Abstract
The soybean cyst nematode (SCN), Heterodera glycines, is a major factor limiting soybean yield. Experiments were conducted in 2009 and 2010 to determine the effects of liquid swine manure and chemical fertilizer PK on soybean and corn yields, and on SCN population in an SCN-suppressive field (S-Site) and an SCN-conducive field (C-Site) in Minnesota. The experiment was a split-plot design with crop sequences as main plots and fertilizer treatments as subplots. The 2-yr crop sequences were Sus-Sus, Res-Sus, and Corn-Sus, where Sus was SCN-susceptible soybean, and Res was SCN-resistant soybean. The fertilizer treatments were manure, PK, and a nonfertilizer as control. Manure did not reduce SCN egg population density but resulted in 31% lower SCN second-stage juvenile (J2) population density at the S-Site at 45 d after planting (DAP) in 2009. Manure also reduced spiral nematode (Helicotylenchus spp.) population density by 52% compared with PK and nonfertilizer treatments at S-Site at 45 DAP in 2009. The crop sequence of Corn-Sus and Res-Sus reduced the SCN egg and J2 but increased spiral nematode population density at both sites. An increase of 1.4 Mg/ha and 0.5 Mg/ha in yield of susceptible soybean was observed in manure and PK treatments, respectively, at the C-Site in 2009. Corn yield was 2.8 Mg/ha and 5.0 Mg/ha greater when treated with manure than nonfertilizer at the S-Site and C-Site, respectively. This study suggests that soil fertility management may be a useful strategy to alleviate the SCN damage to soybean.
Keywords: Helicotylenchus, Heterodera glycines, management, nematode suppressive soil, swine manure, soybean, soybean cyst nematode
The soybean cyst nematode (SCN), Heterodera glycines, has become a significant problem in soybean production in the United States (Wrather et al., 2003; Monson and Schmitt, 2004; Wrather and Koenning, 2006). Despite the presence of SCN-resistant soybean (Glycine max) cultivars and common annual rotation with corn (Zea mays), SCN management has proven difficult. Successful long-term SCN management strategies should have multiple components and include various means to suppress SCN population density and improve soybean yield. One potential component of SCN management program is to use fertilizer application to improve soil health, which may suppress plant-parasitic nematodes and provide sufficient nutrients for plant growth.
Animal manure is one of most popular organic amendments for soils, because incorporation of animal manure adds nutrients into the soil and can positively affect soil quality by increasing organic matter and improving soil structure (Oka, 2010; Thoden et al., 2011). For example, a 7-yr study showed that the treatment with beef cattle (Bos primigenias) manure increased the average yields of potato tubers by 27% (Kimpinski et al., 2003). In addition, nematicidal compounds generated from decomposition of animal manure may help suppress various soil-borne pathogens, such as the wilt fungus Verticillium dahliae (Lazarovits et al., 2000; Tenuta et al., 2002; Conn et al., 2005). Beef, poultry, and swine (Sus domesticus) manures have been reported as effective in reducing the population densities of lesion (Pratylenchus spp.), root-knot (Meloidogyne spp.), cyst (Heterodera spp.), and other economically important plant-parasitic nematodes in various cropping systems (Kimpinski et al., 2003; Everts et al., 2006; Nahar et al., 2006; Briar et al., 2007; Min et al., 2007; Oka et al., 2007; Mahran et al., 2008a, 2009; Liang et al., 2009). A number of researchers have reported that liquid swine manure had the potential for reducing plant-parasitic nematodes, such as lesion nematodes and SCN (Min et al., 2007; Xiao et al., 2007; Mahran et al., 2008a, 2008b). Xiao et al. (2007) suggested that liquid swine manure controlled SCN effectively within 35 d after application in a greenhouse study, and the mechanism involved volatile fatty acids. The suppressive effect on lesion nematodes increased in liquid swine manure with lower pH levels (Mahran et al., 2008a). However, the effect of liquid swine manure on nematodes was inconsistent in field experiments. A single application of liquid swine manure reduced the population density of plant-parasitic nematodes for up to 3 yr in one field but had little or no effect in another field (Conn and Lazarovits, 2000). Liang et al. (2009) reported significantly greater numbers of cyst nematodes in plots treated with composted swine manure in a long-term field study. In a study by Reynolds et al. (1999), swine manure resulted in greater SCN egg population density at harvest.
The present study represents the first investigation of liquid swine manure’s influence on SCN population dynamics in SCN-suppressive soil (S-Site) as compared with SCN-conducive soil (C-Site). Suppressiveness to SCN at the two field sites used in this study had been demonstrated in greenhouse assays (Chen, 2007; Bao et al., 2011). Nematode populations in the S-Site on susceptible soybean were relatively low compared to the average infestation level in the fields in the region, but SCN population densities were high in the C-Site. Greenhouse studies further demonstrated that this nematode suppressiveness was at least partially attributed to soil microbial activities (Bao et al., 2011).
The long-term goal of this study was to develop sustainable management of SCN by integrating organic amendments into the management program to suppress SCN population densities and improve soybean yield. The specific objectives of this study were (i) to quantify the effects of liquid swine manure and chemical fertilizer (P and K) on SCN population densities, other plant-parasitic nematodes, and crop yield; (ii) to determine the interactive effects of fertilizer treatments and crop rotations on SCN and soybean yield; and (iii) to compare soil chemical properties among fertilizer treatments and crop rotations.
Materials and Methods
Experimental design: Field experiments were conducted in the SCN-suppressive (S-Site, 44° 04′ 21" N, 93° 31′ 24" W) and -conducive (C-Site, 44° 05′ 30" N, 93° 32′ 47" W) fields at Waseca, MN, in 2009 and 2010. The soil at the S-Site was a Nicollet clay loam (fine loamy, mixed, mesic Aquic Hapludoll), and the soil at C-Site was a Webster clay loam (fine loamy, mixed, mesic Endoaquoll). Both sites were planted to SCN-susceptible soybean in 2007 and 2008. For each site, the experiment was a split-plot design with three crop sequences as main plots and three fertilizer treatments as subplots. Each subplot was 6 m long and 3 m wide, and consisted of four rows. Each treatment had four replicates. The crop sequences included Sus-Sus, Res-Sus, and Corn-Sus, where Sus was SCN-susceptible soybean ‘DeKalb 22-52’, Res was SCN-resistant soybean ‘Pioneer Brand R92Y20’, and Corn was ‘DeKalb 50-44’; and the first and second abbreviations represent crops in 2009 and 2010, respectively. All of the soybean and corn cultivars were resistant to glyphosate (2-phosphonomethylamino acetic acid). The fertilizer treatments were manure (liquid swine manure at 37.4 m3/ha or 239 kg total N/ha + 26 kg P/ha + 112 kg K/ha), PK (49 kg P/ha + 93 kg K/ha), and nonfertilizer as control (no fertilizer). The manure was injected into the soil 10 cm under each intended row with 25-cm-wide sweep injector on 22 April 2009, and the corn and soybean were planted on 4 May 2009. The PK fertilizer was broadcast on the soil surface and then incorporated into the soil before planting in 2009. In 2010, all plots were planted to Dekalb 22-52 soybean on 6 May, and no fertilizer was applied. Glyphosate was used for preemergence and postemergence weed control. No insecticide and fungicide were used.
