Abstract
Two new species, Neotobrilus nicsmolae n. sp. and Chronogaster carolinensis n. sp. are described from a small, acidic, temperate, natural lake in North Carolina. N. nicsmolae n. sp. comes close to three members of the genus reported from North America, N. filipjevi, N. longus, and N. hopei. However, N. nicsmolae is unique with in the genus in having a combination of characters: size smaller than 1,700 μm, shorter outer labial and cephalic setae, tail shorter than 250 μm, last ventromedian supplement close (about 5 μm) to cloacal opening, spicule length of 61 to 85 μm, flagelloid sperm, and possession of subterminal setae. Assessment of relationships among clades within the Triplonchida using DNA sequences of the D2D3 expansion segment of the LSU rDNA showed that the family Trichodoridae and the genus Tripyla were recovered as monophyletic. The genus Tobrilus was recovered as monophyletic in the neighbor-joining and maximum likelihood trees, but that was not so in the maximum-parsimony tree. The separation among genera of the Trichodoridae, i.e., Trichodorus and Paratrichodorus, was not clear-cut in all phylograms. Chronogaster carolinensis n. sp. in having one ventral mucro with no spine and vacuolated lateral glandular bodies comes close to C. typica and C. ethiopica but differs from all hitherto known species in a combination of characteristics: in having long cephalic setae, long stoma, crystalloid bodies, vacuolated lateral glandular bodies, and a tail terminus with blunt ventral mucro, and its lack of lateral line.
Keywords: Free-living nematode, freshwater nematode, new species, taxonomy
The Tobrilidae Filipjev, 1918 are denizens of primarily fresh water habitats. Their known distribution shows a tendency to be particularly common in water bodies where oxygen is limited or absent (Nuss, 1984; Jacobs, 1987). Current reports show that there are seven orders, 53 families, 161 genera, and 362 species of nematodes reported from North America (Abebe et al., 2006; 2008). Of these, only ten species belong to the family Tobrilidae: Tobrilus aberrans (Schneider, 1925) Andrassy, 1959; T. affinis Gagarin, 1996; Eutobrilus graciliformis (Altherr and Delamare Deboutteville, 1972) Tsalolikhin, 1981; E. steineri (Micoletzky, 1925) Zullini, 2006; Neotobrilus hopei (Loof and Riemann, 1976) Tsalolikhin, 1981; N. filipjevi (Ebsary, 1982) Tsalolikhi and Shoshin, 2009; N. longus (Leidy, 1852) Tsalolikhin, 1981; Semitobrilus ebsaryi Tsalolikhin, 2000; S. pellucidus (Bastian, 1865) Tsalolikhin, 1981; and Epitobrilus sablensis (Ebsary, 1982) Tsalolikhin, 2001.
Neotobrilus Tsalolikhin, 1981 is a genus composed of 18 nominal species and is thought to have originated in the North American continent (Tsalolikhin, 1983). This genus is absent from Lake Biakal, a tertiary lake and a center of tobrilid diversity hitherto unparalleled by any other freshwater habitat (Tsalolikhin, 1983; Zullini, 2006). The genus is characterized by having typical tobrilid stoma with two pockets each separated by a narrow duct, and males having six protruding supplements of unequal size: three large and three small with a gap in between the two groups. Spicules are long and slender and vaginal musculature is strong (Tsalolikhin and Shoshin, 2009).
Chronogaster Cobb, 1913 is the type and only genus within the Chronogasteridae gagarin, 1975, with 48 nominal species (Holovachov and De Ley, 2006), most of which are thelytokous. It is a cosmopolitan genus found in freshwater, thermal springs, moist soils, fungal mats, and salty habitats including caves (Abebe et al., 2006), and is reported to have great physiological plasticity and tolerance to high level of oxygen fluctuation (Hodda et al., 2006). The taxonomy of the genus was first reviewed by Heyns and Coomans (1980) and later by Abebe and Coomans (1996). The genus is characterized by having a subterminal basal bulb, equipped with longitudinal denticulate ridges; a posterior stegostom section that is long; and an annulated cuticle that may have longitudinal lines, ridges, and spines in some species (Holovachov and De Ley, 2006).
Three species of Chronogaster; C. garcilis Cobb, 1913; C. longicollis von Daday, 1899; and C. typical de Man, 1921, are the only ones reported to have a northern hemisphere distribution including the Nearctic region.
In this study we provide the description of two populations, Neotobrilus and Chronogaster, for which we propose two new species: N. nicsmolae n. sp. and C. carolinensis n. sp. This is part of a wider study of the nematode communities of this small, acidic, natural, temperate lake—Lake Phelps, NC.
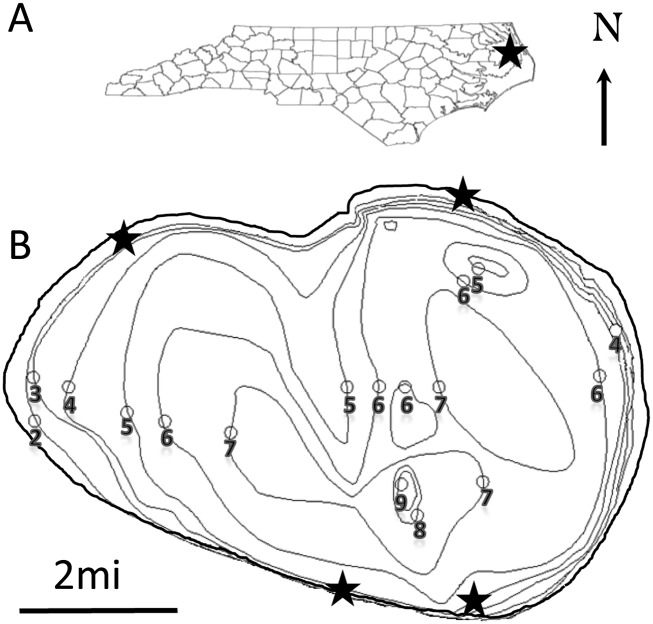
Study site: Lake Phelps, located in North Carolina (N 35° 77.24′, W -76° 45.44′), is the second-largest natural lake in the state with an area of 25 sq. miles (Fig. 1). The lake has an average depth of 1.5 m and a maximum depth of 3 m at an altitude of 4 m above sea level. The water is acidic with a pH of 4.1 to 4.6. Lake water is replenished by precipitation and maintained artificially at 3.0 to 3.5 m by water gates that flow into a canal from the lake. Lake sediment varies from brown and black mud to very fine sand. The mud was confirmed not to be peat but a fine organic detritus matter. The current bathymetric map is Allen et al. (1979), and it is assumed that some movement of top layer of sediment has shifted slightly in the past 33 yr. The lake is reported to have elevated levels of mercury, leading to fish consumption advisories (Pasquotank River Basin: Basinwide Assessment, 2011). The southern shore area, unlike the northern and other parts, is characterized by emergent and submergent vegetation.
Fig. 1.
Lake Phelps. (A) Geographic location within the state of North Carolina. (B) Bathymetric map indicating sampling sites (black stars).
Materials and Methods
Sediment, submerged aquatic vegetation, and algal mat samples were collected from four different sites in Lake Phelps, NC, in June and August 2010 and transported in a cooler to Elizabeth City State University for processing. Upon arrival, nematodes were extracted from sediment following the centrifugal-floatation technique using Ludox (i.e., 50% silicasol colloid solution) and were retained using a 38-μm mesh sieve (i.e., US Std. No. 400 (Heip, 1974; Hodda and Bloemers, 1995)). Plant material was repeatedly washed using a pressure hose immediately after arrival, and live nematodes were collected in a 38-μm mesh sieve (i.e. US Std. No. 400). Washed plant material was left on a Whitehead Tray overnight to collect remaining nematodes (Seinhorst, 1956; Hooper, 1986). For morphological study, specimens were hand-picked, heat-relaxed, fixed in 5% formaldehyde and transferred to anhydrous glycerin (Seinhorst, 1959).
