Abstract
Laboratory mice constitute an extensively used model to study the pathologic and functional outcomes of cerebral ischemic stroke. The middle cerebral artery occlusion (MCAO) model requires surgical intervention, which potentially can result in postsurgical pain and stress. In the present study, we investigated whether buprenorphine and meloxicam, at clinically relevant doses provided pain relief without altering infarct volume in male C57BL/6 mice. Common known side-effects of buprenorphine, including decreased food consumption, were noted after surgery in buprenorphine-treated mice, but these effects were brief and seen only during the treatment period. Fecal corticosterone metabolites did not differ significantly between the groups. In the present study, buprenorphine treatment did not alter infarction volume when compared with that of mice that did not receive analgesia. In contrast, meloxicam treatment significantly reduced infarct volume and may be a confounder if used as an analgesic during MCAO surgery. Furthermore, investigation of behavioral profiles by using an automated behavioral scoring system showed that rearing and sniffing behaviors decreased as infarct volume increased. This suggests that studies of exploratory behavior may aid in developing new markers of short-term stroke-related behavioral deficiencies in laboratory mice.
Abbreviations: FCM, fecal corticosterone metabolites; MCAO, middle cerebral artery occlusion; PC, principal component
In many countries, the recorded incidence of cerebral stroke is high.15 The primary cause of ischemic stroke in humans is obstruction of a cerebral artery, which results in infarction of a localized part of the brain. Because stroke is a vascular disease and because of the complexity of the brain's response to injury, no in vitro systems can presently replace the use of in vivo models in stroke research.23 A frequently used animal model of stroke is the middle cerebral artery occlusion (MCAO). This model resembles the embolic stroke seen in humans63 and results in infarction in the cerebral cortex and caudate putamen.
The use of analgesia in stroke models has been neglected. Pain-sensitive structures in the brain are limited to the cerebral and dural arteries; cranial nerves V, IX, and X; and parts of the dura at the base of the brain;68 these structures rarely are damaged during ischemic stroke. However, in surgical stroke models, the incision through the skin and muscles and damage to the skull and periosteum are likely to cause pain, given that nociceptors are present in these tissues.45 Currently, limited information exists on the degree of pain and stress in laboratory mice after damage to structures affected during surgery to occlude the middle cerebral artery (MCAO model). Even routine procedures can increase corticosterone levels in laboratory mice,5 and anesthesia alone activates the hypothalamic–pituitary–adrenal axis and the sympathoadrenal system.3,65 Therefore, surgical induction of stroke is likely to cause both pain and stress. Stress affects the cardiovascular and metabolic systems and many components of the immune system7 and may significantly alter research outcomes. In the CNS, acute and chronic stress may have structural and neurophysiologic consequences in hippocampus, amygdala, prefrontal cortex, and the paraventricular nucleus of the hypothalamus (for review, see reference 34). Furthermore, pain and stress may increase interanimal variation,37 thereby increasing the risk of type II errors as more animals are needed to obtain statistically significant results of sufficient power.
One of the main concerns with using analgesia in experimental models is the possible risk of the drug confounding the research.55,60 This concern has limited the use of analgesia in several murine models. Studies investigating the neuroprotective effects of high doses of NSAID4 or the neurotoxicity of high doses of opioids42 have fostered the idea that the analgesia may bias experimental results. However, in the studies cited,4,42 analgesic doses much higher than recommended clinical doses were used. Furthermore, most of the studies investigating the effects of analgesia on stroke outcome evaluated brain injury only hours after surgery. However, the short-term consequences of neuroprotection or neurotoxicity may diminish in long-term studies, as has been seen with isoflurane, for example.40,69 In a recent study, buprenorphine was effective in reducing pain in a rat model of global ischemic stroke, and the analgesic treatment did not hamper histologic evaluation of the experimental outcome.37 Whether analgesia at clinically relevant doses is beneficial, detrimental, or without effect on stroke outcome in the laboratory mouse is unknown.23
In the present study, we determined whether 2 analgesic treatments—buprenorphine and meloxicam—at doses clinically relevant in laboratory animal experimentation19,20 could be used to refine a well-validated MCAO model without adverse effects on infarct volume. Both analgesics are commonly used in laboratory animal experimentation.18 Buprenorphine is a highly potent opioid that acts as a partial agonist of the μ receptor subtype and that has reduced pain in laboratory rodents experiencing mildly or moderately invasive surgical procedures.19 The recommended dosage of buprenorphine is by subcutaneous injection every 8 h,20 but analgesia can also be provided by letting mice voluntarily consume the drug.32,38 Meloxicam is a potent inhibitor of cyclooxygenase 2 and has analgesic, antiinflammatory, and antipyretic properties.17
Analgesiometric studies58 have been used frequently to evaluate the clinical effects of analgesia. However, the knowledge obtained from analgesiometric studies may have little relevance in the clinical evaluation of postsurgery pain because the pathology of the pain stimulated in these tests is not comparable to that of postoperative pain.10,58 Clinical studies evaluating the pain of specific surgical procedures are therefore important to refine analgesic treatment and to ensure optimal postsurgical welfare.2 We here evaluated the pain-related response to the MCAO procedure in mice by measuring food consumption, body weight, and fecal corticosterone metabolites (FCM). Previous studies have demonstrated that these parameters may be useful indicators of postsurgical pain in mice,33,70 because surgical procedures induce the release of glucocorticoids to stimulate the hypothalamic–pituitary–adrenal axis and because proinflammatory cytokines affect basic behaviors, such as feeding.24,41 Furthermore, several studies have demonstrated that surgical procedures such as vasectomy, partial hepatectomy, and removal of the mammary fat pad result in behavioral changes indicative of pain in mice.2,33,64 In the present study, behavioral profiles were recorded by using HomeCageScan computer software.59 This methodology has previously been validated in laboratory mice as an effective tool to obtain reliable information on behavioral profiles.14,59 We used behavioral analysis to assess responses to the surgical procedure and the various analgesic treatments and was repeated 8 d later to evaluate behavioral changes due to the infarction. Human patients who survive cerebral stroke often experience various types of pain such as central poststroke pain or shoulder pain, months to years after the incident.36 However, because the pain in these patients is multifactorial in origin and usually develops over considerable time, the assessment plan we used was designed only to investigate surgically induced pain.
We evaluated the potential effect of analgesia on histologic outcome by estimating infarction volume after MCAO in mice.
