Abstract
In the summer and fall of 2010, a series of outdoor-housed rhesus macaques were diagnosed with tularemia. PCR analysis or positive culture confirmed 11 cases, and 9 additional animals with similar clinical signs responded to empiric antibiotic treatment. A serosurvey conducted in the 9 mo after the outbreak found 53% (43 of 81 macaques) seropositivity in the southern outdoor colony, which had an average population of 700 animals. A prospective survey of small mammal reservoirs and arthropod vectors was conducted during the late summer and fall of 2011. PCR analyses of tissues from all 135 mice, 18 ground squirrels, 1 rat, 3 raccoons, 2 cats, and 3 jackrabbits and their fleas were negative for DNA of Francisella tularensis. Conventional PCR evaluation of stored DNA from affected macaques identified the causative organism as F. tularensis subsp. holartica. DNA evaluated from historic cases of tularemia in nonhuman primates confirmed that the organism that infected the colony during the late 1980s likewise was F. tularensis subsp. holartica. The macaque tularemia epizootic of 2010 appears to have been an extreme example of the periodic resurgence of tularemia. No evidence of rodent disease was found in the immediate vicinity during the 2011 interepizootic period. The concurrent widespread seropositivity (53%) and low incidence of clinical disease (2.7%) in 2010 suggests that this strain of Francisella has low pathogenicity in macaques.
Abbreviations: RD, region of difference
Francisella tularensis is a small gram-negative coccobacillus and is the causative agent of tularemia. Also known as rabbit fever and deer fly fever, tularemia is a multisystemic disease that affects many animal species including humans.6,11,30,34,37 F. tularensis can be transmitted to humans and other animals by the bite of arthropods, aerosol, ingestion, or broken-skin contact with infective materials. In the United States, ticks and biting flies are the most common arthropods involved in the transmission of tularemia.9,11,30 Early signs of disease can include ulceration at the site of infection and regional lymphadenopathy; in some cases, local disease progresses to potentially fatal disseminated disease. Pneumonia is associated with the highest case fatality rate and can occur with aerosol exposure or hematogenous spread.27,30 F. tularensis has a low infectious dose: exposure to as few as 1 to 10 organisms can cause fatal disease in humans.27 Because of its potential for transformation into a bioterrorism weapon, F. tularensis is a US Department of Health and Human Services Tier 1 Select Agent. Isolation of the organism from any source must be reported to the Centers for Disease Control within 24 h.6,33
Tularemia was first identified in Tulare county in California and has caused large die-offs of rodents and rabbits during epizootics.12,17,19,24,25 It is enzootic in much of the northern hemisphere.8,37 Most human cases of tularemia are caused by either of 2 subspecies of F. tularensis, tularensis and holartica, which are both present in the United States. F. tularensis subsp. tularensis can be further divided into type A1, found in the eastern United States, and type A2 in the western United States, with A1 having greater case fatality.20,29 F. tularensis subsp. holartica can be divided into biovars I, II, and III (also known as japonica).9,11,36 The 2 other subspecies of F. tularensis are not commonly associated with human disease in the United States. F. tularensis subsp. mediaasiatica is found only in central Asia.12,19 F. tularensis subsp. novicida has worldwide distribution but low pathogenicity and typically is associated with disease in immunocompromised patients.4,19 Subspecies tularensis and holartica have both been reported to cause disease in nonhuman primates, with holartica being more common in captive primates in the United States.20
Tularemia had been diagnosed 3 times in outdoor-housed animals at the California National Primate Research Center between 1987 and 1990. No additional cases were observed until 2010, when a series of young rhesus macaques presented with fever, lethargy, and pronounced mesenteric lymphadenopathy. Eleven cases of tularemia were confirmed by culture or PCR analysis at necropsy, and an additional 9 animals with similar clinical signs were treated with antibiotics and recovered. The clinical and pathologic findings strongly suggested ingestion as the route of exposure. The cases were clustered primarily in the facility's southern outdoor housing area, with a single confirmed case occurring in the northern outdoor housing area. All clinical and confirmed cases occurred in animals younger than 4 y.
To investigate this outbreak of tularemia and assess the risk for ongoing disease, we prospectively surveyed local small mammals and arthropods. In addition, we investigated the outbreak in macaques by conducting a retrospective cross-sectional serosurvey of the macaques by using serum banked before and after the outbreak. A representative set of samples was selected from the populations in both the northern and southern colonies to evaluate the effect of age and housing location on the risk for exposure. We hypothesized that seroprevalence would be higher in macaques in the southern colony than the northern colony, given the greater clinical incidence among animals in the southern enclosures. Additional molecular diagnostics were used to identify the subspecies of F. tularensis involved in the recent epizootic, and new techniques were developed to identify the subspecies in historic cases.
Materials and Methods
Rodents were live-trapped and macaque samples were collected under IACUC-approved protocols at an AAALAC-accredited institution (California National Primate Research Center, Davis, CA). All animals were cared for in accordance with the Animal Welfare Act2 and the Guide for the Care and Use of Laboratory Animals.16 All wild-animal work and carcass salvage was done according to a California Department of Fish and Game permit.
Animal collection.