Measurements and illustrations: Measurements were taken and drawings made using a camera lucida attached to a light microscope (LM) (Olympus BX51) with DIC option.
Pictures and digital multifocal images: Pictures and digital multi-focal images (DMI) in the form of video clips of the various body parts were taken using an imaging system composed of an Olympus BX51 microscope with DIC option and a DP71 digital camera controlled by Olympus DP Controller Software version 3.3.1.292. DMI in avi format file are made openly accessible at http://nematodes.ecsu.edu/.
Scanning electron microscopy: Glycerin processed nematodes were first transferred into pure distilled water, postfixed overnight in a 4.0% Osmium tetraoxide (OsO4) solution and dehydrated in a concentration gradient of ethanol. Dehydrated nematodes were dried with a critical point dryer, attached to a self-adhesive stub and were coated for 1 to 3 min with a 25 nm layer of gold palladium in a Cressington® (Cressington, Watford, UK) 108 Auto sputter coater. Specimens were observed using a Philips XL30-FEG Scanning Electron Microscope (SEM) operated at 10 kV.
Molecular characterization: Total genomic DNA was extracted from a single female using a DNeasy Blood and Tissue Kit (Qiagen, Inc., Valencia, CA). The D2/D3 region of the LSU rDNA were PCR amplified using the primers D2A: 5’-ACAAGTACCGTGAGGGAAAGTTG-3’ (forward) and D3B: 5-TCGGAAGGAACCAGCTACTA-3’ (reverse) (Tenente et al., 2004). All PCR reactions were conducted in a Gradient Thermocycler, PTC-200 (MJ Research, Inc., Watham, MA) with the cycle profile: 1 cycle of 95°C for 15 min followed by 35 cycles of 94°C for 60 sec, 55°C for 60 sec and 72°C for 60 sec. The last step was 72°C for 10 min. Ten 10 μl of the amplification product was electrophoresed on a 1.8% agarose gel and stained with ethidium bromide. The sizes of amplified products were determined by comparison with a 1 Kb molecular weight ladder (Invitrogen Life Technologies, Grand Island, NY). For direct sequencing, PCR products were run on a gel, purified with the QIAquick PCR purification kit (Qiagen) and sequenced at the University of Florida Core DNA Sequencing Facility. Sequences of the complete ITS array were aligned to previously NCBI published sequences of the LSU rDNA using the profile alignment option of Clustal X (Thompson et al., 1997), optimized manually in MacClade 4.05 (Maddison and Maddison, 2002), and then compared with those in Genbank by means of a BLAST search.
The evolutionary history was inferred using a combination of methods; neighbor-joining (Saitou and Nei, 1987), maximum likelihood based on the Tamura-Nei model (Tamura et al., 2004), and the maximum parsimony method. The evolutionary distances were computed using the maximum composite likelihood method (Tamura et al., 2004) and are in the units of the number of base substitutions per site. Phylogenetic analysis was done using the Molecular Evolutionary Genetics Analysis 5 (MEGA 5) software (Tamura et al., 2011). Nucleotide sequences were aligned using the multiple sequence alignment method (MUSCLE) (Edgar, 2004; Tamura et al., 2011). All phylograms were results of 1,000 bootstrap value repetitions using the Tamura-Nei model (Tamura and Nei, 1993). Dorylaimus stagnalis was used as an out-group taxon.
Neotobrilus nicsmolae n. sp
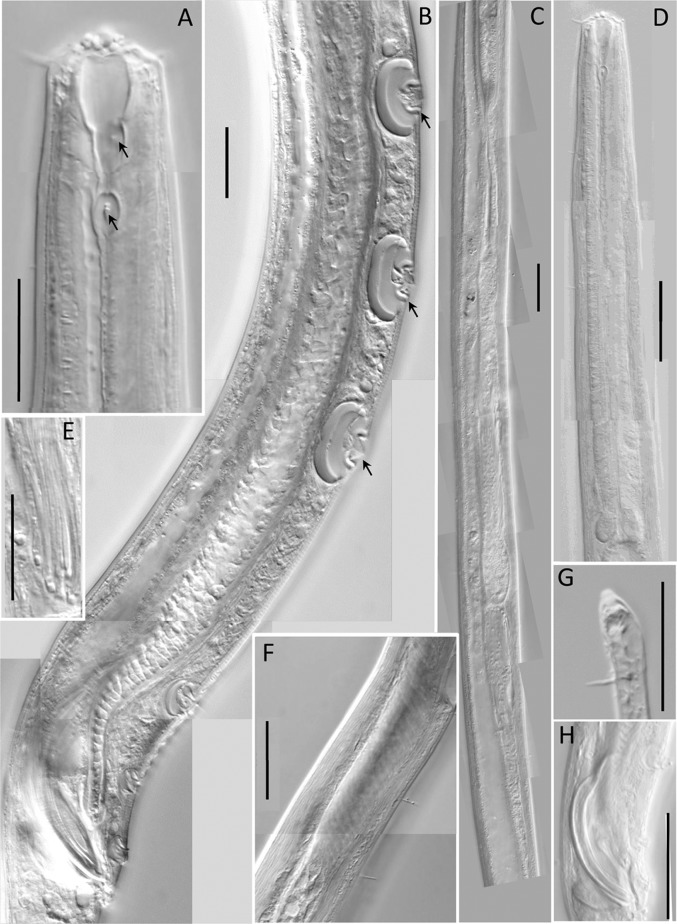
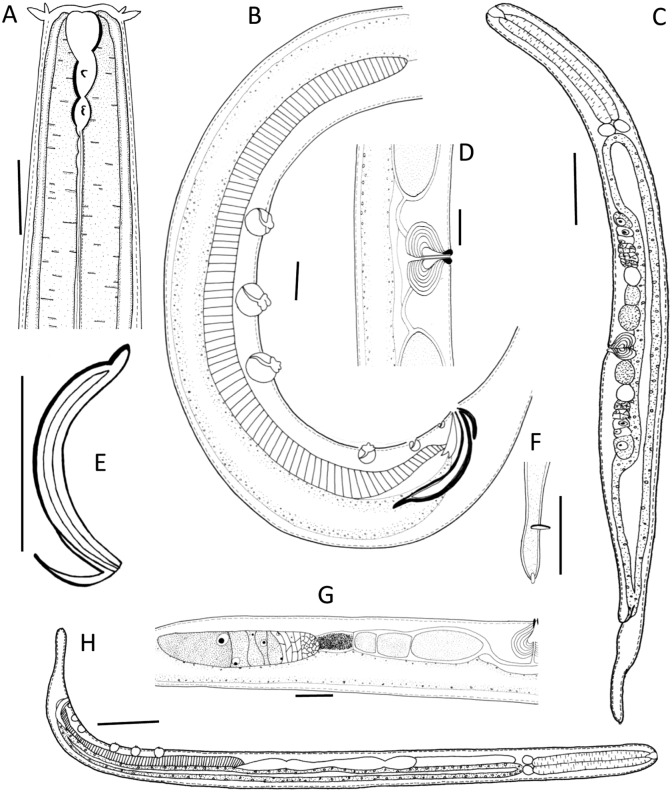
Fig. 2.
Light microscopic pictures of the male of Neotobrilus nicsmolae n. sp. (A) Lip region (arrows at anterior and posterior teeth). (B) Ventromedian supplements. (C) Reproductive system. (D) Pharyngeal region. (E) Flagelloid sperm. (F) Ductus ejaculatoris. (G) Subterminal setae. (H) Spicules. Scale: A, B, G = 20 μm; C–D, F = 40 μm; E = 30 μm; H = 50 μm.
