Abstract
Pristionchus bucculentus n. sp. was isolated from a shining mushroom beetle, Episcapha gorhami, associated with white rot on a decaying log in Hokkaido, Japan. The new species is distinguished by its stomatal morphology, which includes three regularly shaped, conical left subventral denticles and a vacuolated cheilostom with weak internal sclerotization. Also distinguishing P. bucculentus n. sp. are male sexual characters, including arrangement of genital papillae, a rounded and ventrally skewed manubrium, and a gubernaculum with a large, deep posterior curvature and a short, shallow anterior curvature. Morphological and molecular evidence support the new species as being close to P. elegans, which was previously the most basal known species of the genus. Comparative morphology of basal Pristionchus species is supported by a molecular phylogeny inferred from a partial small subunit ribosomal rRNA gene and 25 ribosomal protein-coding genes. Description of P. bucculentus n. sp. provides a new point of comparison for reconstructing the evolution of stomatal characters in the comparative model system of Pristionchus.
Keywords: Evolution, morphology, morphometrics, ribosomal protein genes, new species, phylogeny, stoma, taxonomy
The genus Pristionchus Kreis, 1932 is amenable to detailed mechanistic studies of biological phenomena (Sommer, 2009), including the development of stomatal form (Bento et al., 2010). A macroevolutionary context for model systems research, with P. pacificus Sommer, Carta, Kim, and Sternberg, 1996 at its focus, has been recently advanced by a resolved phylogeny of Diplogastridae Micoletzky, 1922 (Mayer et al., 2007, 2009) and whole-genomic resources for another Pristionchus species, P. exspectatus Kanzaki, Ragsdale, Herrmann, Mayer, and Sommer, 2012 (Rödelsperger et al., unpubl.). A comparative approach to morphological development depends on a sound phylogenetic framework that includes species with recognizable differences in the morphology of interest. Recent description of two species basal in the genus, P. elegans Kanzaki, Ragsdale, Herrmann, and Sommer 2012 and P. fissidentatus Kanzaki, Ragsdale, Herrmann, and Sommer 2012, revealed unusual variations in stomatal morphology within the genus. In this study, we describe a closer sister group to P. elegans, P. bucculentus n. sp., which provides further insight into the evolution of stomatal characters in basal Pristionchus species. To support comparative morphology of these species, a phylogeny including P. bucculentus n. sp. was inferred from a partial small subunit (SSU) rRNA gene and sequences of 25 ribosomal protein-coding genes.
The genus Pristionchus currently includes 36 valid species (Sudhaus and Fürst von Lieven, 2003; Herrmann et al., 2006b; Kanzaki et al., 2012a, 2012c), more than half of which have been placed in a molecular phylogeny. The genus has been dramatically expanded by discovery of species associated with insects, especially scarab beetles (Coleoptera: Scarabaeidae) (Völk, 1950; Fedorko and Stanuszek, 1971; Herrmann et al., 2006a, 2006b, 2007, 2010; Kanzaki et al., 2012a) and stag beetles (Lucanidae) (Kanzaki et al., 2011, 2012a). Sampling from an insect group not previously known to carry Pristionchus species, the shining mushroom beetles (Scaphidiidae), has now yielded P. bucculentus n. sp.
Materials and Methods
Nematode isolation and cultivation: Pristionchus bucculentus n. sp. was isolated from the shining mushroom beetle Episcapha gorhami Lewis, 1879 (Coleoptera: Scaphidiidae), which was collected from a white rot fungus on a rotting log in Sapporo, Hokkaido, Japan. The carrier beetle was dissected on a 2.0% agar plate, after which the plate was kept at room temperature for several weeks. Nematodes proliferated on bacteria associated with the host beetle cadavers. Individuals were transferred thereafter to nematode growth medium (NGM) agar plates seeded with Escherichia coli OP50, and have been since kept in laboratory culture on this medium.
Morphological observation and preparation of type material: One- to two-week-old cultures of P. bucculentus n. sp. provided material for morphological observation. Observations by light microscopy (LM) were conducted using live nematodes, which were hand-picked from culture plates. Because of the clarity and integrity of its morphology, live material was used for morphometrics. To prepare type material, nematodes were isolated from type strain cultures, rinsed in distilled water to remove bacteria, heat killed at 65°C, fixed in TAF to a final concentration of 5% formalin and 1.5% triethanolamine, and processed through a glycerol and ethanol series using Seinhorst’s method (see Hooper, 1986). Nomarski micrographs were taken using a Zeiss Axio Imager Z.1 microscope and a Spot RT-SE camera supported by the program MetaMorph v.7.1.3 (Molecular Devices, Sunnyvale, CA).
Molecular characterization and phylogenetic analysis: For species diagnosis and phylogenetic analysis, we extracted and amplified a 1-kb fragment of the SSU rRNA gene using the primers SSU18A (5’-AAAGATTAAGCCATGCATG-3’) and SSU26R (5’-CATTCTTGGCAAATGCTTTCG-3’) (Floyd et al., 2002). The sequence has been deposited in the GenBank database under accession number KC463832.