Soil sampling: A composite soil sample consisting of 20 soil cores (2 cm in diam. and 15 to 20 cm deep) was collected from the two central rows of each four-row plot at four different times: prior (to fertilizer application in 2009 or planting in 2010), 45 d after planting (DAP) in 2009 (no sampling at 45 DAP in 2010), midseason (around 2 mon after planting), and harvest. The soil was placed in a plastic bag, stored in a cool room (approximately 10°C), and processed within 2 d.
Soil chemical analysis: Soil samples taken prior to fertilizer application and harvest in 2009, and at harvest in 2010 were analyzed in the University of Minnesota Soil Test Laboratory for pH, organic matter, and concentrations of P, K, Zn, Fe, Cu, and Mn according to Recommended Chemical Soil Test Procedures for the North Central Region (Brown, 1998). In addition, NO3--N (Willis and Gentry, 1987) and NH4+-N (Keeney and Nelson, 1982) levels were determined at 45 DAP in 2009.
Nematode extraction and counting: The soil in each sample was thoroughly mixed before nematode extraction. The SCN egg population density was determined from a subsample of 100 cm3 of soil. Cysts were extracted from the soil using an elutriator (Byrd et al., 1976) and centrifugation in 63% (w/v) sucrose solution for 5 min at 1,500 g. Eggs were released from the cysts by breaking the cysts with a mechanical device (Faghihi and Ferris, 2000) and then collected in a 50-ml tube. They were stored at 4°C before being counted within 2 wk. Another subsample of 100-cm3 soil was used to extract vermiform nematodes using the hand-decanting and sucrose centrifugal-flotation technique (Jenkins, 1964) to determine the densities of SCN second-stage juveniles (J2) and other plant-parasitic nematodes.
Yield measurement: Soybean and corn were harvested in October each year from 6.1 m of the two central rows of each plot. Soybean seed yield was computed at 13.0% water, and corn grain yield was computed at 15.5% water.
Data analysis: The general linear model (GLM) procedures in Statistical Analysis System (SAS) Version 9.2 (SAS Institute, Cary, NC) were used to perform the split-plot analysis of variance (ANOVA). Dependent variables were evaluated for normality and transformed as necessary before performing the ANOVA. The nematode population densities were square-root transformed, while yield data were not transformed. Crop sequences (three levels), fertilizer treatments (three levels), and replicates (four) were treated as whole plots, subplots, and blocks, respectively. Across the two sites, a mixed model ANOVA with site, crop, and fertilizer as fixed factors was used in GLM procedure. The interactions of site with crop and/or fertilizer treatments were significant in most dependent variables, so the data from each site were analyzed separately without the site factor. Means of the treatment of crop sequences and fertilizers within each site were compared with least significant difference (LSD) test at P < 0.05 in GLM procedure.
Results
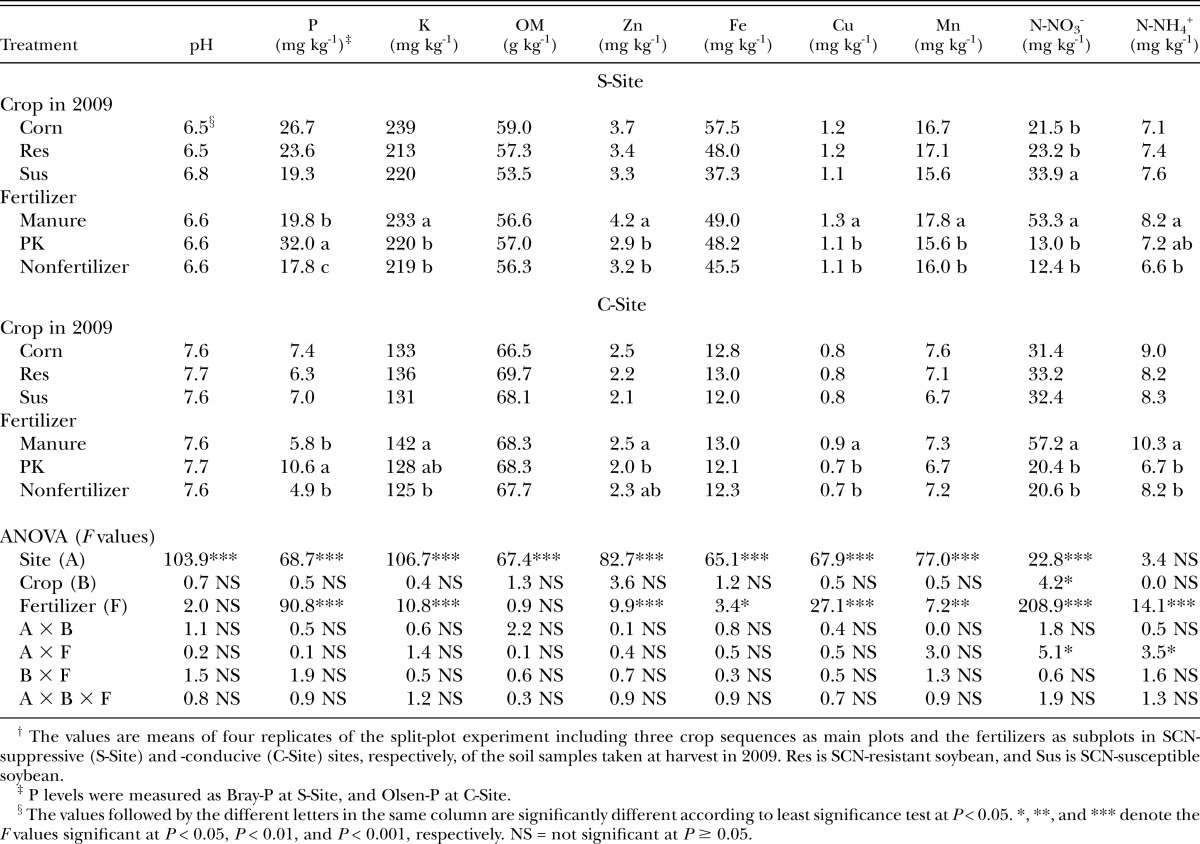
Soil chemical properties: Soil chemical properties were similar among the treatments prior to the fertilizer application within each site with the exception that K concentration was greater in the corn plots than susceptible and resistant soybean plots (data not shown). All of the soil chemical properties were different between the two sites at harvest in 2009 (Table 1). The concentration of nitrate nitrogen at 45 DAP in the treatment that received manure was 53.3 mg/kg and 57.2 mg/kg at the S-Site and C-Site, approximately four and three times of that obtained with the PK or nonfertilizer treatment, respectively (Table 1). The ammonium nitrogen also increased in manure treatments as compared with nonfertilizer at both sites and with PK treatment at the C-Site (Table 1). Manure also increased the concentration of Cu and K in the soil at both sites, and Zn and Mn at the S-Site (Table 1). The PK treatment almost doubled P concentration in the soil at the harvest as compared with nonfertilizer treatment at both sites (Table 1). The manure also slightly increased P level at the S-Site. No treatment effects on soil pH, organic matter, and Fe concentrations were detected at harvest in 2009. There was no fertilizer treatment effect on the organic matter and soil elements at harvest in 2010, except that the P levels in the manure and PK treatments were higher than that in nonfertilizer treatment (data not shown).