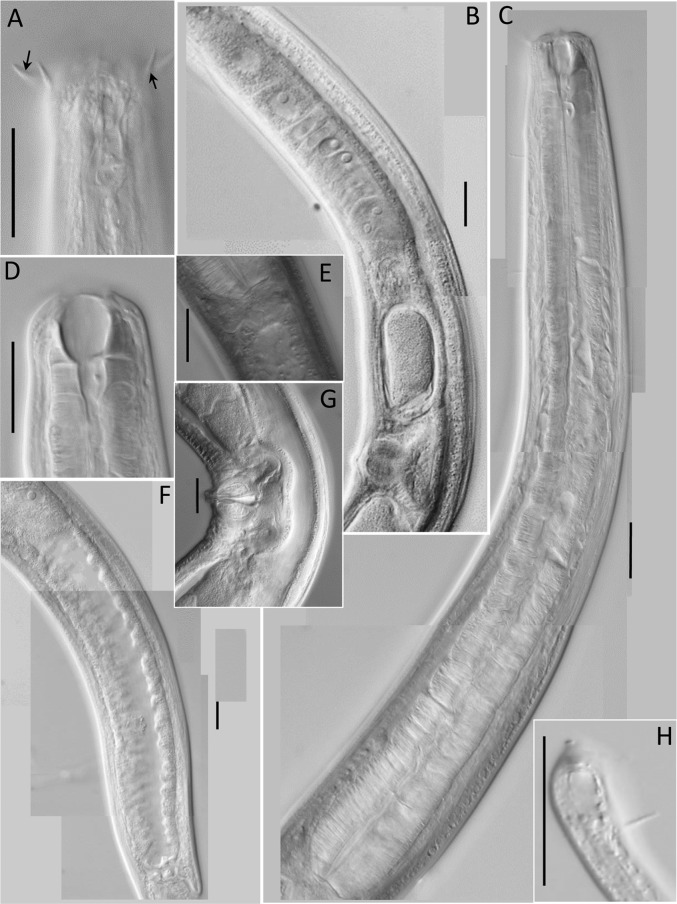
Fig. 3.
Light microscopic pictures of female of Neotobrilus nicsmolae n. sp. (A) Lip region showing cephalic (upward arrow) and labial setae (downward arrow). (B) Reproductive system. (C) Pharyngeal region. (D) Lip region showing stoma structure. (E) Cardia. (F) Posterior gut. (G) Vulval region. (H) Subterminal setae. Scale: A–H = 20 μm.
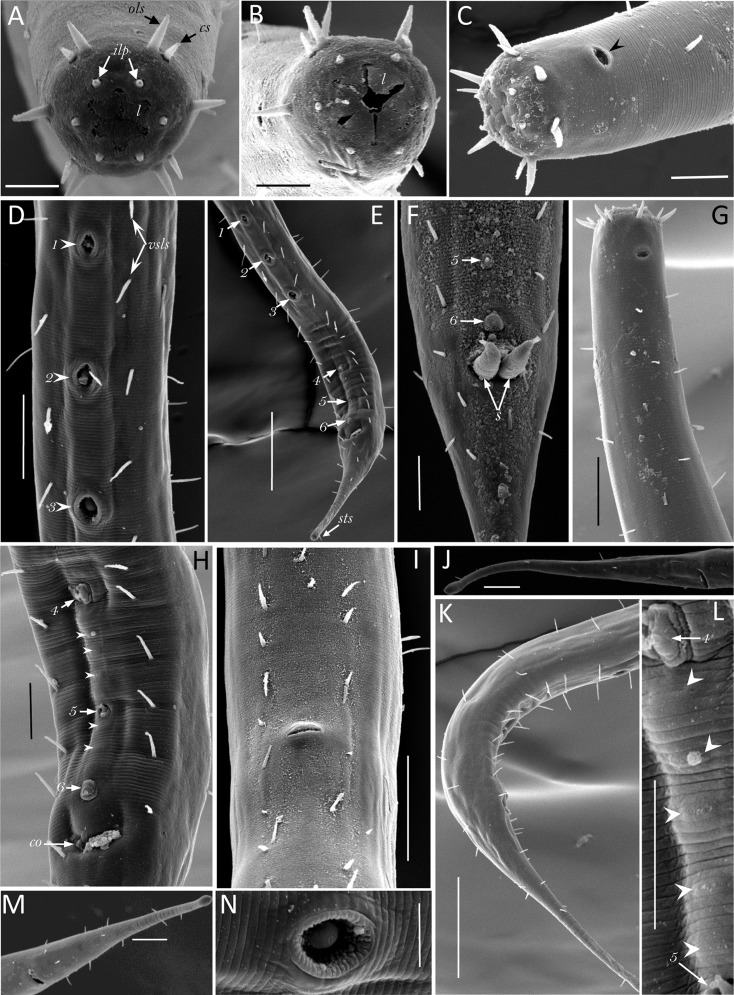
Fig. 4.
Scanning electron micrograph of Neotobrilus nicsmolae n. sp. (A) Anterior end with mouth closed showing cephalic setae (cs), outer labial setae (ols), inner labial papillae (ilp), and six liplets (l). (B) Anterior end with mouth partly open showing the six separate liplets (l). (C) Lateral view of anterior end showing cuticular striations, and amphidial opening (arrowhead). (D) Posterior part of male showing the most anterior three ventromedian supplements (numbered arrows where 1 represents most anterior) and ventrosublateral setae (vsls). (E) Posterior ventral part of male showing the six ventromedian supplements (numbers 1–6), ventrosublateral setae, and the subterminal seta (sts). (F) Cloacal region of male showing protruding spicules (s) and the most posterior two ventromedian supplements (5 and 6). (G) Lateral view showing setae distribution. (H) Ventral side of male posterior end showing most posterior three ventromedian supplements (4, 5, and 6), micropapillae (arrowheads), and cloacal opening (co). (I) Ventral side of mid-body of female showing vulva and ventrosublateral setae. (J and M) Female tail. (K) Male posterior part showing setae distribution. (L) Close-up of the region between the 4th and 5th ventromedian supplements showing five micropapillae (arrowheads). (N) Close-up of the third ventromedian supplement Scale bar: A, B, C, F, H, L, N = 10 μm; D, G, I, J, M = 20 μm; and E, K = 50 μm.
Fig. 5.
Neotobrilus nicsmolae n. sp. (A) Anterior end showing stoma. (B) Ventromedian supplements. (C) Entire female. (D) Vulval region. (E) Spicule. (F) Tail tip showing terminal setae. (G) Female posterior gonad. (H) Entire male. Scale: A, B, D = 20 μm; C, H = 100 μm; E, G = 50 μm; F = 10 μm.
Table 1.
Morphometrics of Neotobrilus nicsmolae n. sp. from Lake Phelps, NC.
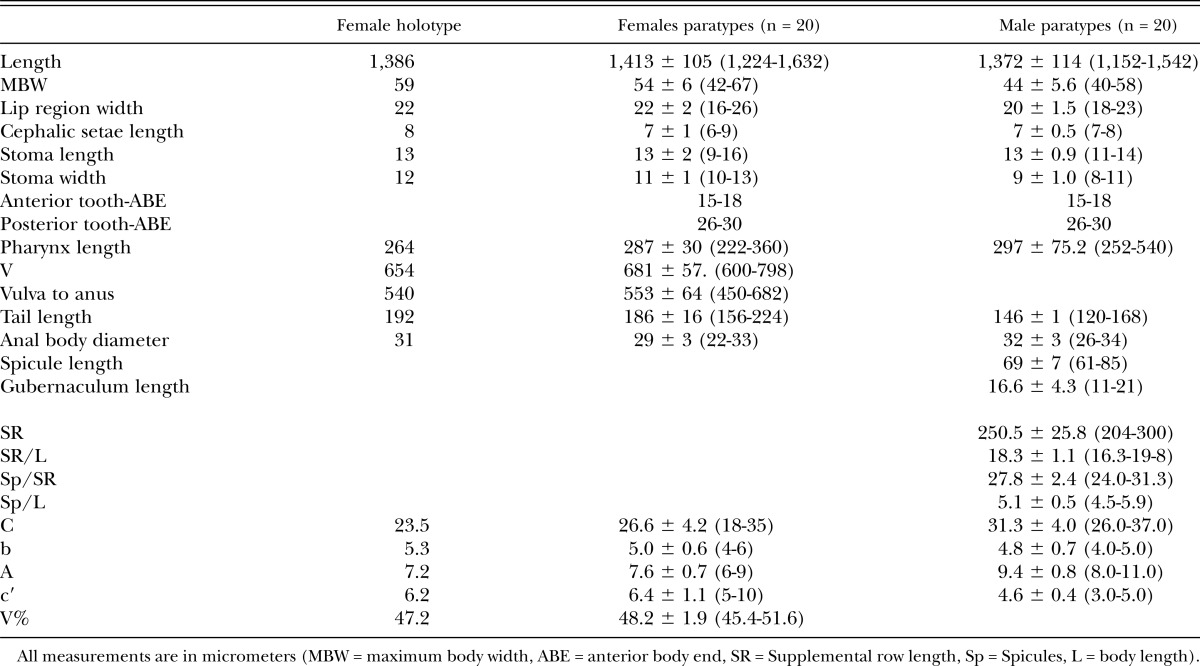
Table 2.
