Abstract
We study and describe the life cycle of Plectus murrayi, a free-living, bacterivorous soil nematode endemic to terrestrial Antarctica. The study was performed at 15°C, a temperature identified as optimal for growth rate studies in the laboratory. Under these conditions, we observed that the first molt occurs in the egg, and second-stage juveniles hatch 12 to 14 d after egg laying. Individuals undergo three subsequent molts to become adults 23 to 26 d after hatching with a final average length of 950 μm. Egg-laying begins 41 to 43 d after hatching, resulting in an egg-to-egg life cycle ranging from 53 to 57 d under our experimental conditions. Considering that the average soil temperature during austral summers in the McMurdo Dry Valleys is only a few degrees above freezing, it is highly likely that many, if not most of these animals, require more than 1 yr to complete their entire life cycle. Our study supports other research that establishes P. murrayi as an important model organism for studying adaptation to extreme environmental stress.
Keywords: Anhydrobiosis, McMurdo Dry Valleys, physiology, soil fauna
The McMurdo Dry Valleys of Antarctica (77°S, 163°E) are considered one of the most extreme desert ecosystems on earth, with less than 100 mm of precipitation per year, a mean annual air temperature of -20°C, and as few as 6.2 degree-days above freezing per year. (Priscu, 1998; Fountain et al., 1999; Doran et al., 2002). In the ice-free areas of these valleys, nematodes contribute to a wide range of soil processes (Barrett et al., 2008), playing an important role in soil nutrient and carbon cycling. Since nematodes constitute the top of the soil food webs in the Dry Valleys (Wall and Virginia, 1999), it is critical to study their communities in great detail if we are to understand their role in soil processes.
Five species of nematodes are present in the Dry Valleys, Scottnema lindsayae, Eudorylaimus antarcticus, Plectus frigophilus, Plectus murrayi, and Geomonhystera antarcticola, all of which are endemic to Antarctica (Andrássy, 1998; Adams et al., 2006). All of these invertebrates survive extreme cold temperatures, desiccated soils, and frozen, dark winters (Freckman and Virginia, 1997). Most of these species are highly specialized and well adapted to harsh conditions (Treonis and Wall, 2005; Pugh and Convey, 2008; Adhikari et al., 2009) but differ in their suitability to various habitats across the landscape. For example, the nematode S. lindsayae, a microbial feeder occurring in drier, saltier desert soils, is the most widely distributed animal of continental Antarctica (Andrássy, 1998; Adams et al., 2006) and, as with other nematodes, undergoes anhydrobiosis (Treonis et al., 2000; Courtright et al., 2001). Some researchers (Porazinska et al., 2002; Weicht and Moorhead, 2004) have suggested that because of this survival strategy, it may take several decades for this species to complete its life cycle. Longer life cycles such as this, which suggest that nematode juvenile and adult stages have survival strategies to last through long polar winters, have spurred research on nematode survival strategies in field (Treonis et al., 2000) and, for P. murrayi, in laboratory studies (Freckman and Virginia, 1997; Treonis et al., 2000; Treonis and Wall, 2005; Adhikari et al., 2010a). Plectus murrayi occurs across the Dry Valley landscape in moist areas along meltstreams, near lake edges, and in snowmelt areas, where there is higher soil moisture, higher organic carbon, and lower soil salinity compared with the distribution of S. lindsayae (Treonis et al., 1999; Adams et al., 2006). Results from field and laboratory life cycle studies of P. murrayi are scarce. Yeates et al. (2009), studying the size distribution of P. murrayi from Cape Hallett field samples, suggested that there was an annual life cycle. The intent of the present study was to describe the life cycle of P. murrayi from the McMurdo Dry Valleys under laboratory conditions, and discuss the possible impact of environmental conditions in the duration of its life cycle in the field.
Materials and Methods
We collected the soil samples for this experiment in the Antarctic Dry Valleys. The samples were taken using clean plastic scoops to 10-cm depth, placed in sterile 24-oz Whirlpack® bags, and shipped frozen at -20°C to Colorado State University. The protocol for nematode culturing has been described in detail elsewhere (Adhikari et al., 2010b). Briefly, soil samples were initially stored in freezers at -20°C at Colorado State University, and defrosted progressively in two 24-hr steps: from -20°C to -10°C, and from -10°C to 4°C. Female nematodes were handpicked from the soil solution under an Olympus SZ×10 stereomicroscope and placed in individual 60-mm petri dishes containing Bold’s Modified Basal Freshwater Nutrient Media (BMBFN, Sigma-Aldrich) with Ottawa Sand, referred to in this paper as BB+O. No bacteria were added to the plates.