Materials and Methods
The experiments performed in this study were approved by the Animal Experiments Inspectorate under the Danish Ministry of Justice (license number 2006/561 to 1263). All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals31 in a fully AAALAC-accredited facility.
Animals.
Male C57BL/6 mice (n = 60; age, 8 to 10 wk; Taconic, Ry, Denmark) were used in the study. Mice were vendor-designated as SPF for mouse hepatitis virus, mouse minute virus, mouse parvovirus, mouse rotavirus, encephalomyelitis virus, pneumonia virus of mice, Sendai virus, lymphocytic choriomeningitis virus, murine norovirus, ectromelia virus, Hantaan virus, mouse adenovirus types 1 and 2, mouse cytomegalovirus, respiratory enteric virus III, K virus, lactic dehydrogenase elevating virus, polyoma virus, thymic virus, β-hemolytic Streptococcus spp., Bordetella broncoseptica, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Klebsiella oxytoca, Klebsiella pneumonia, Mycoplasma spp., Pasteurella pneumotropica, other Pasteurella spp., Pseudonomonas aeruginosa, Salmonella spp., Staphylococcus aureus, Streptococcus pneumonia, cilia-associated respiratory bacillus, Helicobacter hepaticus, Helicobacter bilis, Pneumocystis carinii, and endo- and ectoparasites. The mice were housed individually on arrival at the facility and acclimated to the new surroundings for at least 7 d before entering the study. Individual housing was used in the experimental setup to enable individual measurements of food consumption and fecal corticosterone levels and to control the dosing of buprenorphine in the voluntary ingestion strategy. Mice were housed in polycarbonate cages (Tecniplast, Varese, Italy) with food pellets (no. 1319, Altromin, Lage, Germany) and acidified tap water provided ad libitum. Wood chips (Tapvei Oy, Kortteinen, Finland) were used as bedding. Bite bricks (Tapvet, Tapvei, Kortteinen, Finland), a small handful of nesting material (about 13 to 16 g; Enviro-dri , Shepherd Specialty Papers, Milford, NJ), and a cardboard house (Brogaarden, Gentofte, Denmark) were provided as environmental enrichment. Room temperature was maintained at 20 ± 2 °C, air humidity was within the range of 30% to 60%, and lighting was regulated as a 12:12-h light:dark cycle (lights on, 0630).
Groups.
The mice were randomly divided into 6 groups of 10 mice each. One group (ANEST) underwent isoflurane anesthesia only. Another group (SHAM) underwent the same anesthesia and surgical manipulations (except for occlusion of the middle cerebral artery) as did those that underwent the MCAO procedure.
The remaining 40 mice underwent MCAO. One group (MNA) of these mice were not given any analgesia except for local analgesia at the incision site; 10 mice (MMEL) were given meloxicam (5 mg/kg SC; Metacam, Boehringer Ingelheim Vetmedica, St Joseph, MO) about 1 h before surgery and again at 24 h after the MCAO procedure; 10 mice (MBUPsc) received subcutaneous buprenorphine (0.05 mg/kg; Temgesic, Schering-Plough Europe, Brussels, Belgium) at 1 h before and again at 8, 16, 24, 32 and 48 h after surgery; and 10 mice (MBUPvol) were provided buprenorphine by voluntary ingestion as described previously.1,32 In short, 2 d before surgery, these mice were habituated to chocolate–hazelnut spread by offering them a small amount of the spread on a piece of adhesive tape placed 2 cm above bedding height. The mice then received 0.5 mg/kg buprenorphine as treated spread (5 g/kg in Nutella [Ferrero, Pino Torinese, Italy]) on the day of surgery and again 24 h later. All mice ate the entire dose within minutes after administration.
All mice were euthanized 8 d after the procedure.
Surgery.
Surgery was performed between 0900 and 1230. The same person anesthetized and performed surgery on all mice, in a randomized order. The surgeon was well experienced with the procedures and blinded to the analgesic treatment schedule. Mice were placed in an induction chamber, and anesthesia was induced with 5% isoflurane (Forene, Abbot Scandinavia, Stockholm, Sweden) delivered in pure oxygen. Once the paw withdrawal reflex was absent, mice were shaved at the incision site and attached to an anesthetic face mask for spontaneous respiration. Isoflurane was maintained at approximately 2.5% to ensure adequate anesthesia. Mice were placed on a heating pad (HB101, Panlab, Cornella, Spain), and rectal body temperature was maintained at 36 to 38 °C. The skin was disinfected with 80% ethanol. All mice that underwent surgery received subcutaneous lidocaine (10 mg/mL, Glostrup Apotek, Glostrup, Denmark) at the incision site before the incision was made through the skin. The skin incision was made through the temporal muscle between the lateral part of the orbit and the external auditory meatus. A hole (diameter, 1 to 2 mm) was drilled directly over the distal part of the right MCA, the dura mater was removed, and the MCA was occluded by applying bipolar forceps coupled to an electrosurgical unit (ERBE VIO 100 C, Mediplast NC Nielsen, Balling, Denmark). The skin was closed with interrupted sutures of 6-0 polyglactin 910 (Vicryl, Ethicon, St Stevens Woluwe, Belgium). The time of the procedure, from induction of anesthesia to regaining righting reflex was 10 to 15 min. The mice were allowed to recover in a quiet room, in which filming of the behavioral analyses was performed. Anesthetized mice without surgery (ANEST group) were anesthetized by using 5% isoflurane delivered in pure oxygen for induction and were maintained for 10 to 15 min at 2.5% isoflurane in oxygen. Sham surgery consisted of the same surgical approach as the MCAO procedure, except for coagulation of the MCA.
Immediately after the procedure, 2 mice in each experimental group underwent intracardiac blood sampling during anesthesia. Blood samples were analyzed for pH, pCO2, pO2, sO2, Hgb, Hct, K+, Na+, Ca2+, glucose, lactate, HCO3–, and base excess (ABL 700, Radiometer A/S, Brønshøj, Denmark). After blood samples were obtained, anesthetized mice were euthanized by cervical dislocation.
Data collection.
