Abstract
Cucumis melo var. texanus, a wild melon commonly found in the southern United States and two accessions, Burleson Co. and MX 1230, expressed resistance to Meloidogyne incognita in preliminary experiments. To characterize the mechanism of resistance, we evaluated root penetration, post-penetration development, reproduction, and emigration of M. incognita on these two accessions of C. melo var. texanus. Additionally, we evaluated 22 accessions of C. melo var. texanus for their reaction against M. incognita in a greenhouse experiment. Fewer (P ≤ 0.05) J2 penetrated the root system of C. melo var. texanus accessions (Burleson Co. and MX 1230) and C. metuliferus (PI 482452) (resistant control), 7 days after inoculation (DAI) than in C. melo ‘Hales Best Jumbo’ (susceptible control). A delayed (P ≤ 0.05) rate of nematode development was observed at 7, 14, and 21 DAI that contributed to lower (P ≤ 0.05) egg production on both accessions and C. metuliferus compared with C. melo. Though J2 emigration was observed on all Cucumis genotypes a higher (P ≤ 0.05) rate of J2 emigration was observed from 3 to 6 DAI on accession Burleson Co. and C. metuliferus than on C. melo. The 22 accessions of C. melo var. texanus varied relative to their reaction to M. incognita with eight supporting similar levels of nematode reproduction to that of C. metuliferus. Cucumis melo var. texanus may be a useful source of resistance against root-knot nematode in melon.
Keywords: Cucumis melo; C. melo var. texanus; C. metuliferus, emigration; melon; Meloidogyne incognita, post-penetration development; resistance; and root-knot nematode
The root-knot nematode, Meloidogyne incognita, is an important plant-parasitic nematode on melon, Cucumis melo, in the United States (Sasser, 1979; Lamberti, 1979; Heald et al., 1988; Ploeg and Phillips, 2001; Sikora and Fernandez, 2005). Few management options are available to growers to manage M. incongita on melon. The use of fumigant nematicides has been successful (Hamill and Dickson, 2005); however, the use of fumigants or other nematicides are becoming limited or banned by legislation because of potential hazards to environmental or public health (Ristaino and Thomas, 1997; Nyczepir and Thomas, 2009). The development of root-knot nematode resistant varieties have been successful in cotton, peanut, pepper, tomato, and tobacco (Ogallo et al., 1997; Simpson and Starr, 2001; Starr et al., 2002; Thies et al., 2003; Starr and Mercer, 2009). Currently, no commercially available melon varieties are resistant to M. incognita. Sources of resistance in C. melo are lacking though many closely related species and botanical varieties have been evaluated for resistance to M. incognita (Thomason and McKinney, 1959; Fassuliotis and Rau, 1963; Fassuliotis, 1967; Nugent and Dukes, 1997). A high level of resistance to M. incognita was reported in C. metuliferus (Fassuliotis, 1970; Wehner et al., 1991; Walters et al., 2006), but numerous attempts to incorporate this resistance into C. melo have been unsuccessful due to cross-incompatibility (Fassuliotis, 1977; Norton and Granberry, 1980; Chen and Adelberg, 2000). Thus, identifying resistance to M. incognita within C. melo subspecies or botanical varieties would be beneficial for plant breeders and plant pathologist to introgress resistance into melon varieties.
Cucumis melo is a diverse species composed of tropical and subtropical botanical varieties that are potential sources of resistance to melon diseases. A wild botanical variety, C. melo var. agrestis has been reported to have resistance to a closterovirus that causes melon yellowing disease (Sorie et al., 1996) and var. texanus has been reported to be a putative source of resistance to M. incognita in preliminary greenhouse trials (Faske, 2010). Cucumis melo var. texanus is commonly found in agricultural fields in the southern United States and Mexico. Though widely distributed in North America, little is known about this source of resistance to M. incognita. Thus, characterizing the mechanism of resistance in C. melo var. texanus to M. incognita and evaluating the reaction of several accessions of C. melo var. texanus against M. incognita would further our understanding of this potential source of resistance to root-knot nematodes.
The mechanism of resistance to root-knot nematodes in C. metuliferus has been characterized in a few studies (Fassuliotis, 1970; Haynes and Jones, 1976; Walters et al., 2006). Fassuliotis (1970) reported that the penetration rate of M. incognita was similar between C. metuliferus and C. melo, ‘Hales Best Jumbo’, and a similar observation was reported between C. metuliferus (PI 482452) and C. sativus, ‘Sumter’ (Walters et al., 2006). Post-penetration development of M. incognita was delayed because of abnormal development of the feeding site for optimum nematode development, thus contributing to lower nematode reproduction on C. metuliferus as compared with the susceptible control. Further, no hypersensitivity or necrosis was associated with the infection of M. incognita in the root system of C. metuliferus (Fassuliotis, 1970; Walters et al., 2006. Though a few studies have investigated resistance related to the biology of M. incognita in C. metuliferus, it has yet to be determined if J2 emigration contributes to the mechanism of resistance.
The objectives for this study was (i) to evaluate the penetration rates, post-penetration development, and fecundity of M. incognita on C. melo var. texanus; (ii) evaluate the behavior of M. incognita J2 following root penetration on C. metuliferus and C. melo var. texanus; and (iii) compare reproduction of M. incognita on several North American accessions of C. melo var. texanus.
