Abstract
Sotraustaurin (STN), a small molecule, targeted PKC inhibitor that prevents T lymphocyte activation via a calcineurin-independent pathway, is currently tested in Phase II renal and liver transplantation clinical trials. We have documented the key role of activated T cells in the inflammation cascade leading to liver ischemia/reperfusion injury (IRI). This study explores putative cytoprotective functions of STN in a clinically relevant rat model of hepatic cold ischemia followed by orthotopic liver transplantation (OLT). Livers from SD rats were stored for 30h at 4° in UW solution, and then transplanted to syngeneic recipients. STN treatment of liver donors/recipients or recipients only prolonged OLT survival to >90% (vs. 40% in controls), decreased hepatocellular damage, and improved histological features of IRI. STN treatment decreased activation of T cells, and diminished macrophage/neutrophil accumulation in OLTs. These beneficial effects were accompanied by diminished apoptosis, NF-κB/ERK signaling, depressed pro-apoptotic cleaved caspase-3, yet upregulated anti-apoptotic Bcl-2/Bcl-xl and hepatic cell proliferation. In vitro, STN decreased PKCθ/IκBα activation and IL-2/IFN-γ production in ConA-stimulated spleen T cells, and diminished TNF-α/IL-1β in macrophage-T cell co-cultures. This study documents positive effects of STN on liver IRI in OLT rat model that may translate as an additional benefit of STN in clinical liver transplantation.
Keywords: Liver transplantation, Ischemia/reperfusion injury, Sotrastaurin, Protein Kinase C
Introduction
Sotraustaurin (STN; AEB071), a novel molecular weight synthetic immunosuppressant, is a targeted pan-protein kinase C (PKC) inhibitor that prevents early T cell activation via a calcineurin-independent pathway (1,2). Treatment with STN as monotherapy or adjunct to other immunosuppressive agents prolonged cardiac and renal allograft survival in rats and non-human primates (3–5). Both preclinical safety and tolerability studies did not reveal major adverse effects. STN oral dosing regimen was well tolerated and highly effective in patients with psoriasis (6). Currently, there are several ongoing Phase II randomized multi-center clinical trials analyzing effects of STN in renal and liver transplant patients (7,8). Based on the efficacy data from autoimmune disease (9), allotransplant preclinical models (3–5), and the safety profiles from Phase I clinical studies, STN, the first oral PKC inhibitor, represents a promising new immunosuppressive agent with a distinct mechanism of action that differs from currently approved immunosuppressive agents in organ transplantation.
Ischemia/reperfusion injury (IRI), an exogenous Ag-independent component of the organ “harvesting” insult, remains a significant clinical problem causing early as well as late transplant failure (10). Despite improved preservation and surgical techniques, IRI often leads to primary graft non-function, predisposes to chronic rejection, and contributes to the acute donor organ shortage. We have long been interested in dissecting mechanisms of IR-induced hepatocellular damage in liver IRI models. Although triggered by activation of IRF-3-dependent, MyD88-independent pathway downstream of TLR4 (11), recent data suggest an important contribution of adaptive T cell mechanism in liver IRI (12). Having shown the resistance of CD4 T cell-deficient mice to IRI, we have provided evidence that alloreactive CD4 T cells are capable of enhancing tissue inflammation/injury via CD154-dependent mechanism (13). In agreement with the emerging role of early T cell activation, we have documented the requirement for T cell-dependent TIM-1 pathway in the mechanism of liver IRI (14),
This study was designed to evaluate the potential benefit of STN against liver IRI in a clinically relevant rat model of extended (30h) hepatic cold ischemia followed by orthotopic liver transplantation (OLT). To focus on putative STN-mediated cytoprotection due to depressed T cell activation after PKC inhibition, and to exclude Ag-driven “rejection” response, these experiments were performed in syngeneic OLT recipients.
Materials and Methods
Animals
Male Sprague-Dawley (SD) rats (230–250g; Harlan Sprague-Dawley, Inc., San Diego, CA) were used throughout. Animals were housed in UCLA animal facility under specific pathogen-free conditions, and received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985).