Data were obtained daily between 0800 and 1030. For each mouse, body weight, food consumption, body temperature, and amount of FCM were recorded for 3 d before the procedures and for 7 d after surgery or anesthesia. Daily food consumption was calculated by subtracting the measured weight of food from the amount measured the previous day. Body temperatures were obtained by using a temperature probe (BAT12, Physitemp Instruments, Clifton, NJ). At the time of weighing, all bedding was removed from each cage and frozen at −21 °C, for later separation and collection of fecal pellets. The nesting material, mouse hut, and cage were reused for each mouse throughout the experiment to maintain individual olfactory signals within the mouse's environment, thereby minimizing stress associated with bedding change. FCM were quantified as described previously.39,61 In short, corticosterone metabolites were extracted by incubating feces in 96% ethanol (5 mL/g feces) overnight. FCM levels were analyzed in duplicate by using DRG-Diagnostics Corticosterone ELISA (EIA4164, DRG Instruments, Marburg, Germany) in accordance with the manufacturer's instructions. Standards included in the kit were replaced by a custom 9-point standard curve prepared in 96% ethanol from analytical-grade corticosterone (catalog no. 46148, Sigma-Aldrich, St Louis, MO) in concentrations from 50 to 0.19 ng/mL. The kit has been verified to have a crossreactivity equivalent of 7.4% with progesterone, 3.4% with deoxycorticosterone, 1.6% with 11-dehydrocorticosterone, 0.3% with cortisol and pregnenolone, and less than 0.1% with other steroids. The absorbencies were recorded at 450 nm (Thermo Fisher Scientific, Waltham, MA). Results are presented as total nanograms FCM excreted per 24 h.26
Behavioral observations were obtained by video recording with digital cameras (GZ MG6800, JVC, Yokohama, Japan). The camera was fixed on a tripod and placed so that the entire cage could be seen at all times. At 10 min before video recording, all enrichment items were removed so the mouse could be fully viewed at all times. Each mouse was filmed for 20 min. On the day of surgery the filming began 4 h after the mice had regained the righting reflex.
On day 8 after surgery, mice were moved to the filming area at 0730. Mice were allowed to acclimate to the room for 2 h, enrichment items were removed, and the 20-min videorecording session began 10 min later. For recording, one person entered the room, turned on the camera (one camera per cage), and then left the room. No other persons entered the room during the recording. Each video was analyzed at the end of the study by using the HomeCageScan system (CleverSys, Reston, VA).
Histologic preparation.
At 8 days after surgery, all mice were euthanized by intraperitoneal injection of pentobarbital (0.1 to 0.2 mL; 200 mg/mL). Lidocaine hydrochloride (20 mg/mL, Glostrup Apotek, Glostrup, Denmark) was added to the pentobarbital solution to reduce intraperitoneal pain due to the alkaline properties of the barbiturate. When the paw reflex was absent, mice were perfused transcardially with isotonic saline until blood was cleared from the circulation, after which 4% paraformaldehyde (VWR International, Leuven, Belgium) was infused for 10 min. Brains were postfixed in 4% paraformaldehyde for at least 24 h before being embedded in paraffin. At 400-µm intervals, 7 coronal slices (thickness, 4 µm) were made at each of 10 levels; 1 of each group of 7 slices was stained with hematoxylin and eosin.
Estimation of infarct volume.
Infarct volumes were estimated according to the Cavalieri principle,57 which states that the volume of any arbitrarily shaped object can be estimated by sectioning it into a set of parallel planes with known spacing and measuring the cross-sectional area ai of the object in each of the m planes. The estimated volume V is:
where t is the known distance between sections and m is the number of slices into which the object is sectioned. A blinded observer estimated cross-sectional areas by using a 2-dimensional nucleator on hematoxylin–eosin-stained coronal brain sections, and CAST-GRID software (Olympus Denmark A/S, Ballerup, Denmark).
Statistical analysis.
Data were analyzed by using PASW Statistics version 18 (SPSS, Chicago, IL) or Prism 5.01 (GraphPad Software, La Jolla, CA). P values less than 0.05 were considered significant. Data were tested for normality by using Q-Q-plots and the Shapiro–Wilks test, and log transformations were used when appropriate. Blood measurements were analyzed by one-way ANOVA with the Tukey posthoc test. Differences in food consumption, body weight, core body temperature, and FCM between groups were tested separately, both for effects across the entire studied period (7 d) by using repeated-measures ANOVA and for immediate effects after surgery (or anesthesia without surgery) on day 1 by using one-way ANOVA. To improve the models, average values of the variables for presurgical days (−3 to −1) were offered as explanatory covariates, the anesthetized-only mice were used as the reference category, FCM data were log-transformed, and, to avoid listwise exclusion of data, an expectation–maximization algorithm was used to replace missing values for repeated-measures ANOVA. All missing values could be considered as missing completely at random. Behavioral data were analyzed for latent variables by using principal component analysis. Differences between groups in duration in these latent behavioral trends were tested by using repeated-measures multivariate ANOVA.
When scored by the blinded observer, no mice that were anesthetized without surgery or underwent the sham procedure had infarcts. Therefore, only MCAO mice were included in the one-way ANOVA of log-transformed values for infarction volume.
Results
Blood measurements.
No differences were seen between treatment groups in pH, pCO2, pO2, sO2, Hgb, Hct, K+, Ca2+, glucose, lactate, HCO3–, or base excess. However Na+ differed significantly (F5,11 = 4.714, P = 0.043) between groups, with MBUPsc mice having lower (P = 0.037) levels compared with those in SHAM mice.
Food consumption, body weight, body temperature, and FCM.
Repeated-measures ANOVA revealed within-subjects effects of the different treatments on food intake (F30,246 = 5.1, P < 0.001), body weight (F30,246 = 2.8, P < 0.001), and body temperature (F30,246 = 1.7, P < 0.05). The within-subjects effects mainly occurred between the first day(s) after surgery and the overall experimental period. No significant between-subjects effects were found when the entire period was considered.