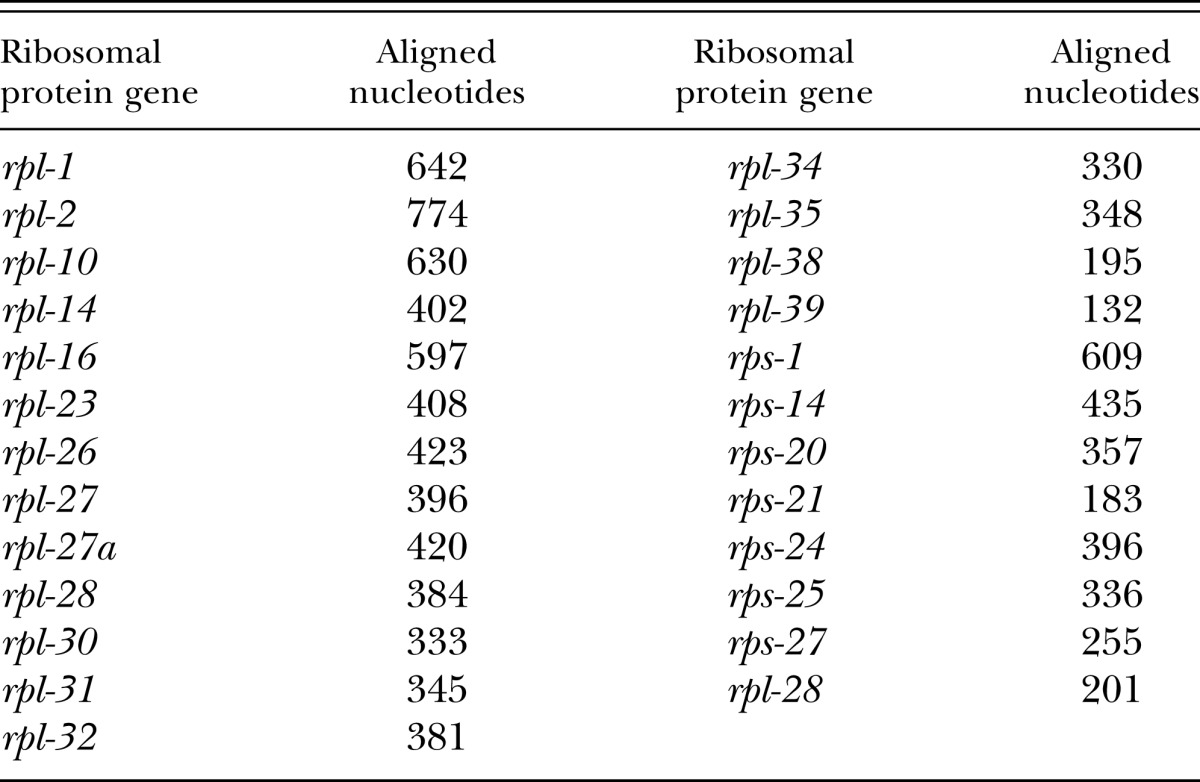
Phylogenetic analysis was performed on the partial SSU rRNA gene and 25 ribosomal protein genes. The analyzed fragment of the SSU rRNA gene was an alignment of 851 positions. The dataset of ribosomal protein genes comprised a total of 9,912 aligned coding nucleotides. Ribosomal protein genes used and lengths of the fragments analyzed are given in Table 1. Ribosomal protein gene sequences for P. bucculentus n. sp. that were longer than 200 bp have been deposited in GenBank under accession numbers KC463833–KC463854. All information regarding primers and PCR conditions for ribosomal protein genes is given in Mayer et al. (2007). Phylogenetic analysis included all nominal Pristionchus species for which orthologous sequences of the analyzed genes were publicly available. Based on previously published analyses (Mayer et al., 2009; van Megen et al., 2009), Koerneria was the closest outgroup for which all orthologous sequences were available, and thus Koerneria sp. RS1982 was chosen as an outgroup for the present analysis.
Table 1.
Ribosomal protein genes comprising the 9,912 aligned coding nucleotides used for phylogenetic analysis, with amplification and sequencing primers following Mayer et al. (2007).
The concatenated dataset of the partial SSU rRNA and ribosomal protein genes was aligned using MUSCLE (Edgar, 2004), followed by manual alignment and removal of ambiguously aligned positions in MEGA5.05 (Tamura et al., 2011). The alignment was partitioned four ways: three subsets corresponded to codon positions for the concatenated ribosomal protein genes and one subset contained the partial SSU rRNA gene. The phylogeny was inferred under the criteria of maximum likelihood (ML) and Bayesian inference, as implemented in RAxML v.7.2.8 (Stamatakis, 2006) and MrBayes 3.2 (Ronquist et al., 2012), respectively. Bayesian analyses were initiated with random starting trees and were run with four chains for 4 × 106 generations. Markov chains were sampled at intervals of 100 generations. Two runs were performed in the analysis. After confirming convergence of runs and discarding the first 1 × 106 generations as burn-in, remaining topologies were used to generate a 50% majority-rule consensus tree with clade credibility values given as posterior probabilities (PP). The ML analysis was performed under a general time reversible model with a gamma-shaped distribution of rates across sites, whereas the Bayesian analysis allowed a mixed model of substitution with a gamma-shaped distribution. Model parameters were unlinked across character partitions in both analyses. Node support in the ML tree was evaluated by 1,000 bootstrap pseudoreplicates.
Results
Molecular characterization and phylogenetic analysis: A diagnostic fragment of the SSU rRNA gene, namely 400 bp of the 5' end (Herrmann et al., 2006a, 2006b), was unique for P. bucculentus n. sp. This sequence differed from the closest inferred species, P. elegans, by 21 nucleotides.
