PubMed] [Google Scholar]
- [49].Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, Elcombe CR, Henderson CJ, Wolf CR, Moffat GJ, Itoh K, Yamamoto M, Hayes JD. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem J. 2002;365:405–16. doi: 10.1042/BJ20020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–9. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- [51].Dore S, Snyder SH. Neuroprotective action of bilirubin against oxidative stress in primary hippocampal cultures. Ann N Y Acad Sci. 1999;890:167–72. doi: 10.1111/j.1749-6632.1999.tb07991.x. [DOI] [PubMed] [Google Scholar]
- [52].Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- [53].Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free Radic Biol Med. 2004;37:1995–2011. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [54].van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, de Vries HE. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med. 2008;45:1729–37. doi: 10.1016/j.freeradbiomed.2008.09.023. [DOI] [PubMed] [Google Scholar]
- [55].Ferrante RJ, Shinobu LA, Schulz JB, Matthews RT, Thomas CE, Kowall NW, Gurney ME, Beal MF. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann Neurol. 1997;42:326–34. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- [56].Cuadrado A, Rojo AI. Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr Pharm Des. 2008;14:429–42. doi: 10.2174/138161208783597407. [DOI] [PubMed] [Google Scholar]
- [57].Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–38. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- [58].Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22925–36. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- [59].de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375–83. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [60].Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–9. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005;4:267–81. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- [62].Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–12. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–6. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- [65].Sagara J, Makino N, Bannai S. Glutathione efflux from cultured astrocytes. J Neurochem. 1996;66:1876–81. doi: 10.1046/j.1471-4159.1996.66051876.x. [DOI] [PubMed] [Google Scholar]
- [66].Bronstein DM, Perez-Otano I, Sun V, Mullis Sawin SB, Chan J, Wu GC, Hudson PM, Kong LY, Hong JS, McMillian MK. Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res. 1995;704:112–6. doi: 10.1016/0006-8993(95)01189-7. [DOI] [PubMed] [Google Scholar]
- [67].Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 1996;16:2553–62. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tanaka J, Toku K, Zhang B, Ishihara K, Sakanaka M, Maeda N. Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species. Glia. 1999;28:85–96. doi: 10.1002/(sici)1098-1136(199911)28:2<85::aid-glia1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- [69].Van Uden E, Sagara Y, Van Uden J, Orlando R, Mallory M, Rockenstein E, Masliah E. A protective role of the low density lipoprotein receptor-related protein against amyloid beta-protein toxicity. J Biol Chem. 2000;275:30525–30. doi: 10.1074/jbc.M001151200. [DOI] [PubMed] [Google Scholar]
- [70].Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–6. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- [71].Lee MK, Borchelt DR, Kim G, Thinakaran G, Slunt HH, Ratovitski T, Martin LJ, Kittur A, Gandy S, Levey AI, Jenkins N, Copeland N, Price DL, Sisodia SS. Hyperaccumulation of FAD-linked presenilin 1 variants in vivo. Nat Med. 1997;3:756–60. doi: 10.1038/nm0797-756. [DOI] [PubMed] [Google Scholar]
- [72].Marshak DR, Pesce SA, Stanley LC, Griffin WS. Increased S100 beta neurotrophic activity in Alzheimer’s disease temporal lobe. Neurobiol Aging. 1992;13:1–7. doi: 10.1016/0197-4580(92)90002-f. [DOI] [PubMed] [Google Scholar]
- [73].Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging. 2006;27:252–61. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- [74].Shaftel SS, Griffin WS, O’Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci. 1997;17:2653–7. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang X, Su B, Perry G, Smith MA, Zhu X. Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer disease. Free Radic Biol Med. 2007;43:1569–73. doi: 10.1016/j.freeradbiomed.2007.09.007. [DOI] [PubMed] [Google Scholar]
- [77].Raina AK, Templeton DJ, Deak JC, Perry G, Smith MA. Quinone reductase (NQO1), a sensitive redox indicator, is increased in Alzheimer’s disease. Redox Rep. 1999;4:23–7. doi: 10.1179/135100099101534701. [DOI] [PubMed] [Google Scholar]
- [78].Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT, Jordan-Sciutto KL. Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol. 2007;66:75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–54. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- [80].Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23:134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- [81].Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–94. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- [82].Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging. 1998;19:33–6. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- [83].Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1-42. J. Neurochem. 2001;78:413–6. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- [84].Kanninen K, Malm TM, Jyrkkanen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M, Yla-Herttuala S, Levonen AL, Koistinaho J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci. 2008;39:302–13. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- [85].Resende R, Moreira PI, Proenca T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–7. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- [86].Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- [87].Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- [88].Wruck CJ, Gotz ME, Herdegen T, Varoga D, Brandenburg LO, Pufe T. Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Mol Pharmacol. 2008;73:1785–95. doi: 10.1124/mol.107.042499. [DOI] [PubMed] [Google Scholar]