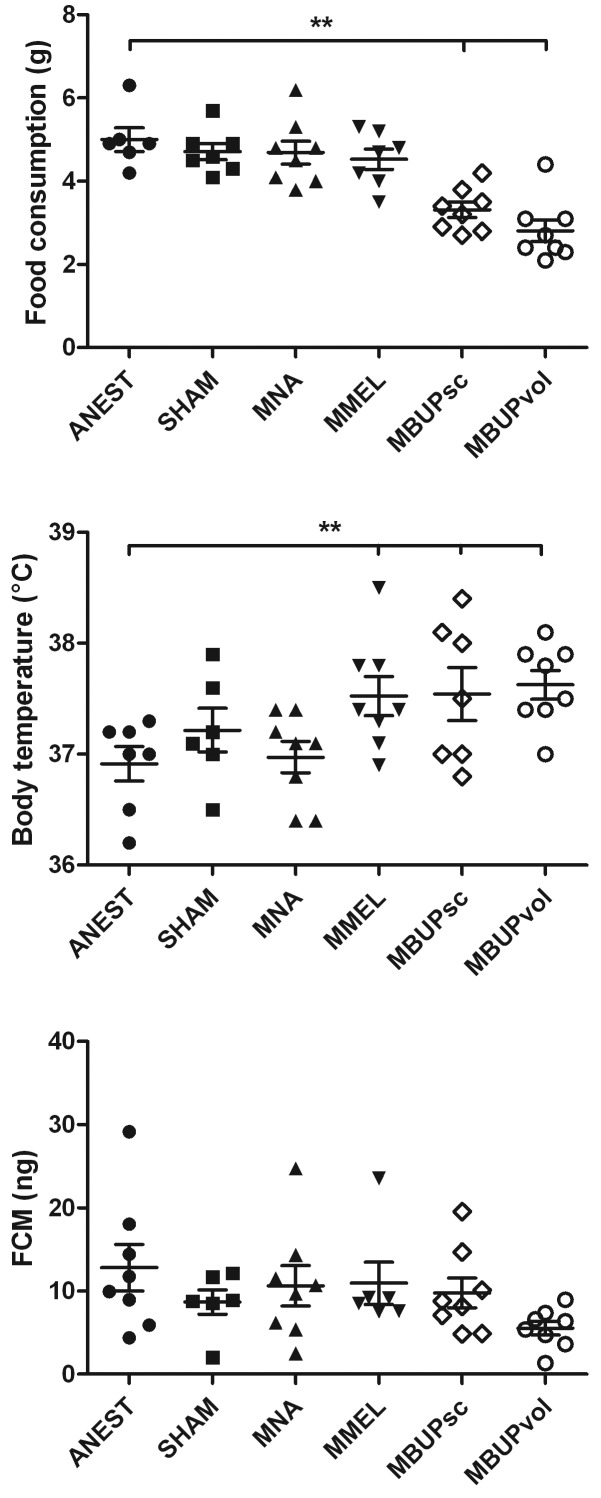
One-way ANOVA revealed differences on day 1 in food consumption (F5,41 = 9.7, P < 0.001), and body temperature (F5,41 = 4.6, P < 0.01). Buprenorphine-treated mice consumed less (P < 0.01 for both MBUPsc and MBUPvol) feed than did ANEST mice, and meloxicam- (MMEL) and buprenorphine- (MBUPsc and MBUPvol) treated mice exhibited significantly (P < 0.01 for all 3 groups) increased body temperatures compared with those of ANEST mice (Figure 1). The decrease in food consumption caused body weight on the first day after surgery to show a trend (F5,41 = 2.4, P = 0.052) toward being lower in the buprenorphine-treated mice than anesthesia-only mice. FCM on day 1 tended (F5,41 = 2.1, P = 0.08) to be lowest in MBUPvol mice (Figure 1).
Figure 1.
Food consumption, body temperature, and fecal corticosterone metabolites the day after isoflurane anesthesia only (ANEST), sham surgery (SHAM), or occlusion of the middle cerebral artery (M). Mice in the MNA group received no analgesia except lidocaine infiltration at the incision site; those in the MMEL group received meloxicam (5 mg/kg SC); mice in the MBUPsc received subcutaneous buprenorphine (0.05mg/kg); and those in the MBUPvol group received 0.5 mg buprenorphine mixed in 5 mg/kg chocolate–hazelnut spread for voluntary ingestion as analgesic treatment. Error bars, SEM; *, significant (P < 0.05) difference between groups.
Behavior.
The duration of 29 behaviors recorded reduced to 5 latent variables that explained 68.9% of the variance in the data (Table 1), by using principal components (PC) extraction with a varimax rotation. The extracted components could be described as collecting general activity (PC1), climbing behavior (PC2), rearing/exploratory behavior (PC3), low-activity behavior (PC4), and feeding/static behaviors (PC5). The durations of the behaviors did not differ significantly (F25,170 = 0.091, P = 0.99) between groups. However, when buprenorphine analgesia was used as an explanatory variable (both injections and voluntary ingestion) and infarct volume was included as a covariate, an explanatory model could be constructed. Buprenorphine had a between-subjects effect (F5,33 = 3.1, P < 0.05), whereas time (F5,33 = 4.8, P < 0.01) and infarct volume (infarction size × time: F5,33 = 3.6, P < 0.05) were found to assert within-subjects effects. Buprenorphine-treated mice exhibited more low-activity behavior soon (approximately 4 h) after surgery (F1,37 = 7.0, P < 0.01) than on day 8 (buprenorphine × time: F1,37 = 7.4, P < 0.01); in other words, the effect was present only while mice were receiving analgesia. Moreover, buprenorphine-treated mice exhibited increased feeding/reduced static behavior throughout the experiment (F1,37 = 4.5, P < 0.05). Infarct size exerted a within-subjects effect on rearing/exploratory behavior soon after surgery but not on day 8 (infarction size × time: F1,37 = 5.5, P < 0.05); the larger the infarct, the fewer the rearing and sniffing behaviors. Overall, all mice exhibited increased climbing behavior (effect of time: F1,37 = 8.5, P < 0.01) and decreased (effect of time: F1,37 = 5.4, P < 0.05) static behavior/increased feeding behavior on day 8 compared with soon after surgery.
Table 1.
Behavioral observations evaluated through principal component analysis
| Principal component explainable variable | Description | Behavioral categories | Approximately 4 h after surgery | Day 8 after surgery |
| 1 | General activity | RearUp, ComeDown, Walk, Turn, RemainRearUp, WalkSlow, ComeDowntoPartialRear, RearUpfromPartialRear, Jump | ||
| 2 | Climbing behavior | HangCuddled, HangVerticalfromHangCuddled, RemainHangVertical, RemainHangCuddled, HangVerticalfromRearUp, LandVertical | ANEST↑ | |
| SHAM↑ | ||||
| MNA↑ | ||||
| MMEL↑ | ||||
| MBUPsc↑ | ||||
| MBUPvol↑ | ||||
| 3 | Rearing/exploratory behavior | ComeDownfromPartialRear, RearUptoPartialRear, Sniff, RemainPartialRear | ||
| 4 | Low-activity behavior | Twitch, Sleep, Awaken, RemainLow | MBUPsc↑ | |
| MBUPvol↑ | ||||
| 5a | Feeding/reduced static behaviors | Eat, Groom, Drink, Stationary | MBUPsc↑ | ANEST↑ |
| MBUPvol↑ | SHAM↑ | |||
| MNA↑ | ||||
| MMEL↑ | ||||
| MBUPsc↑ | ||||
| MBUPvol↑ |
illustrates an increase in the PC variable.