Comparison of Neotobrilus nicsmolae n. sp. with five closely related species.
Description:
Female: Body after fixation straight, variously shaped: most gently curved ventrad, rarely curved dorsad; tapering toward both ends; maximum at midbody. Cuticle 2.0-μm thick, layers faintly distinct; finely annulated with LM. Somatic setae numerous and in six rows along most of body until tail: two ventro-sublateral, two dorso-sublateral, and lateral rows. Most somatic setae in ventro-lateral and dorso-lateral rows; lateral setae fewer than in other rows. Metanemes distinct only in few specimens; nonoverlapping, five to seven dorsolateral, type-II loxometanemes on each side of body; the first in anterior pharynx and the last just before tail; length of frontal and caudal filament about 50 and 20 μm, respectively. Crystalloid bodies fine; situated most probably in pseudocoelom. Lip region narrow, bluntly rounded, continuous with the rest of body. Sensilla in two circles: inner ring of six labial papillae and an outer ring of four, short (4.0 to 5.0 μm) cephalic and six slightly longer (7.0 to 8.0 μm) labial setae. Amphids stirrup-shaped with transverse oval aperture with circular rim, 20.0 to 22.0% CBW; situated 0.7 to 0.9 LRW from ABE; fusus amphidialis just behind fovea, as long as or slightly longer than fovea. Cheilostome distinct, with weakly sclerotized lining. Rest of stoma consisting of three parts, an anterior and larger, cup-shaped part followed by two smaller, ventrosublateral pouches of which the first is situated on the left and the second on the right side. Pouch wall well-sclerotized; each pouch supplied with a well-sclerotized tooth. Cup-shaped part 1.2 to 1.3 times longer than wide. First tooth situated 15 to 18 μm from ABE, second tooth situated 10 to 14 μm from the first. Pharynx cylindrical and muscular. Nerve ring prominent, at about one-third to two-fifths of pharyngeal length. Cardia 15 μm long, with three prominent cells situated at the junction of the cardia with pharynx base. Excretory pore situated at the level of nerve ring. Ventral gland cells not observed. Cells of intestinal wall filled with brown granules. Rectum longer than anal body diameter.
Reproductive system didelphic, amphidelphic: Gonoducts situated ventral to the intestine; direction of flexure variable. Spermatheca distinct. Ovejector with narrow lumen; wall internally lined by highly plicate layer. Vagina about 19% CBW; wall without placation; with 6 to 8 concentric rings of strongly developed sphincter muscles. Vulva slightly anterior to or at midbody. Up to four eggs in uterus. Tail elongate-conoid, about 6 to 7 rectal lengths long, almost always straight, gradually narrowing for most part with the last about 30-μm-long posterior end being slightly swollen and club-shaped. Posterior swollen part containing the spinneret invariably pointed dorsad. Seven to eight setae on one side of tail; subterminal setae invariably present. Caudal glands three, prominent; terminating in a short, tubular terminal spinneret outlet.
Male: Resembling female in many respects except the following. Maximum body width often at level of the testis. Reproductive system diorchic with posterior testis reflexed posteriad; situated ventral to intestine. Spicules strongly curved and banana-shaped, without median stiffening piece. Gubernaculum complex with strongly cuticularized dorsal side and weakly cuticularized ventral pieces; dorsal median piece with a curved extension; side wings of dorsal side less cuticularized. Vas deferens long, extending anterior to midbody; more granular closer to the cloaca. Spermatozoa flagellate; 60 to 70 μm long. Ductus ejaculatoris about 150 μm anterior to the most anterior ventromedian supplement. Ventromedian supplements in two groups with a gap without supplements in between: anterior three larger, when retracted often wider than long, when protruded supplements bulb-shaped; posterior three smaller, as long as wide and always bulb-shaped. Posterior three supplements varying in size; gradually smaller closer to cloacal opening; the one closest to cloacal opening smallest. Micropappillae in intersupplemental space present: the between supplement 6 and 5, five between supplement 5 and 4, and one between supplement 4 and 3 (numbering of supplements starts with the most anterior as number 1 and number 6 is the one closest to cloacal opening.
Each supplement with 2 to 3 internal canals that form a common duct leading to the anterior wall of the ampulla. Distance between supplements variable, the largest distance (34 to 37 μm) is between supplement 3 and 4. This distance separates the anterior three from the posterior three. The distance among the three anterior supplements is about 20 μm; the distance among the most posterior three is about half that distance (about 10 μm). The most posterior supplement is about 5 μm from cloacal opening (Table 1). Tail conoid in its anterior one-third; drastically narrowing at about half its length; narrow, cylindrical in its posterior half. Six to eight setae on each side of tail, one subterminal seta invariably present.
Type locality and habitat: Algal mat collected from 0.5-m water depth at an acidic, temperate lake—Lake Phelps, NC (+35° 44' 2.99", -76° 26' 26.79").
Type designation and deposition: Holotype male and five female and five male paratypes deposited in the collection of the University of California, Riverside, CA (slide number 30797). DMI in avi format file are made openly accessible at http://nematodes.ecsu.edu/.
Differential diagnosis: Neotobrilus nicsmolae n. sp., in having concentric muscular layers in the vaginal wall and a ductus ejaculatoris extending anterior beyond the supplement row comes close to three species: N. longus, N. filipjevi, and N. hopei. Also, N. nicsmolae n. sp is similar to N. filipjevi; N. hopei, N. diversipappilatus (Daday, 1905) Tsalolikhin, 1981; and N. longior (Altherr, 1963) Tsalolikhin, 1981 in that it has subterminal setae. However, N. nicsmolae is unique in having a combination of characters: size smaller than 1,700 μm, shorter outer labial and cephalic setae, tail shorter than 250 μm, last ventromedian supplement close (about 5 μm) to cloacal opening, spicule length 61 to 85 μm, flagelloid sperm, and possession of subterminal seta.
Comparison with N. longus: N. nicsmolae n. sp. differs from N. longus in that our assessment of more than 75 adults shows that it invariably possesses subterminal seta; N. longus lacks subterminal seta (Tsalolikhin and Shoshin, 2009), the outer labial setae and cephalic setae are both shorter in the new species (Outer labial setae = ♂: 7 to 8 μm, ♀: 6 to 9 μm, cephalic setae = ♂: 4 to 5 μm, ♀: 4 to 5 μm in N. nicsmolae n. sp vs. Outer labial setae = ♂: 10 to 11 μm, ♀: 11, cephalic setae = ♂: 6 μm, ♀: 6 μm in N. longus). Body ratio “c” is smaller in N. nicsmolae n. sp (c = ♂: 8 to 11, ♀: 6 to 9 in N. nicsmolae n. sp vs. c = ♂: 11.5, ♀: 9 to 10 in N. longus).