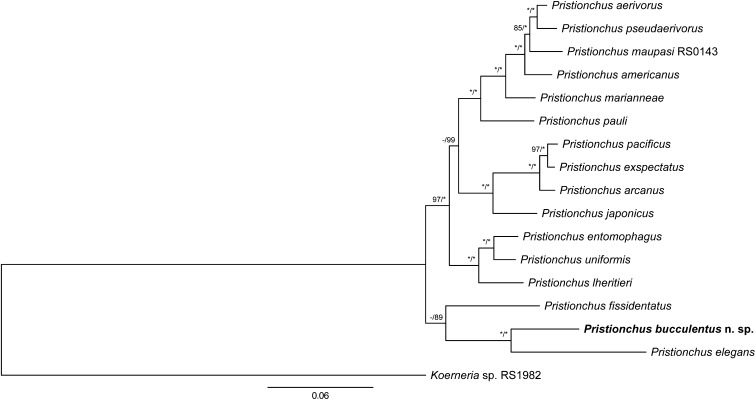
For the phylogenetic analysis, the final concatenated alignment comprised 1697 parsimony-informative sites. Tree topologies from ML and Bayesian analyses were identical. Only the ML tree is shown, along with support values from both analyses (Fig. 1). The phylogeny, which was inferred from 25 ribosomal protein genes and an approximately 850-bp fragment of the SSU rRNA, placed P. bucculentus n. sp. as a sister taxon to P. elegans. This sister-group relationship was well supported in both analyses (100% BS and PP). In addition to P. bucculentus n. sp. and P. elegans, P. fissidentatus was also basal to all other Pristionchus species. However, the precise phylogenetic position of P. fissidentatus was not resolved: the species was placed as a sister group to P. bucculentus n. sp. + P. elegans with only negligible support (< 50% BS, 89% PP).
Fig. 1.
Phylogenetic tree of Pristionchus species inferred under maximum likelihood (ML) from the partial SSU rRNA gene and 25 ribosomal protein genes. Ribosomal protein genes analyzed are listed in Table 1. The tree with the highest log likelihood (-43269.50328) is shown. The proportion of trees in which the associated taxa clustered together in 1,000 bootstrap pseudoreplicates is shown next to the nodes (left value). Tree topology is identical with that from Bayesian inference, and posterior probabilities (PP) for corresponding nodes are also displayed (right value) on tree. Bootstrap support values above 50% are shown. Asterisk indicates 100% support. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site.
Pristionchus bucculentus* n. sp.
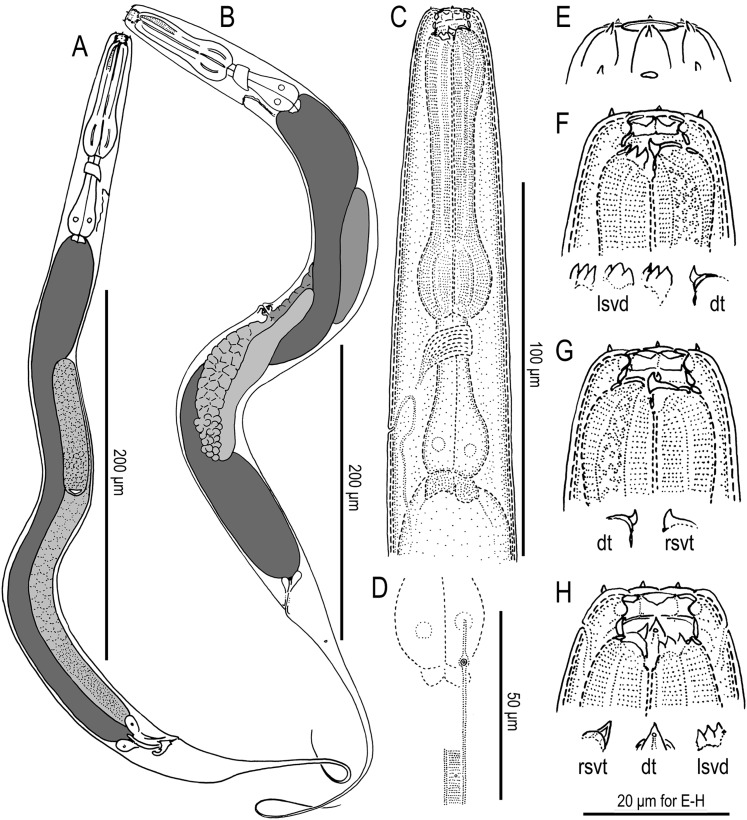
Fig. 2.
Eurystomatous adults of Pristionchus bucculentus n. sp. (A) Whole body of male in right lateral view. (B) Whole body of female in left lateral view. (C) Neck region of female in left lateral view. (D) Deirid and postdeirid in right lateral view. (E) Lip region of male in lateral view. (F) Stomatal region of female in left lateral view, showing dorsal tooth (dt) and variation of left subventral denticles (lsvd). (G) Stomatal region of female in right lateral view, showing dorsal tooth and right subventral tooth (rsvt). (H) Stomatal region of female in ventral view.