The factor loadings in component 5 revealed a positive correlation between eating and drinking behavior (PC5 factor loadings: Eat, 0.795; Drink, 0.559), both of which were negatively correlated with grooming and stationary behavior (PC5 factor loadings: Groom, –0.649; Stationary, –0.485). The component was named Feeding/reduced static behaviors to illustrate the covered behaviors.
Infarct volume.
Infarct size was dependent on the postsurgical treatment (F3,28 = 3.371, P = 0.032), with MMEL mice presenting significantly (Tukey post hoc, P = 0.019) smaller infarcts than those in MCAO mice without analgesia (Figure 2).
Figure 2.
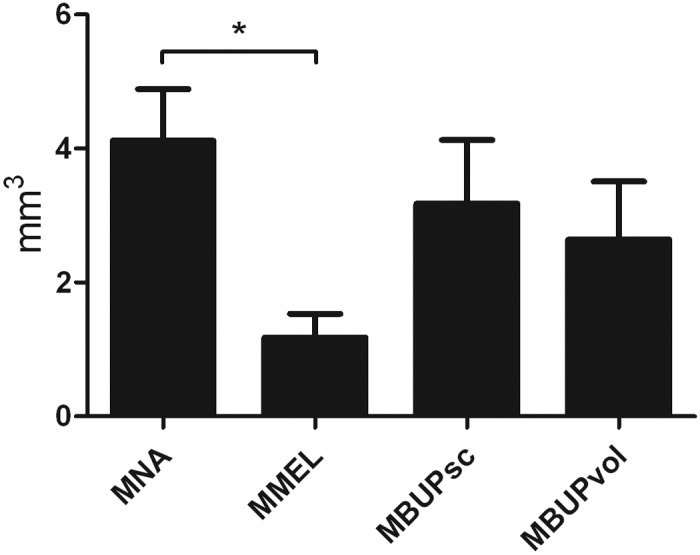
Infarct volume in mice that underwent MCAO. Mice in the MNA group received no analgesia except lidocaine infiltration at the incision site; those in the MMEL group received meloxicam (5 mg/kg SC); mice in the MBUPsc received subcutaneous buprenorphine (0.05mg/kg); and those in the MBUPvol group received 0.5 mg buprenorphine mixed in 5 mg/kg chocolate–hazelnut spread for voluntary ingestion as analgesic treatment. Error bars, SEM; *, significant (P < 0.05) difference between groups.
Discussion
The current study demonstrated that automatic scoring of behavior in mice by using commercially available software has the potential to be a relevant tool in stroke research. In particular, the analysis of the behavioral profiles of our mice showed that exploratory behavior was related to infarct size. Analgesia with meloxicam at a clinically relevant dose reduced infarct volume, whereas mice with MCAO and buprenorphine treatment did not differ significantly from MCAO mice without analgesia. This finding indicates that clinically relevant doses of buprenorphine can be used in male C57BL/6 mice that undergo MCAO without adverse effects on infarct volume. Furthermore, mice that received buprenorphine through the voluntary ingestion method tended to have the lowest levels of FCM.
The present study showed that food consumption, and consequently body weight, was lower in mice treated with buprenorphine the day after surgery than in other groups. This difference was not unexpected, because reduced food intake is a common side effect of the opioid.27 Analysis of food consumption across all days in the present study revealed no differences between groups. The same phenomenon was seen for body temperature: the day after surgery, mice that received buprenorphine or meloxicam analgesia had higher body temperatures than did anesthetized-only mice, but over the entire study period, there was no difference between the 6 groups. Human patients often show increases in body temperature after cerebral stroke54 and body temperature is known to be a major determinant of infarct size in animal models.49 However, only prolonged duration or biologically significant hyperthermia affects infarct volume,11 and given that mean values in all groups remained within the range of published reference values of body temperature for mice,53 the temperature increase in the analgesia-treated groups the day after surgery likely is clinically irrelevant. As mice rarely habituate to procedures,28,29 the temperature monitoring in the present study may have been a significant stressor to the mice and thereby may have biased the data obtained through repeated measurements. This stressor might also have affected FCM levels, thereby obscuring beneficial effects of reduced postsurgical stress in mice treated with analgesia. This potential effect may be apparent in the group of mice that received buprenorphine by the voluntary ingestion method, whose FCM tended to be (but were not quite significantly) lower than those of other groups for several days after the procedure. Because all mice that underwent surgery received local analgesia with lidocaine, pain perception in regard to the skin and muscle incision likely was inhibited. However, it is unlikely that lidocaine affected nociceptors in the periosteum of the skull. The lack of difference in FCM between groups therefore may reflect low sensitivity of this parameter, and therefore calls into question the reliability of FCM as biomarker of moderate pain in mice. Furthermore, given that variations in FCM measurements are often greater than those of blood levels of the hormones, larger group sizes may be needed to obtain sufficient statistical power for this parameter.
In mice, behavioral assessment of pain and stress is complicated by the species’ natural behavior as a prey animal and its adaption to situations that might compromise their welfare. Even when used to follow disease development and to characterize model-based behavioral parameters, behavioral testing requires large sample sizes and consistent testing conditions and may be highly influenced by sex, age, strain, time of observation, the surrounding environment, and so forth44,66 These constraints have led some authors to assume that behavioral observations are either too difficult to include as or too subjective to be part of scientific evaluations. However, several recent studies have demonstrated that behavioral observations were effective in assessing postsurgical pain, both when scored manually and with the use of computer software.33,59,70 In addition to being the only noninvasive parameter that gives immediate information regarding the welfare of the animals, behavioral parameters, when used correctly, are useful to describe model-induced changes. In 2009, the Stroke Therapy Academic Industry Roundtable recommended that behavioral analyses, as supplement to histologic or molecular outcomes, are essential for moving experimental therapies in laboratory animals forward to human trials.16 Several available behavioral tests, including the corner, cylinder, and adhesive-tape tests, have proven somewhat successful in differentiating the effects of brain ischemia,22,46 but the results obtained are highly variable.13 Furthermore, limited information exists on the correlation between histologic and behavioral test outcomes,47,67 and interpretation of cognitive data in stroke research may be problematic, because brain infarction is often associated with sensorimotor deficits that may be confounding factors in complex behavioral tests.13 Furthermore, because strain-associated differences in responses to pain assays51,52 and traditional behavioral testing,56 observations of natural, unprovoked behavior may be less biased and more generally representative of the animals’ condition and may therefore be a useful tool in characterizing the effects of cerebral infarction.
