Abstract
The renin–angiotensin system (RAS) exercises fundamental control over sodium and water handling in the kidney. Accordingly, dysregulation of the RAS leads to blood pressure elevation with ensuing renal and cardiovascular damage. Recent studies have revealed that the RAS hormonal cascade is more complex than initially posited with multiple enzymes, effector molecules, and receptors that coordinately regulate the effects of the RAS on the kidney and vasculature. Moreover, recently identified tissue-specific RAS components have pleomorphic effects independent of the circulating RAS that influence critical homeostatic mechanisms including the immune response and fetal development. Further characterization of the diverse interactions between the RAS and other signaling pathways within specific tissues should lead to novel treatments for renal and cardiovascular disease.
Keywords: Renin, Angiotensin, Kidney
Introduction
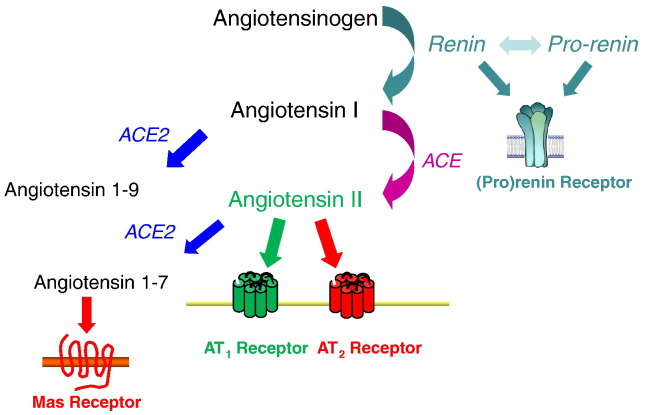
The renin–angiotensin system (RAS) is a master regulator of blood pressure and fluid homeostasis. As shown in Fig. 1 , this system is a multi-enzymatic cascade in which angiotensinogen, the major substrate, is processed in a two-step reaction by renin and angiotensin-converting enzyme (ACE), resulting in the sequential generation of angiotensin I and angiotensin II. In recent years, several new enzymes, peptides, and receptors in this system have been identified, manifesting a complexity that was previously unappreciated. Although appropriate activation of the RAS is vital for preventing circulatory collapse and maintaining intravascular fluid balance, dysregulation and/or persistent RAS activation can lead to inappropriate blood pressure elevation, target organ damage, and even reduced survival [1]. Accordingly, pharmacological agents that inhibit the synthesis or activity of angiotensin II are effective and widely used anti-hypertensive agents [2] that can ameliorate morbidity and mortality in cardiovascular diseases including congestive heart failure [3], [4] and slow the progression of a wide range of progressive kidney diseases including diabetic nephropathy [5]. Angiotensin receptor blockers (ARBs), which block type 1 (AT1) receptors, are similarly effective for treating these disorders [6], [7], [8]. Below, we will review selected recent advances in understanding the physiology of the RAS with an emphasis on actions impacting the kidney.
Fig. 1.
Schematic of the renin–angiotensin system.
Renin and its putative receptor
The aspartyl protease renin is synthesized as a precursor protein, prorenin [9]. Active renin is then generated by removal of an N-terminal peptide fragment, presumably by proteases in the kidney. Active renin specifically cleaves the 10 amino acids from the N-terminus of angiotensinogen to form angiotensin I. As angiotensinogen and angiotensin converting enzyme (ACE) are present in excess, at least in the circulation, the level of renin is a key rate-limiting step determining the level of angiotensin II and thus the activity of the RAS. The primary source of renin in the circulation is the kidney, where its expression and secretion are tightly regulated at the juxta-glomerular (JG) apparatus by a renal baroreceptor [10] and sodium chloride delivery to the macula densa, which is sensed by chloride flux through the NKCC2 transporter expressed on the luminal side of macula densa cells [11], [12]. Recent studies confirm that the pathway linked to triggering renin release by the macula densa involves generation of PGE2 by cyclo-oxygenase (COX)-2 with subsequent activation of the EP4 receptor for PGE2 [13]. Of note, activity of the pathway is unaffected by the absence of either of the two putative microsomal PGE synthase enzymes indicating a possible role for atypical or perhaps non-enzymatic pathways for PGE2 synthesis. Microarray studies have affirmed the unique identity of the renin-producing JG cell [14] and have also elucidated a role for micro-RNAs to maintain the phenotype of JG cells and regulate their emergence from precursors among smooth muscle cells in the renal arteriole [15], [16]. At a transcriptional level, Liver X receptors enhance renin generation within JG cells through interactions with the renin promoter [17] and can even induce differentiation of mesenchymal stem cells into renin-secreting “JG-like” cells [18]. However, other studies suggest that renin may also be generated in epithelial cells of the proximal, connecting, and/or collecting tubule of the nephron [19], [20]. While there is some controversy regarding the physiological relevance of renin in the distal nephron, the impact of dietary salt, blood pressure and angiotensin II levels on its expression seems to be paradoxical compared to renin at the JGA. For example, angiotensin II suppresses renin at the JGA but stimulates renin mRNA and protein formation in the collecting duct [20], an effect that appears to be independent of blood pressure [21].
Along with the enzymatic actions of renin in the RAS, a receptor that binds prorenin and renin has been identified in the kidney glomerulus and the vasculature [22]. In cultured cells, activation of this receptor stimulates profibrotic and pro-inflammatory pathways independently of angiotensin II generation [23]. In addition, studies using a putative antagonist peptide synthesized from the handle-region of the pro-renin molecule suggest a role for the pro-renin receptor to promote kidney diseases such as diabetic nephropathy [24], [25]. However, the effectiveness of this peptide appears to be inconsistent and its ability to block signaling at the pro-renin receptor has been questioned [26].
