Abstract
Background
Hexachlorobenzene (HCB) is a ubiquitous environmental contaminant that, even at low doses, causes destruction of ovarian primordial germ cells in experimental studies. However, its potential for reproductive toxicity in humans exposed to background levels has not been fully evaluated. Here we examined the association between maternal levels of HCB and their infants’ birth weight.
Methods
HCB was measured in milk samples from a subset of women in the Norwegian Human Milk Study (HUMIS), 2003–2006. 300 subjects were randomly chosen from the cohort and 26 from all small for gestational age (SGA) children. Additional information was obtained through questionnaires and the Medical Birth Registry.
Results
Overall, HCB was associated with birth weight (adjusted b=−90 g per eight μg/kg milk fat, 95 % CI −275 to 8) and with SGA (OR 1.8, 95 % CI 0.9–3.7 per eight μg/kg milk fat (difference between the 10th and the 90th percentile)). After stratification, however, the association was present only among smokers. For birth weight for past or current smokers: b=−282, CI −467 to −98; for never smokers: b=0.5, CI −149 to 150, p-value for interaction: 0.01. Similar results were observed for head circumference, crown-heel length, and SGA.
Conclusions
We saw a moderate association between HCB and markers of impaired fetal growth among past and current smokers. This finding may be non-causal and due to underlying genetic variants tied to both growth and breakdown of HCB or to confounding by unmeasured toxicants that coexist in exposure sources. It may, however, also result from HCB exposure.
Keywords: Hexachlorobenzene, small for gestational age, birth weight, ponderal index, POPs
Introduction
Hexachlorobenzene (HCB) is a widespread environmental contaminant regulated by the Stockholm convention (1). Following the ban on its use as a fungicide, environmental levels of HCB have declined by more than 90%. However, HCB is still used as an industrial chemical and it is an unintended by-product from several processes, such as production of chlorinated solvents (2). Therefore, population exposure to HCB is likely to continue at current levels (3).
The most disturbing report on HCB toxicity dates back to the 1950s, when part of the Turkish population was accidentally exposed to high levels through intake of HCB-treated seeds. Breastfed children of exposed mothers developed the lethal disease “Pembe yara” (“pink sore”)(4). After the incident there were reports of villages without children below the age of five years (5). If these anecdotal reports are even partly true, HCB would qualify as among the most potent reproductive toxicants identified.
The reproductive toxicity of HCB has been confirmed in experimental studies. Destruction of ovarian primordial germ cells and changes in the surface of the ovarian germinal epithelium was observed in monkeys and rats exposed to HCB (6–8). These effects were dose-dependent, and seen at low doses (7).
Adverse reproductive outcomes after exposure to background levels of HCB in human populations have been examined in a number of studies, with inconsistent findings. While some studies have shown a positive association with spontaneous abortion (9), low birth weight (3), decreased crown-rump length (10), and reduced gestational length (11), others have reported no association (12–14), or non-linear associations with the reproductive outcome studied (15). The reason for this inconsistency is not clear, but lack of statistical power, residual confounding and differences in susceptibility between populations may explain some of the variation. Because exposure levels varied greatly between studies it would be of interest to examine whether the inconsistent findings could be due to differential response to varying exposure levels, e.g. threshold effects.
In conclusion, surprisingly few studies have examined HCB effects on reproductive health, and 50 years after the mass exposure in Turkey, its potential toxicity for human populations exposed to background levels has not been fully evaluated.
The aim of this study was to investigate the association between level of exposure to HCB and a number of reproductive outcomes in a Norwegian birth cohort. We aimed to take into consideration a number of methodological issues that may have contributed to the heterogeneity of previous findings by exploring the possibility of threshold effects and effect modification, while controlling for coexisting environmental contaminants.
Methods and Materials
Study population
We used data from the “Norwegian Human Milk Study” (HUMIS), an ongoing multi-center birth-cohort of mothers and their newborns. Recruitment took place in five counties, which represent the northern, southern, western, and eastern parts of Norway (Rogaland, Telemark, Troms, Finmark and Oppland). Health Visitors routinely see all families in Norway approximately two weeks after a birth, and participants were recruited at these visits. Mothers who were fluent in Norwegian, and who were resident in the geographical area under the responsibility of the recruiting Health Visitors were eligible. Nearly all Norwegian women initiate breastfeeding (98–99% (16, 17)) thus breastfeeding was not set as an eligibility criterion. Mothers included in this analysis gave birth between February 2003 and October 2006. Overall, 36% of the invited women declined to participate in the study. Participating mothers were sent containers for breast milk and a questionnaire as soon as a written informed consent was received.
Mothers were asked to save a hand-milked 25 ml sample on the morning of each of eight days before the child was two months of age. The sampling on multiple days was done to reduce within-subject variability in estimated level of exposure. The milk was kept in a 250 ml container kept in the freezer. Minor changes in sampling protocol and milk samples collected by pump were accepted. Date and time of collection were recorded for each sample, as well as whether a milk pump had been used. When the container had been filled, participants mailed it by regular mail to the Norwegian Institute of Public Health in Oslo, where it was stored at −20 °C upon arrival.
Data on last menstrual date, date of delivery estimated by ultrasound, head circumference, and length of the newborn were obtained from the Medical Birth Registry of Norway (MBR) (18). Information on demographic and other factors was obtained from a HUMIS questionnaire filled in by the mother when the child was approximately one month old. Information on maternal smoking at the beginning of pregnancy was obtained from the MBR. Women bring their pregnancy record to the delivery unit, where data on smoking are transferred by the midwives to the MBR notification form. Information on past smoking among current non-smokers is not available in MBR, however it was obtained in HUMIS. Information from HUMIS was also used if MBR had missing information on smoking during pregnancy (23 never smokers, one occasional smoker). In case of discrepancy, anyone who reported being a smoker in either source was classified as such (11 occasional smokers and 4 daily smokers, according to HUMIS data, but not in MBR), except in the sensitivity analysis in which only the prospective data on smoking during pregnancy was used (MBR data). Information on alcohol consumption during pregnancy was obtained from the MoBa study (19) for 209 subjects (64.1%) who participated in both studies. Information on alcohol use was available for 91 % of these 209 subjects. The study was approved by the Norwegian Data Inspectorate and the Regional Ethics Committee for Medical Research. As of November 2006, 2,146 mothers had agreed to participate in the HUMIS study, but 6.5% could not provide milk and 24.5% did not return milk samples for other or unspecified reasons. Altogether 662 mothers were lost (31 %).