Comparison with N. filipjevi: The new species differs from N. filipjevi in having smaller spicules (61 to 85 μm in N. nicsmolae n. sp vs. 91 to 112 μm in N. filipjevi). Although N. filipjevi is shown to have a sleeve-like gubernaculum in Tsalolikhin and Shoshin (2009; their Fig. 2F), our study of one male paratype of the species from the Canadian National Collection of Insects, Arachnids and Nematodes and the original description by Ebsary (1982) did not reveal such a structure.
Comparison with N. hopei: N. nicsmolae n. sp. differs from N. hopei in its small size (length = ♂: 1,152 to 1,542 μm, ♀: 1,224 to 1,632 μm in N. nicsmolae n. sp vs. ♂: 2,720 μm, ♀: 2,800 to 3,200 μm in N. hopei), having shorter spicules (61 to 85 μm vs. 122 μm in N. hopei), shorter setae (outer labial setae = ♂: 7 to 8 μm, ♀: 6 to 9 μm, cephalic setae = ♂: 4 to 5 μm, ♀: 4 to 5 μm, vs. outer labial setae = 14 to 16 μm and cephalic setae = 8 to 9 μm in N. hopei), smaller values for the ratios “a”, “c”, “c′” (ratios ♂: a = 26 to 37, c′ = 3 to 5, ♀: a = 18 to 35, c = 4-6, c′ = 6 to 9, respectively, in N. nicsmolae n. sp. vs. ♂: a = 47, c = 12.4, c′ = 5.8, ♀: a = 36 to 58, c′ = 8 to 9.7 in N. hopei). N. hopei is characterized by having 14 to 19 micropapillae between the 3rd and 4th ventromedian supplement and 6 micropapillae between the 4th and 5th ventromedial supplements (N. nicsmolae n. sp has only one micropapilla between the 3rd and 4th ventromedian supplement, and five micropapillae between the 4th and 5th supplement). Also, the ratio SR/L is larger in N. nicsmolae n. sp (SR/L = 16.3 to 19.8 in N. nicsmolae n. sp. vs. SR/L = 13.0 in N. hopei).
Comparison with N. diversipappilatus and N. longior: N. nicsmolae n. sp differs from both N. diversipappilatus and N. longior in having vagina with conspicuous internal concentric muscular layers instead of the external radial muscular layer.
N. diversipappilatus also differs in containing clavate spermatozoa instead of flagelloid. Body length in N. nicsmolae n. sp. is longer than that of N. diversipappilatus (body length = ♂: 1,152 to 1,542 μm, ♀: 1,224 to 1,632 μm in N. nicsmolae n. sp vs. ♂: 1,420 to 1,940 μm, ♀: 1,695 to 2,241 μm in N. diversipappilatus). N. nicsmolae n. sp. has longer spicules (61 to 85 μm vs. 41 to 48 μm in N. diversipappilatus). In terms of body ratios, “c” is smaller and the position of the vulva (V%) in N. nicsmolae n. sp. is more posterior (c = ♂: 8 to 11, ♀: 6 to 9 in N. nicsmolae n. sp vs. ♂: 13 to 16, ♀: 8.8 to 10 in N. diversipappilatus). SR/L, Sp/SR, and Sp/L ratios are all larger in N. nicsmolae n. sp. (SR/L = 16.31 to 19.76, Sp/SR = 24.0 to 31.3, Sp/L = 4.5 to 5.9 in N. nicsmolae n. sp. vs. SR/L = 12, Sp/SR = 22, Sp/L = 3, respectively, in N. diversipappilatus).
N. nicsmolae n. sp. differs from N. longior in having flagelloid sperm (sperm clavate in N. longior), smaller body (body length = ♂: 1,152 to 1,542 μm, ♀: 1,224 to 1,632 μm in N. nicsmolae n. sp. vs. ♂: 1,800 to 3,180, ♀: 1,660 to 3,180 μm in N. longior). Male tail in N. nicsmolae n. sp. is also shorter than that of the male population of N. longior (120 to 168 μm in N. nicsmolae n. sp. vs. 316 to 324 μm in N. longior). Body ratios (a, b, c) in N. nicsmolae n. sp. are smaller (body ratios = ♂: a = 26 to 37, b = 4 to 5, c = 8 to 11, ♀: a = 18 to 35, b = 4 to 6, c = 6 to 9 in N. nicsmolae n. sp. vs. ♂: a = 44 to 51, b = 6.4 to 6.7, c = 14 to 18, ♀: a = 40, b = 7, c = 11, respectively, in N. longior). The cephalic setae are shorter in N. nicsmolae n. sp. (Cephalic setae = ♂: 4 to 5 μm, ♀: 4 to 5 μm in N. nicsmolae n. sp vs. ♂: 6 μm in N. longior). SR/L ratio is larger in N. nicsmolae n. sp. (SR/L = 16.31 to 19.76 in N. nicsmolae n. sp vs. SR/L = 12 in N. longior), and in N. nicsmolae n. sp, the vulva is more posterior than in N. longior (V% = 45.4 to 51.6 vs. 39 in N. longior).
Etymology: The species is named in honor of Nic Smol for her more than 20 years of outstanding service as a mentor, trainer, and coordinator of the Postgraduate International Course of Nematology at Ghent University, Belgium, that trained more than 250 nematologists from developing nations all over the world.
Molecular data: We sequenced the D2D3 region of large subunit (LSU) rDNA (Genbank accession number JX888469) to evaluate the placement of the genus Neotobrilus within the Triploinchida Cobb, 1920. De Ley et al. (2005) enumerated the use and benefits of the D2D3 segment of the LSU: has a high sequence divergence between related species, and the conserved region shows consistence across phyla, provides a robust signal at various taxonomic level from species to family, and primers provide a high success rate. Nevertheless, the absence in public databases of DNA sequences from other species of the genus and genera within the Tobrilidae, except for an unidentified Tobilius sp. made it difficult to assess intra-generic phylogeny using the generated molecular data.
The order Triplonchida is one enoplid group established combining molecular and morphological data (De Ley and Blaxter, 2002) and comprises ecologically diverse groups including those entirely terrestrial such as the Diphtherophorina and exclusively aquatic forms such as the Tobrilina and Tripylina (Zullini, 2006). Recent phylogenetic studies based on molecular data (Bik et al., 2010) showed three distinct groups within the Triplonchida: Tripylidae and Tobrilidae together as one group, Prismatolaimidae and Bastianidae a second group, and Diphtherophorina (Trichodoridae and Diphtherophoridae) as the third monophyletic group. Bik et al. (2010) also reported a strong support for the grouping of the suborder Tripylina within the Enloplida, and indicated that the previous placing within the Triplonchida was “based on homoplasious morphological characters.” Including Tripylina in our analysis did not improve the resolution of the remaining Triplonchid groups. As a result, our current report shows the result after excluding from reanalysis sequences of the three genera Tripylina, Tripylella, and Trischistoma.