Materials and Methods
Nematode culture and inoculum: Meloidogyne incognita was isolated from cotton (Gossypium hirsutum) and maintained in the greenhouse on Solanum lycopersicum L. ‘Rutgers’. Eggs were collected from infected tomato roots with 0.5% NaOCl (Hussey and Barker, 1973) and J2 were collected in a hatching chamber (Vrain, 1977). Only 24-hr-old J2 were used in this study.
Nematode biological response experiments: Three separate experiments were conducted to evaluate penetration and post-penetration development, emigration of J2, and fecundity of M. incognita on four Cucumis genotypes. Two accessions (Burleson Co. and MX 1230) of C. melo var. texanus had lower nematode reproduction in preliminary trials; therefore, they were used in these nematode biological response experiments. The resistant control, C. metuliferus (PI 482452), and susceptible control, C. melo ‘Hales Best Jumbo’, were used throughout this study.
A time course study was used to evaluate nematode penetration and post-penetration development. Germinated seed from each Cucumis genotype was planted into 85-cm3-celled planter flats containing pasteurized sand to peat (6:1 v/v) soil mix. Seedlings were inoculated at the first true leaf stage, 2 to 3 wk after seeding, with approximately 165 J2 evenly distributed among three 2-cm-deep cavities around the seedling. This experiment was arranged in a randomized complete block design (RCBD) and repeated once. In each experiment, treatments were replicated four times per sample date. Root systems were harvested at 7, 14, and 21 d after inoculation (DAI) and washed free of soil. Nematodes were stained with acid fuchsin (Byrd et al., 1983) and classified into four stages of development; vermiform J2, sausage-shaped juveniles, female without eggs, and female with eggs.
Emigration of J2 was evaluated in a hydroponic system. Germinated seeds were planted into 85-cm3-celled planter flats containing pasteurized sand to peat (6:1 v/v) soil mix. Seedlings were inoculated at the first true leaf stage with approximately 2,000 J2, evenly distributed among three 2-cm-deep cavities around the seedling. Each Cucumis genotype was replicated five times in a RCBD and the experiment was conducted once. Roots systems were collected 2 DAI and washed free of soil. Seedlings were transferred into individual 230-cm3 plastic containers filled with distilled water and fitted with a plastic tube attached to a small air-pump. Air was pumped through the water to provide enough oxygen to keep roots healthy. Second-stage juveniles that emigrated from the roots were collected daily from 3 to 6 DAI and enumerated.