Live trapping was conducted for 7 d in September 2011 by using 40 extra-large traps (HB Sherman Traps, Tallahassee, FL) and 12 squirrel-size traps (Tomahawk Live Traps, Hazelhurst, WI). Traps were set in areas with evidence of rodent activity and within a few hundred meters of the primate housing. A paste of peanut butter and oatmeal was used as bait in all traps. Squirrel-size traps were set for the entire day and night, with monitoring at least once every 3 h during daylight. Extra-large Sherman traps were set in the evening and then checked the following morning. The solid-sided Sherman traps were closed in the morning to prevent capture of animals during the heat of the day.
Captured rodents were manually restrained in a mesh capture bag and sedated with ketamine (40 mg/kg) and xylazine (4 mg/kg)31 according to their estimated weight. Rodents trapped within 100 m of the primate enclosures were euthanized with an overdose of sodium pentobarbital. Euthanized animals were placed individually in sealed plastic bags and stored at 4 °C until ectoparasites and tissue collection could be performed. Rodents trapped outside the perimeter fence and 100 to 300 m from primate enclosures were released after sample collection. Ectoparasites and blood were collected in the field, individually numbered metal ear tags were placed, recovery from anesthesia was monitored, and the rodents were released in the location they were trapped.
The personal protective equipment worn by personnel during live trapping was based on the close proximity to the primate enclosures and exceeded the campus requirement for protection from hantavirus when live trapping rodents in open spaces. Personnel involved in trapping wore facility-dedicated scrubs and shoes and disposable sleeves, nitrile gloves, surgical masks, and face shields. Leather gloves were worn while manually restraining rodents for sedation. Rodent carcasses also were obtained from the facility pest control program. Samples of additional species were obtained by salvaging carcasses found on the facility grounds and the bordering roads. Carcass collection was conducted from July to midNovember 2011, coinciding with the time of year that the macaque tularemia cases were seen during 2010.
In addition to inspecting animals for the presence of ticks and other arthropods, the areas surrounding the primary enclosures were ‘flagged’ for ticks. A 1-m2 white cloth was dragged over vegetation and inspected for ticks every 10 to 15 m. Flagging was performed 4 times during November and December 2011. Flagging started once the rainy season began and on days when the temperature was above 50 °F, when Dermacentor ticks might be active.
Sample collection.
Necropsies were performed in a class II A/B biosafety cabinet. Carcasses were examined externally for ectoparasites and pathologic lesions. All ectoparasites were collected with forceps and placed in 70% ethanol in 1.5-mL microcentrifuge tubes. The abdomen and thorax of each carcass were opened and further examined for pathology. Blood was collected from the heart, large vessels, or chest cavity and placed in plastic microcentrifuge or vacuum tubes for serum collection. The spleen, liver, mesenteric lymph nodes, urine, and feces were collected and each placed in 1.5-mL tubes. If any gross lesions were present, samples were saved both in formalin and frozen in 1.5-mL tubes. Instruments and work surfaces were washed and disinfected with 10% sodium hypochlorite or Envirocide (Metrex, Orange, CA) between animals. Samples were stored at −80 °C.
PCR analysis for presence of F. tularensis.
DNA was extracted from spleen samples of each collected small mammal18 by using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) and the manufacturer's spin-column protocol for spleen. The tissue samples were handled and initial DNA extraction steps were completed in a class II A biosafety cabinet. Because most abdominal and thoracic organs were missing from one jackrabbit carcass, a mesenteric lymph node was used for DNA extraction. DNA also was extracted from tissue from any ulcerative or abscessed lesions.1 The manufacturer's instructions were followed, with the addition of an extra drying spin after the washes. The final elution step was performed with 50 µL molecular-grade H2O to maximize the DNA concentration. The extraction was done according to the same procedure used with the macaque tissues during the 2010 outbreak.
Fleas from each carcass were sorted and then pooled (maximum, 3 fleas) by genus. The dorsum of each flea was incised with a clean razor blade and then incubated in lysis buffer (DNeasy Blood and Tissue Kit, Qiagen) and proteinase K overnight. After the lysis steps, the exoskeleton of each flea was reserved for mounting and species identification; DNA was extracted from the remainder as done for the animal tissues.
Real-time PCR detection of F. tularensis was performed as described1 by using a StepOnePlus PCR machine (Applied Biosystems, Grand Island, NY). Primers and probe specific for the outer membrane protein gene fopA were used. Briefly, 12 µL PCR mixture contained 6 µL TaqMan mix (Applied Biosystems), 4.4 µL PCR-quality H2O, 0.6 µL of the primer–probe mixture, and 1 µL of extracted DNA. All PCR runs were performed with 3 negative control wells, with H2O added instead of DNA and a positive control of DNA from a macaque affected in the 2010 outbreak. Cycle threshold values below 40 were considered positive.
PCR for subspecies identification of F. tularensis.