Among the remaining 1,491 mothers, 300 were randomly selected, stratifying by county of residence, to have their milk samples analyzed for contaminants. In addition, due to preliminary results suggesting an association between HCB and birth weight, we included 26 mothers of small for gestational age (SGA) infants (the number limited by the availability of funding), randomly selected among all the remaining mothers of SGA infants.
Outcome Variables
We examined five continuous outcomes (gestational age, birth weight, head circumference, length, and ponderal index) and two binary outcomes (small for gestational age (SGA) and diagnosis of fetal growth restriction as reported by the mothers). Gestational age was calculated based on the last menstrual period. In Norway, ultrasound is routinely performed in the second trimester, and we used the ultrasound-based estimate only if the discrepancy between the two exceeded 14 days (20, 21). Ponderal index was defined as birth weight divided by the cube of crown-heel length (kg/m3) (22, 23). SGA was defined as having a birth weight below the 10th percentile at the attained gestational age. Sex-specific Norwegian standards were used, based on data from the MBR (18). Intrauterine growth restriction was based on whether the mother reported “yes” to “fetal growth restriction confirmed by ultrasound” among the problems she encountered during pregnancy.
Chemical analysis
The absence of contaminants in the containers used for milk sampling was verified before use. Concentrations of hexachlorobenzene (HCB), beta-hexachlorocyclohexane (β-HCH), 1,1-dichloro-2,2-bis (p-chlorophenyl) ethylene (p,p′-DDE), ten non-dioxin-like polychlorinated biphenyls (ndl-PCBs) (IUPAC nos.: 28, −52, −74, −99, −101, −138, −153, −170, −180, and 194), and eight dioxin-like mono-ortho PCBs (mo-PCBs) (IUPAC nos.: −105, −114, −118, −123, −156, −157, −167 and 189) were measured in approximately 15 ml of breast milk at the Norwegian School of Veterinary Science in Oslo. Prior to extraction, the internal standards PCBs 29 and 112 were added. Extraction, lipid clean-up and determination of organochlorine pesticides and ndl-PCBs were performed on a Gas Chromatograph- Electron Capture Detector (GC-ECD) and the eight dioxin-like mono-ortho PCBs (mo-PCBs) (IUPAC nos.: −105, −114, −118, −123, −156, −157, −167 and 189) were determined on a GC coupled to a low resolution mass spectrometer according to previously established methods (24, 25). See online appendix for more details on the chemical analysis. Lower bound values were imputed for non-detected concentrations of chemicals. All samples contained detectable concentrations of HCB.
The association between PCBs and birth weight in the present study will be reported separately.
Data analysis
We divided subjects into 3 categories of HCB exposure using cut-off points set 8 μg/kg lipid apart (chosen because it corresponded to the difference between the 10th and the 90th percentiles) and examined the crude and adjusted associations with outcomes across these categories. We also examined HCB as a continuous variable with reexpression so that the units were per 8 μg/kg lipid increment. Linear regression models were used for the analysis of continuous outcomes and were based on the random study sample of 300 subjects. The 26 extra SGA infants were not included in this model because outcome-dependent sampling results in biased estimates in traditional linear regression models. However, we added these infants when examining SGA and intrauterine growth restriction in logistic regression models, as odds ratios can be correctly estimated in a case-control design (p 170 in Agresti (26)).
In the linear regression models we included a priori the following covariates: gestational age (days), gestational age squared, maternal age at delivery (years), pre-pregnancy BMI (kg/m2) and height (cm), all entered as continuous variables, and sex, smoking (past (before pregnancy) or current smokers (at the beginning of pregnancy): daily less than 10, daily more than 10 cigarettes) and parity (none, one, and more than one previous child), entered in categories. We also considered as potential confounders the number of days from delivery to start of milk sampling, maternal education as a socioeconomic index (less than 12, 12, 13 to 16, and >16 years of education), high fatty fish intake past year (2 or more fatty fish dinner meals per week; seven bread meals with fatty fish was considered equal to one dinner meal), being a participant in the Norwegian Mother and Child Cohort Study (yes, no), Norwegian nationality, county of residence, number of months of exclusive breastfeeding of previous children, β-HCH, p,p′-DDE, sum of all PCB congeners, and sum of 6 PCBs (nos.: −28, −52, −101, −138, −153, −180) by evaluating them one by one in models that included the a priori confounders. The results were very similar whether we adjusted for the sum of all PCBs or the sum of 6 PCBs. To facilitate comparability with other studies, we show estimates adjusted for the sum of 6 PCBs.
After identifying the initial model, we reevaluated each excluded variable. For both steps, the inclusion of a covariate depended on whether it changed the estimate for continuous HCB by 10% or more. For consistency, all variables that were selected in this manner for one or more of the outcomes were kept in all models.
Effect modification of HCB on the outcomes was evaluated for sex and maternal smoking, by using both stratified analysis and cross-product terms in the models.
Missing values of pre-pregnancy BMI (n=5), height (n=3), and child’s age at milk sampling (n=11) were replaced by mean values. We used a separate category for missing values on education (n=6). We show the model including subjects with imputed values, but all final adjusted models were fit with and without imputed values, and the estimates were similar.
We used generalized additive models (GAM) to explore whether the relation between the outcome and HCB was linear (on the logit scale for the binary outcomes) (27). For each outcome, the linear effect of HCB was replaced by a smooth, possibly non-linear function of HCB, followed by a test of deviation from linearity.