To evaluate fecundity, freshly collected eggs were inoculated onto seedlings at the second true leaf stage, 3 to 4 wk after seeding, growing in 1025-cm3 standard pots containing pasteurized sand to peat (6:1 v/v) soil mix. Inoculum concentration of 4,000 eggs was distributed among three 2-cm-deep cavities around each seedling. Each experiment was maintained in a greenhouse where ambient temperature ranged from 22 to 32°C. Each Cucumis genotype was replicated four times in a RCBD and the experiment was repeated once. Roots were harvested 7 wk after inoculation and washed free of soil. Roots were blotted dry with paper towels, rated for galling based on a six-point scale with 0 = no galls and 5 = severe galling. Single egg masses (largest) were collected from each root system and treated with 1.0% NaOCl to extract eggs from each egg mass. Root systems were weighted, cut into 1-cm pieces and treated with 1.0% NaOCl to extract eggs present. Eggs were enumerated with a stereoscope.
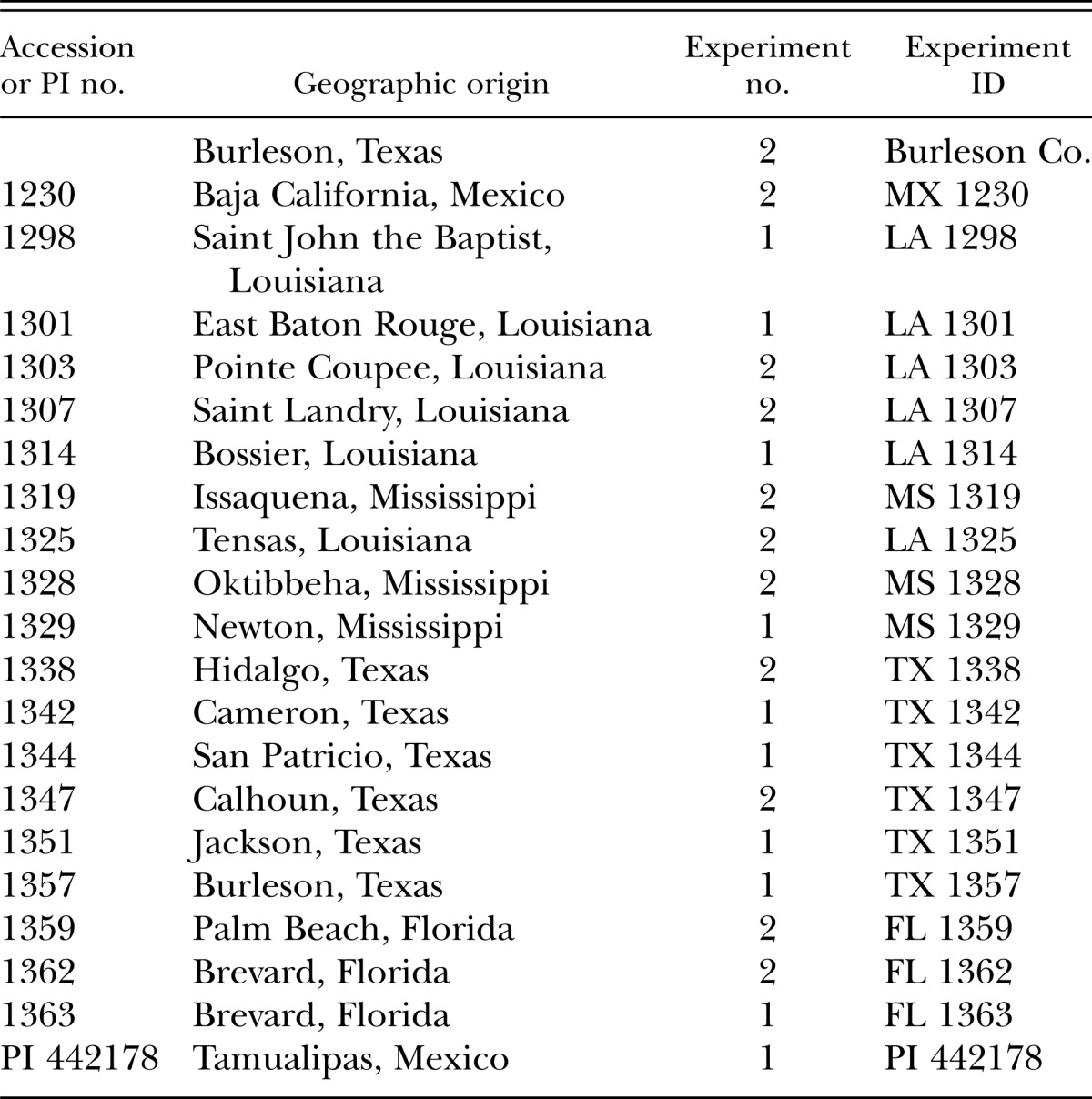
Greenhouse pot experiments: Twenty-two accessions for C. melo var. texanus were compared for their reaction against M. incognita in two separate experiments (Table 1). In each experiment, freshly collected eggs were inoculated onto seedlings at the second true leaf stage, 3 to 4 wk after seeding, growing in 1025-cm3 standard pots containing pasteurized sand to peat (6:1 v/v) soil mix. Inoculum concentration of 4,000 eggs was distributed among three 2-cm-deep cavities around each seedling. Each experiment was maintained in a greenhouse where ambient temperature ranged from 22 to 32°C. Accessions were replicated four times in a RCBD and each experiment was completed once. The resistant control, C. metuliferus (PI 482452), and susceptible control, C. melo ‘Hales Best Jumbo’, was used in this study.
Table 1.
Geographic data of Cucumis melo var. texanus accessions tested for ability to support reproduction of Meloidogyne incognita.
Experiments were harvested 7 wk after inoculation and soil was washed from the root system. Roots were blotted dry with paper towels, rated for galling, weighted, cut into 1-cm pieces and treated with 1.0% NaOCl to extract eggs present. Root galling was based on a six-point scale with 0 = no galls and 5 = severe galling. The eggs were enumerated with a stereoscope.
Statistical analysis: Data from nematode reproduction, fecundity, penetration, and emigration experiments were transformed (ln + 1) to normalize and nontransformed data are reported. Pearson’s correlation coefficient was calculated between root-galling and egg counts. Data were subjected to analysis of variance and mean separations by Tukey’s honestly significant difference (HSD) test, whereas data from post-penetration development experiment were subject to chi square analysis using SPSS 19.0 (SPSS, Inc., Chicago).
Results
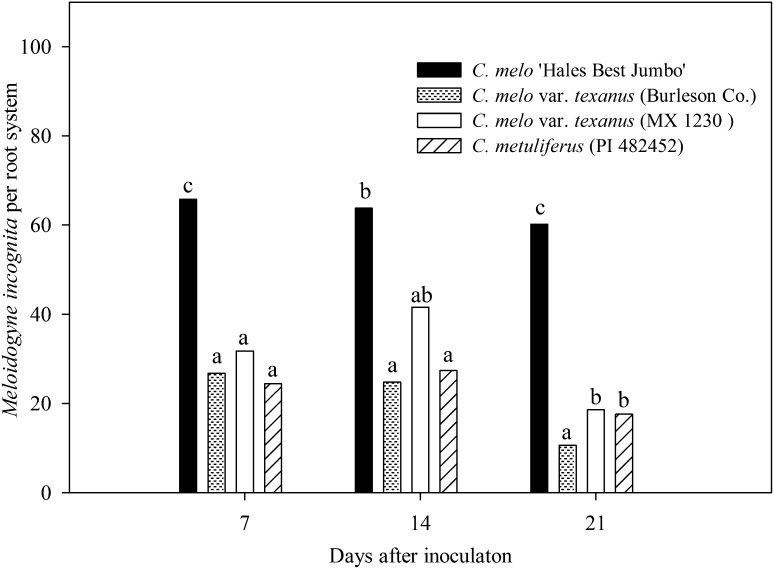
Penetration rates of M. incognita per root system were similar between experiments so data were combined (Fig. 1). Fewer (P ≤ 0.05) J2 were observed at 7 and 21 DAI in both accessions of C. melo var. texanus and C. metuliferus than C. melo, the susceptible control. The average count of M. incognita for all three sample dates was 20.7, 30.6, and 23.1 on the accessions of C. melo var. texanus, Burleson Co., MX 1230, and C. metuliferus, respectively, which was lower (P ≤ 0.05) than 63.1 on C. melo.
