Abstract
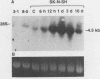
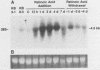
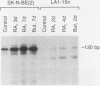
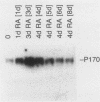
Expression of a multidrug resistance gene (mdr1) and its protein product, P-glycoprotein (Pgp), has been correlated with the onset of multidrug resistance in vitro in human cell lines selected for resistance to chemotherapeutic agents derived from natural products. Expression of this gene has also been observed in normal tissues and human tumors, including neuroblastoma. We therefore examined total RNA prepared from human neuroblastoma cell lines before and after differentiation with retinoic acid or sodium butyrate. An increase in the level of mdr1 mRNA was observed after retinoic acid treatment of four neuroblastoma cell lines, including the SK-N-SH cell line. Western blot (immunoblot) analysis demonstrated concomitant increases in Pgp. However, studies of 3H-vinblastine uptake failed to show a concomitant Pgp-mediated decrease in cytotoxic drug accumulation. To provide evidence that Pgp was localized on the cell surface, an immunotoxin conjugate directed against Pgp was added to cells before and after treatment with retinoic acid. Incorporation of [3H]leucine was decreased by the immunotoxin in the retinoic acid-treated cells compared with the undifferentiated cells. These results demonstrate that whereas expression of the mdr1 gene can be modulated by differentiating agents, increased levels of expression are not necessarily associated with increased cytotoxic drug accumulation.
Full text
PDF


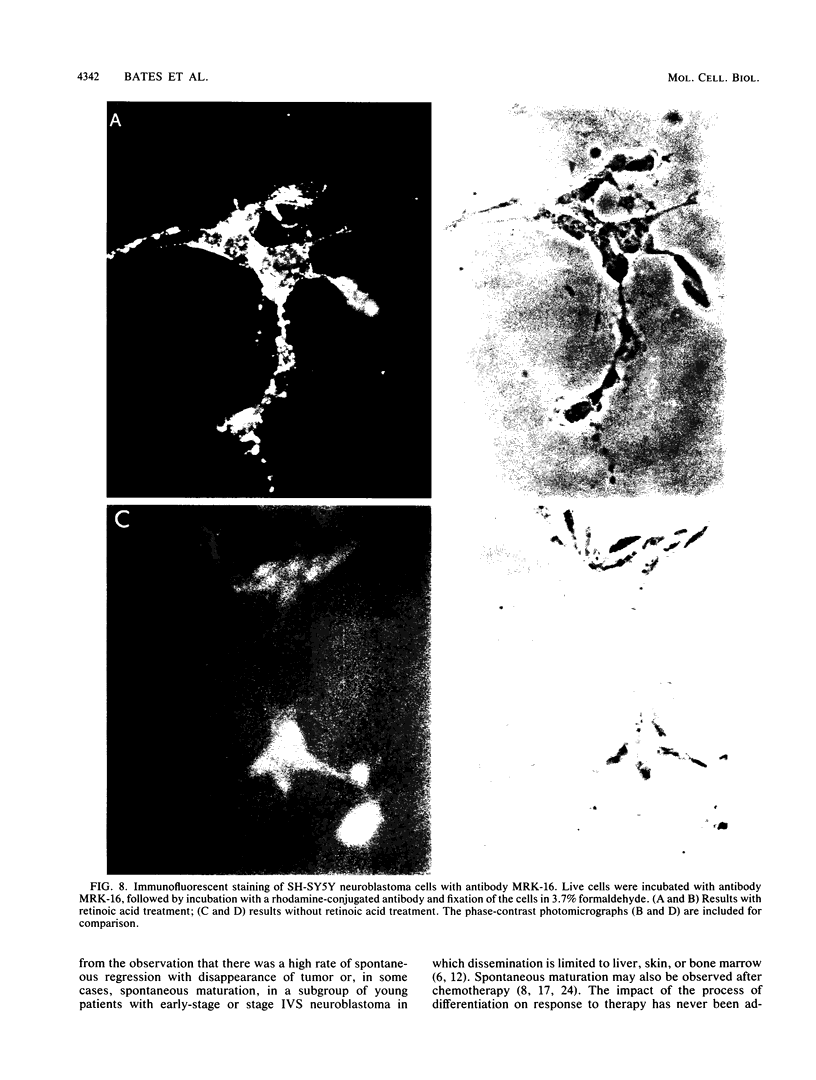


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Fojo A., Hanover J. A., Pastan I., Gottesman M. M. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985 Mar;11(2):117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Biedler J. L., Roffler-Tarlov S., Schachner M., Freedman L. S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978 Nov;38(11 Pt 1):3751–3757. [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Barrett A., Castleberry R. P., D'Angio G., De Bernardi B., Evans A. E., Favrot M., Freeman A. I., Haase G. International criteria for diagnosis, staging and response to treatment in patients with neuroblastoma. Prog Clin Biol Res. 1988;271:509–524. [PubMed] [Google Scholar]
- Choi K. H., Chen C. J., Kriegler M., Roninson I. B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell. 1988 May 20;53(4):519–529. doi: 10.1016/0092-8674(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Ciccarone V., Spengler B. A., Meyers M. B., Biedler J. L., Ross R. A. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989 Jan 1;49(1):219–225. [PubMed] [Google Scholar]
- Cooney D. R., Voorhess M. L., Fisher J. E., Brecher M., Karp M. P., Jewett T. C. Vasoactive intestinal peptide producing neuroblastoma. J Pediatr Surg. 1982 Dec;17(6):821–825. doi: 10.1016/s0022-3468(82)80450-8. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Debenham P. G., Kartner N., Siminovitch L., Riordan J. R., Ling V. DNA-mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol. 1982 Aug;2(8):881–889. doi: 10.1128/mcb.2.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. E., Gerson J., Schnaufer L. Spontaneous regression of neuroblastoma. Natl Cancer Inst Monogr. 1976 Nov;44:49–54. [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Grosfeld J. L., Baehner R. L. Neuroblastoma: an analysis of 160 cases. World J Surg. 1980 Jan;4(1):29–37. doi: 10.1007/BF02393089. [DOI] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J Biol Chem. 1988 Jan 25;263(3):1454–1458. [PubMed] [Google Scholar]