In order to evaluate potential confounding by alcohol use during pregnancy, we repeated our analysis for all outcomes, using the final fully adjusted model, in the subsample of women for whom this information was available (never (n=149), less than monthly (n=24), one to four times per month (n=11). No one reported use of alcohol more often than one to four times per month. We also repeated the analyses using only prospective smoking data (MBR), in order to evaluate a potential effect of recall bias on the results.
Statistical analysis was performed using SPSS 14 software and STATA 9, except for the hierarchical clustering and generalized modeling, which was performed using Splus 7.0.6.
RESULTS
To assess to what extent participants may have differed from the general population, we compared the mothers in our study with the general population of mothers who had recently given birth in Norway, based on data from Norwegian Medical Birth Registry (18) (Table 1). We saw no differences in age, duration of gestation, or child’s birth weight. Participants, however, were less frequently daily smokers or first time mothers. We also examined participants lost to follow up and observed the same pattern: participants were less frequently daily smokers and less often first time mothers. In addition, child’s birth weight and gestational age was higher among participants compared to infants of mothers lost to follow up (median birth weight among the latter was 3541 g and gestational age was 280 days). We also obtained data on education level in the general population from the National Statistics Bureau (http://www.ssb.no/english/) and observed that 40 % of the general population of women in the age range 25 to 35 years have more than 12 years education (17), compared to 68 % in the corresponding age range in our study population (Age range HUMIS 16–44, 10 to 90 percentile 24 to 36 years).
Table 1.
Characteristics of cases and controls, compared to other HUMIS study participants and to the general population of birth-giving mothers in Norway (median values shown for continuous data).
| Random cohort n=300 |
Sampled SGA n=26 |
HUMIS not analyzed n=1165 |
p-valuea |
General populationb n=126182 |
|
|---|---|---|---|---|---|
| Maternal age (years) | 29 | 29 | 30 | 0.45 | 29 |
| Childs birth weight (g) | 3705 | 2797 | 3660 | 0.00 | 3570 |
| Gestational age (days) | 282 | 282 | 281 | 0.65 | 282 |
| Maternal BMI (kg/m2)c | 23 | 22 | 23 | 0.20 | - |
| Maternal height in cm | 168 | 164 | 168 | 0.01 | - |
| Age of child at sampling start (days) | 33 | 34 | 33 | 0.55 | - |
| Maternal parity (%) | 0.02 | ||||
| First child | 31.3 | 61.5 | 38.3 | 43.8 | |
| Second child | 45.0 | 23.1 | 38.7 | 35.3 | |
| Third child or more | 23.7 | 15.4 | 23.0 | 20.8 | |
| Maternal smokingd (%) | 0.07 | ||||
| Never | 60.2 | 38.5 | 54.3 | - | |
| Previous smoking | 23.4 | 38.5 | 30.9 | - | |
| Occasional | 6.4 | 3.8 | 4.5 | 1.1 | |
| Daily | 10.0 | 19.2 | 10.3 | 12.1 | |
| Child sex (%) | 0.68 | ||||
| Female | 49.3 | 42.3 | 47.0 | 48.7 | |
| Male | 50.7 | 57.7 | 53.0 | 51.2 | |
| Maternal education (%) | 0.05 | ||||
| <12 years | 13.6 | 34.6 | 14.9 | - | |
| 12 years | 22.8 | 15.4 | 23.4 | - | |
| 13 to 16 years | 41.8 | 34.6 | 35.9 | - | |
| More than 16 years | 21.8 | 15.4 | 25.9 | - | |
| Nationality (%) | 0.33 | ||||
| Norwegian | 90.6 | 88.5 | 87.5 | - | |
| Not Norwegian | 9.4 | 11.5 | 12.5 |
Differences between cases, controls and remaining HUMIS participants evaluated through Kruskal Wallis test for continuous variables and chi-square test for categorical variables.
Information on the general population of recent mothers was obtained through the Norwegian Medical Birth Registry (which records all births in Norway).32 Mothers who had given birth between 2001 and 2003 were selected and their characteristics compared to the HUMIS participants
Pre-pregnancy BMI.
Smoking status at the beginning of pregnancy. One mother participates with two children.
Among the mothers included in this analysis, sampling of milk usually started when the child was about one month old (median 33, mean 35, range: 2–88 days). Samples were usually collected on eight separate occasions (median 8, mean 7, range: 1– 8), over a period of eight days (median 8, mean 12, range: 1–55 days), and only 26% of subjects had used a milk-pump.
Factors associated with HCB level
The median concentration of HCB in the samples from the 300 randomly selected mothers was 11.5 (mean 12.0) μg/kg milk fat, ranging from 4.4 to 42.0. There was an 8 μg/kg difference in the concentration of HCB between the 10th and the 90th percentiles.
In a multiple linear regression model, HCB level was associated with older age, non-Norwegian nationality, and lower parity (data not shown). The above factors accounted for 18% of the variation in HCB levels. We saw no differences in HCB level as a function of maternal height, pre-pregnancy BMI, education, smoking, sex, infant age when milk sampling started, or intake of fatty fish.
Associations between HCB and outcomes studied
Gestational age
In the crude analysis, the longest gestational age was observed for the middle category of HCB (Table 2). This pattern was also observed in the adjusted analysis (Table 2). However, when HCB was entered continuously, we saw an inverse association between HCB and gestational age (Table 2). These results were strongly influenced by one subject and, when this subject was removed, there was no association between continuous HCB and gestational age (b= −0.8, 95% CI −4.4 to 2.9). Removing the same outlier also changed the adjusted estimate for the highest exposure category of HCB exposure (OR= 2.5, 95 % CI −4.5 to 9.5).
Table 2.