Fig. 1.
Number of Meloidogyne incognita at 7, 14, and 21 d after inoculation in four Cucumis genotypes. Genotypes consisted of a resistant control, C. metuliferus (PI 482452); susceptible control, C. melo ‘Hales Best Jumbo’; and two accessions of C. melo var. texanus (Burleson Co. and MX 1230). Initial population density of M. incognita was 200 J2/100-cm3 soil. Different letters over bars indicate significant differences at α = 0.05 according to Tukey’s HSD test.
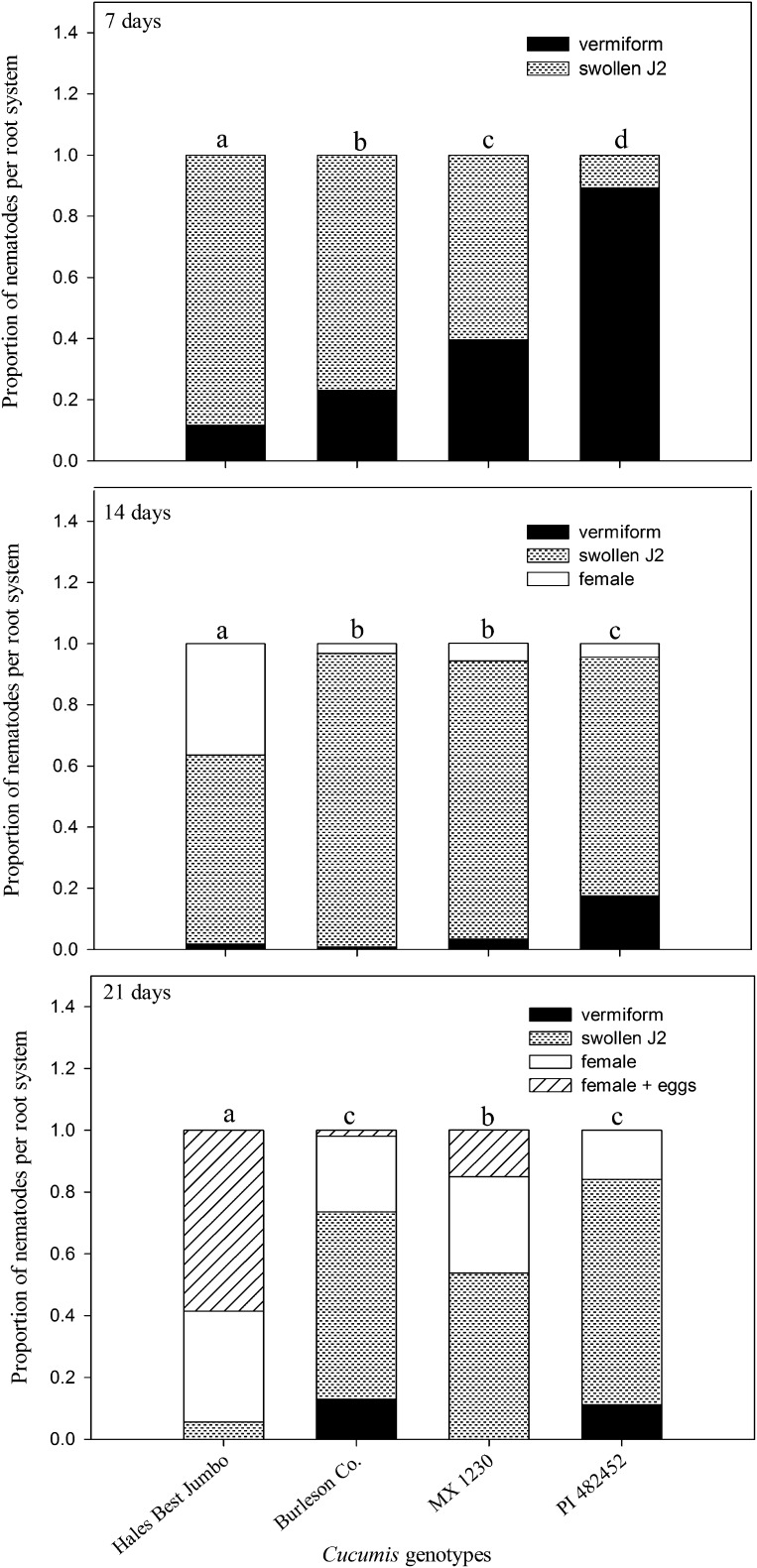
Development of M. incognita was delayed (P ≤ 0.05) at all sample dates in both accessions of C. melo var. texanus and C. metuliferus as compared with C. melo (Fig. 2). The nematode population present as females at 14 DAI and egg-laying females at 21 DAI was lower (P ≤ 0.05) on both accessions of C. melo var. texanus and C. metuliferus than C. melo. The proportion of females and egg-laying females at 14 and 21 DAI on C. melo was 0.40 and 0.58, respectively. No egg-laying females were observed on C. metuliferus and empty galls were observed on both accessions of C. melo var. texanus and C. metuliferus.
