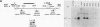Abstract
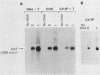
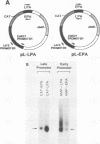
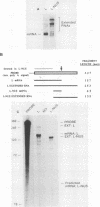
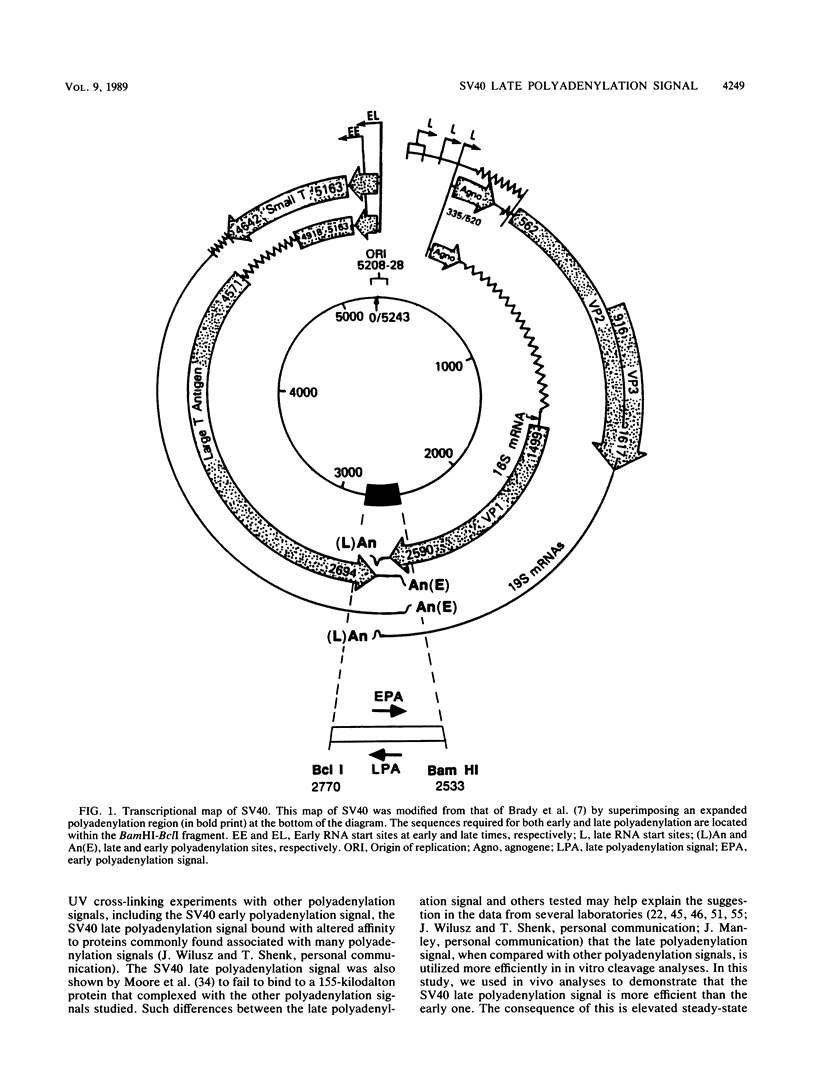
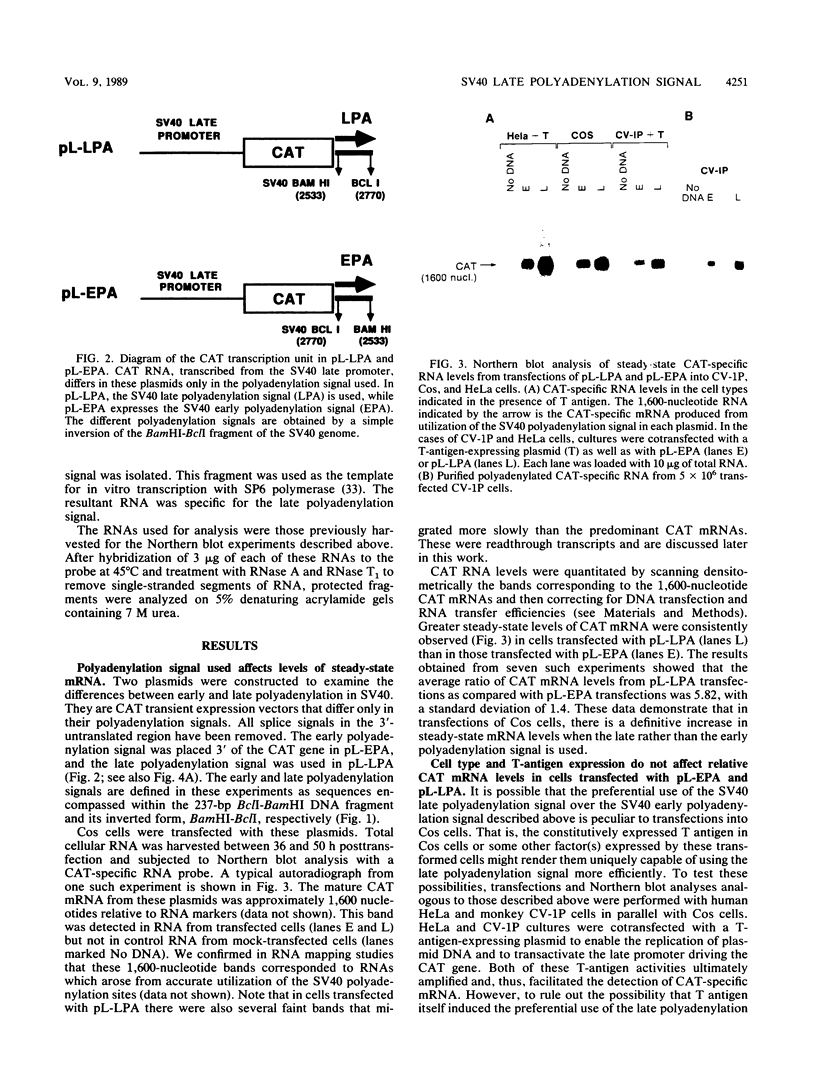
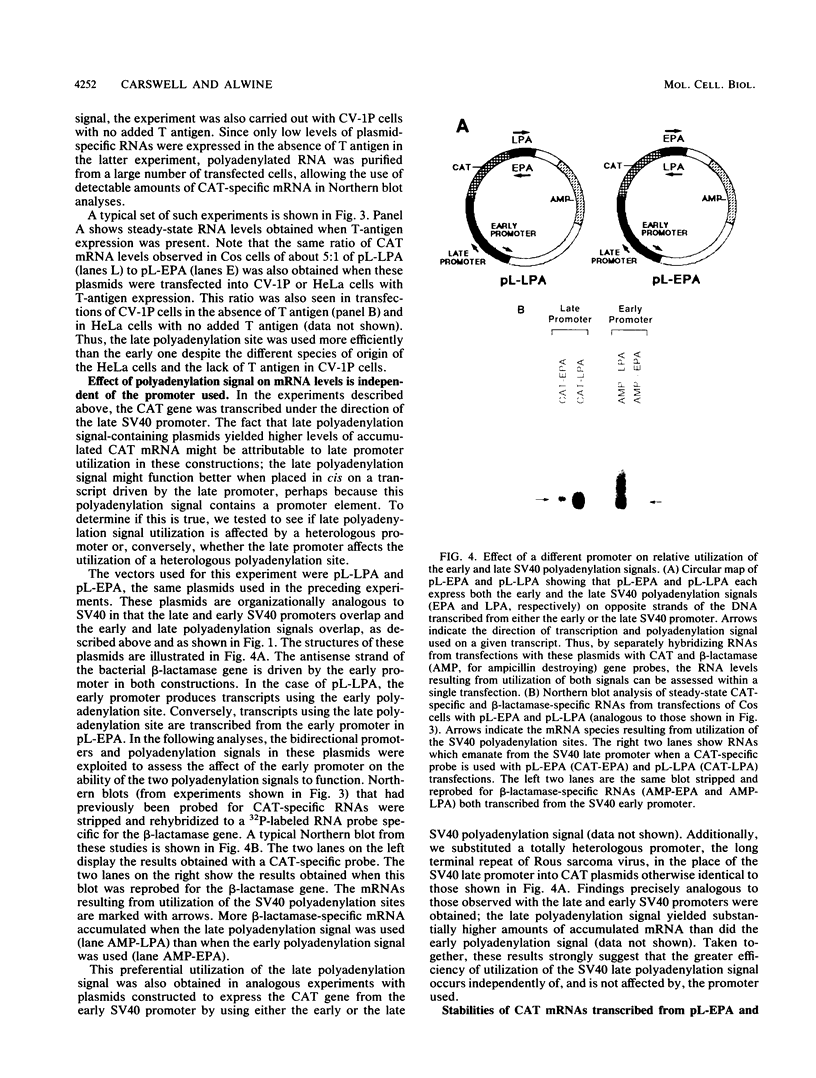
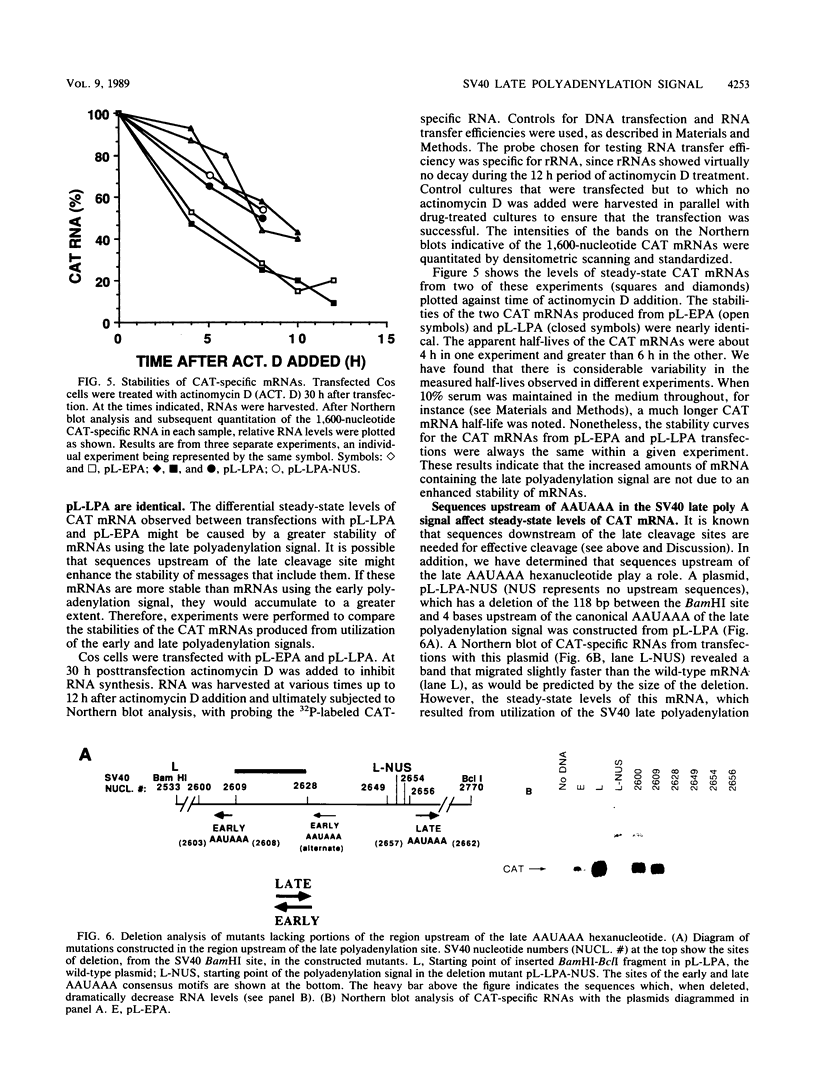
The late polyadenylation signal of simian virus 40 functions with greater efficiency than the early polyadenylation signal, in turn affecting steady-state mRNA levels. Two chloramphenicol acetyltransferase (CAT) transient expression vectors, pL-EPA and pL-LPA, that differ only in their polyadenylation signals were constructed by using the early and late polyadenylation signals, respectively. In transfections of Cos, CV-1P, or HeLa cells and subsequent Northern blot analysis of CAT-specific RNA, approximately five times more steady-state CAT mRNA was produced in transfections with pL-LPA than with pL-EPA. The basis for this difference was not related to the specific promoter used or to RNA stability. Overall, the difference in steady-state mRNA levels derived from the two plasmids appeared to be attributable to intrinsic properties of the two polyadenylation signals, resulting in distinctly different cleavage and polyadenylation efficiencies. Additionally, we found that the utilization of the late polyadenylation site was dramatically reduced by deletion of sequences between 48 and 29 nucleotides 5' of the AAUAAA hexanucleotide. This reduction of mRNA levels was shown not to be caused by altered stability of mutant precursor RNAs or mRNAs, suggesting that these upstream sequences constitute an element of the late polyadenylation signal and may cause, at least to some extent, the greater efficiency of utilization of the late polyadenylation site.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Evans R. M., Rosenfeld M. G. Calcitonin/calcitonin gene-related peptide transcription unit: tissue-specific expression involves selective use of alternative polyadenylation sites. Mol Cell Biol. 1984 Oct;4(10):2151–2160. doi: 10.1128/mcb.4.