Samples of extracted DNA from 4 macaques cases confirmed to be positive for F. tularensis by real-time PCR were available for subspecies identification. Conventional PCR was performed on the extracted DNA by using primers that targeted 3 of the regions of difference (RD) between subspecies.1,5,13,28,29 PCR was performed on each sample by using primers designed against regions RD1, RD3, and RD6 of F. tularensis.29 RD1 can be used to differentiate all subspecies of F. tularensis according to the size of the PCR product. The PCR product from RD1 primers is 1522 to 1523 bp for F. tularensis subsp. tularensis, 1453 bp for F. tularensis subsp. mediaasiatica, 924 bp for F. tularensis subsp. holartica (biovar I or II), 1135 bp for F. tularensis subsp. holartica biovar III (japonica), and 3322 bp for F. tularensis subsp. novicida.5 Primers for RD3 produce a 570-bp product from F. tularensis subsp. tularensis A2, F. tularensis subsp. novicida, and F. tularensis subsp. holartica, whereas those for RD6 produce a 396-bp product with F. tularensis subsp. tularensis A1, F. tularensis subsp. novicida, and F. tularensis subsp. holartica. When used in combination, the results from RD3 and RD6 enable differentiation between F. tularensis subsp. tularensis A1 and A2 and differentiation of F. tularensis subsp. tularensis (either biovar) from both subsp. novicida and holartica.29 Briefly, a 25-µL PCR mixture containing GoTaq Green Master Mix (Promega, Madison, WI) was used according to the manufacturer's instructions with 0.5 µM forward primer, 0.5 µM reverse primer, and 2.5 µL extracted DNA. For RD1, the initial denaturation was performed at 94 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 60 s. For RD3 and RD6, the initial denaturation was performed at 94 °C for 5 min followed by 30 cycles of 95 °C for 45 s, 53 °C for 45 s, and 72 °C for 45 s.
PCR products were separated electrophoretically on a 1% agarose gel stained with GelStar (Lonza, Rockland, ME) and visualized under UV transillumination. Positive bands were excised from the gel and prepared for sequencing by using the QIAquick Gel Extraction Kit (Qiagen) according to the manufacturer's instructions.
Automated DNA sequencing with PCR primers (Davis Sequencing, Davis, CA) was performed on the DNA extracted from each band. Electropherograms were evaluated visually, and high-quality sequences were compared with other available sequences in the NCBI GenBank database.26 Subspecies identification was confirmed when a 100% match was obtained.
PCR for subspecies identification of Francisella tularensis in historic cases.
DNA was extracted from stored samples from 3 historic culture-positive cases of tularemia at the facility: a squirrel monkey in 1987, a rhesus macaque from 1989, and a rhesus macaque from 1990. DNA was obtained from scrolls cut from 2 to 6 paraffin blocks containing biopsy or necropsy specimens for each case. The scrolls were incubated with lysis buffer (Qiagen) and proteinase K at 70 °C for 10 min and then centrifuged at 4 °C for 3 min. Wax then was removed from the samples, and DNA extraction was completed according to the DNeasy Blood and Tissue Kit (Qiagen) spin-column protocol.
The presence of F. tularensis DNA was confirmed by real-time PCR. A set of nested primers was developed to increase the sensitivity of subspecies identification by conventional PCR. Primers were created by using 20 bp internal to the sequenced products from the 2010 macaque cases. The primers created were: RD1i, 5′ CAT TAT TAA AGA CAT CGC AC 3′ (RD1iF) and 5′ TAC AGC AAT CGT CAT TCT AC 3′ (RD1iR); RD3i, 5′ GGT ATC CAT TAA TCG TGG TA 3′ (RD3iF) and 5′ ATA CTG AGA CTC ATC CAT AC 3′ ( RD3iR); and RD6i, 5′ CCT AAT GCG GAA ACA TAT TG 3′ (RD6iF) and 5′ CTT GCC AGC CTA ATA ATT AC 3′ (RD6iR ). The RD1i primers produce a DNA fragment that is 198 bp shorter than that for the RD1 primers, yielding 1324 to 1325 bp for F. tularensis subsp. tularensis, 1255 bp for F. tularensis subsp. mediaasiatica, 726 bp for F. tularensis subsp. holartica (biovar I or II), 937 bp for F. tularensis subsp. holartica biovar III, and 3124 bp for F. tularensis subsp. novicida. The RD3i primers produce a 474-bp segment, and the RD6i primers generate a 341-bp segment; the results from RD3i and RD6i were interpreted together, as done for RD3 and RD6. In samples containing F. tularensis DNA, the presence of a band from RD3i primers only is diagnostic for F. tularensis subsp. tularensis A2, and the presence of a band from RD6i primers only is diagnostic for F. tularensis subsp. tularensis A1. If bands are generated from both RD3i and RD6i primers, the results are consistent with either F. tularensis subsp. novicida or holartica.29
The DNA from the historic primate tularemia cases was amplified by using primers against RD1, RD3, and RD6, as described in the previous section. The second round of amplification was performed by using 1 µL of the first-round amplification product. For RD1i primers, initial denaturation was performed at 94 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 60 s. For RD3i and RD6i primers, initial denaturation was performed at 94 °C for 5 min followed by 30 cycles of 95 °C for 45 s, 53 °C for 45 s, and 72 °C for 45 s. Electrophoresis and DNA sequencing were performed as described in the previous section.
Flea mounting and identification.