Fig. 2.
Number of Meloidogyne incognita development stages at 7, 14, and 21 d after inoculation in four Cucumis genotypes. Genotypes consisted of a resistant control, C. metuliferus (PI 482452); susceptible control, C. melo ‘Hales Best Jumbo’; and two accessions of C. melo var. texanus (Burleson Co. and MX 1230). Initial population density was 200 J2/100-cm3 soil. Different letters over bars indicates a significant difference at P ≤ 0.05 according to chi-square analysis applied in pairs of genotypes.
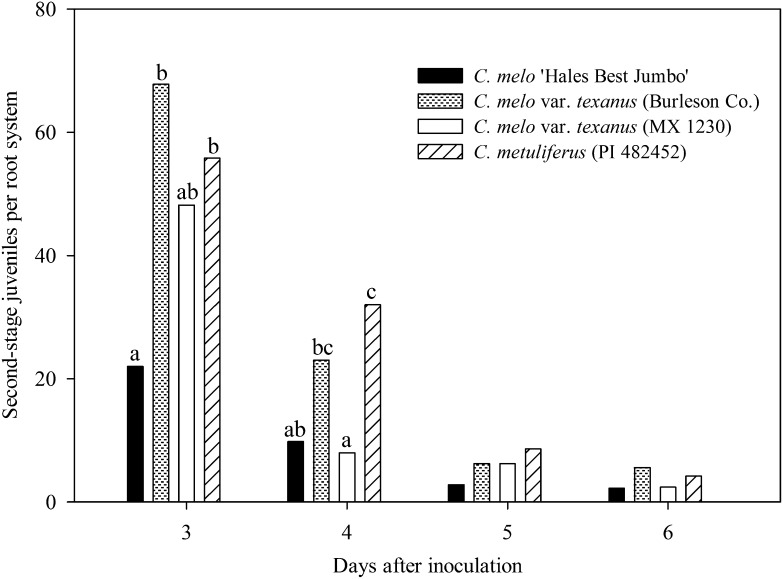
Second-stage juveniles emigrated at all sample dates from the root system of all Cucumis genotypes. More (P ≤ 0.05) J2 emigrated 3 DAI from the root system of accession Burleson Co. and C. metuliferus than C. melo (Fig. 3). The average count of J2 for all four sample dates was 102.6 and 100.6 from accession Burleson Co. and C. metuliferus, respectively, which was more (P ≤ 0.05) than 36.8 from C. melo.
Fig. 3.
Number of Meloidogyne incognita second-stage juveniles emigrating from roots of four Cucumis genotypes. Genotypes consisted of a resistant control C. metuliferus (PI 482452); susceptible control, C. melo ‘Hales Best Jumbo’; and two accessions of C. melo var. texanus (Burleson Co. and MX 1230). Initial population density of M. incognita was 2400 J2/100-cm3 soil. Different letters over bars indicate significant differences at α = 0.05 according to Tukey’s HSD test.
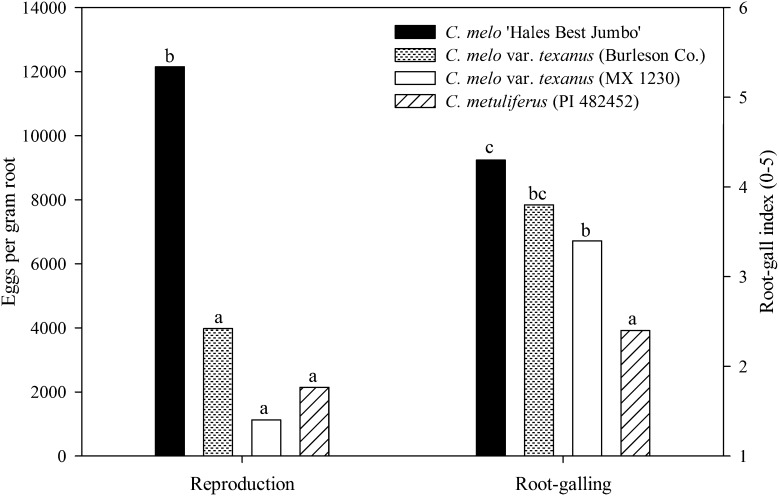
Nematode reproduction on Cucumis root systems was similar between experiments so data were combined (Fig. 4). Fewer (P ≤ 0.05) eggs were produced by M. incognita on both accessions of C. melo var. texanus and C. metuliferus than on C. melo (Fig. 4). A lower (P ≤ 0.05) root-gall rating was observed on accession MX 1230 and C. metuliferus as compared with C. melo. Fecundity of mature females remaining in the root system was similar among Cucumis genotypes with an average 432 eggs/egg mass for all genotypes.
Fig. 4.