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Brady J., Bolen J. B., Radonovich M., Salzman N., Khoury G. Stimulation of simian virus 40 late gene expression by simian virus 40 tumor antigen. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2040–2044. doi: 10.1073/pnas.81.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. The Role of the poly(A) sequence in mammalian messenger RNA. CRC Crit Rev Biochem. 1981;10(1):1–38. doi: 10.3109/10409238109114634. [DOI] [PubMed] [Google Scholar]
- Breathnach R. Selective amplification in methotrexate-resistant mouse cells of an artificial dihydrofolate reductase transcription unit making use of cryptic splicing and polyadenylation sites. EMBO J. 1984 Apr;3(4):901–908. doi: 10.1002/j.1460-2075.1984.tb01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell S., Resnick J., Alwine J. C. Construction and characterization of CV-1P cell lines which constitutively express the simian virus 40 agnoprotein: alteration of plaquing phenotype of viral agnogene mutants. J Virol. 1986 Nov;60(2):415–422. doi: 10.1128/jvi.60.2.415-422.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway L., Wickens M. A sequence downstream of A-A-U-A-A-A is required for formation of simian virus 40 late mRNA 3' termini in frog oocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3949–3953. doi: 10.1073/pnas.82.12.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Mercer J. F. Isolation and sequence of a cDNA clone which contains the complete coding region of rat phenylalanine hydroxylase. Structural homology with tyrosine hydroxylase, glucocorticoid regulation, and use of alternate polyadenylation sites. J Biol Chem. 1986 Mar 25;261(9):4148–4153. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M. B., Dworkin-Rastl E. Changes in RNA titers and polyadenylation during oogenesis and oocyte maturation in Xenopus laevis. Dev Biol. 1985 Dec;112(2):451–457. doi: 10.1016/0012-1606(85)90417-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Galli G., Guise J., Tucker P. W., Nevins J. R. Poly(A) site choice rather than splice site choice governs the regulated production of IgM heavy-chain RNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2439–2443. doi: 10.1073/pnas.85.8.