Abstract
That mutations in the SOD1 enzyme underlie familial form of the motor neuron disease ALS is clear. But there seems to be more than one answer to the question of what are the consequences of such mutations.
Amyotrophic lateral sclerosis (ALS) is a neurological disorder that is characterized by selective, premature degeneration and death of motor neurons, which control voluntary actions such as breathing and walking. It initiates in mid-adult life, and the ensuing progressive paralysis is typically fatal within a few years, mainly due to a failure of the respiratory system. Reporting in Genes & Development, Nishitoh et al.1 provide a new insight into molecular events that provoke ALS, linking pathogenesis to a well established stress pathway within the endoplasmic reticulum.
Although most ALS cases are sporadic, in roughly 10% of instances, the disease is dominantly inherited — that is, carrying even one copy of disease-associated mutation is sufficient to cause fatal paralysis. A landmark discovery2 in 1993 initiated the molecular era of ALS research by finding that mutations in the gene encoding superoxide dismutase 1 (SOD1) are responsible for 20% of inherited ALS cases. (Normal SOD1 is an important intracellular antioxidant, as it facilitates the clearance of the potentially toxic superoxide radical.)
Mice expressing various ALS-related mutants of SOD1 have recapitulated the fatal paralysis seen in human patients, and studies in these animals have established that ALS is caused by one or more acquired toxic effects of SOD1 mutation, rather than by reduced activity of this enzyme3. Selective silencing of the mutant gene in specific cell types has also proven that the effects of the ubiquitously expressed mutant SOD1 include damage within motor neurons and their non-neuronal, neighbouring cells. Whereas damage within motor neurons is associated with ALS onset, damage within astrocytes and microglia — the innate immune cells of the spinal cord — sharply accelerates disease progress4,5. But it is a sobering reality that, 15 years after the discovery of SOD1 mutation in familial ALS, no consensus has yet emerged as to the direct molecules and pathways affected by the toxicity of mutant SOD1 within motor neurons or their neighbours.
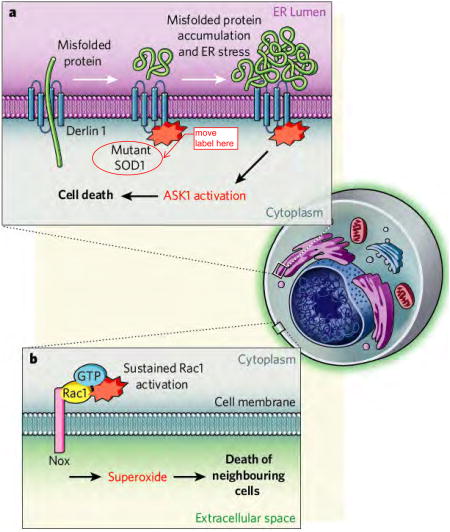
Nishitoh and colleagues1 now show that mutant SOD1 inhibits ERAD, the cell's machinery for eliminating proteins that fail to fold properly in the endoplasmic reticulum (ER). The first crucial step for ‘ERADication’ of misfolded proteins is their transport from the lumen of the ER to the cytoplasm, where they are marked by covalent attachment of ubiquitin, a signal for their degradation by the proteasome protein complex.
Nishitoh et al. find that three different SOD1 mutants, but not the normal enzyme, can interact with an ERAD component, Derlin-1, which is instrumental both for dislocation and degradation of misfolded proteins. By binding to Derlin-1, mutant SOD1 inhibits the ERAD pathway, causing both improper functioning of the ER (ER stress), and activation of a kinase enzyme called ASK1, which was already known to be involved in driving programmed cell death. These observations provide a putative, direct molecular link between mutations in SOD1 and a main pathological hallmark of ALS, the presence of ubiquitinated inclusions in the cytoplasm.
It is not known exactly in which cells Derlin-1 and mutant SOD1 interact, although the likely candidates are astrocytes, and not motor neurons, because deletion of the ASK1 gene extended survival of an SOD1-mutant mouse by slowing disease progress, without affecting its onset. But a perplexing finding is that interaction between Derlin-1 and mutant SOD1 is not detectable before the disease symptoms appear, suggesting that, in vivo, it is secondary to some unidentified initiating event.
Nishitoh and colleagues' observations follow several other — quite different — recent findings. Earlier this year, it was reported6 that both normal and mutant SOD1 associate with Rac1, an activator of the enzyme NADPH oxidase (Nox) at the cell membrane.