Root-gall rating and reproduction of Meloidogyne incognita on four Cucumis genotypes. Genotypes consisted of a resistant control, C. metuliferus (PI 482452); susceptible control C. melo ‘Hales Best Jumbo’; and two accessions of C. melo var. texanus (Burleson Co. and MX 1230). Plants were harvested 7 wk after inoculation of 400 eggs/100-cm3 soil. Different letters over bars indicate significant differences at α = 0.05 according to Tukey’s HSD test.
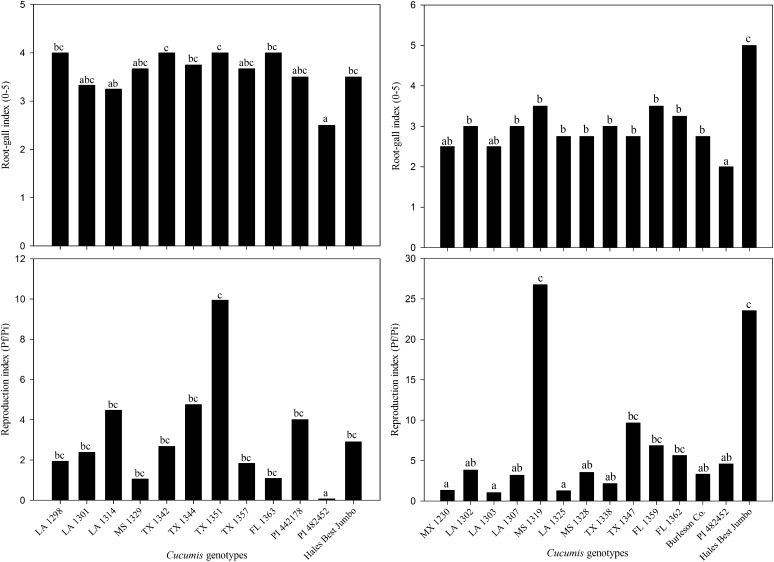
In the two greenhouse experiments, a lower (P ≤ 0.05) nematode reproduction index and root-gall rating was observed on the resistant control, C. metuliferus (PI 482452), than on the susceptible control, C. melo ‘Hales Best Jumbo’ (Fig. 5). In both experiments, galling was positively correlated (r = 0.41 and r = 0.49) with eggs produced by M. incognita. A few accessions of C. melo var. texanus, MX 1230, LA 1302, LA 1303, LA 1307, LA 1325, MS 1328, TX 1338, and Burleson Co. expressed a lower (P ≤ 0.05) root-gall rating and supported lower (P ≤ 0.05) levels of egg production than the susceptible control (Fig. 5). Further, egg production was numerically lower on these eight accessions compared with the resistant control. Numerically, the lowest gall rating and egg production was observed on MX 1230, LA 1303, and LA 1325 among these eight accessions of C. melo var. texanus. The average egg production on these three accessions was less than 5% of the nematode reproduction of the susceptible control. Alternately, nematode reproduction was higher on accession LA 1314, MS 1319, PI 442178, TX 1344, and TX 1351 than the susceptible control. Finally, there was no interaction between nematode reproduction and geographical origin of accessions.
Fig. 5.
Root-gall rating and reproduction of Meloidogyne incognita on 22 accessions of Cucumis melo var. texanus from various locations across North America. Resistant control was C. metuliferus (PI 482452) and susceptible control was C. melo ‘Hales Best Jumbo’. Plants were harvested 7 wk after inoculation of 400 eggs/100-cm3 soil. Different letters over bars indicate significant differences at α = 0.05 according to Tukey’s HSD test.
Discussion
Cucumis melo var. texanus is a wild melon commonly found in agricultural fields in the southern United States. There is limited data on this melon as a potential source of resistance to M. incognita. These data herein suggest that C. melo var. texanus is resistant to M. incognita and the mechanism of resistance is similar to that of C. metuliferus. The mechanism of resistance observed in C. melo var. texanus was related to three different effects on M. incognita. The first effect of resistance was a reduction in root penetration in C. melo var. texanus and C. metuliferus relative to the susceptible control. These results differ from those reported for M. incognita penetration rates in C. metuliferus (Fassuliotis, 1970; Walters et al., 2006) where the number of J2 did not differ between C. metuliferus and the susceptible control. Nematode penetration rates were estimated later than previous studies and nematode emigration was observed 3 DAI for all Cucumis genotypes, which may have affected recorded penetration rates (Herman et al., 1991). A higher rate of nematode emigration was observed from the root system of both accessions of C. melo var. texanus and C. metuliferus. Thus, the second effect was that most of the J2 failed to establish a feeding site and emigrated from the roots. Differences in J2 emigration have been reported between resistant and susceptible genotypes of peanut and soybean (Herman et al., 1991; Bendezu and Starr, 2003; Dhandaydham et al., 2008). It is possible the J2 were responding to cucurbitacians similar to those reported in C. sativus (Haynes and Jones, 1976). The third effect was a delayed rate in the development of M. incognita to reach maturity, which is consistent with earlier studies of a reduced or delayed development of root-knot nematodes (Fassuliotis, 1970; Walters et al., 2006). This effect was observed as early as 7 DAI, which contributed to fewer egg-laying females but had little effect on fecundity. Thus, post-penetration factors that suppressed nematode development had little or no effect on individual nematode that reach maturity.