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimmi E. R., Soprano K. J., Rosenberg M., Reff M. E. Deletions in the SV40 late polyadenylation region downstream of the AATAAA mediate similar effects on expression in various mammalian cell lines. Nucleic Acids Res. 1988 Sep 26;16(18):8977–8997. doi: 10.1093/nar/16.18.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Hart R. P., McDevitt M. A., Ali H., Nevins J. R. Definition of essential sequences and functional equivalence of elements downstream of the adenovirus E2A and the early simian virus 40 polyadenylation sites. Mol Cell Biol. 1985 Nov;5(11):2975–2983. doi: 10.1128/mcb.5.11.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. A small nuclear ribonucleoprotein associates with the AAUAAA polyadenylation signal in vitro. Cell. 1986 May 23;45(4):581–591. doi: 10.1016/0092-8674(86)90290-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Activation of the SV40 late promoter: direct effects of T antigen in the absence of viral DNA replication. Cell. 1984 Feb;36(2):381–389. doi: 10.1016/0092-8674(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Analysis of an activatable promoter: sequences in the simian virus 40 late promoter required for T-antigen-mediated trans activation. Mol Cell Biol. 1985 Aug;5(8):1859–1869. doi: 10.1128/mcb.5.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. M., Beckendorf R. C., Westhafer M. A., Nordstrom J. L. Requirement of A-A-U-A-A-A and adjacent downstream sequences for SV40 early polyadenylation. Nucleic Acids Res. 1986 Jun 25;14(12):4939–4952. doi: 10.1093/nar/14.12.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. M., Westhafer M. A., Carson D. D., Nordstrom J. L. Polyadenylation at a cryptic site in the pBR322 portion of pSV2-neo: prevention of its utilization by the SV40 late poly(A) signal. Nucleic Acids Res. 1987 Jan 26;15(2):631–642. doi: 10.1093/nar/15.