Change in gestational age, birth weight and ponderal index according to HCB levels in human milk among 300 newborns.
| Crude model |
Adjusted model |
|||||
|---|---|---|---|---|---|---|
| N | Mean | β | (95 % CI) | β | (95 % CI) | |
|
|
||||||
| Gestational age (days) | ||||||
| HCB level μg/kg lipid: | ||||||
| 4 to 11.99 | 172 | 280 | ref | ref | ||
| 12 to 19.99 | 110 | 282 | 2.2 | (−0.6 to 5.1) | 3.3 | (−0.05 to 6.7) |
| >20 | 18 | 278 | −2.0 | (−7.8 to 3.9) | 0.5 | (−6.5 to 7.4) |
| Per 8 units increase in HCB: | −2.3 | (−4.8 to 0.3) | −2.8 | (−6.1 to 0.51) | ||
| Birth weight (g) | ||||||
| HCB level μg/kg lipid: | ||||||
| 4 to 11.99 | 172 | 3695 | ref | ref | ||
| 12 to 19.99 | 110 | 3727 | 32 | (−100 to 164) | −50 | (−181 to 80) |
| >20 | 18 | 3497 | −198 | (−466 to 70) | −253 | (−522 to 15) |
| Per 8 units increase in HCB: | −109 | (−224 to 6) | −90 | (−275 to 8) | ||
| Ponderal Index (g/cm3) | ||||||
| HCB level μg/kg lipid: | ||||||
| 4 to 11.99 | 167 | 2.84 | ref | ref | ||
| 12 to 19.99 | 109 | 2.81 | −0.04 | (−0.11 to 0.02) | −0.08 | (−0.20 to −0.004) |
| >20 | 18 | 2.74 | −0.08 | (−0.21 to 0.05) | −0.14 | (−0.29 to 0.01) |
| Per 8 units increase in HCB: | −0.05 | (−0.10 to 0.01) | −0.08 | (−0.15 to −0.004) | ||
The model with gestational age as an outcome was adjusted for parity, maternal smoking, age, height, pre-pregnancy BMI, education, county of residence and the sum of 6 PCB congeners (CB-28, −52, −101, −138, −153, −180). All other models were in addition adjusted for gestational age (plus gestational age squared) and infant’s sex. Only 299 subjects were included in the adjusted analysis due to one subject with missing value for smoking status, while missing values were imputed for 11 subjects (4%).
Birth weight, head circumference, crown-heel length and ponderal index
Adjusted birth weight was lower among newborns exposed to the highest levels of HCB, however the confidence intervals were wide (Table 2).
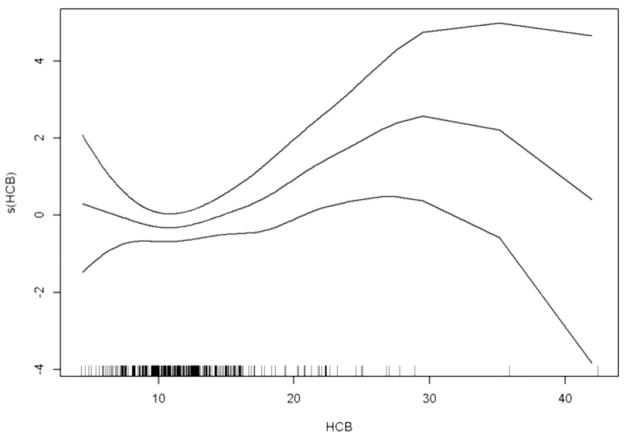
An adjusted GAM plot demonstrates a clear linear dose-response relationship between HCB and birth weight except in the lower and upper borderline areas of HCB levels where there were few observations (Figure 1). The GAM analysis did not reveal any significant deviation from linearity (p value=0.22).
Figure 1.
Estimated smooth effect (solid line) of HCB (μg/kg milk fat) on birth weight by generalized additive models, linearly adjusted for gestational age, gestational age square, sex, parity, maternal smoking, age, height, pre-pregnancy BMI, education, county of residency and sum of the six PCB congeners: CB-28, 52, 101, 138, 153, 180, with 95% confidence interval (dashed lines). The horizontal tick marks at the x-axis indicates the data points.
We saw no evidence of an association between HCB and head circumference and crown-heel length (data not shown). In contrast, we observed a small inverse association between HCB and ponderal index (Table 2).
SGA and reported growth restriction
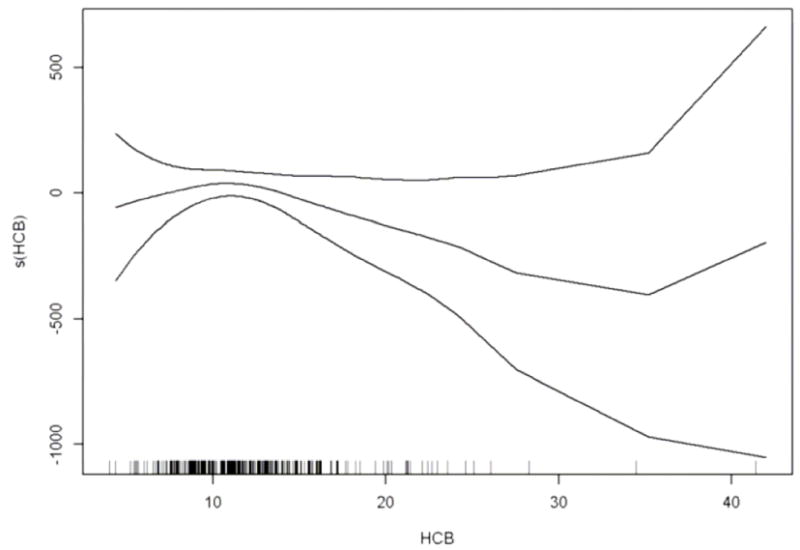
We observed a significant linear trend between categories of HCB and proportion of SGA (Table 3), which was evident in both the crude and adjusted models. A positive association with SGA was also apparent when HCB was entered continuously, although the confidence interval included the null value (Table 3). The GAM analysis supported a linear relationship between HCB and SGA (Figure 2), with no significant deviation from linearity (p value= 0.30) between HCB and SGA, although again the confidence intervals were wide in the tails of the HCB distribution.
Table 3.
