Abstract
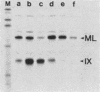
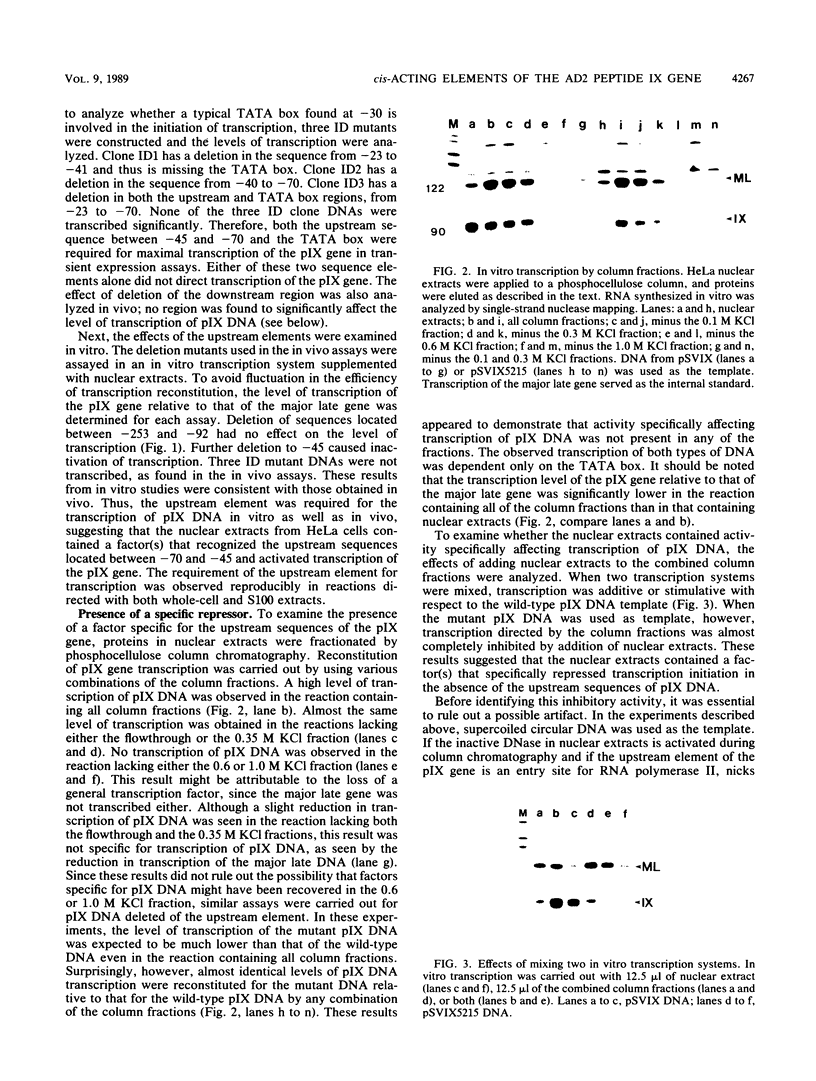
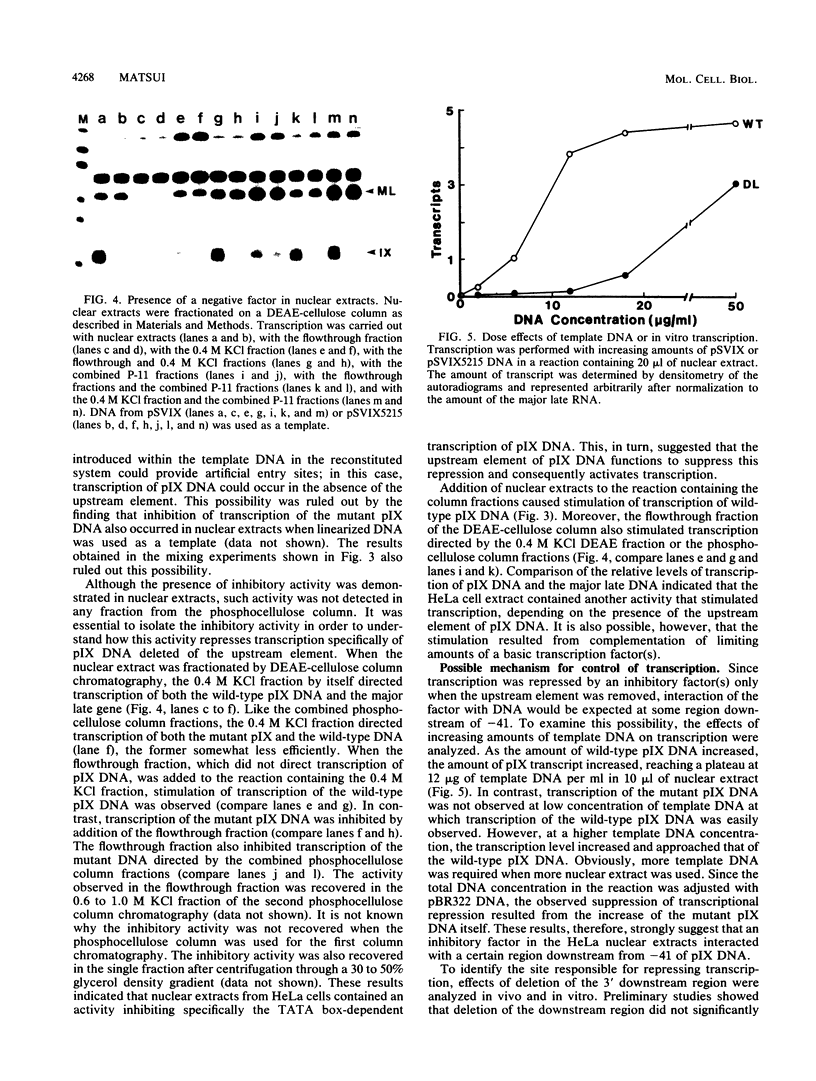
cis-Acting elements involved in transcription of the peptide IX (pIX) gene of adenovirus 2 were identified by using in vivo transient expression assays and two in vitro transcription systems. Deletion of either the sequence between positions -45 and -70 or the TATA box abolished the initiation of pIX gene transcription in vivo and transcription with HeLa cell nuclear extracts in vitro. These results initially suggested the presence of a positive factor acting on the upstream element. However, when proteins in the nuclear extract were fractionated by column chromatography and analyzed by reconstitution of transcription in vitro, it was found that a certain fraction could direct TATA box-dependent transcription initiation even in the absence of the upstream element. Furthermore, activity inhibiting TATA box-dependent transcription was found in the nuclear extract. In contrast, inhibition of TATA box-dependent transcription was suppressed by deletion of a downstream sequence between positions +33 and +122. These results indicate that the TATA box of the pIX gene by itself has the ability to direct initiation of constitutive transcription but that the function of this element is under negative control by a repressor acting on a downstream sequence. Thus, the upstream element of the pIX gene appears to have a novel function: suppression of the transcriptional repression exerted by a downstream sequence, leading to a net transcription activation. Possible mechanisms for transcription initiation of pIX DNA are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison M. L. Enhancers: mechanisms of action and cell specificity. Annu Rev Cell Biol. 1988;4:127–153. doi: 10.1146/annurev.cb.04.110188.001015. [DOI] [PubMed] [Google Scholar]
- Coffino P., Knowles B., Nathenson S. G., Scharff M. D. Suppression of immunoglobulin synthesis by cellular hybridization. Nat New Biol. 1971 May 19;231(20):87–90. doi: 10.1038/newbio231087a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. A., Wu F. K., Mitsuyasu R., Gaynor R. B. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 1987 Dec 1;6(12):3761–3770. doi: 10.1002/j.1460-2075.1987.tb02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Greenberg A., Ber R., Kra-Oz Z., Laskov R. Extinction of expression of immunoglobulin genes in myeloma X fibroblast somatic cell hybrids. Mol Cell Biol. 1987 Feb;7(2):936–939. doi: 10.1128/mcb.7.2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T. W., Horikoshi M., Roeder R. G., Green M. R. Analysis of the role of the transcription factor ATF in the assembly of a functional preinitiation complex. Cell. 1988 Sep 23;54(7):1043–1051. doi: 10.1016/0092-8674(88)90119-5. [DOI] [PubMed] [Google Scholar]