The pro-renin receptor appears to have other functions independent of the renin–angiotensin system. For example, it is found as part of a complex required for the normal function of V-ATPase in several cell lineages including cardiac myocytes [27]. The pro-renin receptor acts as an adaptor between the Wnt receptor and V-ATPase in a Wnt/β-catenin signaling complex required for normal CNS development [28]. Thus deletion of the pro-renin receptor gene causes a lethal phenotype at a very early embryonic stage, which contrasts significantly with the phenotype of renin knockouts [29], indicating important functions of the receptor that are independent of its actions in the RAS. Two groups have recently described studies of mouse lines in which the pro-renin receptor was deleted specifically from podocytes. In both cases, there was a similar, dramatic phenotype characterized by disruption of the glomerular filtration barrier, with marked proteinuria and abnormal podocyte structure, perhaps due to dysregulated autophagy [30], [31]. Thus, while this molecule appears to play a critical role in the kidney, much remains to be learned about its functions in normal kidney physiology and disease, including the extent to which these functions are influenced by renin or pro-renin binding.
Regulatory actions of angiotensinogen
Angiotensinogen, the substrate for renin, is the source of all angiotensin peptides. While it has been suggested that there is an excess of angiotensinogen substrate in human plasma relative to renin, other studies suggest that alterations of plasma angiotensinogen levels can affect the relative activity of the RAS. For example, a variant of the human AGT gene that is associated with higher plasma levels of angiotensinogen is also associated with the development of hypertension [32]. In addition, gene titration studies in transgenic mice engineered to carry from 0 to 4 copies of the Agt gene demonstrated a positive correlation between the number of Agt gene copies, plasma levels of angiotensinogen, and blood pressure [33]. Furthermore, a recent study suggests that changes in conformation of the angiotensinogen molecule caused by exposure to oxidative stress can significantly modify the kinetics of its cleavage by renin [34]. These data indicate that oxidative stress may exert independent control of the activity of the RAS by facilitating the generation of angiotensin I.
Angiotensinogen in the circulation is derived primarily from synthesis in the liver but it is also produced by other tissues including the brain, the immune system, and the kidney [35]. Changes in levels of angiotensinogen in these tissues may impact the activity of local renin–angiotensin systems through mechanisms that are quite independent of angiotensinogen in the circulation. For example, in the kidney, synthesis of angiotensinogen in proximal tubule may be augmented by angiotensin II [36] as part of a local, intra-renal RAS that is regulated independently of the systemic RAS. This apparent “feed-forward” system may impact the function of epithelial cells along the nephron to enhance sodium reabsorption and hypertension. Accordingly, urinary angiotensinogen may be used to monitor RAS activation in the kidney in patients with hypertension and some forms of chronic kidney disease [37], [38].
Novel functions for angiotensin converting enzyme and its homologue
Angiotensin converting enzyme (ACE) is a carboxyl dipeptidase that generates the vasoactive peptide angiotensin II by cleaving 2 amino acids from the c-terminus of the inactive precursor angiotensin I [39]. ACE inhibitors were the first small molecule therapeutic agents targeting the RAS and have become an essential component for treatment of a wide range of cardiovascular and kidney diseases. Polymorphisms of the human ACE gene have been linked to differing susceptibilities to hypertension, cardiovascular and renal diseases [40], [41]. The insertion (I) allele containing an Alu repeat is associated with lower circulating levels of ACE compared to the so-called deletion (D) allele without the repeat. The D allele has been linked to enhanced risk for renal and cardiovascular disease [42], [43]. It has been suggested that the mechanism underlying this increased risk is related to the higher levels of circulating ACE associated with the D allele. However, gene titration studies failed to show an effect of differences in circulating levels of ACE between 62 and 144% on blood pressure in mice [44]. On the other hand, incremental differences ACE levels may be sufficient to affect local generation of angiotensin II in specific target tissues such as the kidney or may impact metabolism of other target peptides such as bradykinin. In this regard, experiments in mice using conventional gene targeting are consistent with a gene dosage effect for Ace in STZ diabetes [45].
Along with angiotensin II, there are other peptide substrates of ACE including the vasodilator peptide bradykinin, which is degraded by ACE into an inactive peptide. Accordingly, enhanced activity of bradykinin appears to contribute to blood pressure lowering by ACE inhibition [46]. Recently, the relatively promiscuous carboxyl dipeptidase activity of ACE has been implicated in regulation of the immune system [47]. Bernstein and associates showed that ACE participates in processing peptides within antigen presenting cells (APCs) for loading onto MHC class I antigens and as such, plays a role in influencing immune surveillance and development of the T cell repertoire. By editing prospective antigens before their presentation to CD8+ T lymphocytes in the context of MHC class I, ACE can alter not only the set of self-antigens that drive deletion and/or tolerization of autoreactive T cells but also the foreign antigens that shape the acquired cell-mediated response to an exogenous immune stimulus. Other recent studies (discussed below) have highlighted a role of the RAS to influence immune responses that are relevant to the development of hypertension and kidney injury.
ACE2, a homologue of ACE identified several years ago, is a monocarboxyl peptidase with affinity for angiotensin II and other angiotensin peptides [48]. ACE2 has now been implicated in a variety of disorders including hypertension, heart failure, atherosclerosis, and diabetic nephropathy [49], [50], [51], [52]. With regard to the RAS, ACE2 can influence its activity through at least two pathways. First, angiotensin II is a substrate of ACE2, and thus ACE2 represents a metabolic pathway for angiotensin II to extinguish its activity [49]. In addition, removal of one amino acid from the carboxyl-terminus of angiotensin II generates angiotensin 1–7, which has a number of putative biological actions [53]. For example, accumulating evidence indicates that this peptide causes vasodilation, natriuresis and may promote reduced blood pressures [54] via activation of the Mas receptor [55]. On the other hand, administration of recombinant ACE2 degrades plasma angiotensin II and lowers blood pressure independently of any effects on angiotensin 1–7 generation [56]. Thus, the functions of ACE2 may be determined by its distinct actions to metabolize angiotensin II and to generate angiotensin 1–7. Moreover, exploitation of the salubrious effects of angiotensin 1–7 may offer therapeutic potential for the treatment of progressive kidney disease [57].