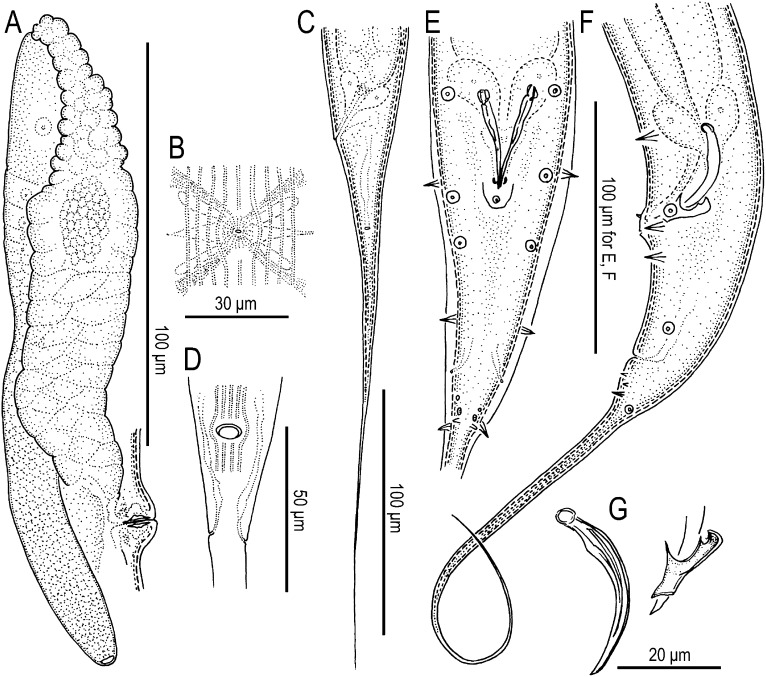
Fig. 3.
Adult females (A–D) and males (E–G) of Pristionchus bucculentus n. sp. (A) Anterior gonad branch in right lateral view. (B) Vulva in ventral view. (C) Tail in left lateral view. (D) Anus in ventral view. (E) Tail in ventral view. (F) Tail in left lateral view. (G) Spicule and gubernaculum in left lateral view.
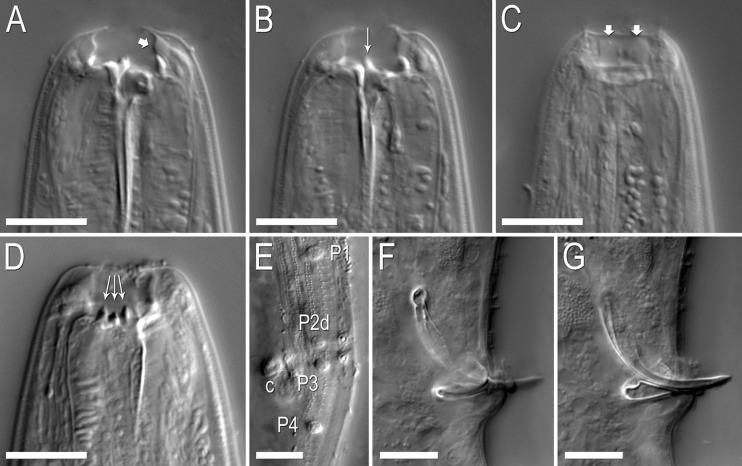
Fig. 4.
Nomarski micrographs of Pristionchus bucculentus n. sp. Scale bars are 10 μm. (A–C) Stoma of a single eurystomatous female in right lateral view through three focal planes. (A) Dorsal plane, showing dorsal tooth and bulging cheilostom (arrow) with thin internal (medial) walls. (B) Right subventral plane, showing right subventral tooth with a narrow, elevated apex. (C) Right subventral plane at right lateral margin of stoma, showing cheilostomatal bulges separated by very weak incision. (D) Left subventral plane of the stoma of a eurystomatous female in left lateral view, showing three regularly shaped, conical denticles. (E) Cuticular surface at male tail in oblique left-ventral view, showing the diagnostic arrangement of genital papillae P1–P4, including with respect to the cloacal opening (c). (F–G) The copulatory organ of a single male individual in right lateral view through two focal planes. Rounded, ventrally skewed manubrium is highlighted in (F). The short, shallow terminal curvature of the gubernaculum is highlighted in (G).
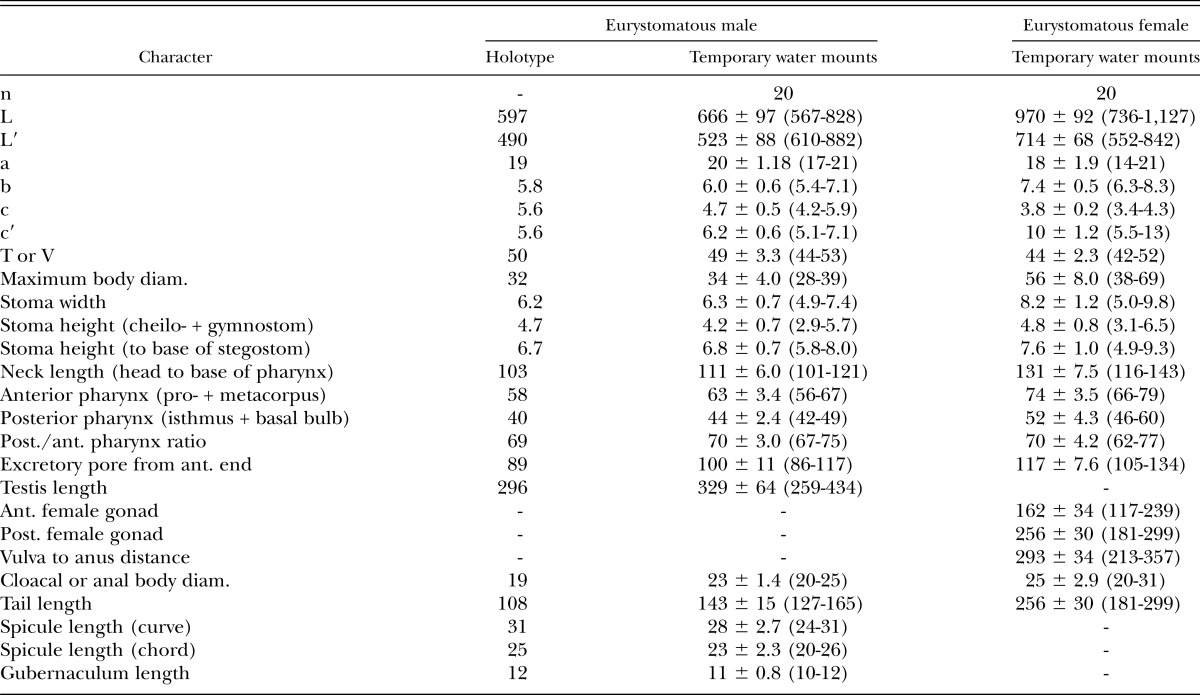
Measurements: See Table 2.