Table 1.
Soil chemical properties as affected by treatments of fertilizers and crop sequences.†
SCN population density: The SCN egg and J2 population density at the C-Site was generally greater than that at the S-Site (Tables 2,3). There were no effects of fertilizer treatments on the SCN egg population density prior to planting, 45 DAP, and at harvest in 2009, and at harvest in 2010 (Table 2). When the data from the two sites were pooled together, the midseason egg population densities in 2009 were slightly greater in the manure and PK treatments than nonfertilizer treatment, but the treatment was not significant when the data were analyzed by site (Table 2). At the S-Site, the SCN egg population density at planting in 2010 was greater in soil treated with PK than with nonfertilizer, and intermediate with manure. At the S-Site, susceptible soybean in 2009 supported greatest SCN egg population densities from midseason 2009 to midseason 2010 (Table 2). The greater SCN egg population density in SCN-susceptible soybean treatment as compared with corn and SCN-resistant soybean was observed only at harvest in 2009 and midseason in 2010 at the C-Site (Table 2). Manure and PK resulted in greater SCN egg population density at the midseason of 2010 than nonfertilizer treatment at the C-Site (Table 2).
Table 2.
Effects of fertilizer and crop sequence on Heterodera glycines egg population density.†
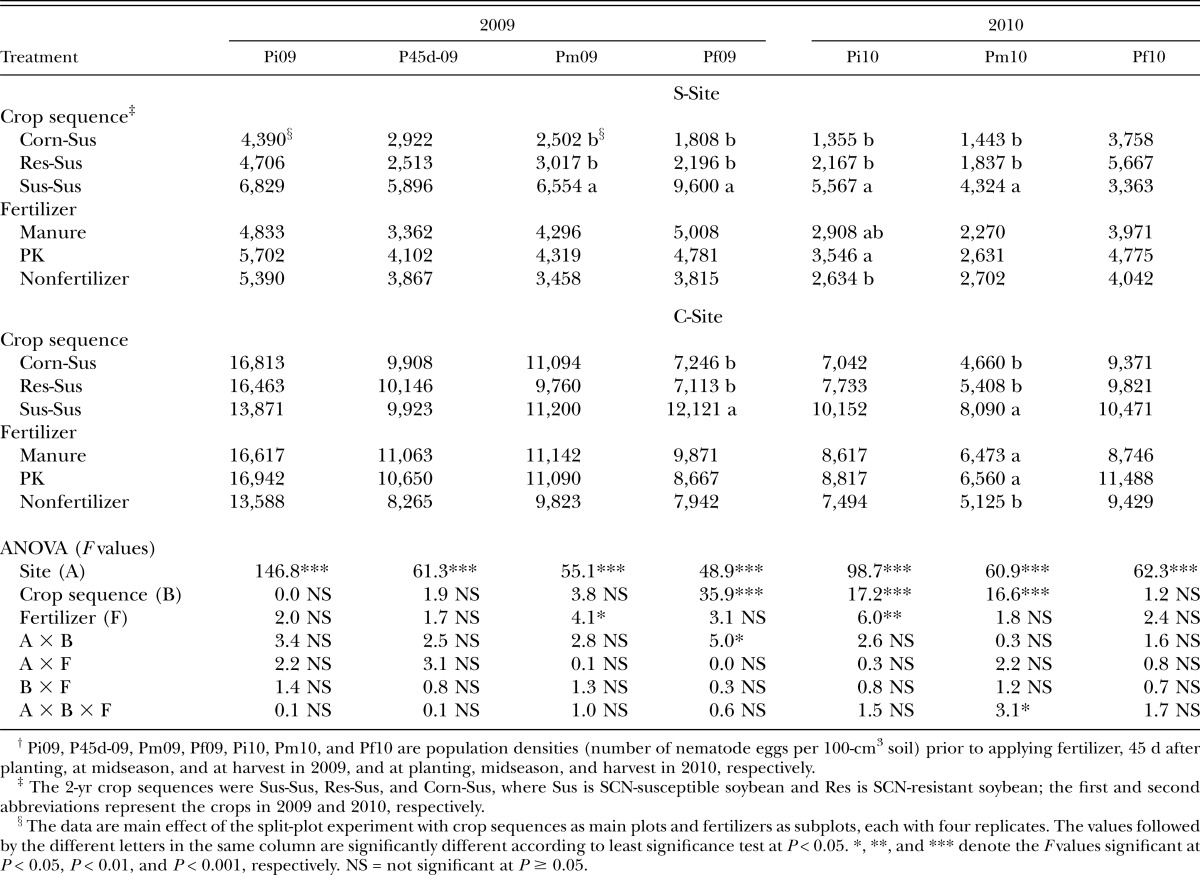
Table 3.
Effects of fertilizer and crop on the Heterodera glycines second-stage juvenile population density.†
The application of manure resulted in 31% and 46% lower SCN J2 population density at 45 DAP in 2009 as compared with nonfertilizer and PK treatment, respectively, at the S-Site (Table 3). In contrast, a greater SCN J2 population density at harvest in 2009 and at planting in 2010 was observed in C-Site with the manure treatment (Table 3). At both sites, the SCN J2 population densities were greater from 45 DAP in 2009 through early season (C-Site) or midseason (S-Site) in 2010 in the plots with susceptible soybean than with resistant soybean or corn in 2009 (Table 3).
