Abstract
The seed gall nematode, Anguina agrostis, feeds and reproduces within the developing ovaries of bentgrass seeds and overwinters in seed galls as anhydrobiotic juveniles. These dormant juveniles can survive within the seed gall for many years. In this dehydrated state, they are more tolerant to extreme environmental conditions than are their hydrated counterparts. Nematodes in seed galls were exposed to various high temperatures (80 to 160°C) for time intervals of 5 to 30 min. Survival decreased as time and temperature increased. Remarkably, these nematodes survived exposure to 155°C for 5 min, higher than that recorded for any other metazoan. In contrast, seed galls that had been stored at room temperature and humidity for 5 yr also survived exposure to extreme temperatures; however, their survival rates were not as high as those for freshly collected galls. Juveniles within the seed gall were coiled and grouped together conforming to the shape of the seed gall. The gross morphology of the cuticle of the juveniles was very smooth and relatively undistorted by the shrinkage from the loss water from their body tissues. Wherever the nematodes were cut with a razor blade, a small amount of their contents oozed out of the opening and coalesced with that of other nearby specimens and appeared gel-like. Elucidation of the mechanisms that enable these nematodes to remain viable after exposure to extreme heat remains a mystery. Understanding the changes that occur in these nematodes as they rehydrate and return to life from an ametabolic state may have major impacts on the life sciences, including insights into the answer of the age-old question: “What is life?”
Keywords: Agrostis stolonifera, ametabolic, Anguina agrostis, anhydrobiosis, desiccation, extremist, feeding site, host-parasitic relationship, longevity, survival, thermal death point, morphology, thermophilic
A seed gall nematode, Anguina agrostis (Steinbuch, 1799) Filipjev, 1936, which was found parasitizing redtop creeping bentgrass (Agrostis stolonifera L.) (Eisenback and Roane, 2006), overwinters in an anhydrobiotic state. Anhydrobiosis, “the ability to survive the cessation of metabolism due to water loss” (Crowe and Madin, 1975), was first reported in Anguina tritici (Steinbuch, 1799) Filipjev, 1936 by Needham in 1743 (Needham, 1743). This loss of water and the subsequent state of suspended animation is often correlated with the ability of ametabolic organisms to survive extreme environmental conditions including cold, heat, X-ray radiation, very low vacuum, very high pressure, and immersion in alcohol and other toxins (Wharton, 2002). Anhydrobiotic nematodes have been reported to survive 105°C for 2 min (Crowe and Madin, 1975). Hydrated living nematodes are more sensitive to temperature. The thermophilic nematode, Aphelenchoides parietinus (Bastian, 1865) Steiner, 1932, was found actively living in a natural hot spring in New Zealand at a temperature of 61.3°C, giving it the record for all metazoans (Rahm, 1937). The thermal death point, however, was not determined (Rahm, 1937). The thermal death point for most mesophiles that are metabolically active is approximately 46°C (Dwinell, 1990); however, anhydrobiotic forms can survive much higher temperatures. Anhydrobiotic tardigrades and rotifers hold the record for surviving exposure to high temperature (Horikawa, 2007). They can survive 151°C for several minutes (Wharton, 2002). To see if the seed gall nematodes could survive similar temperatures, we tested their ability to survive very high temperatures as anhydrobiotes and determined their thermal death point.
Many nematodes become coiled during the dehydration part of the initiation of anhydrobiosis (Demeure et al., 1979; Wharton and Marshall, 2002). Often shrinkage of the body during dessication is noticeable from wrinkles in the cuticle (Freckman et al., 1977). To observe the physical condition of the juveniles of A. agrostis within the seed gall, we cut through the wall with a razor blade and examined the exposed nematodes with a scanning electron microscope (SEM). We also sliced through the nematodes within the untreated seed gall at perpendicular and diagonal angles to observe the nature of the contents of the dehydrated nematodes.