Table 2.
Morphometrics of eurystomatous male holotype (in glycerin) and male and female specimens of Pristionchus bucculentus n. sp. (temporary water mounts). All measurements made in μm and in the form: mean ± sd (range).
Description: Adults: Cuticle thick, with fine annulation and clear longitudinal striations. Lateral field consists of two lines, only weakly distinguishable from body striation. Head without apparent lips, with six short and papilliform labial sensilla. Four small, papilliform cephalic papillae present in males, as typical for diplogastrid nematodes. Amphidial apertures located at level of posterior end of cheilostomatal plates. Eurystomatous (wide-mouthed) form occurs in both males and females. Stenostomatous (narrow-mouthed) form not observed. Dorsal pharyngeal gland clearly observed, penetrating dorsal tooth to gland opening. Anterior part of pharynx (pro- and metacorpus) 1.5 times as long as posterior part (isthmus and basal bulb). Procorpus very muscular, stout, occupying one-half to two-thirds of corresponding body diam. Metacorpus very muscular, forming well-developed median bulb. Isthmus narrow, not muscular. Basal bulb glandular. Pharyngo-intestinal junction clearly observed, well-developed. Nerve ring usually surrounds middle region of isthmus. Excretory pore not conspicuous, usually near posterior part of isthmus to middle of basal bulb, excretory duct extending anteriad and reflexed back to position of pore. Hemizonid not clearly observed. Deirid observed laterally at level of pharyngo-intestinal junction. Postdeirids present and observed laterally, with positions inconsistent among individuals: 5 to 8 for males and 9 to 10 for females being confirmed by LM observation.
Eurystomatous form: Cheilostom apparently consists of six per- and interradial plates, but incisions between plates almost indistinguishable by LM observation. Cheilostomatal epidermis appears vacuolated, cheilostomatal walls thin and membranous in appearance, forming cuticular ring-like anterior end that medially overlaps the posterior cheilostom. Anterior end of each plate rounded and elongated to stick out from stomatal opening and form a small flap. Cheilostom and gymnostom short and about same depth. Gymnostom is thicker posteriorly, forms cuticular ring-like anterior end overlapping cheilostom internally. Stegostom bears a large, claw-like dorsal tooth; a large, claw-like right subventral tooth with a narrow, elevated apex; and a row of three robust, regularly shaped conical left subventral denticles. Dorsal and right subventral teeth movable. Movement of left subventral plate or its associated denticles not observed.
Males: Ventrally arcuate, strongly ventrally curved at tail region when killed by heat. Testis single, ventrally located, anterior part reflexed to right side. Spermatogonia arranged in three to five rows in reflexed part, then well-developed spermatocytes arranged in usually three rows in anterior two-thirds of main branch, then mature amoeboid spermatids arranged in multiple rows in remaining, proximal part of gonad. Vas deferens not clearly separated from other parts of gonad. Testis and intestine join at level of anterior end of spicules to form cloaca. Three (two subventral and one dorsal) cloacal glands observed around posterior end of testis and intestine. Spicules paired, separate. Spicules smoothly curved in ventral view, adjacent or very close to each other for distal third of their length, each smoothly tapering to pointed distal end. Spicule in lateral view smoothly ventrally arcuate, giving spicule about 100° curvature, rounded, ventrally skewed manubrium present at anterior end; lamina/calomus complex clearly expanded one-third of length from anterior end, then smoothly tapering to pointed distal end. Gubernaculum conspicuous, about one-third of spicule in length, broad anteriorly such that dorsal wall is slightly recurved and that dorsal and ventral walls separate at a 45° angle at posterior end. Dorsal side of gubernaculum bears a single, membranous, anteriorly directed process and a lateral pair of more sclerotized, anteriorly and ventrally directed processes. In lateral view, anterior half of gubernaculum with two serial curves separated by anteriorly and ventrally directed process and with small, often shallow anterior terminal curvature less than one-fifth of gubernaculum length and with large, deep posterior curvature of almost half of gubernaculum length and dorsoventrally more open than diameter of spicule passing through it; posterior half forms a tube-like process enveloping spicules. Thick cuticle around tail region, sometimes falsely appearing like a narrow leptoderan bursa in ventral view. Cloacal opening slit-like in ventral view. One small, ventral, single genital papilla on anterior cloacal lip. Nine pairs of genital papillae and a pair of phasmids present and arranged as <P1, (P2d, P3 C), P4, P5d, Ph, (P6, P7, P8), P9d> (= <v1, (v2d, v3, C), v4, ad, Ph, (v5, v6, v7), pd> in nomenclature of Sudhaus and Fürst von Lieven, 2003), whereby P3 is closer to P4 than to P1; P4 is anterior and thus closer to P2d and P3 than to P5; phasmid (Ph) and P6 are clearly apart; Ph is midway between P5d and P6 or closer to P6; P6–P8 are arranged linearly or in an acute triangle, i.e., P7 is sometimes slightly ventral compared with P6 and P8; and position of P9d is slightly posterior to or overlapping P8. P1–P4 papillae of almost equal size, rather large and conspicuous; P5d slightly smaller than P1–P4; P6 and P7 very small, sometimes difficult to observe by LM; P8 and P9d small but larger than P6 and P7, i.e., intermediate between P5d and P6/P7 in size. Tip of P7 papillae split into two small papilla-like projections. P6 and P7 cylindrical, projecting from socket-like base, other papillae conical, projecting directly from body surface. Tail conical, with long spike, about four to five cloacal body diam. long. Bursa or bursal flap absent.