The mechanism of resistance to M. incognita in C. metuliferus has also been related to an increased stimulation of juveniles toward maleness (Fassuliotis, 1970). Empty galls were observed in the biological response experiments on both accessions of C. melo var. texanus and C. metuliferus, which may have contained males that left the root. Potentially, contributing to fewer eggs produced by M. incognita on the root system of these accessions.
These data suggest the resistance to M. incognita in a few accessions of C. melo var. texanus is similar in magnitude to that of C. metuliferus. Cucumis metuliferus was the most resistant Cucumis genotype in this study. These results are consistent with other reports of resistance to M. incognita in C. metuliferus (Wehner et al., 1991; Walters et al., 1993, 1999). A few accessions, MX 1230, LA 1302, LA 1303, LA 1307, LA 1325, MS 1328, TX 1338, and Burleson Co. were moderately resistant to M. incognita. Further, an average reproduction factor of 1.2 for accessions, MX 1230, LA 1303, and LA 1329 is the lowest of all botanical varieties or subspecies of C. melo yet reported. Nugent and Dukes (1997) reported an average reproduction factor of 5.5 at a similar inoculum level for two accessions, PI 183311 and PI 140471, of C. melo subsp. melo. In this study, two accessions TX 1351 and MS 1319 were highly susceptible and, in comparison with the very resistant accessions (MX 1230, LA 1303, and LA 1329), would suggest a major effect in resistance. A similar effect was identified in C. sativus var. hardwickii as a single gene for resistance to M. javanica (Walters et al., 1997). This single resistance gene in C. sativus var. hardwickii has been reported to be effective against M. javanica and M. arenaria (Walters et al., 1996, 1999) whereas the resistance in C. metuliferus has been reported to be effective on these species and M. incognita (Wehner et al., 1991; Walters et al., 1993). Thus, this new source of resistance in C. melo var. texans may confer resistance to other species of Meloidogyne.
Cucumis melo var. texanus was not highly resistant to M. incognita, but it appears to be a potential source of resistance. Little progress has been made to integrate the resistance in C. metuliferus into melon (C. melo) because of cross-incompatibility (Fassuliotis, 1977; Norton and Granberry, 1980; Chen and Adelberg, 2000). Cucumis melo var. texanus is closely related to melon and could be used to develop root-knot nematode resistant varieties. Development of root-knot nematode resistant varieties would be beneficial in the management of root-knot nematode in commercial melon production.
Literature Cited
- Bendezu IF, Starr JL. Mechanism of resistance to Meloidogyne arenaria in the peanut cultivar COAN. Journal of Nematology. 2003;35:115–118. [PMC free article] [PubMed] [Google Scholar]
- Byrd DW, Jr, Kirkpatrick T, Barker KR. An improved technique for clearing and staining tissues for detection of nematodes. Journal of Nematology. 1983;15:142–143. [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Adelberg J. Interspecific hybridization in Cucumis-progress, problems, and perspectives. HortScience. 2000;35:11–15. [Google Scholar]
- Dhandaydham M, Charles L, Zhu H, Starr JL, Huguet T, Cook DR, Perosperi J, Opperman C. Characterization of root-knot nematode resistance in Meloidogyne truncatula. Journal of Nematology. 2008;40:46–54. [PMC free article] [PubMed] [Google Scholar]
- Faske TR. Identification and characterization of resistance to Meloidogyne incognita in wild species of Cucurbitaceae. Phytopathology. 2010;100:S35. [Google Scholar]
- Fassuliotis G. Species of Cucumis resistant to the root-knot nematode, Meloidogyne incognita acrita. Plant Disease Reporter. 1967;51:720–723. [Google Scholar]
- Fassuliotis G. Resistance of Cucumis spp. to the root-knot nematode, Meloidogyne incognita acrita. Journal of Nematology. 1970;2:174–178. [PMC free article] [PubMed] [Google Scholar]
- Fassuliotis G. Self-fertilization of Cucumis metuliferus Naud. and its cross-compatibility with C. melo L. Journal of the American Society for Horticultural Science. 1977;102:336–339. [Google Scholar]
- Fassuliotis G, Rau GJ. Evaluation of Cucumis spp. for resistance to the cotton root-knot nematode, Meloidogyne incognita acrita. Plant Disease Reporter. 1963;47:809. [Google Scholar]
- Hamill JE, Dickson DW. Effects of application strategies of fumigant and nonfumigant nematicides on cantaloupe grown in deep sand soils in Florida. Journal of Nematology. 2005;37:281–284. [PMC free article] [PubMed] [Google Scholar]
- Haynes RL, Jones CM. Effects of the Bi locus in cucumber on reproduction, attraction, and response of the plant to infection by the southern root-knot nematode. Journal of the American Society for Horticultural Science. 1976;101:422–424. [Google Scholar]