Materials and Methods
Heat tolerance: Redtop creeping bentgrass seeds infected with seed gall nematode were collected on 24 August 2011 from a naturally infested site on Butt Mountain Lookout in Giles County, VA. Seed galls were soaked in purified drinking water (Sam’s Choice®, Walmart Stores, Inc., Bentonville, AR) for 24 hr to evaluate the survival of juvenile nematodes at room temperature (20°C). Additional seed galls were exposed to high temperatures in a glass test tube immersed in hot water for 5, 10, 15, and 30 min at 80, 90, and 100°C for each time interval. A glass tube with an analog thermometer inserted inside was placed into a beaker of water and allowed to reach the desired temperature. The seed gall was added to the tube and the temperature was monitored during the desired time interval. The temperature usually exceeded the desired temperatures by 1 to 2 degrees during the treatment, but it did not drop below the targeted temperature. After the desired time interval was complete, the seed gall was removed from the test tube and cooled in a Bureau of Plant Industry (BPI) dish. After 5 to 10 min, 20 ml of purified water was added to the dish and the dish was maintained in a moist chamber at room temperature for 24 hr. For temperatures higher than the boiling point of water, seed galls were placed in a BPI dish in a thermostatically controlled oven maintained at 110, 120, 130, 140, 150, 155, and 160°C for varying times ranging from 5 to 30 min. The temperature was monitored with an analog thermometer placed through a hole in the wall of the oven during the entire period of exposure and usually exceeded the desired temperature by 1 to 2 degrees but did not fall under the targeted temperature. The BPI dish with the treated gall was removed from the oven and allowed to cool to room temperature before 20 ml of purified water was added; the treated gall was then allowed to soak for 24 hr, after which the nematodes were freed from the gall with sharply pointed forceps and counted with an inverted light microscope. Nematodes in each gall were counted as living if they were moving, or as dead if they were not. All treatments were replicated six times and the means of each was compared for statistical significance difference at the 0.05 level with Tukey’s HSD (honestly significant difference) mean comparisons tests as executed with JMP 10 ® software (SAS, Cary, NC).
Effects of aging on heat tolerance: Samples that were collected on 21 August 2006 and stored in an open plastic bag in a laboratory cabinet for more than 5 yr were compared with freshly collected specimens (24 August 2011) from the same population of A. agrostis. Seed galls were soaked in tap water for 24 hr to evaluate the survival of juvenile nematodes. Additional seed galls were exposed to high temperatures in a glass test tube immersed in hot water for 5, 10, 15, and 30 min at 80, 90, and 100°C each. The treated galls were placed into tap water for 24 hr at 20°C, after which the nematodes were freed from the gall with sharply pointed forceps. All treatments were replicated six times and the means of each was evaluated for statistical significance as previously reported. All of the nematodes in each gall were counted and they were considered as living if they were moving, or as dead if they were not.
SEM of juveniles inside the seed gall: Seed galls were selected from the seed heads of infected redtop bentgrass and untreated galls were cut with a razor blade in cross, diagonal, and longitudinal sections. They were mounted onto aluminum stubs for SEM with double sticky tape, coated with approximately 240 Å of gold/paladium with an Emitech SC 7620 sputter coater operating at 20 mA for 90 sec, and imaged with a JEOL Neoscope JCM-5000 operating at 10 kV.