The experiment was conducted at a temperature of 15°C, a temperature identified as optimal for growth rate studies in the laboratory. The Plectus females on culture plates were checked weekly with the stereomicroscope until gravid specimens were found; pregnant females were then handpicked and transferred to additional petri dishes containing BB+O media. A total of 64 petri dishes containing one gravid female each were numbered and inspected daily under the stereomicroscope at ×48 magnification to determine when eggs were laid. After the eggs hatched, 58 juveniles were transferred to individual petri dishes with media. The juveniles were photographed daily under a CKX 41 Olympus inverted microscope at ×40 to ×100 magnification, and their length and width were measured using Olympus’s MicroSuiteTM Five software (Olympus Soft Imaging Solutions Corp.) until the specimens reached their adult size and laid eggs.
Figure 1 illustrates the technique used to estimate body volume. Researchers have routinely used Andrássy’s formula (Andrássy, 1956; Freckman, 1982) as a means to estimate body weight, especially from images of elongated, rod-like specimens with the same growth stage. Andrássy’s formula can be written as
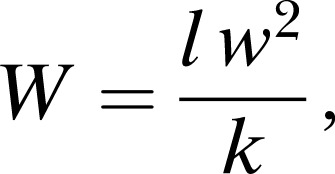 |
where W is the weight in μg, l is the total length of the nematode in μm, w is the width of the nematode at its widest, and k is a constant with usual values in the range of (1.6–1.7) × 106. This constant accounts for the specific gravity of the nematode and for the deviation of its shape from that of cylinder. Ecologists interested in estimating nematode biomass generally average the weight calculated from measurements of a relatively large number (about 100 or more) of specimens (Freckman, 1982; Nether, 1999).
Fig. 1.
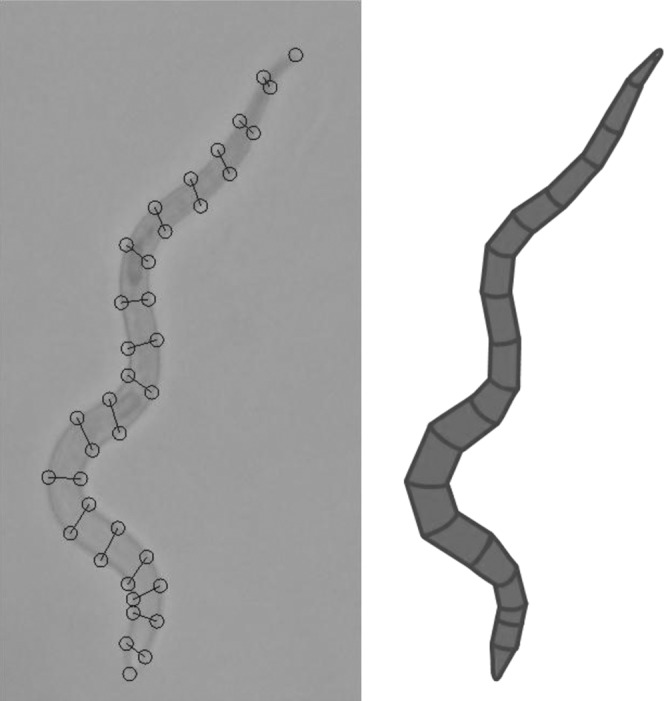
Illustration of the segmentation technique used to estimate body volume. Cross-sections are selected manually on calibrated photographs (left), and volume is calculated automatically by approximating body sections by truncated cones (right).