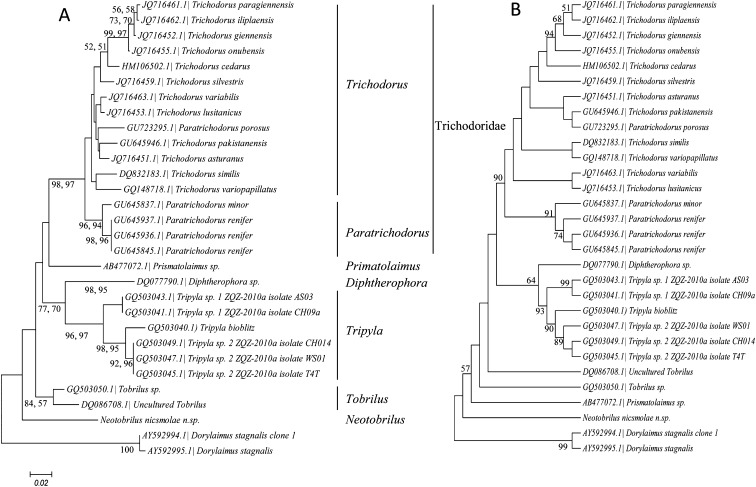
Trees constructed using the neighbor-joining or maximum likelihood algorithms were identical in their structure and resolution (Fig. 6A). In both cases the overall frame of the 28S phylogenetic tree separated the six genera roughly: Trichodorus, Paratrichodorus, Prismatolaimus, Diphtherophora, Tobrilus, and Neotobrilus. In all phylogenetic trees, the genus Tripyla and the family Trichodoridae emerged as monophyletic with strong bootrstap support (Fig. 6A). A difference between the two trees (Figs. 6A,B) is that Tobrilus is monophyletic in the neighbor-joining or maximum likelihood trees but not so in the maximum parsimony tree. In all the three methods used the genera Trichodorus and Paratrichodorus within the Trichodoridae were not cleanly separated from each other. The species Paratrichodorus porosus consistently grouped with the genus Trichodorus including in the maximum parsimony tree (Fig. 6B).
Fig. 6.
Phylogram of Triplonchida showing relationship of Neotorbilus and Tobrilidae. (A) Neighbor-joining and maximum likelihood phylogram. Bootstrap values on branches represent (from left to right) neighbor-joining and maximum likelihood, respectively. (B) Maximum parsimony phylogram. Both trees are based on Clustal W alignment of the D2D3 expansion segment with 1,000 bootstrap value repetitions using the Tamura-Nei model.
Without losing focus on the fact that the used sequence data was limited in relation to the morphology-based diversity of the family and that there are more unresolved parts than resolved ones, the three sequences of the family Tobrilidae did not group together in any of the trees, and Neotobrilus was at the base of the Trionchid tree. Also, when we took only representative sequences per each genus, the resulting tree topology was different from what we attained when we included all sequences species in that Primsatolaimus seems to group with uncultured Tobrilus with a 100% bootstrap support (data not shown).
Families within the Triplonchida have well-defined morphological characteristics on which their taxonomic identity is established and, as a result, recovering those groups as monophyletic clades in molecular studies is not entirely unexpected. The lack of robust support to some genera or families in our analysis, therefore, could simply be a result of the limited 28S sequence data available for analysis.
Description
Morphometric measurements of Chronogaster carolinensis n. sp. (Figs. 7–9) are given in Table 3.
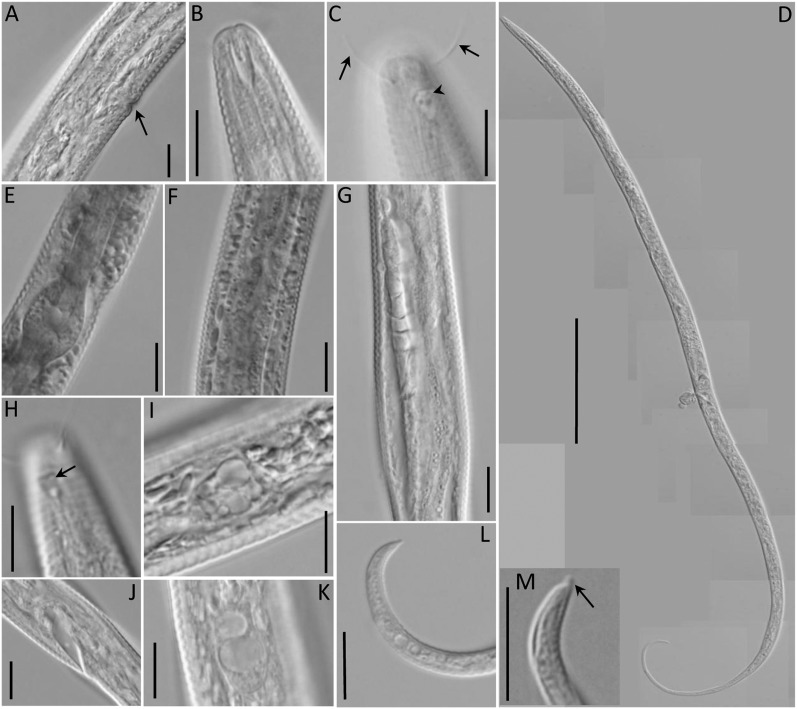
Fig. 7.
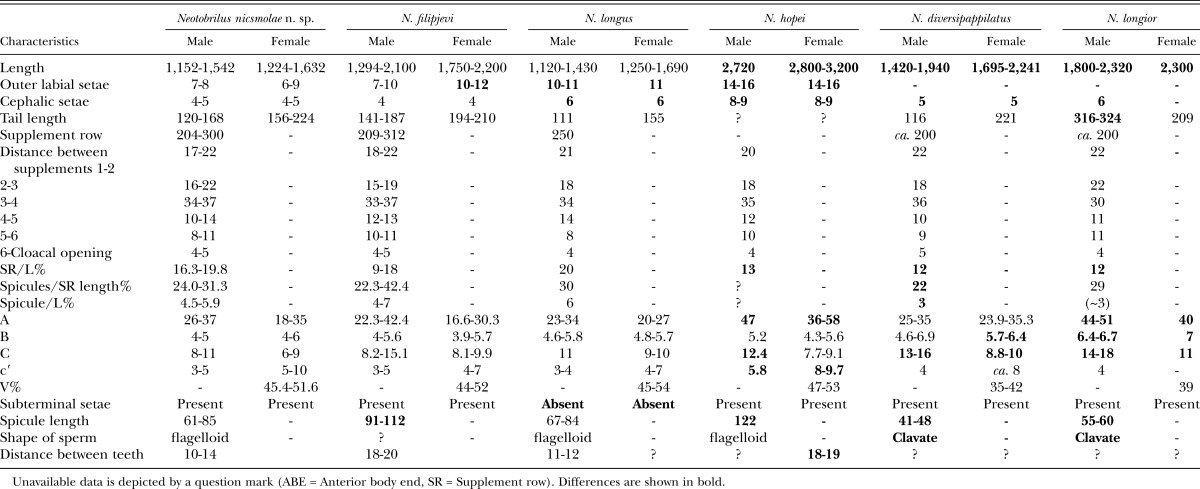
Light microscopic pictures of Chronogaster carolinensis n. sp. (A) Vulva. (B) Lip region, median view. (C) Lip region showing amphid (arrowhead) and setae (arrows). (D) Female body. (E) Pharyngeal bulb. (F) Crystalloid bodies. (G) Reproductive system. (H) Amphid (arrow). (I) Crystalloid bodies. (J) anus region. (K) Vacuolated lateral glandular bodies. (L) Tail tip. (M) Enlarged view of tail tip showing ventral mucro. Scale: A– C, E–L = 10 μm; D = 100 μm.
Fig. 8.