Results
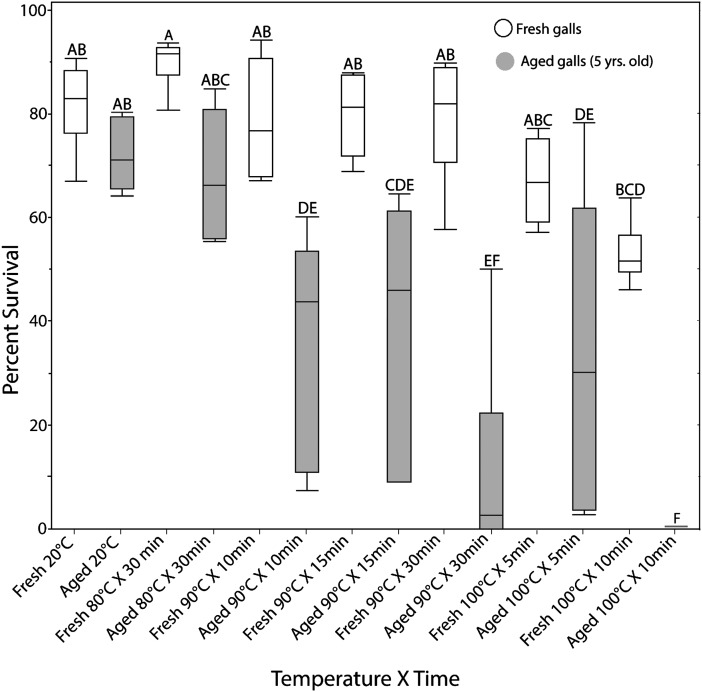
The number of juveniles per gall varied from 297 to 1,280. On average (n = 6), fresh galls contained 630 nematodes, of which 82% were alive, whereas, 5-yr-old seed galls had 694 nematodes, of which 72% were living (Fig. 1). The survival of nematodes in galls that were heat-treated at 80°C for 30 min was 90% in fresh galls and only 68% in 5-yr-old galls. Fresh galls exposed to 90°C for 30 min had survival rates of 68%; but in 5-yr-old galls, only 10% of the individuals survived. All of the nematodes were killed in 5-yr-old galls that were treated at 100°C for 15 min; however, 31% survived exposure for 5 min. In contrast, there was 72% survival in fresh galls that were treated at 100°C for 5 min, and 35% survival after exposure for 30 min.
Fig. 1.
Comparison of the survival rates of 5-yr-old and fresh Anguina agrostis (Steinbuch, 1799) Filipjev, 1936 anhydrobiotic juveniles at 20°C and 80–100°C in time increments of 5 to 30 min. Box plots with different letters have means that are statistically different at the 0.05 level according to Tukey’s HSD (honestly significant difference) means comparisons tests.
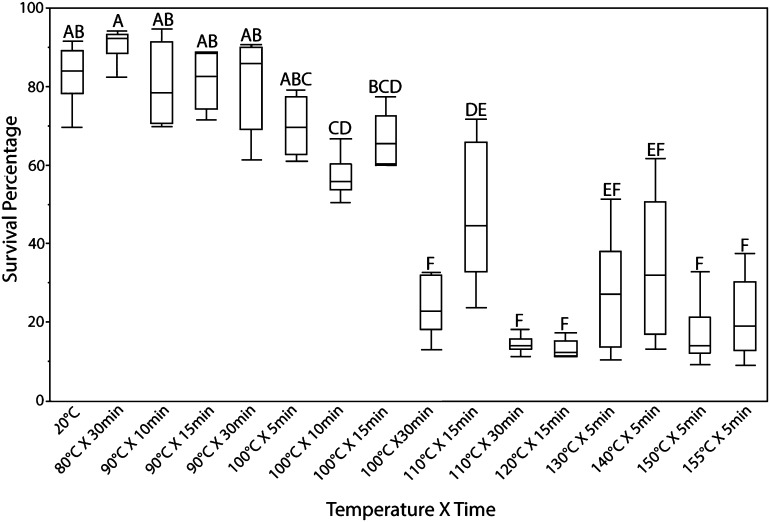
Nematodes from fresh galls had 42% survival at 110°C for 15 min and 6% for 30 min (Fig. 2). Five percent survived 120°C for 15 min; 20% survived after 5 min at 130°C; 28% survived at 140°C for 5 min; 7% survived after 5 min at 150°C; and 13% survived 155°C for 5 min. None survived 160°C for 5 min.
Fig. 2.
Survival of anhydrobiotic seed gall nematode, Anguina agrostis (Steinbuch, 1799) Filipjev, 1936 at 20°C and 80–155°C in time increments of 5 to 30 min. Box plots with different letters have means that are statistically different at the 0.05 level according to Tukey’s HSD (honestly significant difference) means comparisons tests.