Effect of HCB on small for gestational age and intrauterine growth restriction (as reported by the mothers, see text) among 46 SGA cases and 280 controls, according to HCB levels in maternal human milk
| Outcome | Crude model |
Adjusted model |
|||||
|---|---|---|---|---|---|---|---|
| No | Yes | % | OR | (95 % CI) | ORa | (95 % CI) | |
|
|
|||||||
| SGA | |||||||
| HCB level μg/kg lipid: | |||||||
| 4 to 11.99 | 166 | 20 | 10.8 | ref | ref | ||
| 12 to 19.99 | 100 | 18 | 15.3 | 1.5 | (0.8–3.0) | 1.4 | (0.6 to 3.5) |
| >20 | 14 | 8 | 36.4 | 4.7 | (1.8–12.7) | 8.6 | (1.9 to 39) |
| Per 8 units increase in HCB: | 1.9 | (1.2–3.0) | 1.8 | (0.9–3.7) | |||
| Growth restriction | |||||||
| HCB level μg/kg lipid: | |||||||
| 4 to 11.99 | 182 | 4 | 2.2 | ref | (0.7–8.8) | ref | |
| 12 to 19.99 | 112 | 6 | 5.1 | 2.4 | (0.8–26) | 1.4 | (0.3 to 7.7) |
| >20 | 20 | 2 | 9.1 | 4.6 | 5.1 | (0.3 to 79) | |
| Per 8 units increase in HCB: | 2.5 | (1.3 to 4.8) | 2.0 | (0.7 to 5.4) | |||
Adjusted for parity, maternal smoking, age, height, pre-pregnancy BMI, education, county of residence and the sum of 6 PCB congeners (CB-28, −52, −101, −138, −153, −180). Only 325 subjects were included in the adjusted analysis due to one subject with missing value for smoking status, while missing values have been imputed for 13 subjects (4%).
Figure 2.
Estimated smooth effect on the logit scale (solid line) of HCB (μg/kg milk fat) on SGA by generalized additive models, linearly adjusted for parity, maternal smoking, age, height, pre-pregnancy BMI, education, county of residency and sum of the six PCB congeners: CB-28, −52, −101, −138, −153, −180, with 95% confidence interval (dashed lines). The horizontal tick marks at the x-axis indicates the data points.
We also observed a higher percentage of maternally reported intrauterine growth restriction with increasing levels of HCB in both the crude and adjusted analyses. These results were, however, based on small numbers (Table 3).
Effect modification of HCB-effect by maternal smoking and child’s sex
Smoking modified the association between HCB and birth weight. Among babies of past or current smokers (smoking status at the beginning of pregnancy), a decrease of −282 gram (95% CI= −467 to −98) in birth weight was estimated for an eight units increase in HCB, while we saw no association among infants of never-smokers (Table 4). Restricting the analysis to only current smokers yielded similar results.
Table 4.
The association of HCB with studied outcomes according to maternal smoking status at the beginning of pregnancy. The association is shown for an eight units increase in HCB levels in human milk (μg/kg milk fat).
| Current or previous smoker |
Nonsmoker |
||||
|---|---|---|---|---|---|
| β | 95 % CI | β | 95 % CI | p-value interaction | |
|
|
|||||
| Birth weighta | −282 | (−467 to −98) | 0.50 | (−149 to 150) | 0.01 |
| Head Circumferencea | −0.58 | (−1.11 to −0.05) | 0.37 | (−0.06 to 0.80) | 0.00 |
| Crown-heel lengtha | −0.81 | (−1.58 to −0.05) | 0.33 | (−0.29 to 0.94) | 0.01 |
| Ponderal Indexa | −0.11 | (−0.22 to −0.01) | −0.05 | (−.0.13 to 0.03) | 0.30 |
| Gestational ageb | −1.60 | (−6.5 to 3.3) | −2.76 | (−6.7 to 1.2) | 0.70 |
| OR | 95 % CI | OR | 95 % CI | p-value interaction | |
|
|
|||||
| SGAb | 2.7 | (0.99 to 7.6) | 1.5 | (0.6 to 3.3) | 0.28 |
| Growth restrictionb | 3.3 | (0.85 to 12.5) | 1.4 | (0.4 to 5.0) | 0.32 |
Each estimate derives from a separate adjusted model.
Adjusted for gestational age (plus gestational age squared), infant’s sex, parity, maternal age, height, pre-pregnancy BMI, education, county of residence and the sum of 6 PCB congeners (CB-28, −52, −101, −138, −153, −180). Missing values have been imputed for 11 subjects. The analysis were based on 299 subjects for birth weight, 294 for ponderal index, and 295 for head circumference and crown-heel length, due to missing values on smoking or the respective outcomes.
Adjusted for the same variables except gestational age (and gestational age square) and infant’s sex.. One subject was excluded due to missing values for smoking status thus the analysis with SGA and growth restriction were based on 325 subjects, while gestational age was based on 299 subjects. Missing values have been imputed for 13 subjects.
Head circumference, crown-heel-length and ponderal index were also inversely associated with HCB among past or current smokers (Table 4). Similar findings were observed for SGA and intrauterine growth restriction, with stronger associations among past or current smokers, although the confidence interval included the null value (Table 4). No association was observed between HCB and any of the above outcomes among non-smokers (Table 4). The interaction terms for smoking were statistically significant for birth weight, head circumference and crown-heel-length (Table 4). There was no apparent effect modification by smoking for the association between HCB and gestational age.
We saw no evidence of effect modification by sex on the association of HCB with birth weight (p=0. 94), ponderal index (p=0.23), SGA (p=0.69), or gestational age (p=0.82).
Sensitivity analysis
We repeated the fully adjusted final analysis for all outcomes in the subsample of subjects with available data on alcohol consumption during pregnancy and similar results were obtained when adjusting for alcohol use (e.g., the HCB coefficient for birth weight was −.184 when not adjusted for alcohol consumption vs. −.168 when adjusted).
Furthermore, we repeated our analysis using only prospective smoking data, which yielded similar results for all models. A p-value of p=0.011 was obtained for the interaction term between smoking and HCB in the model with birth weight as outcome.