Studies are now emerging that also highlight important roles for ACE2 in tissues other than the kidney and heart impacting homeostatic and/or immune surveillance mechanisms. In this regard, like other RAS components, ACE2 is required for normal gestational development as maternal ACE2 deficiency leads to elevated levels of angiotensin II in the placenta and fetal growth restriction [58]. Also analogous to other RAS components, actions of ACE2 in the central nervous system impact autonomic homeostasis, regulating levels of oxidative stress in the paraventricular nucleus and rostral ventrolateral medulla, which may in turn influence susceptibility to hypertension, presumably due to effects on sympathetic tone [59]. Finally, ACE2 was identified as the receptor in pulmonary tissues for the coronavirus that causes severe acute respiratory syndrome (SARS). Accordingly, ACE2-deficiency protects mice from pulmonary injury induced by SARS coronavirus inoculation [60].
Angiotensin receptors
The angiotensin receptors can be divided into 2 pharmacological classes of 7 trans-membrane receptors: type 1 (AT1) and type 2 (AT2), based on their differential affinities for various non-peptide antagonists. Studies using these antagonists suggested that most of the classically recognized functions of the RAS are mediated by AT1 receptors including vasoconstriction, release of aldosterone from the adrenal glomerulosa, vascular smooth muscle contraction, stimulation of hypothalamic thirst sensors, regulation of tubular glomerular feedback, and stimulation of renal tubular sodium reabsorption [61]. Gene targeting studies described have confirmed these conclusions [62].
In rodents, 2 isoforms of the AT1 receptor, AT1A and AT1B, have been identified. AT1A receptors predominate in most organs, except the adrenal gland and regions of the CNS, where AT1B expression may be more prominent [63]. Although the AT1B receptor has a unique role to mediate thirst responses [64], AT1A receptors have the predominant role in determining the level of blood pressure and in mediating vasoconstrictor responses [65]. Thus, the AT1A receptor is widely considered to be the closest functional homologue to the single human AT1 receptor.
AT1 receptors are present within a number of tissues that coordinately determine the level of blood pressure, including the kidney, the vasculature, and the central and sympathetic nervous systems. Moreover, the relative contributions of AT1 receptors in these different systems to control of blood pressure and the pathogenesis of hypertension are difficult to distinguish. A kidney cross-transplantation approach using wild-type and AT1A receptor-deficient mice illustrated equal and non-redundant contributions of AT1 receptors in the kidney and in systemic tissues to the maintenance of normal blood pressure [66], suggesting that AT1 receptors in many of these tissue sites are utilized to protect body fluid volumes in order to prevent circulatory collapse. By contrast, other studies using this cross-transplantation approach confirmed a dominant contribution of AT1 receptors in the kidney to promote sodium retention and blood pressure elevation in hypertension [67]. More recently, conditional deletion of AT1A receptors selectively within epithelial cells of the proximal tubule in the kidney demonstrated an important role for AT1 receptors in the proximal nephron in maintaining blood pressure homeostasis and in the pathogenesis of angiotensin II-dependent hypertension. These actions are mediated by modulating fluid reabsorption in the proximal tubule through effects on sodium transporter activity in that segment including the NHE3 sodium–proton exchanger [68].
AT1 receptors also appear to modulate solute and fluid reabsorption in the distal nephron. For example, angiotensin II acting via AT1 receptors stimulates sodium–hydrogen exchange in the cortical and outer medullary collecting tubule by increasing the density of the vacuolar sodium–hydrogen ATPase in the apical membrane of the type A intercalated cell, which in turn leads to an increase in bicarbonate reabsorption [69]. On the apical membrane of the principal cells in the cortical collecting duct (CCD), luminal angiotensin II stimulates amiloride-sensitive sodium transport by increasing activity of the epithelial sodium channel (ENaC) through an AT1 receptor-dependent mechanism [70]. As the distal nephron ultimately determines urine flow and composition, actions of angiotensin II to modulate sodium handling at this site may impact blood pressure homeostasis [70], [71], a hypothesis that will require testing through targeted in vivo studies.
Previous studies have indicated a role for the RAS to modulate urinary concentrating mechanisms through the AT1 angiotensin receptor. For example, mice with complete deficiency of AT1A receptors have a urinary concentrating defect with an attenuated increase in urine osmolality after water deprivation or vasopressin administration [72]. However, the precise mechanism of this effect was not clear. To determine whether direct actions of AT1 receptors in epithelial cells of the collecting duct regulate water reabsorption by the kidney, mice were generated with AT1A receptors deleted from the epithelium of the collecting duct with Cre-Loxp technology. Following water deprivation or vasopressin administration, urine osmolalities were consistently lower in the mice lacking AT1A receptors in collecting duct. Likewise, after water deprivation, levels of aquaporin-2 (AQP2) protein in inner and outer medulla were significantly diminished, whereas localization to the apical membrane was unaffected [73]. These results indicate direct effects of AT1A receptors in collecting duct epithelial cells to regulate water reabsorption by modulating AQP2 levels. These actions are required to achieve maximal urinary concentration.
Pharmacological and genetic studies have thus shown that most of the classically recognized functions of the RAS are mediated by AT1 receptors. However, exploring the physiological role of AT2 receptors has become a burgeoning area of more recent research particularly as a new specific AT2 receptor agonist has been identified [74]. AT2 receptors are found in abundance during fetal development [75], [76] but their expression generally falls after birth. Nevertheless, persistent AT2 receptor expression can be detected in several adult tissues including the kidney, adrenal gland and the brain, and absolute levels of AT2 receptor expression may be modulated by angiotensin II and certain growth factors [77]. In this regard, activation of the AT2 receptor in the brain promotes axonal regeneration [78].