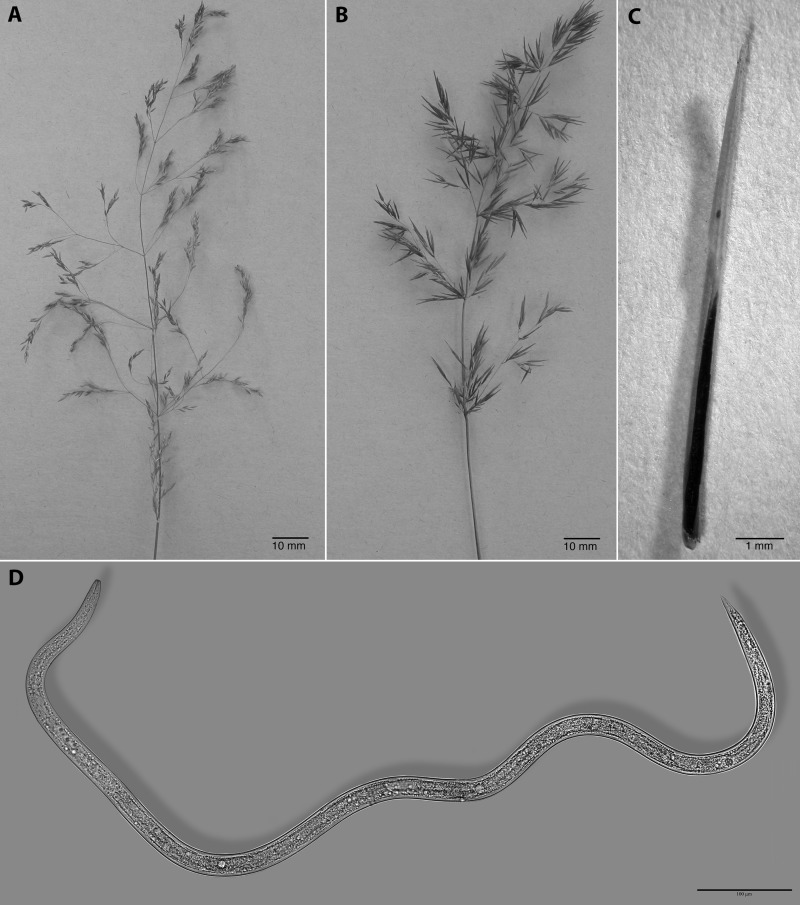
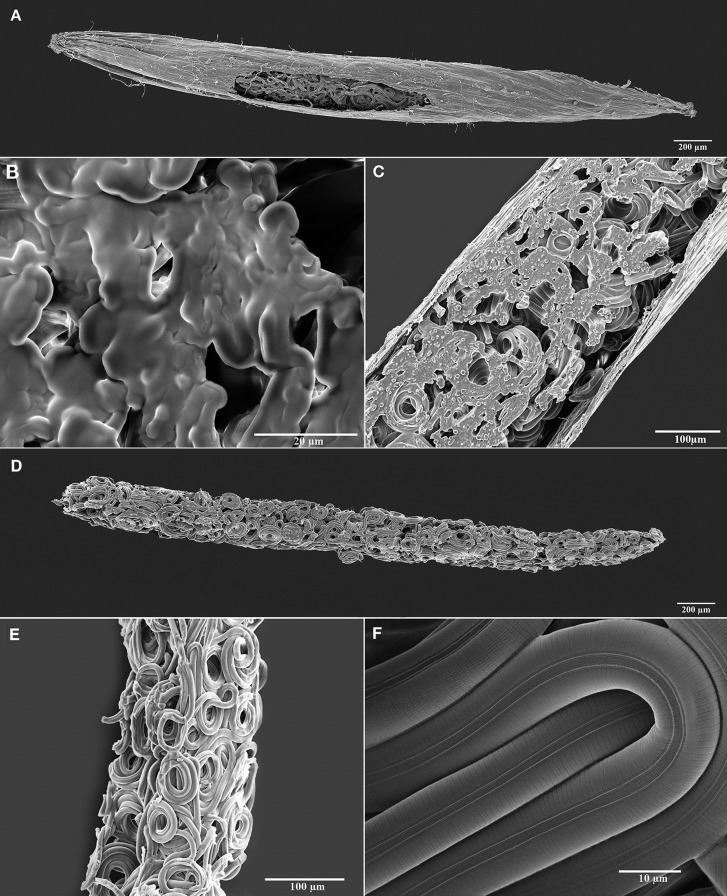
The seed galls induced by A. agrostis on redtop bentgrass were extremely elongated compared with the normal rounded seed (Fig. 3). Cutting the gall with a razor blade resulted in a clean, crisp cut of the plant tissues and revealed the coiled second-stage juveniles (Fig. 4). However, in the SEM, the cut surface of the nematodes allowed the body contents to ooze out into what appeared to be a smooth, amorphous gel-like substance (Fig. 4). The contents from surrounding nematodes or from surfaces cut through adjacent body sections, likewise, fused together. Inside the gall, the coiled nematodes were conformed to the elongated shape of the chamber and often intertwined with each other. Their cuticle was smooth and not wrinkled from the shrinkage caused by a large loss of free water in the body (Fig. 4). Regular body annulations were smooth and the lateral field was clearly marked by four incisures.