Finally, all the final models were reanalyzed adjusting for number of months of exclusive breastfeeding of previous children, and similar results were obtained for all outcomes.
Discussion
In this analysis, we saw an association between HCB and birth weight, both when examined continuously and when using indicators of fetal growth restriction. Our analyses suggested that this association may, however, be restricted to current and past smokers. We saw a similar pattern for the other anthropometric measures. A stronger effect of environmental toxicants on birth weight among smokers has been reported for PCBs (14, 28), but this is the first study to note an effect modification by smoking for HCB. Smoking is a well known risk factor for low birth weight and, as such, may be important to consider as an effect modifier. Cigarette smoke contains a number of toxins, such as cadmium, cyanide, sulphides, carcinogenic hydrocarbons, benzene, and nicotine, which all may cause direct cellular damage as well as specific biological effects, as recently reported (29). Still, the specific causal mechanism for the association between smoking and birth weight remains unknown. A mechanism for the modifying effects of smoking, if not a chance finding, is therefore unclear.
The association between HCB and the reproductive outcomes examined was unexpected, considering the relatively low levels of HCB recently observed in human milk in Norway. This point to the possibility of a non-causal association. An underlying genetic variant or an unknown environmental factor that is involved in both the breakdown of HCB and fetal growth could account for the observed inverse association (30). However, it is also possible that the relevant exposure window for HCB on these outcomes may have been at an earlier time point, such as during puberty. The women in this study would have been exposed to much higher HCB levels in their youth, because levels were much higher in the past (3, 31, 32). The current ranking of women for HCB levels may simply reflect how they would have been ranked in the past. A recent study concluded that “a single organochlorine measure provides considerable information on relative ranking at distant times” (33), supporting the possibility that the relative ranking of HCB may be stable over time. This hypothetical explanation could, if true, account for the variation in results across studies, since past HCB exposure levels may differ between countries even when recent levels were similar.
Another potential explanation is the possibility of differential effects on outcome depending on exposure dose. Protective mechanisms could be activated at higher levels, preventing the negative effects that are seen at lower levels (34). However, the GAM model did not reveal any significant threshold effects on birth weight across the range of HCB seen in this study. This is in accordance with the only other study that has looked for a non linear relationship, which was also performed in a population with low HCB levels (14). Although there may be threshold effects at higher HCB levels, there are no studies to date to either support or refute this possibility.
The results for gestational age are difficult to interpret. We saw a suggestion of a nonlinear relationship between categorical HCB and gestational age. However, when the one subject with high influence was removed, the relation appeared to be linear. Overall, we cannot draw conclusions on the relation between HCB and gestational age in these data, in part due to the imprecision of the estimates.
Overview of previous findings
The relation between HCB levels and anthropometric measures at birth has been examined in 7 previous studies (3, 10–12, 14, 15, 35). The highest exposure levels were reported in a study from Spain including 70 subjects, with HCB measured in cord blood (10). No association with birth weight or SGA was observed. However, the authors did note a statistically significant inverse association with crown- rump length, suggesting impaired growth (10). Among 107 Inuit children with relatively high HCB levels measured in human milk, an association between HCB and reduced length at birth was also reported, but no association was seen for birth weight (35). In a third study of 197 subjects, still with relatively high levels of HCB measured in milk, no association with birth weight was observed (12). Length was not assessed in this study.
There are two studies with intermediate levels of HCB. HCB measured in human milk in 246 subjects was associated with lower birth weight in a study from Germany (3). In a study of 385 Latino-workers in the US (11), in whom HCB was measured in maternal serum at approximately 26 weeks of gestation, a decrease in birth weight of 96 g per log10-transformed units HCB μg/kg lipid was observed in the crude analysis, an estimate comparable to the crude associations in our study of 109 g per 8 units of HCB. However, when adjusting for maternal weight gain during pregnancy and other factors, the association was reduced to −23 g and was no longer significant. This study observed a significant association between HCB and preterm birth.
In a cross-sectional study of 815 Australian mothers, with levels slightly higher than ours, the associations with the outcomes were explored after categorization of HCB exposure into tertiles (15). A twofold increased risk of low birth weight (OR 2.6, CI 0.96–7.3) was observed for those in the middle category compared to the lowest exposure category, the two categories which corresponded to the range of HCB in our study. No association between the highest exposure category and low birth weight were seen, which could suggest a threshold effect. However, no association with SGA was noted for any category of HCB exposure. Finally, in a study of 722 healthy newborns from the US, where HCB was measured in cord blood (14), levels were comparable to our study, although they had a high proportion of non-detects (detection level equal to median level). No association between HCB and the anthropometric outcomes studied was present in this study.
Limitations and Strengths
The main limitation of our study is the retrospective assessment of exposure, although this is generally not raised as a major concern in studies on chlorinated persistent organic pollutants, due to their long half lives (36). Postnatal HCB exposure is assumed to represent prenatal exposure, and for most persistent organic pollutants the levels in human milk and cord blood do show a very high correlation. However, our knowledge with regard to HCB specifically is more limited (37).
Maternal recall of pregnancy-related events have been shown to have a high degree of validity (38). We evaluated potential recall bias for smoking, the covariate probably most likely to be affected by recall bias, by reanalyzing all models using prospective smoking data and we observed very similar results.
A weakness of our study is the low final response rate. Women with higher education were more likely to participate in the study, which probably also explains the lower proportion of smokers, since smoking is associated with lower socioeconomic status in Norway (Table 1). The results may therefore not be generalizable to the whole population. However, we believe that it is not likely that the validity of the results is affected by selection bias, since that would require differential selection that is related to both HCB and to SGA,. SGA status could affect selection to the study, since SGA babies may need a prolonged stay at an intensive care unit and thus miss the visit by the health visitor by whom recruitment took place (although the birth weight is not very different in the study sample compared to the whole population Table 1). However, we find it unlikely that mothers HCB levels, which are unknown to them, would directly be associated with selection to the study.