In the present study, we used an automated behavioral scoring system to obtain information on the behavioral profile at 4 h and 8 d after the induction of focal ischemic stroke. The behavioral analysis at 4 h after the procedure was used to assess the pain associated with the surgical procedure, whereas behavioral analyses at 8 d afterward were used to assess the behavioral consequences of ischemic stroke. The behavioral profiles analyzed through principal component analysis demonstrated that buprenorphine-treated mice exhibited more low-activity behavior (for example, sleeping) immediately after surgery than did other mice. This result may reflect the narcotic properties of buprenorphine, although other studies have demonstrated an opposite outcome.12,21 The increased inactive behavior that we noted may also indicate a quicker return to the normal circadian behavioral rhythm, in which inactivity and sleeping are highly prevalent during daytime.62 The analyses showed that infarct size was negatively correlated with the frequency of rearing/exploratory behavior after the operation: the larger the infarct, the fewer the rearing and sniffing behaviors that were noted. This information agrees well with results from rats with MCAO, which showed less rearing and exploratory activity in anxiety tests, such as the open field and elevated plus maze tests.25,67 When the behavioral profiles at 4 h and 8 d after surgery were compared, all mice exhibited increased climbing behavior, decreased static behavior, and increased feeding behavior with time. This result probably reflects increased spontaneous activity due to the 8-d recovery period.
Buprenorphine treatment by subcutaneous injection or voluntary ingestion did not affect the size of the infarct compared with that of mice that did not receive analgesia. This finding is in agreement with other studies in different animal models, showing that buprenorphine at clinical doses does not affect the outcome of the model.12,37,43 In contrast, clinical doses of meloxicam that were administered about 1 h before and 24 h after surgery reduced the volume of infarction. The infarction-reducing capacity of meloxicam likely is related to the cycloxygenase-2–inhibiting properties of the drug. Cyclooxygenase 2 is expressed mainly in cells involved with inflammation, and this enzyme catalyses the first step in the synthesis of prostanoids, which are important mediators of inflammation.50 The protective effects of meloxicam therefore may be due to its ability to inhibit the production of injurious prostaglandins. However, neuronal cells also express cyclooxygenase 2, which contributes to fundamental cerebral functions such as membrane consolidation and synaptic activity, and overexpression of cyclooxygenase 2 may be associated with neuron damage and neurodegeneration.50 In one study, the oxidative damage due to transient global cerebral ischemia was highly related to the expression of cyclooxygenase 2.6 Inhibition of cyclooxygenase 2 seems therefore neuroprotective, even though the results may vary depending on the type of brain damage and drug therapy used.30 Treatment with indomethacin and ibuprofen have previously been shown to reduce infarct size after MCAO with reperfusion but to increase infarct size after permanent occlusion of the artery,8,35 thus indicating that the beneficial effects of cyclooxygenase inhibition occurred during reperfusion. In the current study, meloxicam decreased the infarct volume even though the occlusions were permanent. However, vulnerability to cerebral ischemia differs between mice of various genetic backgrounds,9,48 and additional studies in other strains is warranted.
In conclusion, our current data suggest that buprenorphine, but not meloxicam, can be used as an analgesic in C57BL/6 male mice that undergo permanent MCAO without adverse effects on infarct volume. Buprenorphine ingested voluntarily resulted in the lowest levels of FCM, thereby indicating pain relief, and therefore has the potential to refine the traditional analgesic strategy associated with this model. Side effects associated with buprenorphine, such as reduced food consumption, were of short duration and occurred only during the treatment period. These possibly short-term adverse consequences should be weighed against the potential to reduce postsurgical stress, improve the welfare of the mice, and to refine the model. Furthermore, the behavioral analysis demonstrated that observation of natural, unprovoked behaviors may aid in developing markers of stroke-related behavioral deficiencies in laboratory mice; exploratory behavior may be of particular relevance in studies involving acute neurodegenerative rodent models. Because significant sex- and strain-associated differences occur in behavioral and stroke-related parameters, additional studies are needed to increase our knowledge regarding the effects of analgesia on postsurgical pain and behavioral parameters in stroke research.
Acknowledgments
This work was supported by the following foundations: the Danish Council for Strategic Research, Alice Brenaa Memorial Fund, Engineer Frode V Nyegaard and Wife Fund, Director Emil C Hertz and Inger Hertz Foundation, King Christian Tiendes Fund, Aase and Ejnar Danielsen Foundation, Arvid Nilsson Foundation, Mrs Asta Florida Bolding Born Andersen Memorial Scholarship Fund, Merchants AV Lykfeldt and Spouse Scholarship Fund, and Ivan Nielsen Foundation. We thank Huda Shalahudin Darusman, Trine Marie Nielsen, Helle Porsdal, Marianne Falk, Henrik Hasseldam, and Oksana Dmytriyeva for providing assistance and equipment during the project.