Fig. 3.
A: Healthy redtop creeping bentgrass (Agrostis stolonifera L.) seed head. B: Grass infected with the seed gall nematode, Anguina agrostis (Steinbuch, 1799) Filipjev, 1936. Note the very elongated glumes, lemmas, and paleas on the infected grass. C: An elongated, black seed gall replaces the ovary in infected grass and contains 400 to 1,200 anhydrobiotic infective juveniles. D: An infective juvenile from a seed gall was exposed to 155°C for 5 min while in its ametabolic, anhydrobiotic state. Normal metabolic processes were resumed after soaking for 2 to 3 hr in filtered water, establishing a new record for the metazoan with the highest tolerance to extreme temperatures.
Fig. 4.
Scanning electron micrographs (SEM) of juveniles of Anguina agrostis (Steinbuch, 1799) Filipjev, 1936 dissected from seed galls of Agrostis stolonifera L. A: Seed gall sliced longitudinally to expose the juveniles contained within. B: Cross-section revealing juveniles tightly packed within the seed gall showing the body contents that appear gel-like in the SEM and oozing out of the cut nematodes and coalesce with each other. C: Close-up of a seed gall sliced longitudinally to exposes the anhydrobiotic nematodes and the contents from their bodies. D: Mass of juvenile nematodes dissected from a seed gall in an anhydrobiotic state. E: Close-up showing coiled anhydrobiotic juveniles dissected from the seed gall. F: Close-up of juveniles revealing that the morphology of the body wall remains smooth, undistorted, and free from any artifact normally associated with the loss of all free water molecules.
Discussion
These experiments demonstrated that juveniles of A. agrostis have a remarkable tolerance to heat that gradually declined as they aged, and as temperature and length of exposure was increased. As temperatures increased, survival was negatively correlated with the time of exposure. Treatment at 90°C for 15 min had little effect on recovery compared with recovery at room temperature (approximately 20°C); however, the effect of treatment at 100°C for 5 min was nearly equal in effect to exposure at 90°C for 30 min. The most remarkable result of these tests was the discovery that this nematode survived treatment at the record high temperature of 155°C for 5 min. The nematode, A. agrostis, was resistant to extremes in temperatures that were higher than those recorded for any other metazoan, including tardigrades and rotifers (Rham, 1937). However, A. tritici survived 105°C for 2 min (Bird and Buttrose, 1974).
The analog thermometer recorded the temperature of the air immediately surrounding the seed gall in either the test tube immersed in water or in the dry oven. Since the seed gall was very thin, we assumed that the nematodes reached the same temperature shortly after it was placed in the chamber and that it remained at that temperature for the specified duration until it was removed from the chamber and allowed to gradually cool to room temperature.
Mertens et al. (2008) demonstrated a difference in survival rates of anhydrobiotic eukaryotic organisms that were slowly heated (∼4°C min-1) compared with those that were rapidly heated (∼100°C min-1). Our method of heating was rapid, since the seed galls were placed directly into a preheated glass test tube or dry oven. Perhaps if we had used a slow heating technique, these nematodes may have survived even higher temperatures.
The ecological significance of the survival of these nematodes at these high temperatures is uncertain, but perhaps it enables them to prevail through local fast-burning fires that may be caused by lightening or mankind. However, evidence suggests that these nematodes are not protected from such fires, because they are readily controlled by burning (Hardison, 1976). On the other hand, perhaps the ability to survive high temperatures is a consequence of the physiological changes that occur during the onset of anhydrobiosis, which is the primary long-term survival strategy for these nematodes (Wormsley et al., 1998; Barrett, 2011; Burnell and Tunnacliffe, 2011; Devaney, 2011; Perry and Moens, 2011).