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Dhar R., Khoury G. Mapping the spliced and unspliced late lytic SV40 RNAs. Cell. 1978 Aug;14(4):971–982. doi: 10.1016/0092-8674(78)90351-3. [DOI] [PubMed] [Google Scholar]
- Mason P. J., Elkington J. A., Lloyd M. M., Jones M. B., Williams J. G. Mutations downstream of the polyadenylation site of a Xenopus beta-globin mRNA affect the position but not the efficiency of 3' processing. Cell. 1986 Jul 18;46(2):263–270. doi: 10.1016/0092-8674(86)90743-9. [DOI] [PubMed] [Google Scholar]
- Mather E. L., Nelson K. J., Haimovich J., Perry R. P. Mode of regulation of immunoglobulin mu- and delta-chain expression varies during B-lymphocyte maturation. Cell. 1984 Feb;36(2):329–338. doi: 10.1016/0092-8674(84)90226-5. [DOI] [PubMed] [Google Scholar]
- Meinkoth J. L., Legouy E., Brison O., Wahl G. M. New RNA species is produced by alternate polyadenylation following rearrangement associated with CAD gene amplification. Somat Cell Mol Genet. 1986 Jul;12(4):339–350. doi: 10.1007/BF01570728. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. L., Chen J., Whoriskey J. Two proteins crosslinked to RNA containing the adenovirus L3 poly(A) site require the AAUAAA sequence for binding. EMBO J. 1988 Oct;7(10):3159–3169. doi: 10.1002/j.1460-2075.1988.tb03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. L., Sharp P. A. Accurate cleavage and polyadenylation of exogenous RNA substrate. Cell. 1985 Jul;41(3):845–855. doi: 10.1016/s0092-8674(85)80065-9. [DOI] [PubMed] [Google Scholar]
- Mukai T., Yatsuki H., Arai Y., Joh K., Matsuhashi S., Hori K. Human aldolase B gene: characterization of the genomic aldolase B gene and analysis of sequences required for multiple polyadenylations. J Biochem. 1987 Nov;102(5):1043–1051. doi: 10.1093/oxfordjournals.jbchem.a122142. [DOI] [PubMed] [Google Scholar]