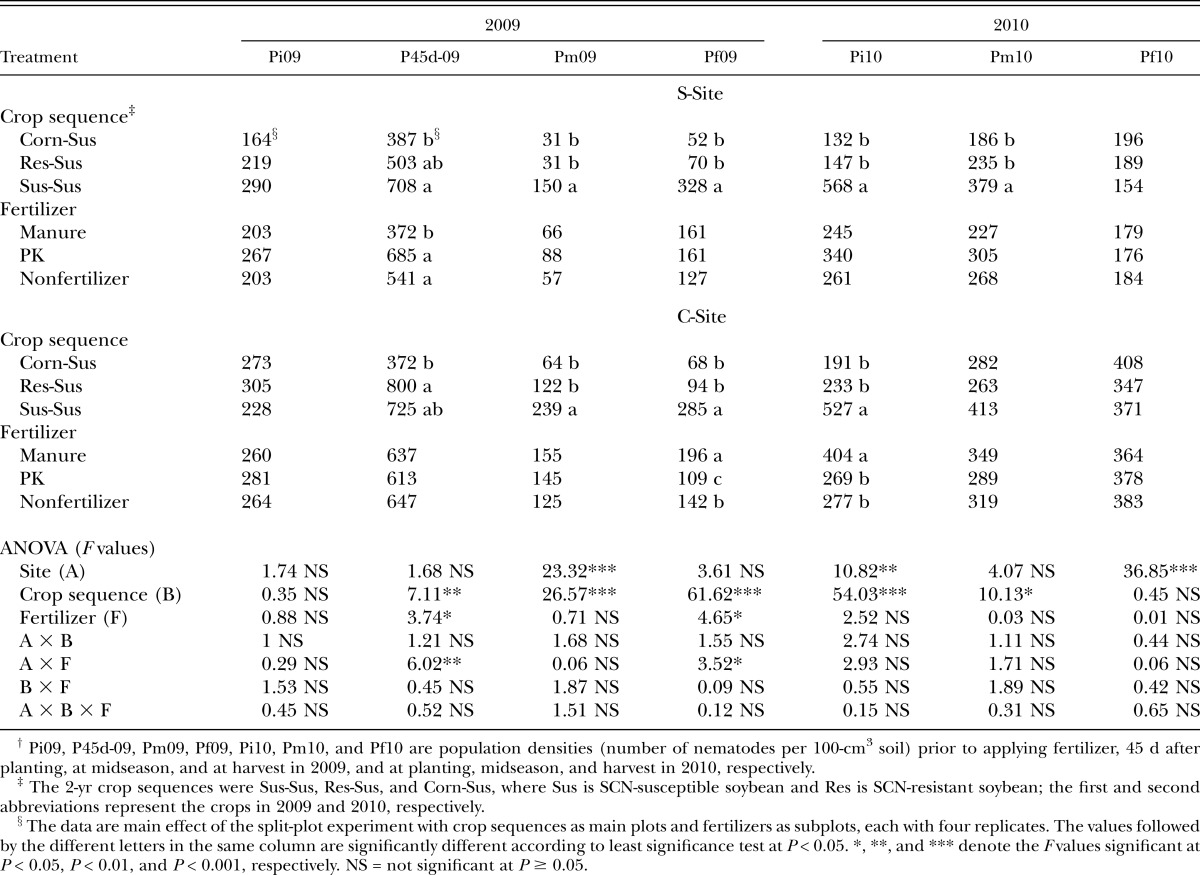
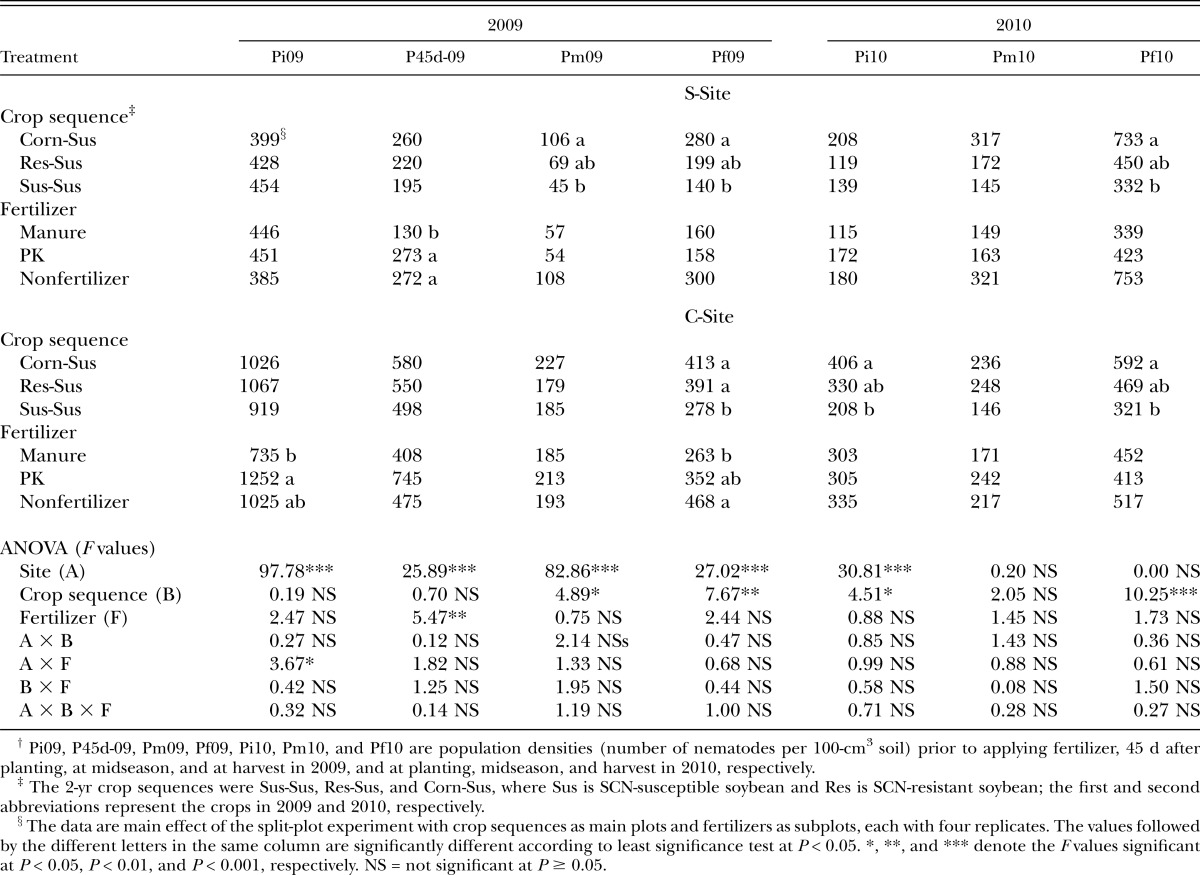
Other plant-parasitic nematodes: For the plant-parasitic nematodes other than SCN, only spiral nematode (Helicotylenchus spp.) was detected from most plots and had enough abundance for statistical analysis. Average population density of spiral nematode was greater at the C-Site than the S-Site, with the initial population densities of 1,004, and 427 per 100 cm3 soil, respectively (data not shown). The manure treatment reduced spiral nematode population density by 52% relative to the PK and nonfertilizer treatment in S-Site at 45 DAP in 2009 (Table 4). The spiral nematode population density at harvest in 2009 was least with manure, intermediate with PK, greatest with nonfertilizer treatment in the C-Site (Table 4). Among three crop sequences, greater population density of spiral nematodes was observed on corn-soybean rotation in both sites at different sampling dates (Table 4).