The mechanisms that allow these organisms to survive these extreme temperatures remain a mystery. Although the production of heat shock proteins and the accumulation of nonreducing disaccharides have been correlated with anhydrobiosis in nematodes (Crowe and Madin, 1975; Behm, 1997; Wormsley et al., 1998; Shannon et al., 2005; Barrett, 2011; Burnell and Tunnacliffe, 2011; Devaney, 2011; Erkut et al., 2012;), a complete understanding of this phenomenon is lacking. Three plausible proposals that help explain the physiological changes that may aid in the stabilization of cell membranes and other cellular components in order to minimize the damage caused by the desiccation of these animals are the water replacement hypothesis, water entrapment, and the vitrification hypothesis; although the three are not mutually exclusive (Crowe et al., 1992; Crowe, 2007). The hypotheses are related to each other because the replacement and entrapment of water causes the vitrification of the body contents. Vitrification results from the formation of an amorphous solid material (glass) resulting from the replacement of water with stabilizing molecules such as trehalose or other sugars (Crowe, 2007).
The SEM revealed the anhydrobiotic juveniles of A. agrostis within the seed gall to be coiled similar to many other nematodes in anhydrobiosis (Freckman et al., 1977; Demure et al., 1979). This coiled posture may be important in reducing the “surface area exposed to the environment” (Bird and Buttrose, 1974). The loss of water from the body is usually associated with marked shrinkage and wrinkling of the nematode cuticle (Freckman et al., 1977); therefore, the unwrinkled appearance of the dehydrated juveniles in the seed gall was unexpected. Several factors may be responsible for the lack of wrinkles, including evidence that Anguina species have considerably less water than other nematode genera, and drying to 5% body water content had only a slight reduction (7% to 8%) in overall body dimensions of length and width compared with hydrated specimens (Bird and Buttrose, 1974). Although “changes in the plane of birefringence …” suggested “that changes in cuticle orientation have taken place in these areas” in transmission electron micrographs of A. tritici (Bird and Buttrose, 1974), SEM failed to reveal evidence of this occurring in A. agrostis. The cuticle appeared to have little interactions at points of contact with each other; no “twisting or kinking” as reported for A. tritici (Bird and Buttrose, 1974) was observed in A. agrostis.
The gel-like appearance of the cut surfaces of the nematodes within the gall supports the idea that in the absence of water the tissues change into a glassy state during desiccation that helps prevent damage to components of the cell (Crowe et al., 1992; Crowe, 2007; Erkut et al., 2012). Trehalose and others sugars have been credited with this phenomenon, although some organisms that undergo anhydrobiosis may not have large amounts of trehalose (Crowe, 2007). Additional research on the morphological and physiological changes that occur in the seed gall nematodes as they dehydrate into an ametabolic state and then return to life may provide insight into the age-old question, “What is life?”, as well as have other major impacts on the life sciences, including medicine (Crowe, 2007), especially for the preservations of cells, tissues, organs, and whole organisms.