- Nadin-Davis S. A., Mezl V. A. Variation in the lack of polyadenylation of the rat milk protein mRNAs during the lactation cycle. Int J Biochem. 1985;17(10):1067–1075. doi: 10.1016/0020-711x(85)90038-2. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Vuocolo G. A. Synthesis of two mRNAs by utilization of alternate polyadenylation sites: expression of SV40-mouse immunoglobulin mu chain gene recombinants in Cos monkey cells. EMBO J. 1984 Apr;3(4):689–699. doi: 10.1002/j.1460-2075.1984.tb01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M. L., Perry R. P. The regulated production of mu m and mu s mRNA is dependent on the relative efficiencies of mu s poly(A) site usage and the c mu 4-to-M1 splice. Mol Cell Biol. 1989 Feb;9(2):726–738. doi: 10.1128/mcb.9.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Raju V. S., Reddy P. R. Inhibition of polyadenylation of mRNA by gonadotropin releasing hormone. Biochem Biophys Res Commun. 1983 Sep 15;115(2):451–455. doi: 10.1016/s0006-291x(83)80165-x. [DOI] [PubMed] [Google Scholar]
- Rao V. N., Papas T. S., Reddy E. S. erg, a human ets-related gene on chromosome 21: alternative splicing, polyadenylation, and translation. Science. 1987 Aug 7;237(4815):635–639. doi: 10.1126/science.3299708. [DOI] [PubMed] [Google Scholar]
- Ryner L. C., Manley J. L. Requirements for accurate and efficient mRNA 3' end cleavage and polyadenylation of a simian virus 40 early pre-RNA in vitro. Mol Cell Biol. 1987 Jan;7(1):495–503. doi: 10.1128/mcb.7.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner L. C., Takagaki Y., Manley J. L. Sequences downstream of AAUAAA signals affect pre-mRNA cleavage and polyadenylation in vitro both directly and indirectly. Mol Cell Biol. 1989 Apr;9(4):1759–1771. doi: 10.1128/mcb.9.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky M., Alwine J. C. Sequences on the 3' side of hexanucleotide AAUAAA affect efficiency of cleavage at the polyadenylation site. Mol Cell Biol. 1984 Aug;4(8):1460–1468. doi: 10.1128/mcb.4.8.