Table 4.
Effects of fertilizer and crop on the spiral nematode (Helicotylenchus spp.) population density.†
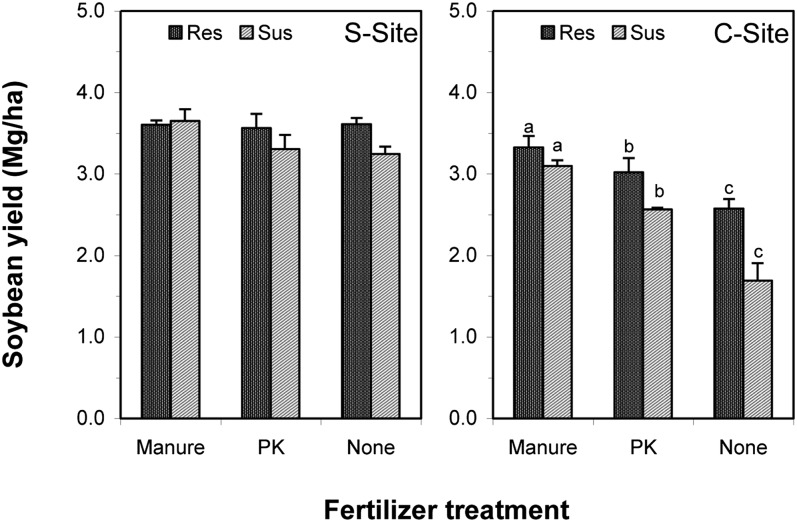
Soybean yield: Soybean yield response to fertilizer application differed dramatically between the two field sites (Table 5 and Figs. 1,2). At the S-Site, average soybean yield in 2009 was 3.50 Mg/ha, but there was no difference in the soybean yield between the SCN-resistant and susceptible cultivars and among the three fertilizer treatments (Fig. 1). At the C-Site, average soybean yield in 2009 was only 2.71 Mg/ha (data not shown). An increase of 1.4 Mg/ha and 0.5 Mg/ha in yield of susceptible soybean was observed in manure and PK treatments, respectively, at the C-Site in 2009 (Fig. 1). The yield advantage of SCN-resistant cultivar over susceptible cultivar was 883 kg/ha for nonfertilizer (P = 0.06), 454 kg/ha for PK, and only 230 kg/ha for manure at the C-Site in 2009 (Fig. 1), although none of the differences were statistically significant (P > 0.05). In manure treatment, the soybean yield in 2009 was similar between the two sites, but in nonfertilizer treatment the yield of susceptible soybean was 1.56 Mg/ha less in the SCN-conducive soil than the SCN-suppressive soil (Fig. 1).
Table 5.
Analysis of variance (F value) for soybean and corn yield response to crop and fertilizer treatment in the soybean cyst nematode suppressive and conducive fields.
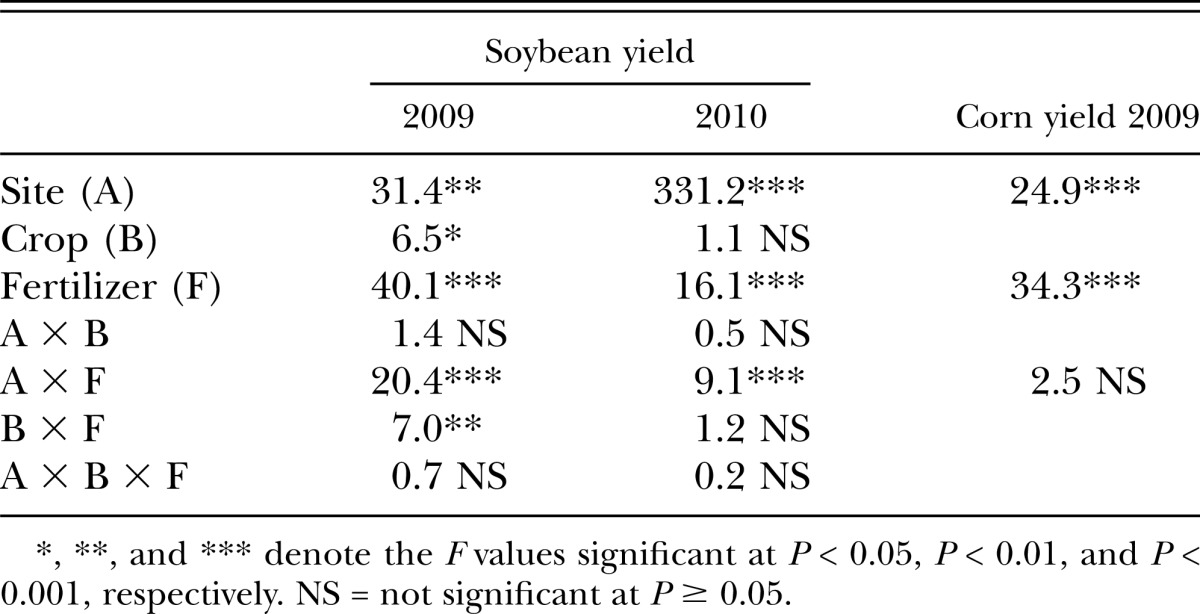
Fig. 1.
Fertilizer treatment effect on yield of SCN resistant (Res), susceptible (Sus) soybean cultivars in the SCN-suppressive (S-Site) and -conducive (C-Site) sites, respectively, in 2009. The bars are mean values of four replicates, and the lines on bars indicate the standard error. Significant differences among the different fertilizer treatments within the same soybean cultivar at C-Site are indicated by different letters above the bars according to unprotected least significance difference test at P < 0.05. No treatment effects were detected in S-Site.