Targeted disruption of the mouse Agtr2 gene did not cause a dramatically abnormal phenotype at baseline. However, these animals clearly manifest increased sensitivity to the pressor actions of angiotensin II [79], [80] and to angiotensin II-induced vascular damage that promotes aortic aneurysm progression [81]. One of the AT2 deficient lines manifested increased baseline blood pressure and heart rate [79]. Interestingly, behavioral changes were also observed in AT2-deficient mice. They had decreased spontaneous movements and rearing activity [79], [80] and impaired drinking response to water deprivation [80]. Transgenic mice that overexpress the AT2 receptor gene under control of a cardiac-specific promoter have decreased sensitivity to AT1-mediated pressor and chronotropic actions [82]. Moreover, the pressor actions of angiotensin II are significantly attenuated in these transgenic mice. Taken together, these data suggest that a primary function of the AT2 receptor may be to negatively modulate the actions of the AT1 receptor.
Studies with the specific AT2 receptor agonist, compound 21 (C21) have highlighted AT2's role to counteract the detrimental effects of AT1 receptor activation on target organ damage. For example, in a myocardial infarction model, treatment with C21 reduced the size of the myocardial scar and limited cardiac inflammation [83]. Subsequent studies have confirmed the beneficial effects of C21 treatment of cerebrovascular disease and hypertensive renal damage [84], [85]. Consistent with its role to balance the pro-inflammatory actions of AT1 receptors, AT2 receptor stimulation appears to limit tissue injury by inhibiting the NF-κB pathway [86].
The RAS and immune system activation in hypertension
In addition to the classical functions of AT1 receptors in the kidney and the vasculature to regulate sodium and water homeostasis, actions of angiotensin II to modulate immune responses can also impact blood pressure and target organ damage in hypertension. Gene expression and ligand-binding studies have demonstrated the presence of AT1 receptors and other RAS components on several mononuclear cell populations [87], [88], but a role for the immune system RAS in hypertension has only recently emerged. In a seminal study, Guzik and colleagues found that the absence of functional lymphocytes in Rag1-deficient mice largely abrogated the chronic hypertensive response to angiotensin II and that reconstitution with T cells but not B cells could restore blood pressure elevation in this model [89]. The exact mechanism through which T cells contribute to angiotensin II-dependent hypertension is not entirely clear but may involve modulation of vascular responses and/or effects on renal sodium handling [89], [90]. Putative antigens responsible for activating the adaptive immune response in hypertension have not been identified. Nevertheless, the ability of T cells to mediate angiotensin II-induced hypertension also requires activation of co-stimulatory pathways in the T cell by B7 molecules on the antigen-presenting cell (“Signal 2”), pointing to an antigen-specific mechanism of classical T cell stimulation [91]. Whether angiotensin II stimulates the immune system through direct activation of AT1 receptors on inflammatory cells remains controversial as AT1 receptor-deficiency on bone marrow-derived cells is protective in angiotensin II-induced hypertension [92], and stimulation of the CNS by angiotensin II can activate T cells indirectly [93].
Activation of the immune system by angiotensin II also potentiates target organ damage in hypertension. Muller and colleagues demonstrated that broad immunosuppression during angiotensin II-induced hypertension could reduce albuminuria, inflammatory cell infiltration in the kidney, and renal structural damage through a blood pressure-independent mechanism [94]. Angiotensin II appears to exacerbate renal injury in this model in part by inducing expression of pro-inflammatory cytokines in the kidney including tumor necrosis factor-α (TNF-α) [95] as TNF-α blockade abrogates angiotensin II-induced renal injury [94]. Although most studies have emphasized that angiotensin II exacerbates tissue damage through immune stimulation, recent experiments show that the induction of immunosuppressive T regulatory cells by angiotensin II can provide cardioprotection and even blunt the hypertensive response [96], [97]. Thus, the functions of the immune system RAS in cardiovascular and renal disease are multifaceted and will require further elucidation.
Conclusions
Recent advances have revealed that the RAS is a far more complex hormonal cascade than was initially considered. Angiotensin II remains a dominant effector molecule of this system, and the coordinated actions of ACE and ACE2 provide exquisite control of angiotensin II levels and its downstream metabolites. Nevertheless, other RAS mediators including prorenin and angiotensin 1–7 have functions independent of angiotensin II that can influence blood pressure regulation and target organ damage. Identifying new nodes in the RAS matrix continues to highlight profound connections between the RAS and other homeostatic systems. For example, signaling functions of the pro-renin receptor unrelated to the RAS are clearly required for sustaining normal fetal development. Moreover, understanding the precise mechanisms through which the local RAS within specific tissues influences blood pressure and end-organ damage should facilitate the development of novel therapeutic interventions for the treatment of renal and cardiovascular disease.