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadofsky M., Connelly S., Manley J. L., Alwine J. C. Identification of a sequence element on the 3' side of AAUAAA which is necessary for simian virus 40 late mRNA 3'-end processing. Mol Cell Biol. 1985 Oct;5(10):2713–2719. doi: 10.1128/mcb.5.10.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., Ryner L. C., Manley J. L. Separation and characterization of a poly(A) polymerase and a cleavage/specificity factor required for pre-mRNA polyadenylation. Cell. 1988 Mar 11;52(5):731–742. doi: 10.1016/0092-8674(88)90411-4. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Villarreal L. A paranuclear extract contains a unique set of viral transcripts late in SV40 infection. Virology. 1981 Sep;113(2):663–671. doi: 10.1016/0042-6822(81)90195-1. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Feig D. I., Shenk T. The C proteins of heterogeneous nuclear ribonucleoprotein complexes interact with RNA sequences downstream of polyadenylation cleavage sites. Mol Cell Biol. 1988 Oct;8(10):4477–4483. doi: 10.1128/mcb.8.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Shenk T. A 64 kd nuclear protein binds to RNA segments that include the AAUAAA polyadenylation motif. Cell. 1988 Jan 29;52(2):221–228. doi: 10.1016/0092-8674(88)90510-7. [DOI] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Transcriptional regulation of the mu-delta heavy chain locus in normal murine B lymphocytes. J Exp Med. 1984 Aug 1;160(2):564–583. doi: 10.1084/jem.160.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkower D., Wickens M. A functionally redundant downstream sequence in SV40 late pre-mRNA is required for mRNA 3'-end formation and for assembly of a precleavage complex in vitro. J Biol Chem. 1988 Apr 25;263(12):5780–5788. [PubMed] [Google Scholar]
- Zhang F., Denome R. M., Cole C. N. Fine-structure analysis of the processing and polyadenylation region of the herpes simplex virus type 1 thymidine kinase gene by using linker scanning, internal deletion, and insertion mutations. Mol Cell Biol. 1986 Dec;6(12):4611–4623. doi: 10.1128/mcb.6.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]