After tissue lysis, flea exoskeletons were cleared in 0.78 M KOH (3 drops saturated solution from a standard disposable Pasteur pipet in 0.75 mL H2O) for 24 h and then dehydrated in 70%, 80%, 95%, and 100% alcohol for at least 30 min at each step. Dehydrated exoskeletons were mounted on glass slides in Euparal (BioQuip Products, Rancho Dominguez, CA), and the slides were dried at 37 °C for at least 3 wk before samples were examined microscopically for species identification.
Fleas were identified to the level of genus by using a key.35 When available, current literature was used to confirm species.15,21 Otherwise, flea species were identified by using an older reference.22
Serology.
Representative samples from the macaque populations in the northern and southern enclosures were obtained by using random sampling, stratified by age and sex. Samples were selected from the routinely banked serum that was collected between August 2010 and April 2011. The preepizootic serum samples were selected in the same fashion from serum banked between August 2009 and April 2010. A total of 324 samples were evaluated, comprising 81 samples from each location before and after the outbreak. Serum samples from all small mammals collected outdoors and 12 mice collected indoors were evaluated also.
Serology was conducted by using slide agglutination with F. tularensis antigen (Becton Dickinson, Sparks, MD) according to the manufacturer's instructions.3 Negative and positive controls were run with each test. Serum was screened at 1:20, and all samples testing positive were retested at a 1:160 dilution.
A positive serologic result was defined as titer of at least 160, and a sample with no agglutination at the 1:20 dilution was considered negative. Any intermediate results were categorized as indeterminate. For some mouse samples, the serum volume available was insufficient to confirm a negative result. For serum-limited samples, the 1:160 dilution was tested first, and lower dilutions were evaluated as serum volume permitted.
Statistics.
Statistical analyses of the results were performed by using StatXact 9 (Cytel Software, Cambridge, MA). The serologic results for each population and time point were compared by using the Fisher exact test. Confidence intervals were generated by estimation of binomial parameters. The results of each population after the epizootic were evaluated for age-related trends in the serologic results by using the Jonckheere–Terpstra test and for sex-associated differences by using the Kruskal–Wallis test. Statistical significance was defined as a P value of less than 0.05.
Results
Animals collected.
For the first 2 d, traps were distributed evenly between the northern and southern enclosures. Due to lack of trapping success and limited evidence of rodent activity around the northern enclosures, 75% of the traps were set around the southern enclosures for the remaining 5 d to maximize animal collection. Animals trapped (Table 1) included a total of 10 mice (Mus musculus) and 7 California ground squirrels (Otospermophilus beecheyi); 3 of the mice were trapped outside of the perimeter fence and were released after ectoparasite examination and serum collection. All trapped animals were collected near the southern colony.
Table 1.
Small mammals collected and ectoparasites present
| No. (%) of animals collected that had |
||||||
| No. of animals collected | Ticks | Fleas | Mites | Lice | Rate of parasitisma | |
| Mus musculus (trapped indoors) | 129 | 0 (0%) | 0 (0%) | 3 (2.3%) | 0 (0%) | 3 (2.3%) |
| Mus musculus (trapped outdoors) | 9 | 0 (0%) | 2 (22.2%) | 2 (22.2%) | 0 (0%) | 3 (33.3%) |
| Otospermophilus beecheyi | 18 | 0 (0%) | 18 (100%) | 1 (5.5%) | 9 (50%) | 18 (100%) |
| Procyon lotor | 3 | 0 (0%) | 1 (33.3%) | 0 (0%) | 0 (0%) | 1 (33.3%) |
| Lepus californicus | 3 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33.3%) | 1 (33.3%) |
| Felis catus | 2 | 0 (0%) | 2 (100%) | 0 (0%) | 0(0%) | 2 (100%) |
| Rattus rattus | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total | 165 | 0 (0%) | 23 (14.0%) | 6 (3.7%) | 11 (6.7%) | 29 (17.7%) |
Total number (percentage) of animals collected that had ectoparasites; coinfestation counted only once.
An additional 128 mouse, 1 rat (Rattus rattus), and 9 ground squirrel carcasses were received from the facility pest control program. The mice and rat were trapped inside buildings, and the ground squirrels were trapped in the southern enclosures. Two mice had abscesses, and another mouse had an ulcerative lesion. Splenic tissue and samples of each lesion were screened for F. tularensis by using real-time PCR. Carcasses salvaged from the grounds and surrounding roads included 2 additional ground squirrels, 3 raccoons (Procyon lotor), 3 jack rabbits (Lepus californicus), and 2 feral cats (Felis catus). Seven of the trapped mice and all 16 of the ground squirrels trapped or received from the pest control program were collected in the immediate vicinity of the southern primate enclosures. None of the carcasses received came from the area immediately surrounding the northern enclosures.
Ectoparasite identification.
Ectoparasites were collected from all host species represented except the single rat (Table 1). The most commonly identified ectoparasites were fleas, followed by lice. Fleas were categorized by species (Table 2), with members of the genera Hoplopsyllus and Echidnophaga having the greatest representation. Flagging the areas adjacent to the macaque enclosures yielded various arthropods, but no ticks were collected.
Table 2.