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Benigni A., Corna D., Zoja C., Sonzogni A., Latini R., Salio M., Conti S., Rottoli D., Longaretti L., Cassis P., Morigi M., Coffman T.M., Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Husain A., Graham R. Harwood Academic; Sydney: 2000. Drugs, Enzymes and Receptors of the Renin–Angiotensin System: Celebrating a Century of Discovery. [Google Scholar]
- 3.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 4.The SOLVD Investigators Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N. Engl. J. Med. 1992;327:725–727. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 5.Lewis E.J., Hunsicker L.G., Bain R.P., Rohde R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 6.Dahlof B., Devereux R.B., Kjeldsen S.E., Julius S., Beevers G., de Faire U., Fyhrquist F., Ibsen H., Kristiansson K., Lederballe-Pedersen O., Lindholm L.H., Nieminen M.S., Omvik P., Oparil S., Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (life): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 7.Lewis E.J., Hunsicker L.G., Clarke W.R., Berl T., Pohl M.A., Lewis J.B., Ritz E., Atkins R.C., Rohde R., Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 8.Brenner B.M., Cooper M.E., de Zeeuw D., Keane W.F., Mitch W.E., Parving H.H., Remuzzi G., Snapinn S.M., Zhang Z., Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 9.Danser A.H., Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension. 2005;46:1069–1076. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- 10.Carey R.M., McGrath H.E., Pentz E.S., Gomez R.A., Barrett P.Q. Biomechanical coupling in renin-releasing cells. J. Clin. Invest. 1997;100:1566–1574. doi: 10.1172/JCI119680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenz J.N., Weihprecht H., Schnermann J., Skott O., Briggs J.P. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am. J. Physiol. 1991;260:F486–F493. doi: 10.1152/ajprenal.1991.260.4.F486. [DOI] [PubMed] [Google Scholar]
- 12.Bell P.D., Lapointe J.Y., Sabirov R., Hayashi S., Peti-Peterdi J., Manabe K., Kovacs G., Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facemire C.S., Nguyen M., Jania L., Beierwaltes W.H., Kim H.S., Koller B.H., Coffman T.M. A major role for the EP4 receptor in regulation of renin. Am. J. Physiol. Renal Physiol. 2011;301:F1035–F1041. doi: 10.1152/ajprenal.00054.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunskill E.W., Sequeira-Lopez M.L., Pentz E.S., Lin E., Yu J., Aronow B.J., Potter S.S., Gomez R.A. Genes that confer the identity of the renin cell. J. Am. Soc. Nephrol. 2011;22:2213–2225. doi: 10.1681/ASN.2011040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequeira-Lopez M.L., Weatherford E.T., Borges G.R., Monteagudo M.C., Pentz E.S., Harfe B.D., Carretero O., Sigmund C.D., Gomez R.A. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J. Am. Soc. Nephrol. 2010;21:460–467. doi: 10.1681/ASN.2009090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medrano S., Monteagudo M.C., Sequeira-Lopez M.L., Pentz E.S., Gomez R.A. Two microRNAs, miR-330 and miR-125b-5p, mark the juxtaglomerular cell and balance its smooth muscle phenotype. Am. J. Physiol. Renal Physiol. 2012;302:F29–F37. doi: 10.1152/ajprenal.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morello F., de Boer R.A., Steffensen K.R., Gnecchi M., Chisholm J.W., Boomsma F., Anderson L.M., Lawn R.M., Gustafsson J.A., Lopez-Ilasaca M., Pratt R.E., Dzau V.J. Liver X receptors alpha and beta regulate renin expression in vivo. J. Clin. Invest. 2005;115:1913–1922. doi: 10.1172/JCI24594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita K., Morello F., Wu Y., Zhang L., Iwanaga S., Pratt R.E., Dzau V.J. Mesenchymal stem cells differentiate into renin-producing juxtaglomerular (JG)-like cells under the control of liver X receptor-alpha. J. Biol. Chem. 2010;285:11974–11982. doi: 10.1074/jbc.M109.099671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M., Harris M.P., Rose D., Smart A., He X.R., Kretzler M., Briggs J.P., Schnermann J. Renin and renin mRNA in proximal tubules of the rat kidney. J. Clin. Invest. 1994;94:237–243. doi: 10.1172/JCI117312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto-Carrasquero M.C., Harrison-Bernard L.M., Kobori H., Ozawa Y., Hering-Smith K.S., Hamm L.L., Navar L.G. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto-Carrasquero M.C., Botros F.T., Pagan J., Kobori H., Seth D.M., Casarini D.E., Navar L.G. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008;51:1590–1596. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen G., Delarue F., Burckle C., Bouzhir L., Giller T., Sraer J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen G., Muller D.N. The biology of the (pro)renin receptor. J. Am. Soc. Nephrol. 2010;21:18–23. doi: 10.1681/ASN.2009030300. [DOI] [PubMed] [Google Scholar]
- 24.Ichihara A., Suzuki F., Nakagawa T., Kaneshiro Y., Takemitsu T., Sakoda M., Nabi A.H., Nishiyama A., Sugaya T., Hayashi M., Inagami T. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1A receptor-deficient mice. J. Am. Soc. Nephrol. 2006;17:1950–1961. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H., Ichihara A., Kaneshiro Y., Inomata K., Sakoda M., Takemitsu T., Nishiyama A., Itoh H. Regression of nephropathy developed in diabetes by (pro)renin receptor blockade. J. Am. Soc. Nephrol. 2007;18:2054–2061. doi: 10.1681/ASN.2006080820. [DOI] [PubMed] [Google Scholar]
- 26.Feldt S., Maschke U., Dechend R., Luft F.C., Muller D.N. The putative (pro)renin receptor blocker HRP fails to prevent (pro)renin signaling. J. Am. Soc. Nephrol. 2008;19:743–748. doi: 10.1681/ASN.2007091030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinouchi K., Ichihara A., Sano M., Sun-Wada G.H., Wada Y., Kurauchi-Mito A., Bokuda K., Narita T., Oshima Y., Sakoda M., Tamai Y., Sato H., Fukuda K., Itoh H. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ. Res. 2010;107:30–34. doi: 10.1161/CIRCRESAHA.110.224667. [DOI] [PubMed] [Google Scholar]