- Helson L., Helson C., Peterson R. F., Das S. K. A rationale for the treatment of metastatic neuroblastoma. J Natl Cancer Inst. 1976 Sep;57(3):727–729. doi: 10.1093/jnci/57.3.727. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kyritsis A., Joseph G., Chader G. J. Effects of butyrate, retinol, and retinoic acid on human Y-79 retinoblastoma cells growing in monolayer cultures. J Natl Cancer Inst. 1984 Sep;73(3):649–654. [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Ikeda K., Tsuda T., Higashi K. Biological characteristics of N-myc amplified neuroblastoma in patients over one year of age. Prog Clin Biol Res. 1988;271:31–39. [PubMed] [Google Scholar]
- Ogita S., Tokiwa K., Arizono N., Takahashi T. Neuroblastoma: incomplete differentiation on the way to maturation or morphological alteration resembling maturity? Oncology. 1988;45(3):148–152. doi: 10.1159/000226552. [DOI] [PubMed] [Google Scholar]
- Påhlman S., Ruusala A. I., Abrahamsson L., Mattsson M. E., Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984 Jun;14(2):135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Reynolds C. P., Biedler J. L., Spengler B. A., Reynolds D. A., Ross R. A., Frenkel E. P., Smith R. G. Characterization of human neuroblastoma cell lines established before and after therapy. J Natl Cancer Inst. 1986 Mar;76(3):375–387. [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979 Dec 25;254(24):12701–12705. [PubMed] [Google Scholar]
- Rosen E. M., Cassady J. R., Frantz C. N., Kretschmar C., Levey R., Sallan S. E. Neuroblastoma: the Joint Center for Radiation Therapy/Dana-Farber Cancer Institute/Children's Hospital experience. J Clin Oncol. 1984 Jul;2(7):719–732. doi: 10.1200/JCO.1984.2.7.719. [DOI] [PubMed] [Google Scholar]
- Ross R. A., Spengler B. A., Biedler J. L. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983 Oct;71(4):741–747. [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Sewell J. L., Meyers M. B., Biedler J. L., Felsted R. L. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J Biol Chem. 1987 Jun 5;262(16):7884–7888. [PubMed] [Google Scholar]
- Seeger R. C., Rayner S. A., Banerjee A., Chung H., Laug W. E., Neustein H. B., Benedict W. F. Morphology, growth, chromosomal pattern and fibrinolytic activity of two new human neuroblastoma cell lines. Cancer Res. 1977 May;37(5):1364–1371. [PubMed] [Google Scholar]
- Shafford E. A., Rogers D. W., Pritchard J. Advanced neuroblastoma: improved response rate using a multiagent regimen (OPEC) including sequential cisplatin and VM-26. J Clin Oncol. 1984 Jul;2(7):742–747. doi: 10.1200/JCO.1984.2.7.742. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Cardarelli C., Hwang J., Cornwell M., Richert N., Ishii S., Pastan I., Gottesman M. M. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986 Jun 15;261(17):7762–7770. [PubMed] [Google Scholar]
- Shimada H., Chatten J., Newton W. A., Jr, Sachs N., Hamoudi A. B., Chiba T., Marsden H. B., Misugi K. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984 Aug;73(2):405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- Sidell N., Altman A., Haussler M. R., Seeger R. C. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp Cell Res. 1983 Oct;148(1):21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- Sidell N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982 Apr;68(4):589–596. [PubMed] [Google Scholar]
- Sidell N., Sarafian T., Kelly M., Tsuchida T., Haussler M. Retinoic acid-induced differentiation of human neuroblastoma: a cell variant system showing two distinct responses. Exp Cell Biol. 1986;54(5-6):287–300. doi: 10.1159/000163368. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C. J., Reynolds C. P., Israel M. A. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. 1985 Jan 31-Feb 6Nature. 313(6001):404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- Tsokos M., Ross R. A., Triche T. J. Neuronal, Schwannian and melanocytic differentiation of human neuroblastoma cells in vitro. Prog Clin Biol Res. 1985;175:55–68. [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]