- Heald CM, Inserra RN, Vovlas N. Parasitism and reproduction of Meloidogyne incognita and Rotylenchulus reniformis on cantaloupe in two soils. Nematropica. 1988;18:53–58. [Google Scholar]
- Herman M, Hussey RS, Boerma HR. Penetration and development of Meloidogyne incognita on roots of resistant soybean genotypes. Journal of Nematology. 1991;23:155–161. [PMC free article] [PubMed] [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;59:1025–1028. [Google Scholar]
- Lamberti F. 1979. Chemical and cultural control. Pp. 403–423 in F. Lamberti and C. E. Taylor, eds. Root-knot nematodes (Meloidogyne species): Systematics, biology, and control. London: Academic Press. [Google Scholar]
- Norton JD, Granberry DM. Characteristics of progeny from an interspecific cross of Cucumis melo with C. metuliferus. Journal of the American Society for Horticultural Science. 1980;105:174–180. [Google Scholar]
- Nugent PE, Dukes PD. Root-knot nematode resistance in Cucumis species. HortScience. 1997;32:880–881. [Google Scholar]
- Nyczepir AP, Thomas SH. 2009. Current and future management strategies in intensive crop production systems. Pp. 410–443 in R. N. Perry, M. Moens, and J. L. Starr, eds. Root-knot nematodes. Cambridge, MA: CABI International. [Google Scholar]
- Ogallo JL, Goodell PB, Eckert J, Roberts PA. Evaluation of NemX, a new cultivar of cotton with high resistance to Meloidogyne incognita. Journal of Nematology. 1997;29:531–537. [PMC free article] [PubMed] [Google Scholar]
- Ploeg AT, Phillips MS. Damage to melon (Cucumis melo L.) cv. Durango by Meloidogyne incognita in southern California. Nematology. 2001;3:151–157. [Google Scholar]
- Ristaino JB, Thomas W. Agriculture, methyl bromide, and the ozone hole, can we fill the gap? Plant Disease. 1997;81:964–977. doi: 10.1094/PDIS.1997.81.9.964. [DOI] [PubMed] [Google Scholar]
- Sasser JN. 1979. Economic importance of Meloidogyne in tropical countries. Pp. 359–374 in F. Lamberti and C. E. Taylor, eds. Root-knot nematodes (Meloidogyne species): Systematics, biology, and control. London: Academic Press. [Google Scholar]
- Sikora RA, Fernandez E. 2005. Nematode parasites of vegetables. Pp. 319–392 in M. Luc, Sikora, R. A. and Bridge, J., eds. Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford, UK: CAB International. [Google Scholar]
- Simpson CE, Starr JL. Registration of ‘Coan’ peanut. Crop Science. 2001;41:918. [Google Scholar]
- Soria C, Sese AIL, Gomez-Guillamon ML. Resistance mechanisms of Cucumis melo var. agrestis against Trialeurodes vaporariorum and their use to control a closterovirus that causes a yellowing disease of melon. Plant Pathology. 1996;45:761–766. [Google Scholar]
- Starr JL, Mercer CF. 2009. Development of resistant varieties. Pp. 326–337 in R. N. Perry, M. Moens, and J. L. Starr, eds. Root-knot nematodes. Cambridge, MA: CABI International. [Google Scholar]
- Starr JL, Bridge J, Cook R. 2002. Resistance to plant-parasitic nematodes: History, current use and future potential. Pp. 1–22 in J. L. Starr, R. Cook, and J. Bridge, eds. Plant resistance to parasitic nematodes. New York: CABI International. [Google Scholar]
- Thies JA, Fery RL, Mueller JD, Miller G, Varne J. Response of bell pepper varieties near-isogenic for the N gene to Meloidogyne incognita in field trials. HortScience. 2003;38:1394–1396. [Google Scholar]
- Thomason IJ, McKinney HE. Reaction of some Cucurbitaceae to root-knot nematodes Meloidogyne spp. Plant Disease Reporter. 1959;43:448–450. [Google Scholar]
- Vrain TC. A technique for collection of Meloidogyne larvae and a comparison of eggs and larvae as inocula. Journal of Nematology. 1977;9:249–251. [PMC free article] [PubMed] [Google Scholar]
- Walters SA, Wehner TC, Barker KR. Root-knot nematode resistance in cucumber and horned cucumber. HortScience. 1993;28:151–154. [PMC free article] [PubMed] [Google Scholar]
- Walters SA, Wehner TC, Barker KR. NC-42 and NC-43: root-knot nematode-resistant cucumber germplasm. HortScience. 1996;31:1246–1247. [Google Scholar]
- Walters SA, Wehner TC, Barker KR. A single recessive gene for resistance to the root-knot nematode (Meloidogyne javanica) in Cucumis sativus var. hardwickii. Journal of Heredity. 1997;88:66–69. [Google Scholar]
- Walters SA, Wehner TC, Barker KR. Greenhouse and field resistance in cucumber to root-knot nematodes. Nematology. 1999;1:279–284. [PMC free article] [PubMed] [Google Scholar]
- Walters SA, Wehner TC, Daykin ME, Baker KR. Penetration rates of root-knot nematodes into Cucumis sativus and C. metuliferus roots and subsequent histological changes. Nematropica. 2006;36:231–242. [Google Scholar]
- Wehner TC, Walters SA, Barker KR. Resistance to root-knot nematodes in cucumber and horned cucumber. Journal of Nematology. 1991;23:611–614. [PMC free article] [PubMed] [Google Scholar]