Fleas on small mammals
| No. (%) of animals collected that had | |||||
| Hoplopsyllus anomalus | Oropsylla montana | Echidnophaga gallinacea | Ctenocephalides felis | Totala | |
| Mus musculus (trapped indoors) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mus musculus (trapped outdoors) | 1 (11%) | 0 (0%) | 1 (11%) | 0 (0%) | 0 (0%) |
| Otospermophilus beecheyi | 17 (94.4%) | 4 (22.2%) | 13 (72.2%) | 0 (0%) | 18 (100%) |
| Procyon lotor | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | 1 (33%) |
| Lepus californicus | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Felis catus | 0 (0%) | 0 (0%) | 1 (50%) | 1 (50%) | 2 (100%) |
| Rattus rattus | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total | 18 (10.2%) | 4 (2.4%) | 15 (9.0%) | 2 (1.2%) | |
Total number (%) of animals collected that had one or more species of flea.
PCR for presence of Francisella tularensis.
All tissue samples from all small mammals collected (Table 1) and pooled samples of fleas (Table 2) tested negative for F. tularensis DNA by using real-time PCR. Each run yielded a positive result from the positive control sample, and no amplification was detected after 50 cycles on all negative controls.
Wildlife serology for tularemia.
A single raccoon was serologically positive; the other 2 raccoons and all other small mammals had no agglutination at the lowest dilution tested (1:20). For 8 of the 12 indoor-mouse samples evaluated, there was insufficient serum available to confirm negative serology, but all were negative at 1:160. The seropositive raccoon had no lesions consistent with active tularemia infection, and its splenic tissue was PCR negative.
Macaque serology for tularemia.
The population of the southern colony averaged 700 animals during the outbreak, so that the 19 clinical cases represent a clinical incidence of 2.7%. The northern colony averages 3000 animals, so that the single case represents a clinical incidence of 0.03%.
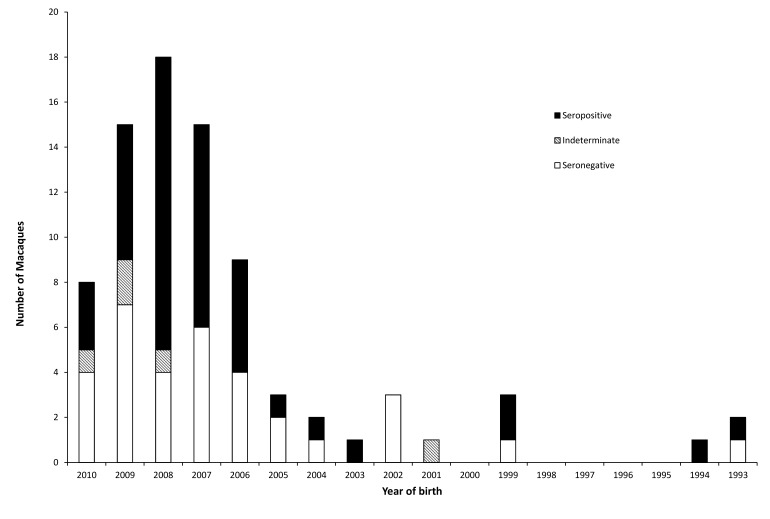
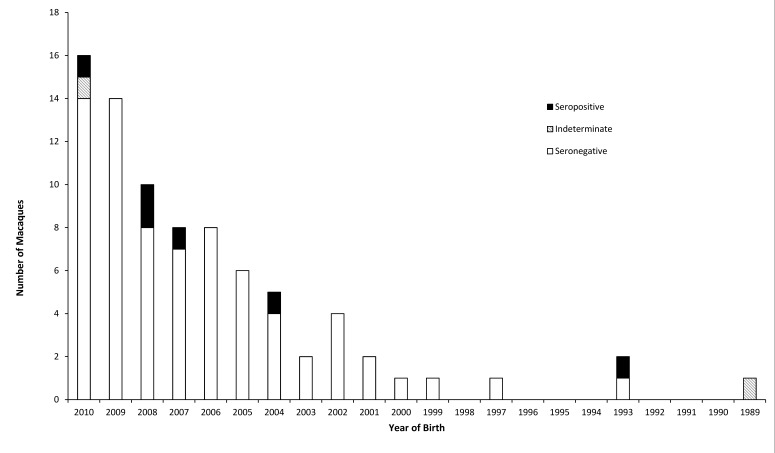
No seropositive animals were identified from the macaque serum samples collected prior to the 2010 epizootic. Samples collected after the epizootic revealed seropositive macaques were in both the northern and southern colonies. The southern colony had a seroprevalence of 0% (95% confidence interval, 0.00% to 4.45%) prior to the epizootic and 53.1% (95% confidence interval, 41.7% to 64.3%) afterward (P < 0.0001). The northern colony had a seroprevalence of 0% (95% confidence interval, 0.00% to 4.45%) prior to the epizootic and 7.4% (95% confidence interval, 2.8% to 15.4%) afterward (P = 0.02839). The seroprevalence after the epizootic was significantly (P < 0.0001) higher in the southern colony than in the northern colony. No relationship was found between birth year and seroprevalence (Jonckheere–Terpstra test; Figures 1 and 2) in either the southern colony (P = 0.89) or northern colony (P = 0.65). A sex-associated difference in seroprevalence was found in the southern colony, with female macaques having greater (P = 0.035) seroprevalence (63.0%) than male macaques (38.2%). No significant difference in seroprevalence was observed between female (6.0%) and male (9.6%) macaques in the northern colony (P = 0.66).