This study has some important advantages compared with studies that have used serum or cord blood to assess exposure. First, human milk enables sampling of large volumes. We used 15 ml for the contaminant analysis, an amount usually not available from either cord blood or maternal serum. The large volume and the high fat content of human milk allow measurement with greater precision, especially for contaminants that are present in low concentrations. This is important, since the varying fat concentrations in biological samples of either type imply that a sample with a non-detectable value cannot be assumed to have a lower concentration than a sample with a detectable value. Furthermore, to the best of our knowledge, this is the first study in which milk has been sampled over a prolonged period of time (eight days). This was done in order to reduce the high within-subject variability that has been demonstrated for these contaminants in lactating women (39).
Another advantage of the present study was the availability of data on coexisting environmental contaminants. In all the adjusted models, the estimates were higher after inclusion of selected environmental contaminants, and the stepwise approach revealed that this effect was primarily tied to including PCBs into the model. Since environmental contaminants coexist in exposure sources and may have additive or antagonistic effects, this finding points to the importance of studying a broad array of contaminants that coexist in a specific population. Most studies on HCB have not adjusted for PCBs. Still, adjustment for other contaminants can only partly be achieved, and we cannot exclude the possibility of confounding by other as yet unmeasured persistent organic pollutants that are strongly correlated with HCB exposure in Norway.
In conclusion, we observed an association between HCB levels measured in maternal milk and size at birth, which was, however, restricted to current and past smokers. This association may be non-causal, or may reflect past exposure. Compromised fetal growth has been associated with increased risk of a number of diseases later in life, and understanding what mechanisms lay behind this association has important public health implications (40). The observed effect modification by smoking is intriguing and, if confirmed, may increase our understanding of the mechanisms of action of these contaminants.
Acknowledgments
Sources of financial support: This study was supported by a grant from the Norwegian Research Council and in part by the intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
The authors thank Sharon Lynn Broadwell for handling and preparing the milk samples for chemical analysis and Katharina Løken for her contribution to the analytical work. We also thank the visiting health visitors who were engaged in recruiting mothers to the study, and the mothers for their great efforts.
Abbreviations
- HCB
Hexachlorobenzene
- DDE
1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene
- HCH
Hexachlorocyclohexane
- PCB
Polychlorinated biphenyls
- HUMIS
The Norwegian Human Milk Study
- SGA
Small for gestational age
- POPs
Persistent Organic Pollutants
- GC-ECD
Gas Chromatograph- Electron Capture Detector
- MBR
Medical Birth Registry of Norway
- HUMIS
Norwegian Human Milk Study
Footnotes
Approvals: The study was approved by the Regional Ethics Committee for Medical Research in Norway 30 May 2002, reference S-02122, project title in Norwegian; ”Mat og miljøgifter. Risikable kostvaner til hvilken pris? Effekten av miljøgifter på barns helse” (”Food and environmental toxicants. Risky food choices at what costs. Effects of environmental toxicants on child health”). The study was also approved by the Norwegian Data Inspectorate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1. [updated 2008; cited]. http://www.pops.int/ [Internet]
- 2.Bailey RE. Global hexachlorobenzene emissions. Chemosphere. 2001;43:167–82. doi: 10.1016/s0045-6535(00)00186-7. [DOI] [PubMed] [Google Scholar]
- 3.Schade G, Heinzow B. Organochlorine pesticides and polychlorinated biphenyls in human milk of mothers living in northern Germany: current extent of contamination, time trend from 1986 to 1997 and factors that influence the levels of contamination. Sci Total Environ. 1998;215:31–9. doi: 10.1016/s0048-9697(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 4.Cam CN. Acquired toxic porphyria cutanea tarda due to hexachlorobenzen. 183. 1963. [PubMed] [Google Scholar]
- 5.Jarrell J, Gocmen A. A review of human and sub-human primate toxicity of hexachlorobenzene. Pure Appl Chem. 2000;72:1015–21. [Google Scholar]
- 6.Iatropoulos MJ, Hobson W, Knauf V, Adams HP. Morphological effects of hexachlorobenzene toxicity in female rhesus monkeys. Toxicol Appl Pharmacol. 1976;37:433–44. doi: 10.1016/0041-008x(76)90205-2. [DOI] [PubMed] [Google Scholar]
- 7.Jarrell JF, McMahon A, Villeneuve D, Franklin C, Singh A, Valli VE, et al. Hexachlorobenzene toxicity in the monkey primordial germ cell without induced porphyria. Reprod Toxicol. 1993;7:41–7. doi: 10.1016/0890-6238(93)90008-u. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez L, Randi A, Alvarez P, Piroli G, Chamson-Reig A, Lux-Lantos V, et al. Reproductive effects of hexachlorobenzene in female rats. J Appl Toxicol. 2000;20:81–7. doi: 10.1002/(sici)1099-1263(200001/02)20:1<81::aid-jat629>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Jarrell J, Gocmen A, Foster W, Brant R, Chan S, Sevcik M. Evaluation of reproductive outcomes in women inadvertently exposed to hexachlorobenzene in southeastern Turkey in the 1950s. Reprod Toxicol. 1998;12:469–76. doi: 10.1016/s0890-6238(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 10.Ribas-Fito N, Sala M, Cardo E, Mazon C, De Muga ME, Verdu A, et al. Association of hexachlorobenzene and other organochlorine compounds with anthropometric measures at birth. Pediatr Res. 2002;52:163–7. doi: 10.1203/00006450-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Fenster L, Eskenazi B, Anderson M, Bradman A, Harley K, Hernandez H, et al. Association of in utero organochlorine pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2006;114:597–602. doi: 10.1289/ehp.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gladen BC, Shkiryak-Nyzhnyk ZA, Chyslovska N, Zadorozhnaja TD, Little RE. Persistent organochlorine compounds and birth weight. Ann Epidemiol. 2003;13:151–7. doi: 10.1016/s1047-2797(02)00268-5. [DOI] [PubMed] [Google Scholar]