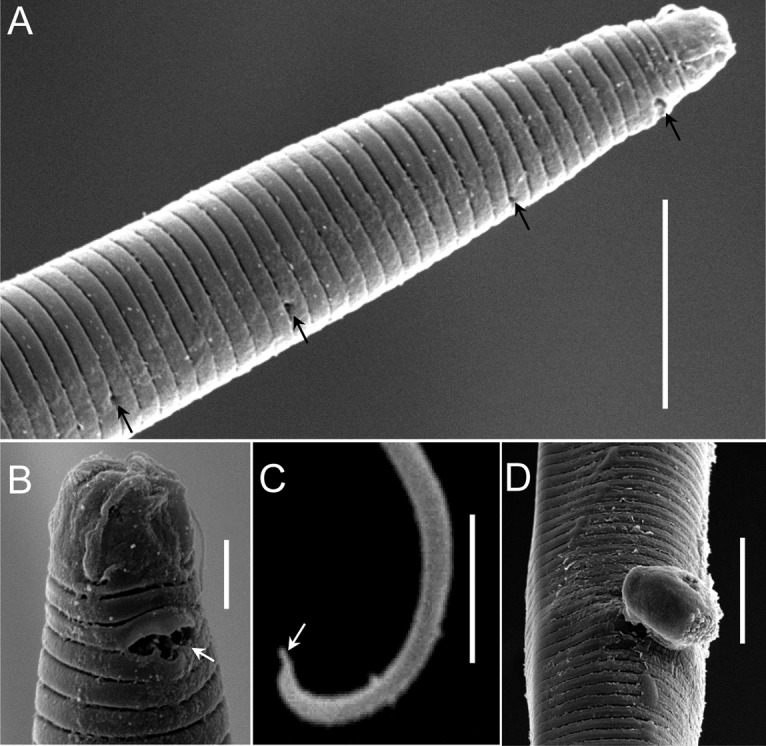
Scanning electron micrograph of Chronogaster carolinensis n. sp. (A) Lateral view of adult female (arrows indicate pores). (B) Lip region showing amphideal fovea (arrow). (C) Tail tip showing ventral mucro (arrow). (D) vulva. Scale: A, C, D = 10 μm; B = 2 μm.
Fig. 9.
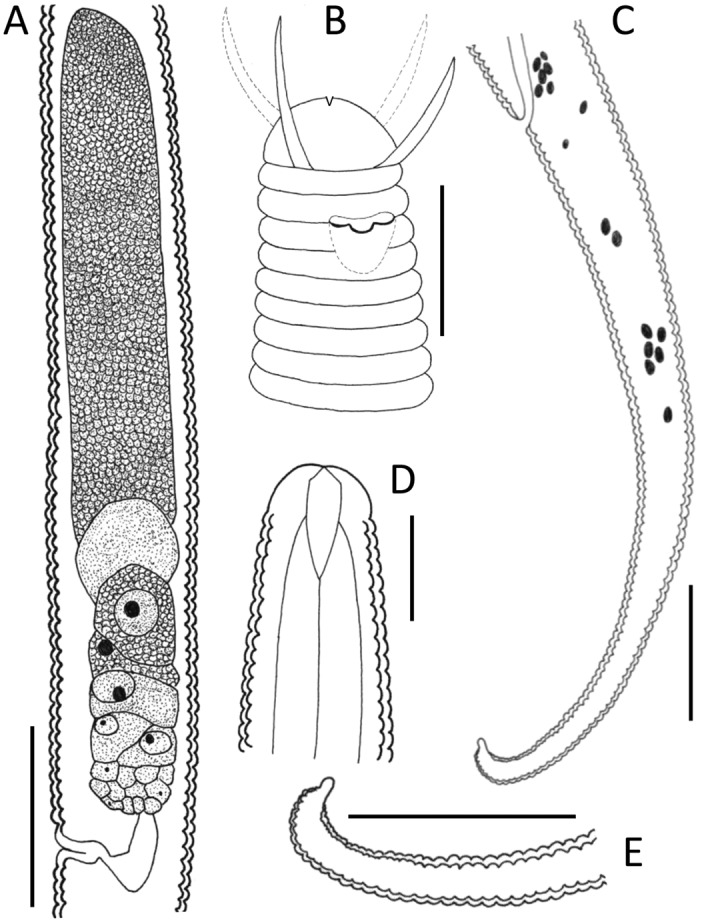
Chronogaster carolinensis n. sp. female. (A) Gonad. (B) Anterior end showing setae and amphideal fovea. (C) Tail region. (D) Anterior end showing stoma. (E) Tail tip showing blunt ventral mucro. Scale: A = 25 μm; B, D, = 10 μm; C, E = 20 μm.
Table 3.
Morphometrics and comparison of Chronogaster carolinensis n. sp. with seven closely related species that have tail terminus with ventral mucro.
Females: Body posture after fixation with a posterior hook similar to the number 6. Cuticle annulated. Annuli width variable: about 0.9 μm close to anterior end, about 1.2 μm close to midbody, and about 1.0 μm at the posterior end. No lateral line. Vacuolated lateral glandular bodies (VLGB) present, very few distinct under LM; five between base of pharynx and anus. Lateral cuticular pores observed with both light microscope and scanning electron microphs; 14 in one specimen on pharyngeal region under light microscope; pores almost one every 10 μm on anterior pharynx under SEM. Crystalloid bodies dense, oval or elongate-ovoid; occurring throughout body. Unstriated lip region wider than long; with four setae. The latter 0.9 to 1.0 LRW long. Amphids uniquely shaped, more or less stirrup like but with the lower lip with two upward projections at equal distance from the center, aperture 2.5 to 3.0 μm wide, situated about 1.0 to 1.1 LRW from anterior body end, between the second and the third annulus. Stoma anteriorly cylindrical, posteriorly narrowing. Radial tubules originating 10.0 μm behind the end of stoma. Nerve ring anterior to middle of pharynx. Pharynx cylindrical for most of its length, with an oval terminal bulb. Basal bulb with longitudinal rows of seven denticles in its anterior half. Postbulbal extension about 1.4 times basal bulb length. Rectum about 2.0 ABWs long. Excretory pore not discernible.
Female reproductive system prodelphic, monodelphic, ovary reflexed, situated on the right side of intestine. Vagina 5.0 μm long. Postvulval uterine sac absent. Vulva posterior to midbody. Tail elongate-conoid; curved ventrad; about 8 to 9 rectal lengths long; terminal part with a convex dorsal side and a ventral side bearing a short blunt mucro.
Males: Males not found.
Type habitat and locality: Algal mat collected from 0.5-m water depth at an acidic, temperate lake—Lake Phelps, NC (+35° 44′ 2.99", -76° 26′ 26.79").
Type material: Holotype female and one female paratype deposited in the collection of the University of California, Riverside, CA (slide number 30798). DMI in avi format file are made openly accessible at http://nematodes.ecsu.edu/.
Diagnosis and Relationships: C. carolinensis n. sp., in having one ventral mucro with no spine, comes close to seven known species within the genus: C. ethiopica Abebe and Coomans, 1996; C. neotypica Tahseen, Ahmad, and Ahmad, 1994; C. typica de Man, 1921; C. bigubernacula Khera 1972; C. tenuis Loof and Jairapuri, 1965; C. boettgeri Kischke, 1956; and C. loofi Chatuverdi and Khera, 1979. It is also similar to C. ethiopica, C. neotypica, and C. loofi, in containing crystalloid bodies, and to C. ethiopica, C. typica, and C. tenuis in containing VLGB. It is, however, closest to C. typica and C. ethiopica. However, the new species differs from all hitherto known species in a combination of characteristics: in having long cephalic setae, long stoma, crystalloid bodies, vacuolated lateral glandular bodies, and a tail terminus with blunt ventral mucro, and its lack of lateral line.
Comparison with C. typica: The new species is most similar to differs from C. typica in lacking longitudinal lines (two longitudinal lines present in C. typica).
Comparison with C. ethiopica: C. caroninensis n. sp. compared with C. ethiopica has longer body (L = 1,146 to 1,287 μm in C. carolinensis n. sp. vs. 1,010, 1,054 μm in C. ethiopica) larger values for the ratios “a”, “b”, and “c′” (“a”, “b”, and “c′” = 42.0 to 57.0, 4.0 to 5.0, 8.0 to 12.0, respectively, in C. carolinensis n. sp. vs. 58.6 to 67.8; 5.3 to 5.6; 15.0 to 17.8, respectively, for C. ethiopica), much posteriorly positioned vulva (V% = 50.2 to 56.5 in C. carolinensis n. sp. vs. = 45.0 to 46.1 in C. ethiopica), longer cephalic setae and stoma (cephalic setae and stoma 7 to 8 μm and 9 to 10 μm long in C. carolinensis n. sp. vs. vs. 6.0 μm and 5.5 μm, respectively, in C. ethiopica).