Normally, Nox mediates superoxide production — for example, in immune cells, such as microglia, to kill bacteria and other pathogens. It emerges that association of normal SOD1 with Rac1 is part of a tightly regulated mechanism that, under reducing conditions, activates Nox. But mutant SOD1 interacts with Rac1 with a higher affinity, ‘locking’ Nox in its active, superoxide-producing form, even under oxidizing conditions. This results in some tenfold increase in superoxide production and its release into the extracellular space.
Paradoxically, therefore, instead of its normal job of removing intracellular superoxide, mutant SOD1 might increase extracellular levels of this radical and so damage motor neurons or other cell types. A Nox inhibitor improved survival of a SOD1-mutant mouse almost exclusively by slowing the onset of ALS (ref. 6). Since mutant SOD1 within motor neurons, but not microglia, is a determinant of timing of disease onset, this finding suggests that the important interaction between mutant SOD1 and Rac1 in provoking disease is activation of the Nox variant (Nox1) in motor neurons, rather than activation of a different variant (Nox2) within microglia.
In ER stress1 and increased production of superoxide6 — both potential triggers of death of motor neurons or their neighbouring cells — do we have the whole story of molecular events underlying ALS? Almost certainly, not. One proposal, based on observations in SOD1-mutant mice and in samples obtained from patients with sporadic ALS, is that a component of damage arises from excitotoxicity. The latter is an excessive firing of motor neurons when the stimulatory neurotransmitter glutamate is not rapidly removed from synaptic junctions. Excessive stimulation of the neurons' glutamate receptors and the accompanying increase in calcium influx, can trigger a cascade of toxic events in the postsynaptic neuron, including damage to mitochondria (the cell's powerhouses and along with the ER the major gatekeepers of intracellular calcium). Loss of the glutamate transporter EAAT2, a synaptic vacuum cleaner for glutamate, from the neighbouring astrocytes contributes to this phenomenon7, but a direct — if any — role of mutant SOD1 in this mechanism remains unclear.
Also, mutant SOD1 has been proposed to directly interact with chromogranins8 (components of neurosecretory vesicles), thereby leading unexpectedly to co-secretion of chromogranins with mutant SOD1, which in turn may trigger damage originating on the outside of either motor neurons or their neighbouring cells.
There is more. Mitochondrial dysfunction has long been implicated in damaging motor neurons in ALS. Misfolded mutant SOD1 is deposited onto the cytoplasmic face of the outer mitochondrial membrane in the spinal cord9. Moreover, mitochondrial dysfunction in motor neurons10 carrying SOD1 mutations is associated with the release of cytochrome c, one of the most potent stimuli of cell death. Finally, mutant SOD1 provokes oxidative stress in mitochondria within astrocytes11, which could accelerate death of the neighbouring neurons.
With all these potential contributors, pathogenesis in inherited ALS might strike some as “curiouser and curiouser”, much as Alice proclaimed of strange happenings in “Alice in Wonderland”. Among the biggest challenges will be to distinguish mechanisms that cause this disease from those that are only epiphenomena. The likeliest possibility in our view is that every set of observations discussed here may be partly right, with age dependent, selective toxicity requiring a convergence of damage to motor neurons and non-neurons alike. So stay tuned: the main pieces of this mystifying puzzle are yet to be revealed.
Figure. Proposed mechanisms of toxicity in SOD1-mediated ALS.
(A) ER-stress: Mutant SOD1 was shown1 to interact with Derlin1, a protein that plays a major role in the transport of misfolded proteins from the endoplasmic reticulum (ER) lumen to the cytosol. This mutant SOD1-Derlin1 interaction results in accumulation of misfolded proteins in the ER-lumen and thereby ER-stress. The latter provokes activation of apoptosis signal-regulating kinase 1 ASK1 by a yet unidentified mechanism. It is not known exactly in which cells Derlin-1 and mutant SOD1 interact. (B) Superoxide production: A strong interaction of mutant SOD1 with Rac1 was recently demonstrated6 to result in keeping Nox in the activating stage. This led to the production of increased amounts of superoxide in the extracellular space that is toxic for neighboring cells. While the Nox pathway is intrinsically active in both motor neurons and glia, the disease relevant cellular site of the Rac1/SOD1 interaction remains unclear.
References
- 1.Nishitoh H, et al. Genes Dev. 2008;22:1451–1464. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen DR, et al. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Bruijn LI, et al. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 4.Boillee S, et al. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka K, et al. Nature Neurosci. 2008;11:251–3. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harraz MM, et al. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 8.Urushitani M, et al. Nature Neurosci. 2006;9:108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 9.Vande Velde C, Miller TM, Cashman NR, Cleveland DW. Proc Natl Acad Sci USA. 2008;105:4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pehar M, et al. J Neurosci. 2007;27:7777–7785. doi: 10.1523/JNEUROSCI.0823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassina P, et al. J Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]