References
- 1.Abelson KS, Jacobsen K, Sundbom R, Kalliokoski O, Hau J. 2012. Voluntary ingestion of nut paste for administration of buprenorphine in rats and mice. Lab Anim 46:349–351 [DOI] [PubMed] [Google Scholar]
- 2.Adamson TW, Kendall LV, Goss S, Grayson K, Touma C, Palme R, Chen JQ, Borowsky AD. 2010. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci 49:610–616 [PMC free article] [PubMed] [Google Scholar]
- 3.Altholtz LY, Fowler KA, Badura LL, Kovacs MS. 2006. Comparison of the stress response in rats to repeated isoflurane or CO2: O2 anesthesia used for restraint during serial blood collection via the jugular vein. J Am Assoc Lab Anim Sci 45:17–22 [PubMed] [Google Scholar]
- 4.Antezana DF, Clatterbuck RE, Alkayed NJ, Murphy SJ, Anderson LG, Frazier J, Hurn PD, Traystman RJ, Tamargo RJ. 2003. High-dose ibuprofen for reduction of striatal infarcts during middle cerebral artery occlusion in rats. J Neurosurg 98:860–866 [DOI] [PubMed] [Google Scholar]
- 5.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51 [PubMed] [Google Scholar]
- 6.Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Alvarez D, Al-Dalain S, Martinez G, Leon OS, Springer JE. 2003. Assessment of the relative contribution of COX1 and COX2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia. J Neurochem 86:545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrousos GP, Gold PW. 1992. The concepts of stress and stress system disorders—overview of physical and behavioral homeostasis. JAMA 267:1244–1252 [PubMed] [Google Scholar]
- 8.Cole DJ, Patel PM, Reynolds L, Drummond JC, Marcantonio S. 1993. Temporary focal cerebral ischemia in spontaneously hypertensive rats—the effect of ibuprofen on infarct volume. J Pharmacol Exp Ther 266:1713–1717 [PubMed] [Google Scholar]
- 9.Connolly ES, Winfree CJ, Stern DM, Solomon RA, Pinsky DJ. 1996. Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia. Neurosurgery 38:523–531 [DOI] [PubMed] [Google Scholar]
- 10.Cooper DM, Hoffman W, Wheat N, Lee HY. 2005. Duration of effects on clinical parameters and referred hyperalgesia in rats after abdominal surgery and multiple doses of analgesic. Comp Med 55:344–353 [PubMed] [Google Scholar]
- 11.Corbett D, Thornhill J. 2000. Temperature modulation (hypothermic and hyperthermic conditions) and its influence on histological and behavioral outcomes following cerebral ischemia. Brain Pathol 10:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J, St Clair M, Everett C, Reitman M, Star RA. 2000. Buprenorphine given after surgery does not alter renal ischemia–reperfusion injury. Comp Med 50:628–632 [PubMed] [Google Scholar]
- 13.DeVries AC, Nelson RJ, Traystman RJ, Hurn PD. 2001. Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neurosci Biobehav Rev 25:325–342 [DOI] [PubMed] [Google Scholar]
- 14.Dickinson AL, Leach MC, Flecknell PA. 2009. The analgesic effects of oral paracetamol in 2 strains of mice undergoing vasectomy. Lab Anim 43:357–361 [DOI] [PubMed] [Google Scholar]
- 15.Feigin VL, Lawes CMM, Bennett DA, Anderson CS. 2003. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case fatality in the late 20th century. Lancet Neurol 2:43–53 [DOI] [PubMed] [Google Scholar]
- 16.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. 2009. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40:2244–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flecknell P. 2009. Laboratory animal anaesthesia. San Diego (CA): Academic Press [Google Scholar]
- 18.Flecknell PA. 1984. The relief of pain in laboratory animals. Lab Anim 18:147–160 [DOI] [PubMed] [Google Scholar]
- 19.Flecknell PA. 2001. Analgesia of small mammals. Vet Clin North Am Exot Anim Pract 4:47–56 [DOI] [PubMed] [Google Scholar]
- 20.Flecknell PA, Waterman-Pearson A. 2005. Pain management in animals. Philadelphia (PA): WB Saunders [Google Scholar]
- 21.Foley PL, Liang HX, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204 [PMC free article] [PubMed] [Google Scholar]
- 22.Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, Schumann-Bard P, Boulouard M. 2009. Behavioral deficits after distal focal cerebral ischemia in mice: usefulness of adhesive removal test. Behav Neurosci 123:224–230 [DOI] [PubMed] [Google Scholar]
- 23.Graham SM, McCullough LD, Murphy SJ. 2004. Animal models of ischemic stroke: balancing experimental aims and animal care. Comp Med 54:486–496 [PubMed] [Google Scholar]
- 24.Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137 [DOI] [PubMed] [Google Scholar]
- 25.Harukuni I, Bhardwaj A, Shaivitz AB, DeVries AC, London ED, Hurn PD, Traystman RJ, Kirsch JR. 2000. σ1 receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine affords neuroprotection from focal ischemia with prolonged reperfusion. Stroke 31:976–982 [DOI] [PubMed] [Google Scholar]
- 26.Hau J, Kalliokoski O, Jacobsen KR, Abelson KSP. 2011. Interpretations of faecal concentrations of corticosteroids. Lab Anim 45:129–130 [DOI] [PubMed] [Google Scholar]
- 27.Hayes KE, Raucci JA, Jr, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23 [PubMed] [Google Scholar]
- 28.Hennessy MB. 1991. Sensitization of the plasma corticosterone response to novel environments. Physiol Behav 50:1175–1179 [DOI] [PubMed] [Google Scholar]
- 29.Hennessy MB, Levine S. 1977. Effects of various habituation procedures on pituitary–adrenal responsiveness in mouse. Physiol Behav 18:799–802 [DOI] [PubMed] [Google Scholar]
- 30.Hurley SD, Olschowka JA, O'Banion MK. 2002. Cyclooxygenase inhibition as a strategy to ameliorate brain injury. J Neurotrauma 19:1–15 [DOI] [PubMed] [Google Scholar]
- 31.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press [Google Scholar]
- 32.Jacobsen KR, Kalliokoski O, Hau J, Abelson K. 2011. Voluntary ingestion of buprenorphine in mice. Anim Welf 20:591–596 [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen KR, Kalliokoski O, Teilmann AC, Hau J, Abelson KSP. 2012. Postsurgical food and water consumption, fecal corticosterone metabolites, and behavior as noninvasive measures of pain in vasectomized BALB/c mice. J Am Assoc Lab Anim Sci 51:69–75 [PMC free article] [PubMed] [Google Scholar]
- 34.Joels M, Karst H, Krugers HJ, Lucassen PJ. 2007. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol 28:72–96 [DOI] [PubMed] [Google Scholar]
- 35.Johshita H, Asano T, Hanamura T, Takakura K. 1989. Effect of indomethacin and a free radical scavenger on cerebral blood flow and edema after cerebral artery occlusion in cats. Stroke 20:788–794 [DOI] [PubMed] [Google Scholar]