- 28.Cruciat C.M., Ohkawara B., Acebron S.P., Karaulanov E., Reinhard C., Ingelfinger D., Boutros M., Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 29.Yanai K., Saito T., Kakinuma Y., Kon Y., Hirota K., Taniguchi-Yanai K., Nishijo N., Shigematsu Y., Horiguchi H., Kasuya Y., Sugiyama F., Yagami K., Murakami K., Fukamizu A. Renin-dependent cardiovascular functions and renin-independent blood–brain barrier functions revealed by renin-deficient mice. J. Biol. Chem. 2000;275:5–8. doi: 10.1074/jbc.275.1.5. [DOI] [PubMed] [Google Scholar]
- 30.Riediger F., Quack I., Qadri F., Hartleben B., Park J.K., Potthoff S.A., Sohn D., Sihn G., Rousselle A., Fokuhl V., Maschke U., Purfurst B., Schneider W., Rump L.C., Luft F.C., Dechend R., Bader M., Huber T.B., Nguyen G., Muller D.N. Prorenin receptor is essential for podocyte autophagy and survival. J. Am. Soc. Nephrol. 2011;22:2193–2202. doi: 10.1681/ASN.2011020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshima Y., Kinouchi K., Ichihara A., Sakoda M., Kurauchi-Mito A., Bokuda K., Narita T., Kurosawa H., Sun-Wada G.H., Wada Y., Yamada T., Takemoto M., Saleem M.A., Quaggin S.E., Itoh H. Prorenin receptor is essential for normal podocyte structure and function. J. Am. Soc. Nephrol. 2011;22:2203–2212. doi: 10.1681/ASN.2011020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeunemaitre X., Soubrier F., Kotelevtsev Y.V., Lifton R.P., Williams C.S., Charru A., Hunt S.C., Hopkins P.N., Williams R.R., Lalouel J.M., Corvol P. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 33.Kim H.S., Krege J.H., Kluckman K.D., Hagaman J.R., Hodgin J.B., Best C.F., Jennette J.C., Coffman T.M., Maeda N., Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc. Natl. Acad. Sci. U. S. A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou A., Carrell R.W., Murphy M.P., Wei Z., Yan Y., Stanley P.L., Stein P.E., Broughton Pipkin F., Read R.J. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickson M.E., Sigmund C.D. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension. 2006;48:14–20. doi: 10.1161/01.HYP.0000227932.13687.60. [DOI] [PubMed] [Google Scholar]
- 36.Kobori H., Harrison-Bernard L.M., Navar L.G. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J. Am. Soc. Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobori H., Alper A.B., Jr., Shenava R., Katsurada A., Saito T., Ohashi N., Urushihara M., Miyata K., Satou R., Hamm L.L., Navar L.G. Urinary angiotensinogen as a novel biomarker of the intrarenal renin–angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konishi Y., Nishiyama A., Morikawa T., Kitabayashi C., Shibata M., Hamada M., Kishida M., Hitomi H., Kiyomoto H., Miyashita T., Mori N., Urushihara M., Kobori H., Imanishi M. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension. 2011;58:205–211. doi: 10.1161/HYPERTENSIONAHA.110.166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corvol P., Williams T.A., Soubrier F. Peptidyl dipeptidase A: angiotensin I-converting enzyme. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- 40.Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayed-Tabatabaei F.A., Oostra B.A., Isaacs A., van Duijn C.M., Witteman J.C. ACE polymorphisms. Circ. Res. 2006;98:1123–1133. doi: 10.1161/01.RES.0000223145.74217.e7. [DOI] [PubMed] [Google Scholar]
- 42.Gardemann A., Weiss T., Schwartz O., Eberbach A., Katz N., Hehrlein F.W., Tillmanns H., Waas W., Haberbosch W. Gene polymorphism but not catalytic activity of angiotensin I-converting enzyme is associated with coronary artery disease and myocardial infarction in low-risk patients. Circulation. 1995;92:2796–2799. doi: 10.1161/01.cir.92.10.2796. [DOI] [PubMed] [Google Scholar]
- 43.Lovati E., Richard A., Frey B.M., Frey F.J., Ferrari P. Genetic polymorphisms of the renin–angiotensin–aldosterone system in end-stage renal disease. Kidney Int. 2001;60:46–54. doi: 10.1046/j.1523-1755.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 44.Krege J.H., Kim H.S., Moyer J.S., Jennette J.C., Peng L., Hiller S.K., Smithies O. Angiotensin-converting enzyme gene mutations, blood pressures, and cardiovascular homeostasis. Hypertension. 1997;29:150–157. doi: 10.1161/01.hyp.29.1.150. [DOI] [PubMed] [Google Scholar]
- 45.Huang W., Gallois Y., Bouby N., Bruneval P., Heudes D., Belair M.F., Krege J.H., Meneton P., Marre M., Smithies O., Alhenc-Gelas F. Genetically increased angiotensin I-converting enzyme level and renal complications in the diabetic mouse. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13330–13334. doi: 10.1073/pnas.231476798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gainer J.V., Morrow J.D., Loveland A., King D.J., Brown N.J. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N. Engl. J. Med. 1998;339:1285–1292. doi: 10.1056/NEJM199810293391804. [DOI] [PubMed] [Google Scholar]
- 47.Shen X.Z., Billet S., Lin C., Okwan-Duodu D., Chen X., Lukacher A.E., Bernstein K.E. The carboxypeptidase ACE shapes the MHC class I peptide repertoire. Nat. Immunol. 2011;12:1078–1085. doi: 10.1038/ni.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gurley S.B., Coffman T.M. Angiotensin-converting enzyme 2 gene targeting studies in mice: mixed messages. Exp. Physiol. 2008;93:538–542. doi: 10.1113/expphysiol.2007.040014. [DOI] [PubMed] [Google Scholar]