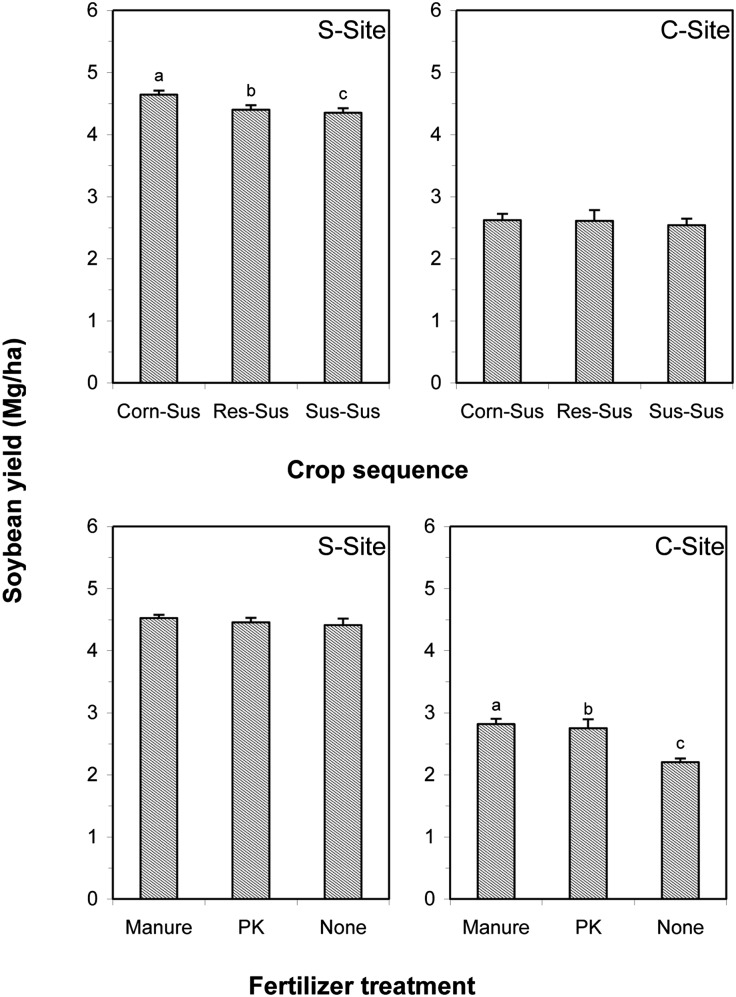
Fig. 2.
Soybean yield in response to the fertilizer treatment and crop sequence in the SCN-suppressive (S-Site) and -conducive (C-Site) sites, respectively, in 2010. The 2-yr crop sequences were Sus-Sus, Res-Sus, and Corn-Sus, where Sus is SCN-susceptible soybean and Res is SCN-resistant soybean; the first and second abbreviations represent the crops in 2009 and 2010, respectively. The bars are means and the lines on the bars are the standard error of main effect with four replicates. The different letters within a graph denote the significant differences according to unprotected least significance difference test at P < 0.05. No letter in the graph indicates no difference.
The manure and PK applied in 2009 resulted in 610 and 545 kg/ha, respectively, greater soybean yield in 2010 as compared with the nonfertilizer at the C-Site. However, there was no difference in the 2010 soybean yield among the fertilizer treatments at the S-Site (Fig. 2). The 2010 soybean yield following corn was slightly greater than following soybean at the C-Site (Fig. 2).
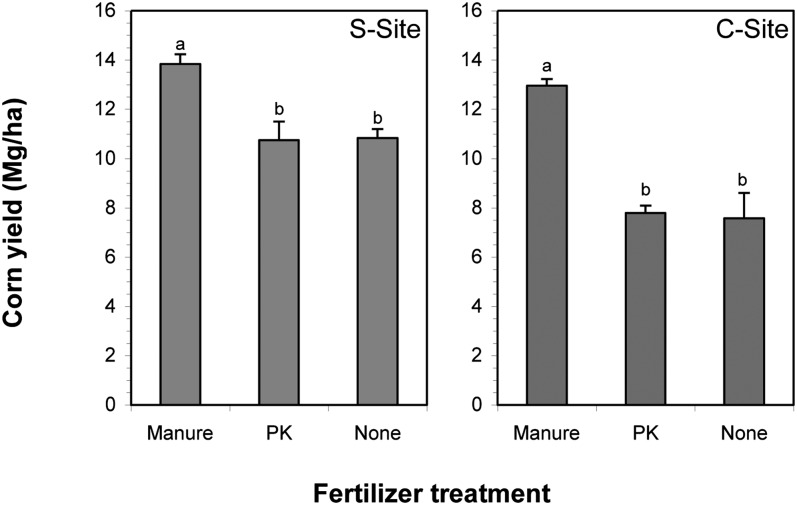
Corn yield: With the manure treatment, the yield of corn was 13.87 Mg/ha at the S-Site and 12.99 Mg/ha at the C-Site (Fig. 3). Corn yield was 2.8 Mg/ha and 5.0 Mg/ha greater when soil treated with manure than nonfertilizer at S-Site and C-Site, respectively (Fig. 3). There was no difference in corn yield between the PK and nonfertilizer treatments at either site (Fig. 3).
Fig. 3.
Fertilizer treatment effect on corn in the SCN-suppressive (S-Site) and -conducive (C-Site) sites in 2009. The bars are mean values of four replicates, and the lines on bars indicate the standard error. Significant differences among the different fertilizer treatments within the same graph are indicated by different letters above the bars according to least significance difference test at P < 0.05.
Discussion
Our study showed that soybean yield responded positively to application of liquid swine manure and chemical fertilizers, and the manure was more effective than the chemical fertilizer PK. This study agrees with the results of a previous study that liquid swine manure application increased seed yield in soybean fields in southern Minnesota (Schmitt et al., 2001). In this study, the soybean yield response to fertilizer treatments was more sensitive in SCN-susceptible than SCN-resistant cultivar and for the SCN-conducive than SCN-suppressive fields. In other words, the soybean yield advantage of using SCN-resistant cultivars in a nematode-conducive soil may at least be partially replaced by fertilizer input, especially liquid swine manure.