- [89].Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Yla-Herttuala S, Tanila H, Levonen AL, Koistinaho M, Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:16505–10. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–98. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- [91].Nichols DP, Ziady AG, Shank SL, Eastman JF, Davis PB. The triterpenoid CDDO limits inflammation in preclinical models of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol. 2009;297:L828–36. doi: 10.1152/ajplung.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–9. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, Musiek ES, Morrow JD, Sporn M, Beal MF. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS One. 2009;4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, Guilarte TR, Yamamoto M, Sporn MB, Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–62. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- [95].Dumont M, Wille E, Calingasan NY, Tampellini D, Williams C, Gouras GK, Liby K, Sporn M, Nathan C, Flint Beal M, Lin MT. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer’s disease. J Neurochem. 2009;109:502–12. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Thomas B, Beal MF. Parkinson’s disease. Hum Mol Genet. 2007;16(Spec No. 2):R183–94. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- [97].Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord. 2008;23(Suppl 3):S548–59. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- [98].Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–93. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- [99].Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- [100].Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson’s but not incidental Lewy body disease. J Neurochem. 1997;69:1326–9. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- [101].Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- [102].Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–6. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Dexter DT, Sian J, Rose S, Hindmarsh JG, Mann VM, Cooper JM, Wells FR, Daniel SE, Lees AJ, Schapira AH, et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 1994;35:38–44. doi: 10.1002/ana.410350107. [DOI] [PubMed] [Google Scholar]
- [104].Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–8. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Innamorato NG, Jazwa A, Rojo AI, Garcia C, Fernandez-Ruiz J, Grochot-Przeczek A, Stachurska A, Jozkowicz A, Dulak J, Cuadrado A. Different susceptibility to the Parkinson’s toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS One. 2010;5:e11838. doi: 10.1371/journal.pone.0011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Burton NC, Kensler TW, Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- [107].Jakel RJ, Townsend JA, Kraft AD, Johnson JA. Nrf2-mediated protection against 6-hydroxydopamine. Brain Res. 2007;1144:192–201. doi: 10.1016/j.brainres.2007.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yamamoto N, Sawada H, Izumi Y, Kume T, Katsuki H, Shimohama S, Akaike A. Proteasome inhibition induces glutathione synthesis and protects cells from oxidative stress: relevance to Parkinson disease. J Biol Chem. 2007;282:4364–72. doi: 10.1074/jbc.M603712200. [DOI] [PubMed] [Google Scholar]
- [109].Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet. 2003;361:1642–4. doi: 10.1016/S0140-6736(03)13304-1. [DOI] [PubMed] [Google Scholar]
- [110].Browne SE, Beal MF. Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–73. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- [111].Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann Neurol. 1996;39:385–9. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- [112].Brennan WA, Jr., Bird ED, Aprille JR. Regional mitochondrial respiratory activity in Huntington’s disease brain. J Neurochem. 1985;44:1948–50. doi: 10.1111/j.1471-4159.1985.tb07192.x. [DOI] [PubMed] [Google Scholar]
- [113].Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, Hyman BT. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13:4181–92. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Beal MF, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman BT. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem. 1993;61:1147–50. doi: 10.1111/j.1471-4159.1993.tb03633.x. [DOI] [PubMed] [Google Scholar]
- [115].Brouillet E, Jenkins BG, Hyman BT, Ferrante RJ, Kowall NW, Srivastava R, Roy DS, Rosen BR, Beal MF. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J Neurochem. 1993;60:356–9. doi: 10.1111/j.1471-4159.1993.tb05859.x. [DOI] [PubMed] [Google Scholar]
- [116].Brouillet E, Conde F, Beal MF, Hantraye P. Replicating Huntington’s disease phenotype in experimental animals. Prog Neurobiol. 1999;59:427–68. doi: 10.1016/s0301-0082(99)00005-2. [DOI] [PubMed] [Google Scholar]