- 49.Gurley S.B., Allred A., Le T.H., Griffiths R., Mao L., Philip N., Haystead T.A., Donoghue M., Breitbart R.E., Acton S.L., Rockman H.A., Coffman T.M. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J. Clin. Invest. 2006;116:2218–2225. doi: 10.1172/JCI16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 51.Wong D.W., Oudit G.Y., Reich H., Kassiri Z., Zhou J., Liu Q.C., Backx P.H., Penninger J.M., Herzenberg A.M., Scholey J.W. Loss of angiotensin-converting enzyme-2 (ACE2) accelerates diabetic kidney injury. Am. J. Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas M.C., Pickering R.J., Tsorotes D., Koitka A., Sheehy K., Bernardi S., Toffoli B., Nguyen-Huu T.P., Head G.A., Fu Y., Chin-Dusting J., Cooper M.E., Tikellis C. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ. Res. 2010;107:888–897. doi: 10.1161/CIRCRESAHA.110.219279. [DOI] [PubMed] [Google Scholar]
- 53.Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J., Godbout K., Parsons T., Baronas E., Hsieh F., Acton S., Patane M., Nichols A., Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 54.Ferrario C., Brosnihan K., Diz D., Jaiswal N., Khosla M., Milsted A., Tallant E. Angiotensin-(1–7): a new hormone of the angiotensin system. Hypertension. 1991;18 doi: 10.1161/01.hyp.18.5_suppl.iii126. III-126–133. [DOI] [PubMed] [Google Scholar]
- 55.Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de Buhr I., Heringer-Walther S., Pinheiro S.V., Lopes M.T., Bader M., Mendes E.P., Lemos V.S., Campagnole-Santos M.J., Schultheiss H.P., Speth R., Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wysocki J., Ye M., Rodriguez E., Gonzalez-Pacheco F.R., Barrios C., Evora K., Schuster M., Loibner H., Brosnihan K.B., Ferrario C.M., Penninger J.M., Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J., Noble N.A., Border W.A., Huang Y. Infusion of angiotensin-(1–7) reduces glomerulosclerosis through counteracting angiotensin II in experimental glomerulonephritis. Am. J. Physiol. Renal Physiol. 2010;298:F579–F588. doi: 10.1152/ajprenal.00548.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bharadwaj M.S., Strawn W.B., Groban L., Yamaleyeva L.M., Chappell M.C., Horta C., Atkins K., Firmes L., Gurley S.B., Brosnihan K.B. Angiotensin-converting enzyme 2 deficiency is associated with impaired gestational weight gain and fetal growth restriction. Hypertension. 2011;58:852–858. doi: 10.1161/HYPERTENSIONAHA.111.179358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia H., Suda S., Bindom S., Feng Y., Gurley S.B., Seth D., Navar L.G., Lazartigues E. ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS One. 2011;6:e22682. doi: 10.1371/journal.pone.0022682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Timmermans P.B., Wong P.C., Chiu A.T., Herblin W.F., Benfield P., Carini D.J., Lee R.J., Wexler R.R., Saye J.A., Smith R.D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol. Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 62.Crowley S.D., Tharaux P.L., Audoly L.P., Coffman T.M. Exploring type I angiotensin (AT1) receptor functions through gene targeting. Acta Physiol. Scand. 2004;181:561–570. doi: 10.1111/j.1365-201X.2004.01331.x. [DOI] [PubMed] [Google Scholar]
- 63.Burson J.M., Aguilera G., Gross K.W., Sigmund C.D. Differential expression of angiotensin receptor 1A and 1B in mouse. Am. J. Physiol. 1994;267:E260–E267. doi: 10.1152/ajpendo.1994.267.2.E260. [DOI] [PubMed] [Google Scholar]
- 64.Davisson R.L., Oliverio M.I., Coffman T.M., Sigmund C.D. Divergent functions of angiotensin II receptor isoforms in the brain. J. Clin. Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito M., Oliverio M.I., Mannon P.J., Best C.F., Maeda N., Smithies O., Coffman T.M. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crowley S.D., Gurley S.B., Oliverio M.I., Pazmino A.K., Griffiths R., Flannery P.J., Spurney R.F., Kim H.S., Smithies O., Le T.H., Coffman T.M. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin–angiotensin system. J. Clin. Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crowley S.D., Gurley S.B., Herrera M.J., Ruiz P., Griffiths R., Kumar A.P., Kim H.S., Smithies O., Le T.H., Coffman T.M. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gurley S.B., Riquier-Brison A.D., Schnermann J., Sparks M.A., Allen A.M., Haase V.H., Snouwaert J.N., Le T.H., McDonough A.A., Koller B.H., Coffman T.M. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levine D.Z., Iacovitti M., Buckman S., Burns K.D. Role of angiotensin II in dietary modulation of rat late distal tubule bicarbonate flux in vivo. J. Clin. Invest. 1996;97:120–125. doi: 10.1172/JCI118378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peti-Peterdi J., Warnock D.G., Bell P.D. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J. Am. Soc. Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 71.Hall J.E. Control of sodium excretion by angiotensin II: intrarenal mechanisms and blood pressure regulation. Am. J. Physiol. 1986;250:R960–R972. doi: 10.1152/ajpregu.1986.250.6.R960. [DOI] [PubMed] [Google Scholar]
- 72.Oliverio M.I., Delnomdedieu M., Best C.F., Li P., Morris M., Callahan M.F., Johnson G.A., Smithies O., Coffman T.M. Abnormal water metabolism in mice lacking the type 1A receptor for Ang II. Am. J. Physiol. Renal Physiol. 2000;278:F75–F82. doi: 10.1152/ajprenal.2000.278.1.F75. [DOI] [PubMed] [Google Scholar]
- 73.Stegbauer J., Gurley S.B., Sparks M.A., Woznowski M., Kohan D.E., Yan M., Lehrich R.W., Coffman T.M. AT1 receptors in the collecting duct directly modulate the concentration of urine. J. Am. Soc. Nephrol. 2011;22:2237–2246. doi: 10.1681/ASN.2010101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wan Y., Wallinder C., Plouffe B., Beaudry H., Mahalingam A.K., Wu X., Johansson B., Holm M., Botoros M., Karlen A., Pettersson A., Nyberg F., Fandriks L., Gallo-Payet N., Hallberg A., Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J. Med. Chem. 2004;47:5995–6008. doi: 10.1021/jm049715t. [DOI] [PubMed] [Google Scholar]