Females: Relaxed or slightly ventrally arcuate when killed by heat. Gonad didelphic, amphidelphic. Each gonadal system arranged from vulva/vagina as uterus, oviduct, and ovary. Anterior gonad right of intestine, with uterus and oviduct extending ventrally and anteriorly on right of intestine and with a totally reflexed (= antidromous reflexion) ovary extending dorsally on left of intestine. Oocytes mostly arranged in two to eight rows in distal two-thirds of ovary and in double or single row in rest of ovary, distal tips of each ovary reaching vulva or oviduct of opposite gonad branch. Anterior part of ovary proximal to flexure is a columnella-like structure with irregularly numbered rows of cells and resembling a bunch of grapes. Middle part of oviduct serves as spermatheca. Eggs in single- to multiple-cell stage at proximal part of oviduct (= uterus), of which the cells are often diamond-shaped in appearance. Mature females usually retaining few (e.g., fewer than eight) eggs. Receptaculum seminis not observed. Vaginal glands present but obscure. Vagina perpendicular to body surface, surrounded by sclerotized tissue. Vulva slightly protuberant in lateral view, pore-like in ventral view. Rectum about one anal body diam. long, intestinal-rectal junction surrounded by well-developed sphincter muscle. Three anal glands (two subventral and one dorsal) present but not obvious. Anus in form of dome-shaped slit, posterior anal lip slightly protuberant. Phasmid about one to two anal body diam. posterior to anus. Tail long, about 10 anal body diam. long, distal end filiform.
Type host (carrier) and locality: The culture from which the type specimens were obtained was isolated from the body of an adult Episcapha gorhami (Coleoptera: Scaphidiidae), which was associated with white rot fungus on a decaying log. The isolate was collected by N. Kanzaki at Hitsujigaoika in Sapporo, Hokkaido Prefecture, Japan (42.9954° N, 141.3934° E) on 1 June 2012.
Type material: Holotype eurystomatous male (accession number 30797), four paratype eurystomatous males, and four paratype eurystomatous hermaphrodites deposited in the University of California Riverside Nematode Collection, CA. Two paratypes each of eurystomatous males and eurystomatous females deposited in the Swedish Natural History Museum, Stockholm, Sweden. Two paratypes each of eurystomatous males and eurystomatous females deposited in the Natural History Museum Karlsruhe, Germany.
Type strain culture: Available in living culture under code RS5596 in the Department of Evolutionary Biology, Max Planck Institute for Developmental Biology, Tübingen, Germany, and can be provided to other researchers upon request.
Nomenclatural registration: The new species binomial has been registered in the ZooBank database (zoobank.org) under the identifier BDBA4148-B141-413E-91FC-E108A3FE730B.
Diagnosis and relationships: Besides its generic characters, Pristionchus bucculentus n. sp. is diagnosed by the unique stoma shape and armature of its eurystomatous (i.e., the only known) form and by the male genital papillae arranged as <P1, (P2d, P3 C), P4, P5d, Ph, (P6, P7, P8), P9d>, whereby P1 is far from P2 and P2–P4 are close together. Pristionchus bucculentus n. sp. is distinguished from all other known Pristionchus species by three regularly shaped, conical left subventral denticles vs. denticles of variable shape and size and often with curved, cusp-like apices. The species is distinguished from all nominal Pristionchus species except P. elegans by having an inwardly bulging and apparently vacuolated cheilostom. It is distinguished from the closest known species, P. elegans, by an anteriorly smooth vs. heavily serrated gymnostom and by a cheilostom with thin and apparently membranous internal walls with weak incisions between plates vs. a deeper cheilostom with thickened cheilostomatal walls and clear incisions between plates. It is further distinguished from P. elegans by the following: an anterior P1, such that P2 is closer to P4 than to P1 vs. a posterior P1, such that P1, P3, and P4 are close together and equidistant; the anterior curvature of the gubernaculum being almost one-half vs. less than one-third of gubernaculum length; a manubrium that is rounded and skewed ventrally vs. being squared or angular at the anterior end. The two species are tentatively distinguished by body size: female length to anus (L′) of P. bucculentus n. sp. (550 to 840 μm) was less than that recorded for P. elegans (850 to 1,280 μm), although future investigation may reveal overlap in size ranges. Pristionchus bucculentus n. sp. is also tentatively distinguished from P. elegans by having only eurystomatous vs. only stenostomatous males and females, although further sampling may reveal the putatively missing form in either species. Finally, P. bucculentus n. sp. and P. elegans can be distinguished by their molecular divergence, namely by 21 nucleotide differences in a 400-bp diagnostic SSU rRNA fragment alone. Pristionchus bucculentus n. sp. is additionally distinguished from the basal Pristionchus species P. fissidentatus n. sp., as well as from P. entomophagus (Steiner, 1928) Sudhaus and Fürst von Lieven, 2003, P. lheritieri (Maupas, 1919) Paramonov, 1952, P. maupasi (Potts, 1910) Paramonov, 1952, and P. pacificus, by a gonochoristic vs. hermaphroditic mode of reproduction.