Andrássy’s formula is indeed utilitarian; however, it requires careful determination of the point at which body diameter is measured, and its use across different species and growth stages requires a careful analysis of k for each case. Although k has been customarily considered as a constant across different groups its value is actually shape-dependent, so using it as a fixed factor has resulted in reported inaccuracies of up to 30% (see, for example, Freckman, 1982). To better understand this point let us briefly consider a simple geometrical construction where we assume that the shape of a nematode of length l and width w can be accurately represented as two cones of height h, joined by a cylinder of length (l – 2h). Approximating the volume of the nematode as 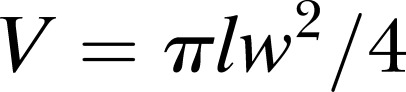 overestimates the actual volume by a factor of (
overestimates the actual volume by a factor of (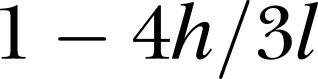 ), meaning that by using Andrássy's formula with a factor of 1.6 for this case we implicitly are assuming that the conical ends of the nematode add up to about 30% of its total length. This assumption may be reasonably accurate for some body shapes, but not so for others. This simple geometrical analysis also indicates that although isometric growth is not actually required for Andrássy's formula to be equally accurate for different growth stages, the h/l ratio needs to remain constant. Since inaccuracies in the determination of the body weight or volume can blur the transition between growth stages in life cycle studies, we chose to approximate the nematode’s shape by a series of 20 to 30 truncated cones, assuming circular cross-sections all along the body. A script written in MATLAB® (The Mathworks Inc.) was used to manually select the cross-sections and automatically calculate the body volume (script available upon request). This approximation worked well even in the case of nonfully expanded specimens, and it may prove to be useful for biomass studies of axisymmetric organisms with shapes other than spindle-like.
), meaning that by using Andrássy's formula with a factor of 1.6 for this case we implicitly are assuming that the conical ends of the nematode add up to about 30% of its total length. This assumption may be reasonably accurate for some body shapes, but not so for others. This simple geometrical analysis also indicates that although isometric growth is not actually required for Andrássy's formula to be equally accurate for different growth stages, the h/l ratio needs to remain constant. Since inaccuracies in the determination of the body weight or volume can blur the transition between growth stages in life cycle studies, we chose to approximate the nematode’s shape by a series of 20 to 30 truncated cones, assuming circular cross-sections all along the body. A script written in MATLAB® (The Mathworks Inc.) was used to manually select the cross-sections and automatically calculate the body volume (script available upon request). This approximation worked well even in the case of nonfully expanded specimens, and it may prove to be useful for biomass studies of axisymmetric organisms with shapes other than spindle-like.
Results and Discussion
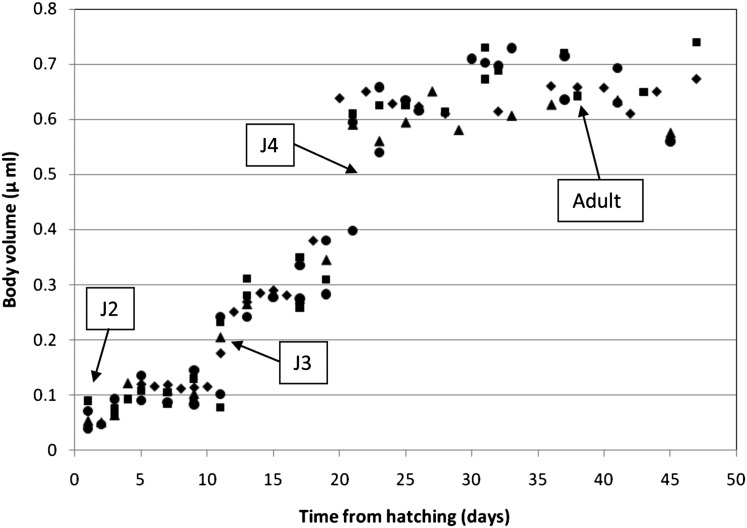
Figure 2 illustrates the ontogeny of P. murrayi body volume over time. The data indicate that the nematodes undergo their first molt in the egg and hatch as J2. The second-stage juveniles hatch 12 to 14 d after the eggs were laid, with an average hatching length of 400 μm. They complete their second molt in about 10 days, becoming J3 between 11 and 13 d after hatching. The third molt lasts from 5 to 6 d, turning into J4 between 19 and 22 d after hatching. After a fourth molt the nematodes become adults, reaching a final average length of 950 μm, with the period from egg to adult taking 36 to 38 d. The nematodes are observed to lay eggs 41 to 43 d after hatching, resulting into a total egg-to-egg life cycle length of 53 to 57 d, or an average of 825 degree-days referenced to 0°C.
Fig. 2.
Body volume ontogeny.
Not much is known of the duration of the life cycle of P. murrayi in the McMurdo Dry Valleys. To our knowledge, the only report of life cycle duration of P. murrayi in Antarctica is based on an analysis of populations from Cape Hallet (Yeates et al., 2009). The study shows that body length distributions as a function of time are indicative of an annual life cycle. However, as noted by the authors, Cape Hallet is somewhat warmer than the extreme climate of the Dry Valleys, and liquid water is available during most of the summer.