- 13.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. PCBs, hexachlorobenzene and DDE are not associated with recurrent miscarriage. Am J Reprod Immunol. 2003;50:485–9. doi: 10.1046/j.8755-8920.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 14.Sagiv SK, Tolbert PE, Altshul LM, Korrick SA. Organochlorine exposures during pregnancy and infant size at birth. Epidemiology. 2007;18:120–9. doi: 10.1097/01.ede.0000249769.15001.7c. [DOI] [PubMed] [Google Scholar]
- 15.Khanjani N, Sim MR. Reproductive outcomes of maternal contamination with cyclodiene insecticides, hexachlorobenzene and beta-benzene hexachloride. Sci Total Environ. 2006;368:557–64. doi: 10.1016/j.scitotenv.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Heiberg EE, Helsing E. Changes in breastfeeding practices in Norwegian maternity wards: national surveys 1973, 1982 and 1991. Acta Paediatr. 1995;84:719–24. doi: 10.1111/j.1651-2227.1995.tb13744.x. [DOI] [PubMed] [Google Scholar]
- 17.[Statistics Norway] [[updated 2009; cited]. Available from: http://www.ssb.no/english/
- 18.Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79:440–9. [PubMed] [Google Scholar]
- 19.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 20.Henriksen TB, Hedegaard M, Secher NJ. Standing and walking at work and birthweight. Acta Obstet Gynecol Scand. 1995;74:509–16. doi: 10.3109/00016349509024380. [DOI] [PubMed] [Google Scholar]
- 21.Blondel B, Morin I, Platt RW, Kramer MS, Usher R, Breart G. Algorithms for combining menstrual and ultrasound estimates of gestational age: consequences for rates of preterm and postterm birth. BJOG. 2002;109:718–20. doi: 10.1111/j.1471-0528.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 22.Koo WW, Walters JC, Hockman EM. Body composition in neonates: relationship between measured and derived anthropometry with dual-energy X-ray absorptiometry measurements. Pediatr Res. 2004;56:694–700. doi: 10.1203/01.PDR.0000142587.59238.BD. [DOI] [PubMed] [Google Scholar]
- 23.The Use and Interpretation of Anthropometry. Report of a WHO expert committee; WHO Geneva, Switzerland: 1995. World Health Organization Expert Committee on Physical Status. [PubMed] [Google Scholar]
- 24.Brevik EM. Gas chromatograhic method for the determination of organochlorine pesticides in human milk. Bull Environ Contam Toxicol. 1978;19:281–6. doi: 10.1007/BF01685799. [DOI] [PubMed] [Google Scholar]
- 25.Polder A, Gabrielsen GW, Odland JO, Savinova TN, Tkachev A, Loken KB, et al. Spatial and temporal changes of chlorinated pesticides, PCBs, dioxins (PCDDs/PCDFs) and brominated flame retardants in human breast milk from Northern Russia. Sci Total Environ. 2008;391:41–54. doi: 10.1016/j.scitotenv.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 26.Agresti A. Categorical Data Analysis. 2. John Wiley & Sons, Inc; Hoboken, New Jersey: 2002. [Google Scholar]
- 27.Hastie TJTRJ. Generalised Additive Models. Chapman & Hall; London: 1990. [Google Scholar]
- 28.Rylander L, Stromberg U, Hagmar L. Decreased birthweight among infants born to women with a high dietary intake of fish contaminated with persistent organochlorine compounds. Scand J Work Environ Health. 1995;21:368–75. doi: 10.5271/sjweh.51. [DOI] [PubMed] [Google Scholar]
- 29.Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83:699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Longnecker MP. Pharmacokinetic variability and the miracle of modern analytical chemistry. Epidemiology. 2006;17:350–1. doi: 10.1097/01.ede.0000222510.59457.7b. [DOI] [PubMed] [Google Scholar]
- 31.Furst P. Dioxins, polychlorinated biphenyls and other organohalogen compounds in human milk. Levels, correlations, trends and exposure through breastfeeding. Mol Nutr Food Res. 2006;50:922–33. doi: 10.1002/mnfr.200600008. [DOI] [PubMed] [Google Scholar]
- 32.Skaare JU. Persistent organochlorinated compounds in Norwegian human milk in 1979. Acta Pharmacol Toxicol (Copenh ) 1981;49:384–9. doi: 10.1111/j.1600-0773.1981.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 33.Vo TT, Gladen BC, Cooper GS, Baird DD, Daniels JL, Gammon MD, et al. Dichlorodiphenyldichloroethane and polychlorinated biphenyls: intraindividual changes, correlations, and predictors in healthy women from the southeastern United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2729–36. doi: 10.1158/1055-9965.EPI-08-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabrese EJ, Baldwin LA. Toxicology rethinks its central belief. Nature. 2003;421:691–2. doi: 10.1038/421691a. [DOI] [PubMed] [Google Scholar]
- 35.Dewailly E, Bruneau S, Ayotte P, Laliberte C, Gingras S, Belanger D, et al. Health status at birth of Inuit newborn prenatally exposed to organochlorines. Chemosphere. 1993;27:359–66. [Google Scholar]
- 36.ATSDR. Toxicological profile for Hexachlorobenzene. Atlanta, GA: Agency for Toxic Substances and Diseases Registry; 2002. [PubMed] [Google Scholar]
- 37.Sala M, Ribas-Fito N, Cardo E, De Muga ME, Marco E, Mazon C, et al. Levels of hexachlorobenzene and other organochlorine compounds in cord blood: exposure across placenta. Chemosphere. 2001;43:895–901. doi: 10.1016/s0045-6535(00)00450-1. [DOI] [PubMed] [Google Scholar]
- 38.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10:774–7. [PubMed] [Google Scholar]
- 39.Skaare JU, Polder A. Polychlorinated biphenyls and organochlorine pesticides in milk of Norwegian women during lactation. Arch Environ Contam Toxicol. 1990;19:640–5. doi: 10.1007/BF01183978. [DOI] [PubMed] [Google Scholar]
- 40.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–9. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]