Discussion
This morphological description of P. bucculentus n. sp. provides a new point of comparison for reconstructing the evolution of stomatal morphology in Pristionchus. Furthermore, it has broadened the known degree of variation of stomatal morphology in the genus. Despite the apparent similarity of most characters traditionally diagnostic in Diplogastridae, description of P. bucculentus n. sp. confirms the diagnostic value of mouthparts in Pristionchus (Kanzaki et al., 2012a, 2012b, 2012c). Unique in P. bucculentus n. sp. is the degree of apparent vacuolation in the cheilostom and the presence of three conical left subventral denticles. The cheilostomatal bulges of P. bucculentus n. sp. differ from those from its closest known species, P. elegans, by being smaller and less internally sclerotized in the former species. However, comparison of the stomata of P. bucculentus n. sp. and P. elegans is necessarily limited by the putative presence of only single, noncorresponding mouth-forms in the two species. Because the stoma is more heavily sclerotized in the eurystomatous form of Pristionchus species that have both forms, the anterior gymnostomatal serration of the stenostomatous P. elegans, if present in P. bucculentus n. sp., would be predicted to be present and possibly more exaggerated in the eurystomatous form of the latter species. Likewise, the sclerotization of and incisions between cheilostomatal plates observed in P. elegans should be at least as distinct if they were present in the eurystomatous form of P. bucculentus n. sp. Because these characters are missing in eurystomatous P. bucculentus n. sp., we hypothesize that these characters represent real differences between P. bucculentus n. sp. and P. elegans.
Phylogenetic relationships inferred from 26 nuclear gene sequences support morphological similarities found among basal Pristionchus species. A clear synapomorphy for P. bucculentus n. sp. and P. elegans, which are strongly supported (100% BS and PP) as sister taxa, is a vacuolated cheilostom. Another synapomorphy is the structure of the gubernaculum: in both P. bucculentus n. sp. and P. elegans, the anterior terminal curvature is short and shallow, particularly with respect to the large posterior curvature at the part enveloping the spicules (Figs. 3,4), whereas in putative outgroups P. fissidentatus and Parapristionchus giblindavisi Kanzaki, Ragsdale, Herrmann, Mayer, Tanaka, and Sommer, 2012, the anterior curvature is closer in size to the posterior curvature. A long, filiform tail in both sexes is putatively synapomorphic between the two species with respect to the same putative outgroups. The closeness of male papillae P2, P3, and P4 to each other and to the cloacal opening (Figs. 3,4) is shared by P. bucculentus n. sp. and P. elegans and is probably symplesiomorphic, considering the similar states in putative outgroups P. fissidentatus and Pa. giblindavisi.
The genus Pristionchus is in part circumscribed by the presence of stomatal dimorphism (Sudhaus and Fürst von Lieven, 2003). However, inspection of hundreds of individuals has so far not revealed the presence of more than one form in either P. elegans or P. bucculentus n. sp. The lack of a stenostomatous form in P. bucculentus n. sp. is especially supported by the absence of this form in males, which are typically highly stenostomatous in other species of Pristionchus (Serobyan et al., 2013) and other diplogastrid genera (Fürst von Lieven and Sudhaus, 2000). Thus, a condition of putative monomorphy characterizes both species, although the nonhomologous morph has been fixed in either case. It is possible that P. elegans and P. bucculentus n. sp. speciated from a dimorphic stem ancestor through niche partitioning, as afforded by fixing alternate forms, although the lack of clearly described niches in Pristionchus species is still an obstacle to testing this idea. Deeper taxon sampling, if possible, will allow evolutionary reconstruction of the dimorphism, such as by determining the age of fixation events and whether species have radiated since any such events. Furthermore, whether there is some common mechanism for phenotypic accommodation in these species will hopefully be tested as a more detailed mechanistic understanding of the dimorphism in Pristionchus is achieved (Bento et al., 2010; Bose et al., 2012).
The description of a new basal species of Pristionchus from a shining mushroom beetle in Japan adds important information with regard to the biogeography and insect association of Pristionchus nematodes. First, P. bucculentus n. sp. represents yet another Pristionchus species with a known range restricted to Japan, highlighting the importance of the Japanese archipelago for the ancestral diversification of the genus. Finally, the presence of P. bucculentus n. sp. on a mushroom beetle suggests another ecological niche for Pristionchus nematodes that can only be fully explored by long-term systematic sampling.
Footnotes
The species epithet is a Latin adjective meaning “full-cheeked” and refers to the bulges of the cheilostom.