- 75.Grady E., Sechi L., Griffn C., Schambelan M., Kalinyak J. Expression of AT2 receptors in the developing rat fetus. J. Clin. Invest. 1991;88:921–933. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Millan M., Carvallo P., Izumi S.-I., Zemel S., Catt K., Aguilera G. Novel sites of expression of functional angiotensin II receptors in the late gestation fetus. Science. 1989;244:1340–1342. doi: 10.1126/science.2734613. [DOI] [PubMed] [Google Scholar]
- 77.Ichiki T., Kambayashi Y., Inagami T. Multiple growth factors modulate mRNA expression of angiotensin II type-2 receptor in R3T3 cells. Circ. Res. 1995;77:1070–1076. doi: 10.1161/01.res.77.6.1070. [DOI] [PubMed] [Google Scholar]
- 78.Lucius R., Gallinat S., Rosenstiel P., Herdegen T., Sievers J., Unger T. The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J. Exp. Med. 1998;188:661–670. doi: 10.1084/jem.188.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hein L., Barsh G.S., Pratt R.E., Dzau V.J., Kobilka B.K. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- 80.Ichiki T., Labosky P.A., Shiota C., Okuyama S., Imagawa Y., Fogo A., Niimura F., Ichikawa I., Hogan B.L., Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 81.Habashi J.P., Doyle J.J., Holm T.M., Aziz H., Schoenhoff F., Bedja D., Chen Y., Modiri A.N., Judge D.P., Dietz H.C. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masaki H., Kurihara T., Yamaki A., Inomata N., Nozawa Y., Mori Y., Murasawa S., Kizima K., Maruyama K., Horiuchi M., Dzau V.J., Takahashi H., Iwasaka T., Inada M., Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J. Clin. Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaschina E., Grzesiak A., Li J., Foryst-Ludwig A., Timm M., Rompe F., Sommerfeld M., Kemnitz U.R., Curato C., Namsolleck P., Tschope C., Hallberg A., Alterman M., Hucko T., Paetsch I., Dietrich T., Schnackenburg B., Graf K., Dahlof B., Kintscher U., Unger T., Steckelings U.M. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin–angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 84.McCarthy C.A., Vinh A., Callaway J.K., Widdop R.E. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke. 2009;40:1482–1489. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- 85.Gelosa P., Pignieri A., Fandriks L., de Gasparo M., Hallberg A., Banfi C., Castiglioni L., Turolo L., Guerrini U., Tremoli E., Sironi L. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J. Hypertens. 2009;27:2444–2451. doi: 10.1097/HJH.0b013e3283311ba1. [DOI] [PubMed] [Google Scholar]
- 86.Rompe F., Artuc M., Hallberg A., Alterman M., Stroder K., Thone-Reineke C., Reichenbach A., Schacherl J., Dahlof B., Bader M., Alenina N., Schwaninger M., Zuberbier T., Funke-Kaiser H., Schmidt C., Schunck W.H., Unger T., Steckelings U.M. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappab. Hypertension. 2010;55:924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 87.Nataraj C., Oliverio M.I., Mannon R.B., Mannon P.J., Audoly L.P., Amuchastegui C.S., Ruiz P., Smithies O., Coffman T.M. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J. Clin. Invest. 1999;104:1693–1701. doi: 10.1172/JCI7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jurewicz M., McDermott D.H., Sechler J.M., Tinckam K., Takakura A., Carpenter C.B., Milford E., Abdi R. Human T and natural killer cells possess a functional renin–angiotensin system: further mechanisms of angiotensin II-induced inflammation. J. Am. Soc. Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 89.Guzik T.J., Hoch N.E., Brown K.A., McCann L.A., Rahman A., Dikalov S., Goronzy J., Weyand C., Harrison D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crowley S.D., Song Y.S., Lin E.E., Griffiths R., Kim H.S., Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1089–R1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vinh A., Chen W., Blinder Y., Weiss D., Taylor W.R., Goronzy J.J., Weyand C.M., Harrison D.G., Guzik T.J. Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537. doi: 10.1161/CIRCULATIONAHA.109.930446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crowley S.D., Song Y.S., Sprung G., Griffiths R., Sparks M., Yan M., Burchette J.L., Howell D.N., Lin E.E., Okeiyi B., Stegbauer J., Yang Y., Tharaux P.L., Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marvar P.J., Thabet S.R., Guzik T.J., Lob H.E., McCann L.A., Weyand C., Gordon F.J., Harrison D.G. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ. Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muller D.N., Shagdarsuren E., Park J.K., Dechend R., Mervaala E., Hampich F., Fiebeler A., Ju X., Finckenberg P., Theuer J., Viedt C., Kreuzer J., Heidecke H., Haller H., Zenke M., Luft F.C. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am. J. Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crowley S.D., Frey C.W., Gould S.K., Griffiths R., Ruiz P., Burchette J.L., Howell D.N., Makhanova N., Yan M., Kim H.-S., Tharaux P.-L., Coffman T.M. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am. J. Physiol. Renal Physiol. 2008;295:F515–F524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kvakan H., Kleinewietfeld M., Qadri F., Park J.K., Fischer R., Schwarz I., Rahn H.P., Plehm R., Wellner M., Elitok S., Gratze P., Dechend R., Luft F.C., Muller D.N. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782. [DOI] [PubMed] [Google Scholar]
- 97.Barhoumi T., Kasal D.A., Li M.W., Shbat L., Laurant P., Neves M.F., Paradis P., Schiffrin E.L. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57:469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]