Figure 1.
Serology results from the southern colony.
Figure 2.
Serology results from the northern colony.
Subspecies identification.
In the 4 macaque cases evaluated, the causative organism was identified as F. tularensis subsp. holartica (biovar I or II) according to the size and sequence of the RD1 PCR product. A product was generated for both RD3 and RD6 primers, thus ruling out F. tularensis subsp. tularensis, and the DNA sequences of these products were consistent with F. tularensis subsp. holartica also.
For the 3 historic macaque cases of tularemia, the causative organism of the 1989 rhesus macaque case was identified as F. tularensis subsp. holartica by sequencing of nested PCR products obtained by RD1and RD1i, RD3 and RD3i, and RD6 and RD6i primers. For the 1987 squirrel monkey case, RD6 and RD6i primers yielded the only clear band, but sequencing confirmed the subspecies to be holartica rather than tularensis. The 1990 macaque case had a weak positive result on real-time PCR, but the nested PCR analysis did not yield useable bands.
Discussion
A tularemia epizootic occurred in outdoor-housed rhesus macaques during 2010 in the context of a serologically naïve population prior to the outbreak. In the year after the outbreak, no cases of tularemia were identified in either the macaques or the local small mammals. However, the serosurvey of the macaque population documented extensive seroconversion among macaques, particularly those in the southern colony, where most clinical cases occurred. The northern and southern housing areas are separated by less than 0.2 mi, but the increased incidence of disease and seropositivity in the southern enclosures suggests differences in exposure to infected vectors and reservoirs. Although not confirmed statistically, considerably less rodent activity was observed during trapping in the vicinity of the northern colony.
Clinical disease was limited to macaques younger than 4 y, although the serosurvey showed that older animals seroconverted at similar rates as those of younger animals in each of the housing locations. This finding is consistent with previous reports of tularemia in outdoor-housed cynomolgus macaques23 and rhesus macaques,10 in which fatal disease occurred primarily in the young animals but older animals had seroconverted. A large proportion of our young animals likely had subclinical disease, given that more than half of the macaques tested in the southern colony showed positive serology after the epizootic, and the 19 clinical cases represented a very small proportion of the population. The subclinical infections could affect research conducted on animals during a tularemia outbreak. Antibodies are present 1 to 2 wk after infection but do not peak for 1 to 2 mo, thereby complicating rapid diagnosis in subclinically infected animals.3 The increased seroprevalence observed in female macaques in the southern colony was unexpected, given that they are housed in mixed-sex groups, and the literature does not report such a sex-associated bias.
Serology for tularemia has the potential to cross react with several bacterial antigens, including Yersinia, Brucella, and Salmonella. Setting the diagnostic threshold to 1:160 diminishes the risk of cross-reactivity.3,11 The absence of positive serologic results in the macaque colony prior to the outbreak supports that cross-reactivity was unlikely and therefore not a possible source of false-positive results.
The subspecies was identified as F. tularensis subsp. holartica in the 2010 macaque cases as well as 2 of the historic nonhuman primate cases. This subspecies has been associated with waterborne transmission, and infection has been documented in the widest variety of species including mammals, birds, amphibians, and invertebrates.11,19 F. tularensis subsp. holartica is the subspecies that has been isolated most frequently from captive nonhuman primates in the United States.20 Although F. tularensis subsp. tularensis generally is considered to have a higher case fatality rate, in the western United States F. tularensis subsp. holartica has a higher case fatality rate than does the local F. tularensis subsp. tularensis A2.20 Rodents are infected with F. tularensis subsp. holartica more commonly than are lagomorphs.20 Although no infected rodents were found in 2011, they very likely were involved in the amplification and transmission of tularemia to the primates in 2010. Primates are not considered to be either donor or recipient hosts12 that would develop sufficiently high bacterial loads to infect vectors. The pattern of lesions seen in the 2010 primate cases was consistent with exposure via ingestion of infective material. Direct transmission from primate to primate potentially could occur via bite wounds but is not consistent with the presence of mesenteric lymphadenopathy. Primate outbreaks of tularemia in zoos often involve contact with wild rodents.12
None of the evaluated rodents were seropositive in the year after the outbreak. Perhaps none of the infected rodents survived or none generated long-lasting or high titers after recovery. An alternative possibility is that the sample size was inadequate to reveal low seroprevalence. We do not suspect that sample size is the reason that no seropositive rodents were found, in light of findings from recent studies that sampled large numbers of rodents. A study of tularemia during an interepizootic period found none of the 177 rodents tested was seropositive on slide agglutination; both rodents and ectoparasites were PCR negative.14 Another study found 183 rodents from an endemic area were negative on serology,18 including 15 that were positive on PCR.