The lack of a reduction of SCN egg population density by manure does not agree with the results from a previous greenhouse study in which manure effectively lowered SCN egg population density (Xiao et al., 2007). However, it is similar to the results from a previous field study (Reynolds et al., 1999). In the greenhouse study, Xiao et al. (2007) planted soybean immediately following application of manure, while in the present field study the soybean was planted 2 wk after application of the manure. The concentrations of fatty acid compounds, which are the major nematicidal compounds, in the soil from the manure application declined rapidly (Mahran et al., 2009). It is possible that these fatty acids are more effective in killing SCN J2 than eggs. The SCN generally does not hatch before planting soybean, and the manure might have less effect on the unhatched SCN eggs under field conditions. In the same greenhouse study, Xiao et al. (2007) found that liquid swine manure became ineffective in lowering SCN eggs population at 61 d after application. It appears that the application time may be a reason for the different effects of manure on SCN eggs between the greenhouse and field experiments.
A combination of potential mechanisms, rather than a single one, was involved in suppression of SCN in soil by the manure treatment. The immediate decrease in SCN J2 population density after manure application may have been due to the preexisting nematicidal compounds in manure, such as ammonia and fatty acids. It is unclear why a significant reduction of SCN J2 was detected only in SCN-suppressive field. It is possible that besides the effect of the nematicidal chemical compounds, manure might affect J2 and vermiform nematode populations through changing microbial activities, depending on soil microbial community and environmental conditions. In the nematode-suppressive soil, some nematophagous fungi including trapping fungi and endoparasites of nematodes have been detected (Chen, 2007). The swine manure might enhance the activities of the nematophagous fungi especially the trapping fungi. Wachira et al. (2009) reported that cow (Bos primigenius) manure and chicken (Gallus gallus domesticus) manure stimulated the occurrence of nematode-destroying fungi in the soil and also reduced plant-parasitic nematodes. When alfalfa leaves (Medicago sativa) were added to soil, both microbivorous nematodes and the nematode-trapping fungus Dactylellina candidum increased (Jaffee, 2006). Further studies are needed to determine whether or not the effect of the swine manure on nematophagous fungi is a major mechanism in suppressing plant-parasitic nematodes.
A number of plant-parasitic nematodes were observed at the two sites, but only spiral nematodes were observed in most samples in addition to SCN. Spiral nematodes can parasitize both corn and soybean, but the pathogenicity was low. At the population density we observed at the two sites, the nematode should cause limited damage, if any, to corn and soybean. Thus, the manure treatment effect on corn yield was probably due to the nutrient effect. The manure provided nitrogen as well as other nutrients for corn growth and resulted in higher yield than both PK fertilizer and nonfertilizer treatments. We realize that N is generally needed for corn production, and the increase of corn yield by manure was mainly because of the nitrogen effect. Considering our emphasis on SCN and soybean yield, we used PK fertilizer treatment without nitrogen for both soybean and corn crops for a balanced experimental design, because chemical nitrogen fertilizer is generally not used for soybean.
Differences in soil chemical properties among fertilizer treatments might have a direct or indirect influence on nematode population dynamics, including free-living nematodes via plant growth or microbial activity (Bulluck et al., 2002; Okada and Harada, 2007). Manure application has been shown to significantly increase the concentrations of nitrate and ammonium in the soil (Jokela, 1992; Mikha and Rice, 2004). The concentration of nitrate was tripled with the manure treatment. More abundant plant nutrition from manure amendment might not only increase crop tolerance to SCN infestation but also provide soil microbes with a new energy source that resulted in the increased diversity and activities of soil microorganism communities. Effectiveness of liquid swine manure in suppressing SCN may be affected by specific soil conditions, such as soil type and pH. For example, the results from Mahran et al. (2008a) indicated that liquid swine manure may be an effective means to kill P. penetrans in slightly acid and neutral soil when application is combined with low rates of acid. In acidic soils, swine manure killed Verticillium dahliae within a day after application but had no effect in neutral or alkaline soils (Lazarovits et al., 2001). The soil pH was near neutral at the S-Site and slightly higher in the C-Site. Whether the soil pH affected control of nematodes by the manure is unclear in the present study.
In conclusion, our results suggest that soil fertility management plays an important role in developing sustainable strategies to manage SCN and improve crop yield in soybean-corn cropping system. The population density of SCN J2 was significantly lower in soil treated with manure in the SCN-suppressive soil, but no reduction of SCN egg population densities by manure was observed in either site. The liquid swine manure treatment, a source of N, P, K, and other macro- and micro-nutrients, and the chemical fertilizer PK treatment, a source of P and K, increased soybean yield in the SCN-conducive soil. Manure also increased corn yield in both sites. The soil fertility management, especially the application of swine manure, can partially replace the yield advantages of SCN-resistant cultivars in the SCN-conducive soil.
Literature Cited
- Bao Y, Neher DA, Chen SY. Effect of biocides and soil disturbance on nematode community and extracellular enzyme activity in soybean cyst nematode suppressive soil. Nematology. 2011;13:687–699. [Google Scholar]
- Briar SS, Grewal PS, Somasekhar N, Stinner D, Miller SA. Soil nematode community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Applied Soil Ecology. 2007;37:256–266. [Google Scholar]
- Brown JR, editor. 1998 Recommended chemical soil test procedures for the North Central region, North Central Research Publication No. 221. Columbia, MO: Missouri Agricultural Experiment Station. 75 pp. http://extension.missouri.edu/publications/DisplayPub.aspx?P=SB1001. [Google Scholar]
- Bulluck LR, Barker KR, Ristaino JB. Influences of organic and synthetic soil fertility amendments on nematode trophic groups and community dynamics under tomatoes. Applied Soil Ecology. 2002;21:233–250. [Google Scholar]