Comparison with C. neotypica: C. carolinensis n. sp. differs from C. neotypica in having a posteriorly positioned vulva (V% = 50.2 to 56.5 in C. carolinensis n. sp. vs. 45.0 to 50.1 in C. neotypica), longer cephalic setae (7 to 8 μm in C. carolinensis n. sp. vs. 3.5 μm in C. neotypica), longer stoma and pharynx (Stoma length 9 to 10 μm in C. carolinensis n. sp. vs. 6 to 8 μm in C. neotypica; pharynx length = 246 to 285 μm in C. carolinensis n. sp. vs. 157 to 242 μm in C. neotypica), and in having VLGB (VLGB absent in C. neotypica).
Comparison with C. loofi: C. carolinensis n. sp. differs from C. loofi in having VLGB and its longer cephalic setae (VLGB absent in C. loofi and cephalic setae = 7 to 8 μm in C. carolinensis n. sp. vs. 4 to 5 μm in C. loofi).
Comparison with C. bigubernacula: C. carolinensis n. sp. differs from C. bigubernacula in having both crystalloid bodies and VLGB (Crystalloid bodies and VLGB absent in C. bigubernacula), and having longer cephalic setae (7 to 8 μm in C. carolinensis n. sp. vs. 5 μm in C. bigubernacula).
Comparison with C. tenuis: C. carolinensis n. sp. differs from C. tenuis in having both crystalloid bodies and VLGB (Crystalloid bodies and VLGB absent in C. tenuis), higher values for the ratio “a” and “c” and lower value for the ratio “c′” (“a”, “c”, and “c′” are 42 to 57, 7.0 to 9.0 and 8 to 12 in C. carolinensis n. sp., respectively, vs. 67 to 75, 4.8 to 5.2, 21 to 22 in C. tenuis). Another difference is that the vulva in Chronogaster n. sp. is more posterior than C. tenuis (V% = 50.2 to 56.5 in C. carolinensis n. sp. vs. 50.2 to 56.5 in C. tenuis).
Comparison with C. boettgeri: C. carolinensis n. sp. differs from C. boettgeri in having crystalloid bodies (Crystalloid bodies are absent is C. boettgeri), being larger (length = 1,146 to 1,287 μm in C. carolinensis n. sp. vs. 880 to 1080 μm in C. boettgeri), smaller values for the ratios “a” and “c” and larger for the ratio “c′” (“a”, “c”, and “c′” are 42 to 57, 7.0 to 9.0, 8.0 to 12.0 in C. carolinensis n. sp., respectively, vs. 65 to 68, 11.2 to 11.4, and 7.0 in C. boettgeri).
Etymology: The species is named after the state of North Carolina, the type locality.
Literature Cited
- Abebe E, Coomans A. Aquatic nematodes from Ethiopia VI: The genera Chronogaster Cobb, 1914, Plectus Bastian, 1865 and Prismatolaimus de Man, 1880 with description of C. ethiopica n. sp. and C. getachewi n. sp. (Chromadorida: Nematoda) Hydrobiologia. 1996;332:41–61. [Google Scholar]
- Abebe E, Decraemer W, De Ley P. Global diversity of nematodes (Nematoda) in freshwater. Hydrobiologia. 2008;595(1):67–78. [Google Scholar]
- Abebe E, Traunspurger W, Andrássy I, editors. 2006. Freshwater nematodes: ecology and taxonomy. Wallingford, UK; CAB International. [Google Scholar]
- Allen R, Crowson B, Riggs S. 1979. The geology of Lake Phelps Washington County, North Carolina. Masters thesis, East Carolina University, Greenville, NC. [Google Scholar]
- Bik HM, Thomas WK, Lunt DH, Lambshead PJD. Low endemism, continued deep-shallow interchanges, and evidence for cosmopolitan distributions in free-living marine nematodes (order Enoplida) BMC Evolutionary Biology. 2010;10:389. doi: 10.1186/1471-2148-10-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley P, Blaxter ML. 2002. Systematic position and phylogeny. Pp. 1–30 in D. L. Lee, ed. The biology of nematodes. London: Taylor and Francis Press. [Google Scholar]
- De Ley P, De Ley TI, Morris K, Abebe E, Mundo M, Yoder M, Heras J, Waumann D, Rocha-Olivares A, Burr J, Baldwin JG, Thomas WK. An integrated approach to fast and informative morphological vouchering of nematodes for applications in molecular barcoding. Philosophical Transactions of the Royal Society of London. 2005;272:1945–1958. doi: 10.1098/rstb.2005.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebsary BA. Canadian Species of Tobrilus (Nematoda: Tobrilidae) with description of three new species. Canadian Journal of Zoology. 1982;60:3048–3062. [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heip C. A rapid method to estimate nematode density. Nematologica. 1974;20:266–288. [Google Scholar]
- Heyns J, Coomans A. Freshwater nematodes from South Africa 5. Chronogaster Cobb, 1913. Nematologica. 1980;26:187–208. [Google Scholar]
- Hodda M, Bloemers GF. A method for extraction of nematodes from a tropical forest soil. Pedobiologica. 1995;39:331–343. [Google Scholar]
- Hodda M, Ocana A, Traunspurger W. 2006. Nematodes from extreme freshwater habitats. Pp. 179–210 in E. Abebe, W. Traunspurger, and I. Andrássy, ed. Freshwater nematodes: Ecology and taxonomy. Wallingford, UK: CAB International. [Google Scholar]
- Holovachov O, De Ley P. 2006. Order Plectida. Pp. 611–647 in E. Abebe, W. Traunspurger, and I. Andrássy, ed. Freshwater nematodes: Ecology and taxonomy. Wallingford, UK: CAB International. [Google Scholar]
- Hooper DJ. 1986. Extraction of free-living stages from soil. Pp. 5-30 in J. F. Southey ed. Laboratory methods for work with plant and soil nematodes. London: HMSO. [Google Scholar]
- Jacobs LJ. 1987. A checklist of the Monhysteridae (nematoda: Monhysterida). Publication C46, Rand Afrikaans University, Johannesburg, South Africa. [Google Scholar]
- Maddison WP, Maddison DR. 2002. MacClade version 4.0. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Nuss B. Ultrastructurelle und okophysiologische Untersuchungen an kristalloiden Einschliissen der Muskelneine sulfid-toleranten limnischen Nematoden (Tobrilus gracilis) Meeresforsch. Brenlerh. 1984;20:3–15. [Google Scholar]
- Pasquotank River Basin: Basinwide Assessment Report. 2011. North Carolina Department of environment and natural resources Division of water quality Environmental sciences section. URL: http://portal.ncdenr.org/c/document_library/get_file?p_l_id=1169848&folderId=722215&name=DLFE-44068.pdf.
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Seinhorst JW. The quantitative extraction of nematodes from soil. Nematologica. 1956;1:249–267. [Google Scholar]
- Seinhorst JW. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica. 1959;4:67–69. [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control regions of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA) 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenente GCMV, De Ley P, Tandingan DI, Karssen G, Vanfleteren JR. Sequence analysis of the D2/D3 region of the large subunit rDNA from different Meloidogyne isolates. Nematropica. 2004;34:1–12. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalolikhin SY. 1983. Nematodes of the families Tobrilidae and Tripylidae of the World Fauna. Nauka, Leningrad 232. [Google Scholar]
- Tsalolikhin SY, Shoshin A. Review of the genus Neotobrilus Tsalolikhin, 1981 (Nematoda, Enoplida: Tobrilidae). Russian Journal of Nematology. 2009;17(107(1):59–774. [Google Scholar]
- Zullini A. 2006. Order Triplonchida. Pp. 293–323 in E. Abebe, W. Traunspurger, and I. Andrássy, ed. Freshwater nematodes: Ecology and taxonomy. Wallingford, UK: CAB International. [Google Scholar]