Although none of the fleas we sampled were positive for F. tularensis, they have been documented as competent vectors for spread among rodents.24 Fleas and lice are not considered to be important vectors for transmission to humans, but these pests can serve to spread tularemia within rodent populations.12,24 In the ground squirrels evaluated, considerable numbers of both fleas and lice were present. Whenever tularemia is introduced into the local ground squirrel population, these ectoparasites can serve to spread disease among the rodents, providing local disease amplification. In addition, disease transmission within a ground squirrel colony is aided by cannibalism of carcasses, which is seen in times of overpopulation.12
Ticks serve as a biologic vector of tularemia and are potentially the reservoir during interepizootic periods.11,12 Although we collected no ticks, the disease may have been introduced to the area when an animal with a greater roaming range than those of the small mammals we evaluated carried infected ticks into the vicinity of the primate center. In the current study, a raccoon was the only seropositive wild animal, indicating previous infection. Raccoons have been implicated in other cases of tularemia.12 A few species of wild mammals have been seen near the primate enclosures but were not collected: opossums (Didelphis virginiana), gray foxes (Urocyon cinereoargenteus), and occasionally coyotes (Canis latrans). Like the sampled mammals, these species have the potential to transmit disease to rodents and arthropods that have direct contact with nonhuman primates.12
Tularemia poses a threat to both the outdoor-housed macaques themselves and the people caring for them. Although human-to-human transmission has not been documented, the disease has been transmitted to people by the bite of an infected animal.12,24 In addition, people are at risk from the same arthropod vectors that can introduce the disease into macaques.7 Although humans are unlikely to ingest infective material, in the setting of a primate center, there is risk for the diagnostic personnel. Clinical pathology and pathology staff have contracted tularemia from exposure to infected tissues or culture isolates.1,8,12
After diagnosis of tularemia in the macaque colony during 2010, the pest control program increased efforts to live trap and euthanize pest species to prevent contact between primates and wild rodents. Employee education regarding tularemia was enhanced, and diagnostic personnel were notified whenever tularemia was a differential diagnosis. Fortunately, no employees developed tularemia. Formalin-fixed and paraffin-embedded tissues were tested successfully by both the real-time and nested standard PCR assays. By reducing the handling of fresh tissues and avoiding culture, the risk of transmission to diagnostic personnel can be minimized.
A single year of sample collection does not provide sufficient data to fully characterize the ecology of F. tularensis during interepizootic periods. Additional studies are in progress to evaluate the risk factors for tularemia in different outdoor-housing locations for nonhuman primates in northern California. Areas for future research include investigation of the roles of biting flies and mosquitoes32 in the transmission of tularemia, of wild rodents in amplification of tularemia during an epizootic, and of local bodies of water in the maintenance of endemic tularemia.
Acknowledgments
We thank Dr Amanda Koehne, Dr Takayuki Tanaka, Dr Philip Kass, and the members of Dr Janet Foley's lab for expert advice and technical support. This work was supported by the NIH R25 training grant RR024232.
References
- 1.Abril C, Nimmervoll H, Pilo P, Brodard I, Korczak B, Markus S, Miserez R, Frey J. 2008. Rapid diagnosis and quantification of Francisella tularensis in organs of naturally infected common squirrel monkeys (Saimiri sciureus). Vet Microbiol 127:203–208 [DOI] [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended. 2008. 7 USC §2131–2159.
- 3.Becton DaC. 2010. Febrile antigens for febrile antigen agglutination tests. Package insert. Sparks (MD): Becton Dicknson [Google Scholar]
- 4.Birdsell DN, Stewart T, Vogler AJ, Lawaczeck E, Diggs A, Sylvester TL, Buchhagen JL, Auerbach RK, Keim P, Wagner DM. 2009. Francisella tularensis subsp. novicida isolated from a human in Arizona. BMC Res Notes 2:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broekhuijsen M, Larsson P, Johansson A, Bystrom M, Eriksson U, Larsson E, Prior RG, Sjostedt A, Titball RW, Forsman M. 2003. Genome-wide DNA microarray analysis of Francisella tularensis strains demonstrates extensive genetic conservation within the species but identifies regions that are unique to the highly virulent F. tularensis subsp. tularensis. J Clin Microbiol 41:2924–2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention and National Institutes of Health 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. Bethesda (MD): Department of Health and Human Services [Google Scholar]
- 7.Dantas-Torres F, Chomel BB, Otranto D. 2012. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol 28:437–446 [DOI] [PubMed] [Google Scholar]
- 8.Evans ME. 1985. Francisella tularensis. Infect Control 6:381–383 [PubMed] [Google Scholar]