Literature Cited
- Bento G, Ogawa A, Sommer RJ. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature. 2010;466:494–497. doi: 10.1038/nature09164. [DOI] [PubMed] [Google Scholar]
- Bose N, Ogawa A, von Reuss SH, Yim JJ, Ragsdale EJ, Sommer RJ, Schroeder FC. Complex small-molecule architectures regulate phenotypic plasticity in a nematode. Angewandte Chemie. (2012);51:12438–12443. doi: 10.1002/anie.201206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko A, Stanuszek S. Pristionchus uniformis sp. n. (Nematoda, Rhabditida, Diplogasteridae), a facultative parasite of Leptinotarsa decemlineata Say and Melolontha melolontha L. in Poland. Morphology and biology. Acta Parastiologica. 1971;19:95–112. [Google Scholar]
- Floyd R, Abebe E, Papert A, Blaxter M. Molecular barcodes for soil nematode identification. Molecular Ecology. 2002;11:839–850. doi: 10.1046/j.1365-294x.2002.01485.x. [DOI] [PubMed] [Google Scholar]
- Fürst von Lieven A, Sudhaus W. Comparative and functional morphology of the buccal cavity of Diplogastrina (Nematoda) and a first outline of the phylogeny of this taxon. Journal of Zoological Systematics and Evolutionary Research. 2000;38:37–63. [Google Scholar]
- Herrmann M, Kienle S, Rochat J, Mayer WE, Sommer RJ. Haplotype diversity of the nematode Pristionchus pacificus on Réunion in the Indian Ocean suggests multiple independent invasions. Biological Journal of the Linnean Society. 2010;100:170–179. [Google Scholar]
- Herrmann M, Mayer WE, Hong RL, Kienle S, Minasaki R, Sommer RJ. The nematode Pristionchus pacificus (Nematoda: Diplogastridae) is associated with the Oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zoological Science. 2007;24:883–889. doi: 10.2108/zsj.24.883. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Mayer WE, Sommer RJ. Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology. 2006a;109:96–108. doi: 10.1016/j.zool.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Mayer WE, Sommer RJ. Sex, bugs and Haldane’s rule: The nematode genus Pristionchus in the United States. Frontiers in Zoology. 2006b;3:14. doi: 10.1186/1742-9994-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DJ. 1986. Handling, fixing, staining and mounting nematodes. Pp. 59–80 in J. F. Southey, ed. Laboratory methods for work with plant and soil nematodes. London: Her Majesty’s Stationary Office.
- Kanzaki N, Ragsdale EJ, Herrmann M, Mayer WE, Sommer RJ. Description of three Pristionchus species (Nematoda: Diplogastridae) from Japan that form a cryptic species complex with the model organism P. pacificus. Zoological Science. 2012a;29:403–417. doi: 10.2108/zsj.29.403. [DOI] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Mayer WE, Tanaka R, Sommer RJ. Parapristionchus giblindavisi n. gen., n. sp. (Rhabditida: Diplogastridae) isolated from stag beetles (Coleoptera: Lucanidae) in Japan. Nematology. 2012b;14:933–947. [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Sommer RJ. Two new species of Pristionchus (Rhabditida: Diplogastridae): P. fissidentatus n. sp. from Nepal and La Réunion Island and P. elegans n. sp. from Japan. Journal of Nematology. (2012c);44:80–91. [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N, Taki H, Masuya H, Okabe K, Tanaka R, Abe F. Diversity of stag beetle-associated nematodes in Japan. Environmental Entomology. 2011;40:281–288. [Google Scholar]
- Kreis HA. Beiträge zur Kenntnis pflanzenparasitischer Nematoden. Zeitschrift für Parasitenkunde. 1932;5:184–194. [Google Scholar]
- Lewis G. On certain new species of Coleoptera from Japan. Annals and Magazine of Natural History. 5th Series. 1879;4:459–467. [Google Scholar]
- Maupas E. Essais d’hybridation chez les nématodes. Bulletin Biologique de la France et de la Belgique. 1919;52:466–498. [Google Scholar]
- Mayer WE, Herrmann M, Sommer RJ. Phylogeny of the nematode genus Pristionchus and implications for biodiversity, biogeography and the evolution of hermaphroditism. BMC Evolutionary Biology. 2007;7:104. doi: 10.1186/1471-2148-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer WE, Herrmann M, Sommer RJ. Molecular phylogeny of beetle associated diplogastrid nematodes suggests host switching rather than nematode-beetle coevolution. BMC Evolutionary Biology. 2009;9:212. doi: 10.1186/1471-2148-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micoletzky H. Die freilebenden Erd-Nematoden. Archiv für Naturgeschichte. Abteiliung A. 1922;87:1–650. [Google Scholar]
- Paramonov AA. Opyt ekologicheskoi klassifikatsii fitonematod. Trudy Gelmintologicheskoi Laboratorii. Akademia Nauk SSSR (Moskva) 1952;6:338–369. [Google Scholar]
- Potts FA. Notes on the free-living nematodes. Quarterly Journal of Microscopical Science. 1910;55:433–484. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serobyan V, Ragsdale EJ, Müller MR, Sommer RJ. Feeding plasticity in the nematode Pristionchus pacificus is influenced by sex and social context and is linked to developmental speed. Evolution & Development. 2013 doi: 10.1111/ede.12030. 15 (in press) [DOI] [PubMed] [Google Scholar]
- Sommer RJ. The future of evo-devo: Model systems and evolutionary theory. Nature Reviews in Genetics. 2009;10:416–422. doi: 10.1038/nrg2567. [DOI] [PubMed] [Google Scholar]
- Sommer RJ, Carta LK, Kim SY, Sternberg PW. Morphological, genetic and molecular description of Pristionchus pacificus n. sp. (Nematoda: Neodiplogastridae) Fundamental and Applied Nematology. 1996;19:511–521. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Steiner G. Diplogaster entomophaga n. sp., a new Diplogaster (Diplogasteridae, Nematodes) found on a Pamphilius stellatus (Christ) (Tenthredinidae, Hymenoptera) Zoologischer Anzeiger. 1928;80:143–145. [Google Scholar]
- Sudhaus W, Fürst von Lieven A. A phylogenetic classification and catalogue of the Diplogastridae (Secernentea, Nematoda) Journal of Nematode Morphology and Systematics. 2003;6:43–90. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Megen H, van den Elsen S, Holterman M, Karssen G, Mooyman P, Bongers T, Holovachov O, Bakker J, Helder J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology. 2009;11:927–950. [Google Scholar]
- Völk J. Die Nematoden der Regenwürmer und aasbesuchenden Käfer. Zoologische Jahrbücher. Abteilung für Systematik. 1950;79:1–70. [Google Scholar]