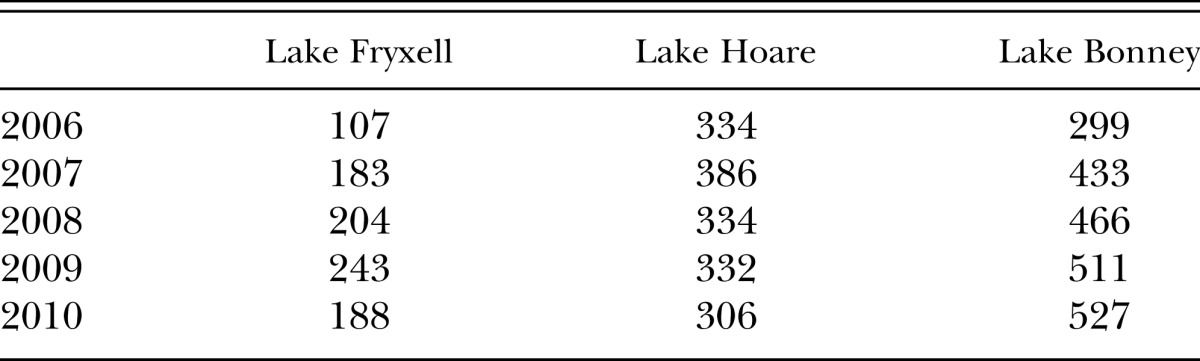
In the Dry Valleys, the temperatures and environmental conditions are more extreme than Cape Hallet. Table 1 shows the annual number of degree-days above freezing based on data from three lake basins in Taylor Valley where P. murrayi has been recovered before (Simmons et al., 2009). Temperatures used for this calculation were sampled by thermistors at 5-cm depth every 15 min at local meteorological stations (McMurdo Dry Valleys LTER 2012). With an average of 825 degree-days for P. murrayi to complete its life cycle, the data in the table suggest that this species may take no less than two, and in many cases several years to complete its life cycle in the Dry Valleys. These data also imply that most other nematodes surviving the environmental conditions of the Dry Valleys are likely to exhibit multi-year life cycles (Overhoff et al., 1993; Porazinska, 2002; Weicht and Moorhead, 2004).
Table 1.
Number of degree-days above freezing for years 2006–2010 in the Fryxell, Hoare, and Bonney basins, in Taylor Valley, Antarctica. Values calculated from soil temperature data at a depth of 5 cm.
This study supports recent research that establishes P. murrayi as an important model organism for studying adaptation to extreme environmental stress (Adhikari et al., 2010b). In the McMurdo Dry Valleys, even small changes in temperature and/or availability of liquid water could have a very large effect on the population dynamics of P. murrayi, which in turn has a cascading effect on the structure and functioning of its ecological community. Further studies on P. murrayi in the field and lab will reveal how the response of P. murrayi to increased moisture and temperature with climate change affect survival strategies and community dynamics in the Dry Valleys.
Literature Cited
- Adams BJ, Bardgett RD, Ayres E, Wall DH, Aislabie J, Bamforth S, Bargagli R, Cary C, Cavacini P, Connell L, Convey P, Fell JW, Frati F, Hogg ID, Newsham KK, O'Donnell A, Russell N, Seppelt RD, Stevens MI. Diversity and distribution of Victoria Land biota. Soil Biology & Biochemistry. 2006;38:3003–3018. [Google Scholar]
- Adhikari BN, Tomasel CM, Li G, Wall DH, Adams BJ. 2010a Culturing the antarctic nematode Plectus murrayi. Cold Spring Harbor Protocols. doi:10.1101/pdb.prot5522. [Google Scholar]
- Adhikari BN, Tomasel CM, Li G, Wall DH, Adams BJ. 2010b The antarctic nematode Plectus murrayi: An emerging model to study multiple stress survival. Cold Spring Harbor Protocols. doi:10.1101/pdb.emo142. [Google Scholar]
- Adhikari BN, Wall DH, Adams BJ. Desiccation survival in an Antarctic nematode: Molecular analysis using expressed sequenced tags. BMC Genomics. 2009;10:69. doi: 10.1186/1471-2164-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrássy I. Die Rauminhalts- und Gewichtsbestimmung der Fadenwürmer (Nematoden) Acta Zoologica. 1956;2:1–14. [Google Scholar]
- Andrássy I. Nematodes in the sixth continent. Journal of Nematode Morphology and Systematics. 1998;1:107–187. [Google Scholar]
- Barret JE, Virginia RA, Wall DH, Adams BJ. Decline in a dominant invertebrate species contributes to altered carbon cycling in a low-diversity soil ecosystem. Global Change Ecology. 2008;14:1734–1744. [Google Scholar]
- Courtright EM, Wall DH, Virginia RA. Determining habitat suitability for soil invertebrates in an extreme environment: The McMurdo Dry Valleys, Antarctica. Antarctic Science. 2001;13:9–17. [Google Scholar]