- [117].Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102:244–9. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Calkins MJ, Townsend JA, Johnson DA, Johnson JA. Cystamine protects from 3-nitropropionic acid lesioning via induction of nf-e2 related factor 2 mediated transcription. Exp Neurol. 2010;224:307–17. doi: 10.1016/j.expneurol.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Stack C, Ho D, Wille E, Calingasan NY, Williams C, Liby K, Sporn M, Dumont M, Beal MF. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington’s disease. Free Radic Biol Med. 2010;49:147–58. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ellrichmann G, Petrasch-Parwez E, Lee DH, Reick C, Arning L, Saft C, Gold R, Linker RA. Efficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington’s disease. PLoS One. 2011;6:e16172. doi: 10.1371/journal.pone.0016172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- [122].Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- [123].Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- [124].Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99:1604–9. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Barbeito LH, Pehar M, Cassina P, Vargas MR, Peluffo H, Viera L, Estevez AG, Beckman JS. A role for astrocytes in motor neuron loss in amyotrophic lateral sclerosis. Brain Res Brain Res Rev. 2004;47:263–74. doi: 10.1016/j.brainresrev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- [126].Goodall EF, Morrison KE. Amyotrophic lateral sclerosis (motor neuron disease): proposed mechanisms and pathways to treatment. Expert Rev Mol Med. 2006;8:1–22. doi: 10.1017/S1462399406010854. [DOI] [PubMed] [Google Scholar]
- [127].Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–9. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sarlette A, Krampfl K, Grothe C, Neuhoff N, Dengler R, Petri S. Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2008;67:1055–62. doi: 10.1097/NEN.0b013e31818b4906. [DOI] [PubMed] [Google Scholar]
- [129].Pehar M, Vargas MR, Robinson KM, Cassina P, Diaz-Amarilla PJ, Hagen TM, Radi R, Barbeito L, Beckman JS. Mitochondrial superoxide production and nuclear factor erythroid 2-related factor 2 activation in p75 neurotrophin receptor-induced motor neuron apoptosis. J Neurosci. 2007;27:7777–85. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kraft AD, Resch JM, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway in muscle and spinal cord during ALS-like pathology in mice expressing mutant SOD1. Exp Neurol. 2007;207:107–17. doi: 10.1016/j.expneurol.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem. 2006;97:687–96. doi: 10.1111/j.1471-4159.2006.03742.x. [DOI] [PubMed] [Google Scholar]
- [132].Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Vargas MR, Pehar M, Diaz-Amarilla PJ, Beckman JS, Barbeito L. Transcriptional profile of primary astrocytes expressing ALS-linked mutant SOD1. J Neurosci Res. 2008;86:3515–25. doi: 10.1002/jnr.21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–81. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M, Liby KT, Risingsong R, Sporn M, Beal MF, Kiaei M. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic Biol Med. 2011;51:88–96. doi: 10.1016/j.freeradbiomed.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Williamson TP, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTP-mediated neurotoxicity. Neurotoxicology. doi: 10.1016/j.neuro.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zipper LM, Mulcahy RT. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J Biol Chem. 2002;277:36544–52. doi: 10.1074/jbc.M206530200. [DOI] [PubMed] [Google Scholar]
- [138].Li X, Zhang D, Hannink M, Beamer LJ. Crystal structure of the Kelch domain of human Keap1. J Biol Chem. 2004;279:54750–8. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- [139].Padmanabhan B, Scharlock M, Tong KI, Nakamura Y, Kang MI, Kobayashi A, Matsumoto T, Tanaka A, Yamamoto M, Yokoyama S. Purification, crystallization and preliminary X-ray diffraction analysis of the Kelch-like motif region of mouse Keap1. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005;61:153–5. doi: 10.1107/S1744309104032506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–71. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci U S A. 2005;102:10070–5. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- [144].Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J Biol Chem. 2005;280:31768–75. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- [145].Ahlgren-Beckendorf JA, Reising AM, Schander MA, Herdler JW, Johnson JA. Coordinate regulation of NAD(P)H:quinone oxidoreductase and glutathione-S-transferases in primary cultures of rat neurons and glia: role of the antioxidant/electrophile responsive element. Glia. 1999;25:131–42. [PubMed] [Google Scholar]
- [146].Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37:139–43. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- [147].Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci U S A. 2006;103:768–73. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–31. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Lin SX, Lisi L, Russo CD, Polak PE, Sharp A, Weinberg G, Kalinin S, Feinstein DL. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro. 2011;3:75–84. doi: 10.1042/AN20100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–92. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- [151].Schilling S, Goelz S, Linker R, Luehder F, Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol. 2006;145:101–7. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Scannevin RH, Chollate S, Jung MY, Shackett M, Patel H, Bista P, Zeng W, Ryan S, Yamamoto M, Lukashev M, Rhodes KJ. Fumarates Promote Cytoprotection of Central Nervous System Cells Against Oxidative Stress via the Nrf2 Pathway. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.111.190132. [DOI] [PubMed] [Google Scholar]