- 9.Farlow J, Wagner DM, Dukerich M, Stanley M, Chu M, Kubota K, Petersen J, Keim P. 2005. Francisella tularensis in the United States. Emerg Infect Dis 11:1835–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrecchia CE, Colgin LM, Andrews KR, Lewis AD. 2012. An outbreak of tularemia in a colony of outdoor-housed rhesus macaques (Macaca mulatta). Comp Med 62:316–321 [PMC free article] [PubMed] [Google Scholar]
- 11.Foley JE, Nieto NC. 2010. Tularemia. Vet Microbiol 140:332–338 [DOI] [PubMed] [Google Scholar]
- 12.Friend M. 2006. Tularemia. Reston (VA): US Geological Survey, circular 1297 [Google Scholar]
- 13.Garcia Del Blanco N, Dobson ME, Vela AI, De La Puente VA, Gutierrez CB, Hadfield TL, Kuhnert P, Frey J, Dominguez L, Rodriguez Ferri EF. 2002. Genotyping of Francisella tularensis strains by pulsed-field gel electrophoresis, amplified fragment length polymorphism fingerprinting, and 16S rRNA gene sequencing. J Clin Microbiol 40:2964–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyuranecz M, Rigo K, Dan A, Foldvari G, Makrai L, Denes B, Fodor L, Majoros G, Tirjak L, Erdelyi K. 2011. Investigation of the ecology of Francisella tularensis during an interepizootic period. Vector Borne Zoonotic Dis 11:1031–1035 [DOI] [PubMed] [Google Scholar]
- 15.Hubbart JA, Jachowski DS, Eads DA. 2011. Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). J Vector Ecol 36:117–123 [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 17.Katz L, Orr-Urteger A, Brenner B, Hourvitz A. 2002. Tularemia as a biological weapon. Harefuah 141 Spec No:78–83, 120 [PubMed] [Google Scholar]
- 18.Kaysser P, Seibold E, Matz-Rensing K, Pfeffer M, Essbauer S, Splettstoesser WD. 2008. Re-emergence of tularemia in Germany: presence of Francisella tularensis in different rodent species in endemic areas. BMC Infect Dis 8:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keim P, Johansson A, Wagner DM. 2007. Molecular epidemiology, evolution, and ecology of Francisella. Ann N Y Acad Sci 1105:30–66 [DOI] [PubMed] [Google Scholar]
- 20.Kugeler KJ, Mead PS, Janusz AM, Staples JE, Kubota KA, Chalcraft LG, Petersen JM. 2009. Molecular epidemiology of Francisella tularensis in the United States. Clin Infect Dis 48:863–870 [DOI] [PubMed] [Google Scholar]
- 21.Lewis RE. 2002. A review of the North American species of Oropsylla Wagner and Ioff, 1926 (Siphonaptera: Ceratophyllidae: Ceratophyllinae). J Vector Ecol 27:184–206 [PubMed] [Google Scholar]
- 22.Lewis RE, Lewis JH, Maser C. 1988. The fleas of the Pacific Northwest. Corvallis (OR): Oregon State University Press [Google Scholar]
- 23.Matz-Rensing K, Floto A, Schrod A, Becker T, Finke EJ, Seibold E, Splettstoesser WD, Kaup FJ. 2007. Epizootic of tularemia in an outdoor-housed group of cynomolgus monkeys (Macaca fascicularis). Vet Pathol 44:327–334 [DOI] [PubMed] [Google Scholar]
- 24.McCoy G, Chapin CW. 1912. Further observations on a plague like disease of rodents with a preliminary note on the causative agent, bacterium tularense. J Infect Dis 10:61–72 [Google Scholar]
- 25.McCoy GW. 1911. A plague-like disease of rodents. Public Health Bulletin 45:53–71 [Google Scholar]
- 26.Medicine NLo [Internet]. BLAST. Standard Nucleotide BLAST. [Cited 30 April 2012]. Available at: http://blast.ncbi.nlm.nih.gov
- 27.Metzger DW, Bakshi CS, Kirimanjeswara G. 2007. Mucosal immunopathogenesis of Francisella tularensis. Ann N Y Acad Sci 1105:266–283 [DOI] [PubMed] [Google Scholar]
- 28.Molins CR, Carlson JK, Coombs J, Petersen JM. 2009. Identification of Francisella tularensis subsp. tularensis A1 and A2 infections by real-time polymerase chain reaction. Diagn Microbiol Infect Dis 64:6–12 [DOI] [PubMed] [Google Scholar]
- 29.Molins-Schneekloth CR, Belisle JT, Petersen JM. 2008. Genomic markers for differentiation of Francisella tularensis subsp. tularensis A.I and A.II strains. Appl Environ Microbiol 74:336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nigrovic LE, Wingerter SL. 2008. Tularemia. Infect Dis Clin North Am 22:489–504 [ix.] [DOI] [PubMed] [Google Scholar]
- 31.Plumb DC. 2011. Plumb's veterinary drug handbook. Ames (IA): Wiley [Google Scholar]
- 32.Ryden P, Bjork R, Schafer ML, Lundstrom JO, Petersen B, Lindblom A, Forsman M, Sjostedt A, Johansson A. 2012. Outbreaks of tularemia in a boreal forest region depends on mosquito prevalence. J Infect Dis 205:297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Select Agents and Toxins. 2008. 42 CFR Part 73.
- 34.Sjostedt A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci 1105:1–29 [DOI] [PubMed] [Google Scholar]
- 35.Stark HE. 1959. The Siphonaptera of Utah: their taxonomy, distribution, host relations, and medical importance. Atlanta (GA): United States Department of Health and Human Services [Google Scholar]
- 36.Tomaso H, Aldahouk S, Hofer E, Splettstoesser W, Treu T, Dierich M, Neubauer H. 2005. Antimicrobial susceptibilities of Austrian Francisella tularensis holarctica biovar II strains. Int J Antimicrob Agents 26:279–284 [DOI] [PubMed] [Google Scholar]
- 37.Zhang F, Liu W, Chu MC, He J, Duan Q, Wu XM, Zhang PH, Zhao QM, Yang H, Xin ZT, Cao WC. 2006. Francisella tularensis in rodents, China. Emerg Infect Dis 12:994–996 [DOI] [PMC free article] [PubMed] [Google Scholar]