- Doran PT, McKay CP, Clow GD, Dana GL, Fountain AG, Nylen T, Lyons WB. Valley floor climate observations from the McMurdo Dry Valleys, Antarctica, 1986–2000. Journal of Geophysical Research. 2002;107:1–12. [Google Scholar]
- Fountain AG, Lyons WB, Burkins MB, Dana GL, Doran PT, Lewis KJ, McKnight DM, Moorhead DL, Parsons AN, Priscu JC, Wall DH, Wharton RA, Virginia RA. Physical controls on the Taylor Valley ecosystem, Antarctica. Bioscience. 1999;49:961–971. [Google Scholar]
- Freckman DW. 1982 Parameters of the nematode contribution to ecosystems. Pp. 81–97 in D. W. Freckman, ed. Nematodes in soil ecosystems. Austin: University of Texas Press. [Google Scholar]
- Freckman DW, Virginia RA. Low-diversity antarctic soil nematode communities: distribution and response to disturbance. Ecology. 1997;78:363–369. [Google Scholar]
- McMurdo Dry Valleys long term ecological research—Meteorology data. 2012. URL: http://www.mcmlter.org/queries/met/met_home.jsp. Lake Bonney: Fountain A., Lake Bonney Meteorological Station Measurements, dataset knb-lter-mcm.7003.7. Lake Fryxell: Dataset knb-lter-mcm.7010.7. Lake Hoare: Dataset knb-lter-mcm.7011.8.
- Nether DA. Nematode communities in organically and conventionally managed agricultural soils. Journal of Nematology. 1999;31:142–154. [PMC free article] [PubMed] [Google Scholar]
- Overhoff A, Freckman DW, Virginia RA. Life cycle of the microbivorous antarctic Dry Valley nematode Scottnema lindsayae (Timm 1971) Polar Biology. 1993;13:151–156. [Google Scholar]
- Porazinska DL, Wall DH, Virginia RA. Age structure of nematodes in the antarctic dry valleys: Perspectives on time, space, and habitat suitability. Arctic, Antarctic, and Alpine Research. 2002;34:159–168. [Google Scholar]
- Priscu JC, editor. 1998 Ecosystem dynamics in polar desert: The McMurdo Dry Valleys of Antarctica. Washington, DC: American Geophysical Union. [Google Scholar]
- Pugh PJA, Convey P. Surviving out in the cold: Antarctic endemic invertebrates and their refugia. Journal of Biogeography. 2008;35:2176–2186. [Google Scholar]
- Simmons BL, Wall DH, Adams BJ, Ayres E, Barrett JE, Virginia RA. Long-term experimental warming reduces soil nematode populations in the McMurdo Dry Valleys, Antarctica. Soil Biology and Biochemistry. 2009;41:2052–2060. [Google Scholar]
- Treonis AM, Wall DH, Virginia RA. Invertebrate biodiversity in Antarctic Dry Valley soils and sediments. Ecosystems. 1999;2:482–492. [Google Scholar]
- Treonis AM, Wall DH, Virginia RA. The use of anhydrobiosis by soil nematodes in the Antarctic Dry Valleys. Functional Ecology. 2000;14:460–467. [Google Scholar]
- Treonis AM, Wall DH. Soil nematodes and desiccation survival in the extreme arid environment of the Antarctic Dry Valleys. Integrative and Comparative Biology. 2005;45:741–750. doi: 10.1093/icb/45.5.741. [DOI] [PubMed] [Google Scholar]
- Wall DH, Virginia RA. Controls on soil biodiversity: Insights from extreme environments. Applied Soil Ecology. 1999;13:137–150. [Google Scholar]
- Weicht TR, Moorhead DL. The impact of anhydrobiosis on the persistence of Scottnema lindsayae (Nematoda): A modeling analysis of population stability thresholds. Polar Biology. 2004;27:504–512. [Google Scholar]
- Yeates GW, Scott MB, Chown SL, Sinclair BJ. Changes in soil nematode populations indicate an annual life cycle at Cape Hallett, Antarctica. Pedobiologia. 2009;52:375–386. [Google Scholar]