- Byrd DW, Jr, Barker KR, Ferris H, Nusbaum CJ, Griffin WE, Small RH, Stone CA. Two semi-automatic elutriators for extracting nematodes and certain fungi from soil. Journal of Nematology. 1976;8:206–212. [PMC free article] [PubMed] [Google Scholar]
- Chen SY. Suppression of Heterodera glycines in soils from fields with long-term soybean monoculture. Biocontrol Science and Technology. 2007;17:125–134. [Google Scholar]
- Conn KL, Lazarovits G. Soil factors influencing the efficacy of liquid swine manure added to soil to kill Verticillium dahliae. Canadian Journal of Plant Pathology. 2000;22:400–406. [Google Scholar]
- Conn KL, Tenuta M, Lazarovits G. Liquid swine manure can kill Verticillium dahliae microsclerotia in soil by volatile fatty acid, nitrous acid, and ammonia toxicity. Phytopathology. 2005;95:28–35. doi: 10.1094/PHYTO-95-0028. [DOI] [PubMed] [Google Scholar]
- Everts KL, Sardanelli S, Kratochvil JR, Armentrout DK, Gallagher LE. Root-knot and root-lesion nematode suppression by cover crops, poultry litter, and poultry litter compost. Plant Disease. 2006;90:487–492. doi: 10.1094/PD-90-0487. [DOI] [PubMed] [Google Scholar]
- Faghihi J, Ferris JM. An efficient new device to release eggs from Heterodera glycines. Journal of Nematology. 2000;32:411–413. [PMC free article] [PubMed] [Google Scholar]
- Jaffee BA. Interactions among a soil organic amendment, nematodes, and the nematode-trapping fungus Dactylellina candidum. Phytopathology. 2006;96:1388–1396. doi: 10.1094/PHYTO-96-1388. [DOI] [PubMed] [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Jokela WE. Nitrogen fertilizer and dairy manure effects of corn yield and soil nitrate. Soil Science Society of America Journal. 1992;56:148–154. [Google Scholar]
- Keeney DR, Nelson DW. 1982 Nitrogen-inorganic forms. Pp. 643–698 in L. Page, ed. Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd ed. Madison, WI: ASA, SSA. [Google Scholar]
- Kimpinski J, Gallant CE, Henry R, Macleod JA, Sanderson JB, Sturz AW. Effect of compost and manure soil amendments on nematodes and on yields of potato and barley: A 7-year study. Journal of Nematology. 2003;35:289–293. [PMC free article] [PubMed] [Google Scholar]
- Lazarovits G, Tenuta M, Conn KL. 2000 Utilization of high nitrogen and swine manure amendments for control of soil-borne diseases: Efficacy and mode of action. Proceedings of the 5th International Symposium on Chemical and Non-Chemical Soil and Substrate Disinfestation, Torino, Italy, 11–15 September. Acta Horticulturae 59–64. [Google Scholar]
- Lazarovits G, Tenuta M, Conn KL. Organic amendments as a disease control strategy for soilborne diseases of high-value agricultural crops. Australian Plant Pathology. 2001;30:111–117. [Google Scholar]
- Liang WJ, Lou YL, Li Q, Zhong S, Zhang XK, Wang JK. Nematode faunal response to long-term application of nitrogen fertilizer and organic manure in Northeast China. Soil Biology & Biochemistry. 2009;41:883–890. [Google Scholar]
- Mahran A, Conn KL, Tenuta M, Lazarovits G, Daayf F. Effectiveness of liquid hog manure and acidification to kill Pratylenchus spp. in soil. Journal of Nematology. 2008a;40:266–275. [PMC free article] [PubMed] [Google Scholar]
- Mahran A, Tenuta M, Hanson ML, Daayf F. Mortality of Pratylenchus penetrans by volatile fatty acids from liquid hog manure. Journal of Nematology. 2008b;40:119–126. [PMC free article] [PubMed] [Google Scholar]
- Mahran A, Tenuta M, Lumactud RA, Daayf F. Response of a soil nematode community to liquid hog manure and its acidification. Applied Soil Ecology. 2009;43:75–82. [Google Scholar]
- Mikha MM, Rice CW. Tillage and manure effects on soil and aggregate-associated carbon and nitrogen. Soil Science Society of America Journal. 2004;68:809–816. [Google Scholar]
- Min YY, Sato E, Shirakashi T, Wada S, Toyota K, Wananabe A. Suppressive effect of anaerobically digested slurry on the root lesion nematode Pratylenchus penetrans and its potential mechanisms. Japanese Journal of Nematolology. 2007;37:93–100. [Google Scholar]
- Monson M, Schmitt DP. 2004 Economics. Pp. 41–53 in D. P. Schmitt, J. A. Wrather, and R. D. Riggs, eds. Biology and management of the soybean cyst nematode. Marceline, MO: Schmitt & Associates of Marceline. [Google Scholar]
- Nahar MS, Grewal PS, Miller SA, Stinner D, Stinner BR, Kleinhenz MD, Wszelaki A, Doohan D. Differential effects of raw and composted manure on nematode community, and its indicative value for soil microbial, physical and chemical properties. Applied Soil Ecology. 2006;34:140–151. [Google Scholar]
- Oka Y. Mechanisms of nematode suppression by organic soil amendments—A review. Applied Soil Ecology. 2010;44:101–115. [Google Scholar]
- Oka Y, Shapira N, Fine P. Control of root-knot nematodes in organic farming systems by organic amendments and soil solarization. Crop Protection. 2007;26:1556–1565. [Google Scholar]
- Okada H, Harada H. Effects of tillage and fertilizer on nematode communities in a Japanese soybean field. Applied Soil Ecology. 2007;35:582–598. [Google Scholar]
- Reynolds DA, Tylka GL, Martinson CA. 1999 Effects of swine manure on soybean cyst nematode. Proceeding of National Soybean Cyst Nematode Conference, Orlando, FL, 16. [Google Scholar]
- Schmitt MA, Schmidt JP, Randall GW, Lamb JA, Orf JH, Gollany HT. Effect of manure on accumulation of dry matter, nitrogen, and phosphorus by soybean. Communications in Soil Science and Plant Analysis. 2001;32:1931–1941. [Google Scholar]
- Tenuta M, Conn KL, Lazarovits G. Volatile fatty acids in liquid swine manure can kill microsclerotia of Verticillium dahliae. Phytopathology. 2002;92:548–552. doi: 10.1094/PHYTO.2002.92.5.548. [DOI] [PubMed] [Google Scholar]
- Thoden TC, Korthals GW, Termorshuizen AJ. Organic amendments and their influences on plant-parasitic and free-living nematodes: A promising method for nematode management? Nematology. 2011;13:133–153. [Google Scholar]
- Wachira PM, Kimenju JW, Okoth SA, Mibey RK. Stimulation of nematode-destroying fungi by organic amendments applied in management of plant parasitic nematode. Asian Journal of Plant Science. 2009;8:153–159. [Google Scholar]
- Willis RB, Gentry CE. Automated method for determining nitrate and nitrite in water and soil extracts. Communications in Soil Science and Plant Analysis. 1987;18:625–636. [Google Scholar]
- Wrather JA, Koenning SR. Estimates of disease effects on soybean yields in the United States 2003 to 2005. Journal of Nematology. 2006;38:173–180. [PMC free article] [PubMed] [Google Scholar]
- Wrather JA, Koenning SR, Anderson TR. Effect of diseases on soybean yields in the United States and Ontario (1999 to 2002) Plant Health Progress (March): 2003:0–16. [Google Scholar]
- Xiao J, Zhu J, Chen S, Ruan W, Miller C. A novel use of anaerobically digested liquid swine manure to potentially control soybean cyst nematode. Journal of Environmental Science and Health Part B-Pesticides Food Contaminants and Agricultural Wastes. 2007;42:749–757. doi: 10.1080/03601230701503724. [DOI] [PubMed] [Google Scholar]