- Heberlein U., Tjian R. Temporal pattern of alcohol dehydrogenase gene transcription reproduced by Drosophila stage-specific embryonic extracts. Nature. 1988 Feb 4;331(6155):410–415. doi: 10.1038/331410a0. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Carey M. F., Kakidani H., Roeder R. G. Mechanism of action of a yeast activator: direct effect of GAL4 derivatives on mammalian TFIID-promoter interactions. Cell. 1988 Aug 26;54(5):665–669. doi: 10.1016/s0092-8674(88)80011-4. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Hai T., Lin Y. S., Green M. R., Roeder R. G. Transcription factor ATF interacts with the TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988 Sep 23;54(7):1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Harney J. W., Moore D. D. Repression mediates cell-type-specific expression of the rat growth hormone gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8283–8287. doi: 10.1073/pnas.83.21.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T. In vitro accurate initiation of transcription on the adenovirus type 2 IVa2 gene which does not contain a TATA box. Nucleic Acids Res. 1982 Nov 25;10(22):7089–7101. doi: 10.1093/nar/10.22.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Murayama M., Mita T. Adenovirus 2 peptide IX gene is expressed only on replicated DNA molecules. Mol Cell Biol. 1986 Dec;6(12):4149–4154. doi: 10.1128/mcb.6.12.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Nir U., Walker M. D., Rutter W. J. Regulation of rat insulin 1 gene expression: evidence for negative regulation in nonpancreatic cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3180–3184. doi: 10.1073/pnas.83.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periman P. IgG synthesis in hybrid cells from an antibody-producing mouse myeloma and an L cell substrain. Nature. 1970 Dec 12;228(5276):1086–1087. doi: 10.1038/2281086a0. [DOI] [PubMed] [Google Scholar]
- Remmers E. F., Yang J. Q., Marcu K. B. A negative transcriptional control element located upstream of the murine c-myc gene. EMBO J. 1986 May;5(5):899–904. doi: 10.1002/j.1460-2075.1986.tb04301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Venkatesh L. K., Chinnadurai G. Activation of the adenovirus 2 protein IX promoter by DNA replication in a transient expression assay. Nucleic Acids Res. 1987 Mar 11;15(5):2235–2250. doi: 10.1093/nar/15.5.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Imler J. L., Chatton B., Schatz C., Wasylyk C. Negative and positive factors determine the activity of the polyoma virus enhancer alpha domain in undifferentiated and differentiated cell types. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7952–7956. doi: 10.1073/pnas.85.21.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Weiss M. C., Chaplain M. Expression of differentiated functions in hepatoma cell hybrids: reappearance of tyrosine aminotransferase inducibility after the loss of chromosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3026–3030. doi: 10.1073/pnas.68.12.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]