- 36.Jonsson AC, Lindgren I, Hallstrom B, Norrving B, Lindgren A. 2006. Prevalence and intensity of pain after stroke: a population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry 77:590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalliokoski O, Abelson KSP, Koch J, Boschian A, Thormose SF, Fauerby N, Rasmussen RS, Johansen FF, Hau J. 2010. The effect of voluntarily ingested buprenorphine on rats subjected to surgically induced global cerebral ischaemia. In Vivo 24:641–646 [PubMed] [Google Scholar]
- 38.Kalliokoski O, Jacobsen KR, Hau J, Abelson KSP. 2011. Serum concentrations of buprenorphine after oral and parenteral administration in male mice. Vet J 187:251–254 [DOI] [PubMed] [Google Scholar]
- 39.Kalliokoski O, Jacobsen KR, Teilmann AC, Hau J, Abelson KSP. 2012. Quantitative effects of diet on fecal corticosterone metabolites in 2 strains of laboratory mice. In Vivo 26:213–221 [PubMed] [Google Scholar]
- 40.Kawaguchi M, Kimbro JR, Drummond JC, Cole DJ, Kelly PJ, Patel PM. 2000. Isoflurane delays but does not prevent cerebral infarction in rats subjected to focal ischemia. Anesthesiology 92:1335–1342 [DOI] [PubMed] [Google Scholar]
- 41.Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. 2003. Cytokine-induced sickness behavior. Brain Behav Immun 17:S112–S118 [DOI] [PubMed] [Google Scholar]
- 42.Kofke WA, Garman RH, Garman R, Rose ME. 1999. Opioid neurotoxicity: fentanyl-induced exacerbation of cerebral ischemia in rats. Brain Res 818:326–334 [DOI] [PubMed] [Google Scholar]
- 43.Krueger KL, Fujiware Y. 2008. The use of buprenorphine as an analgesic after rodent embryo transfer. Lab Anim (NY) 37:87–90 [DOI] [PubMed] [Google Scholar]
- 44.LaBuda CJ, Sora I, Uhl GR, Fuchs PN. 2000. Stress-induced analgesia in µ-opioid receptor knockout mice reveals normal function of the δ-opioid receptor system. Brain Res 869:1–5 [DOI] [PubMed] [Google Scholar]
- 45.Lamont LA, Tranquilli WJ, Grimm KA. 2000. Physiology of pain. Vet Clin North Am Small Anim Pract 30:703–728 [DOI] [PubMed] [Google Scholar]
- 46.Li X, Blizzard KK, Zeng ZY, DeVries AC, Hurn PD, McCullough LD. 2004. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol 187:94–104 [DOI] [PubMed] [Google Scholar]
- 47.Macrae IM. 2011. Preclinical stroke research—advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol 164:1062–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majid A, He YY, Gidday JM, Kaplan SS, Gonzales ER, Park TS, Fenstermacher JD, Wei L, Choi DW, Hsu CY. 2000. Differences in vulnerability to permanent focal cerebral ischemia among 3 common mouse strains. Stroke 31:2707–2714 [DOI] [PubMed] [Google Scholar]
- 49.Meden P, Overgaard K, Pedersen H, Boysen G. 1994. The Influence of body temperature on infarct volume and thrombolytic therapy in a rat embolic stroke model. Brain Res 647:131–138 [DOI] [PubMed] [Google Scholar]
- 50.Minghetti L. 2004. Cyclooxygenase 2 (COX2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol 63:901–910 [DOI] [PubMed] [Google Scholar]
- 51.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. 1999. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80:67–82 [DOI] [PubMed] [Google Scholar]
- 52.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. 1999. Heritability of nociception II. ‘Types’ of nociception revealed by genetic correlation analysis. Pain 80:83–93 [DOI] [PubMed] [Google Scholar]
- 53.Netherlands Centre Alternatives to Animal Use (NCA) [Internet]. 2012. Mouse physiological parameters and normal values, humane endpoints in laboratory animal experimentation. [Cited 27 February 2013]. Available at: http://www.humane-endpoints.info/eng/index.php?option=com_content&view=article&id=234&Itemid=250&lang=en.
- 54.Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, Olsen TS. 1996. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 347:422–425 [DOI] [PubMed] [Google Scholar]
- 55.Richardson CA, Flecknell PA. 2005. Anaesthesia and postoperative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern Lab Anim 33:119–127 [DOI] [PubMed] [Google Scholar]
- 56.Rogers DC, Jones DNC, Nelson PR, Jones CM, Quilter CA, Robinson TL, Hagan JJ. 1999. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of 6 inbred mouse strains. Behav Brain Res 105:207–217 [DOI] [PubMed] [Google Scholar]
- 57.Rosen GD, Harry JD. 1990. Brain volume estimation from serial section measurements: a comparison of methodologies. J Neurosci Methods 35:115–124 [DOI] [PubMed] [Google Scholar]
- 58.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343 [DOI] [PubMed] [Google Scholar]
- 59.Roughan JV, Wright-Williams SL, Flecknell PA. 2009. Automated analysis of postoperative behaviour: assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab Anim 43:17–26 [DOI] [PubMed] [Google Scholar]
- 60.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154 [DOI] [PubMed] [Google Scholar]
- 61.Sundbom R, Jacobsen KR, Kalliokoski O, Hau J, Abelson KSP. 2011. Postoperative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In Vivo 25:335–342 [PubMed] [Google Scholar]
- 62.Tang X, Sanford LD. 2002. Telemetric recording of sleep and home cage activity in mice. Sleep 25:691–699 [PubMed] [Google Scholar]
- 63.Traystman RJ. 2003. Animal models of focal and global cerebral ischemia. ILAR J 44:85–95 [DOI] [PubMed] [Google Scholar]
- 64.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191 [PMC free article] [PubMed] [Google Scholar]
- 65.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. 2005. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:823–828 [DOI] [PubMed] [Google Scholar]
- 66.Van Loo PLP, Kuin N, Sommer R, Avsaroglu H, Pham T, Baumans V. 2007. Impact of ‘living apart together’ on postoperative recovery of mice compared with social and individual housing. Lab Anim 41:441–455 [DOI] [PubMed] [Google Scholar]
- 67.Wahl F, Allix M, Plotkine M, Boulu RG. 1992. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke 23:267–272 [DOI] [PubMed] [Google Scholar]
- 68.Walker WC. 2004. Pain pathoetiology after TBI: neural and nonneural mechanisms. J Head Trauma Rehabil 19:72–81 [DOI] [PubMed] [Google Scholar]
- 69.Warner DS. 2000. Isoflurane neuroprotection. A passing fantasy, again? Anesthesiology 92:1226–1228 [PubMed] [Google Scholar]
- 70.Wright-Williams SL, Courade JP, Richardson CA, Roughan JV, Flecknell PA. 2007. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in 2 strains of laboratory mouse. Pain 130:108–118 [DOI] [PubMed] [Google Scholar]