Literature Cited
- Barrett J. 2011. Biochemistry of survival. Pp. 282–310 in R. N. Perry and D. Wharton, eds. Molecular and physiological basis of nematode survival. Wallingford, UK: CABI Press. [Google Scholar]
- Behm CA. The role of trehalose in the physiology of nematodes. International Journal for Parasitology. 1997;27:215–229. doi: 10.1016/s0020-7519(96)00151-8. [DOI] [PubMed] [Google Scholar]
- Bird AF, Buttrose MS. Ultrastructural changes in the nematode Anguina tritici associated with anhydrobiosis. Journal of Ultrastructure Research. 1974;48:177–189. doi: 10.1016/s0022-5320(74)80075-4. [DOI] [PubMed] [Google Scholar]
- Burnell AM, Tunnacliffe A. 2011 Gene induction and desiccation stress in nematodes. Pp. 126–156 in R. N. Perry, and D. Wharton, eds. Molecular and physiological basis of nematode survival. Wallingford, UK: CABI Press. [Google Scholar]
- Crowe JH. Trehalose as a ‘chemical chaperone’: Fact and fantasy. Advances in Experimental Medicine and Biology. 2007;594:143–158. doi: 10.1007/978-0-387-39975-1_13. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Madin KAC. Anhydrobiosis in nematodes: Evaporative water loss and survival. Journal of Experimental Zoology. 1975;193:323–334. [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annual Review of Physiology. 1992;54:578–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Demeure Y, Freckman DW, Van Gundy SD. Anhydrobiotic coiling in nematodes in soil. Journal of Nematology. 1979;11:189–195. [PMC free article] [PubMed] [Google Scholar]
- Devaney E. 2011 Thermobiotic survival. Pp. 233–255 in R. N. Perry, and D. Wharton, eds. Molecular and physiological basis of nematode survival. Wallingford, UK: CABI Press. [Google Scholar]
- Dwinell DL. Heat-treating southern pine lumber to eradicate Bursaphelenchus xylophilus. Nematologica. 1990;36:346–347. (Abstr.). [Google Scholar]
- Eisenback JD, Roane CW. First report of bentgrass seed gall nematode, Anguina agrostis, in Virginia and Minnesota. Plant Disease. 2006;90:1110. doi: 10.1094/PD-90-1110C. [DOI] [PubMed] [Google Scholar]
- Erkut C, Penhov S, Fahmy K, Kurzchalia TV. How worms survive desiccation: Trehalose pro water. Worm. 2012;1:61–65. doi: 10.4161/worm.19040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freckman DW, Kaplan DT, VanGundy SD. A comparison of techniques for extraction and study of anhydrobiotic nematodes from dry soils. Journal of Nematology. 1977;9:176–181. [PMC free article] [PubMed] [Google Scholar]
- Hardison JR. Fire and flame for plant disease control. Annual Review of Phytopathology. 1976;14:355–379. [Google Scholar]
- Horikawa DD. 2007 Survival of Tardigrades in extreme environments: A model animal for astrobiology. Pp. 205–217 in A. V. Altenbach, J. B. Bernhard, and J. Seckbach, eds. Anoxia: Evidence for eukaryote survival and paleontological strategies. New York: Springer. [Google Scholar]
- Mertens J, Beladjal L, Alcantara A, Fougnies L, van der Straen D, Clegg JS. Survival of dried eukaryotes (anhydrobiotes) after exposure to very high temperatures. Biological Journal of the Linnean Society. 2008;93:15–22. [Google Scholar]
- Needham JT. 1743 Concerning chalky concretions called malm; with some microscopical observations on the farina of red lily, and worms discovered in smutty corn. Philosophical Transactions of the Royal Society, London 42:634–641. [Google Scholar]
- Perry RN, Moens M. 2011 Survival of parasitic nematodes outside the host. Pp. 1–27 in R. N. Perry, and D. Wharton, eds. Molecular and physiological basis of nematode survival. Wallingford, UK: CABI Press. [Google Scholar]
- Rahm G. 1937 Grenzen des lebens. Studien in heissen quellen. [Limits of life. Studies in hot springs.] Forsch Fortschr. 13:318–383. [Google Scholar]
- Shannon AJ, Browne JA, Boyd J, Fitzpatrick DA, Burnell AM. The anhydrobiotic potential and molecular phylogenetics of species and strains of Panagrolaimus (Nematoda, Panagrolaimidae) The Journal of Experimental Biology. 2005;208:2433–2445. doi: 10.1242/jeb.01629. [DOI] [PubMed] [Google Scholar]
- Wharton DA. Life at the limits organisms in extreme environments. Caimbridge, UK: Cambridge University Press; 2002. [Google Scholar]
- Wharton DA, Marshall AT. Changes in surface features during dessication of the anhydrobiotic plant parasitic nematode Ditylenchus dipsaci. Tissue & Cell. 2002;34:81–87. doi: 10.1016/s0040-8166(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Womsersley CZ, Wharton D, Higa LM. 1998. Survival biology. Pp. 271–302 in R. N. Perry, and D. J. Wright, eds. Physiology and biochemistry of plant parasitic and free-living nematodes. Wallingford, UK; CABI Press. [Google Scholar]