IRI/OLT model and STN treatment
Livers from SD rats were stored at 4C in UW solution for 30h, and then transplanted to SD rats with revascularization (15). STN (30mg/kg b.i.d. via oral gavage) was used (3) in two treatment protocols. In Gr. I (n=10), liver STN was given to liver donors (90min prior to organ harvest) and OLT recipients (90min prior to the transplant, and for three days post-OLT). In Gr. II (n=6), STN was administered to OLT recipients only (according to Gr. I protocol). Gr. III controls were treated with PBS (n=10). OLT survival was assessed at day 14. Separate cohorts in Gr. I (n=3–4/gr) were sacrificed at 6h and 24h; OLT and peripheral blood samples were collected for analyses.
Hepatocellular function
Serum alanine transaminase (sALT) levels were measured in blood samples with an autoanalyzer (IDEXX Laboratories, Sacramento, CA).
OLT histology and immunohistochemistry
OLT samples (5-μm) were stained with hematoxylin and eosin (H&E). Histological severity of IRI in was graded blindly using Suzuki’s classification (15) in which sinusoidal congestion, hepatocyte necrosis and ballooning degeneration are graded from 0 to 4. No necrosis, congestion/centrilobular ballooning is given a score of 0, while severe congestion and >60% lobular necrosis is given a value of 4.
For immunohiostochemical staining, primary mAb against rat CD3 (G4.18; BD Pharmingen), or rat CD68 (ED1; Millipore, Temecula, CA) were used on OLT sections. The secondary, biotinylated goat anti-mouse IgG (Vector, Burlingame, CA) was incubated with immunoperoxidase (ABC Kit, Vector). Positive cells were counted blindly in 10 HPF/section (×400).
Evaluation of hepatocyte proliferation
OLT recipients were treated with 5-Bromo-2′-deoxyuridine (100mg/kg b.w. i.p.; Sigma-Aldrich Corp., St. Louis, MO) 1h prior to sacrifice. Liver cell proliferation was assessed using monoclonal anti-BrdU Ab (Sigma). We counted the number of BrdU-positive nuclei per 1000 hepatocytes to calculate the BrdU labeling index.
Myeloperoxidase assay
The presence of myeloperoxidase (MPO) was used as an index of neutrophil accumulation in the liver (14). The change in absorbance was measured spectrophotometrically at 655nm (Bio-tek Instruments, Winooski, VT). One unit of MPO activity (U/g) was defined as the quantity of enzyme degrading 1μmol peroxide per minute at 25°C per gram of tissue.
Quantitative RT-PCR
Quantitative RT-PCR was performed using DNA Engine with Chromo 4 Detector (MJ Research, Waltham, MA) (14). Primer sequences for the amplification of IL-2, IFN-γ, CD25, TNF-α, IL-1β, CXCL-10, MCP-1 and β-actin are shown (Supplemental Table). Target gene expressions were calculated by their ratios to the housekeeping β-actin gene.
Detection of apoptosis
Apoptosis in OLT paraffin sections was detected by TUNEL method (FragEL DNA Fragmentation Detection kit; Calbiochem, Gibbstown, NJ) (14). Negative control was prepared through omission of terminal transferase. Positive controls were generated by treatment with DNase. TUNEL-positive cells were counted in 10 HPF/section (×400).
Western blots
Western blot analysis was performed using cell extracts/OLT proteins (30μg/sample) and polyclonal rabbit anti-rat cleaved caspase-3, ERK1/2, Bcl-2, Bcl-xL, phospho-PKCθ, phospho-IκBα (Cell Signaling Technology, Danvers, MA), NF-κB and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) (14). Relative quantities of protein were determined by densitometer and expressed in absorbance units (AU).
Cell isolations and in vitro cultures/co-cultures
Rat T spleen cells and bone marrow derived-macrophages were isolated and cultured (14). Spleen T cells were incubated for 24–72h with ConA (5μg/mL; Sigma) with or without STN (10nM). Macrophages were co-cultured with T cells (responder/stimulator ratios 1:5) for 24–72h with ConA (5μg/mL), supplemented with or without STN (10nM). IL-2 mRNA expression was analyzed by qRT-PCR. Cell-free supernatants were measured for IFN-γ and TNF-α/IL-1β levels by ELISA (eBioscience, San Diego, CA). Standard curves were performed with serial two-fold dilutions; the optical density (OD) was measured with an ELISA reader.
Statistical analysis
All data are expressed as Mean±Standard Deviation (SD) Animal survival was evaluated by Kaplan-Meier method and log-rank test. Data were analyzed with Student-t test for unpaired data. The p-value of <0.05 was considered statistically significant.
Results
STN ameliorates liver IRI and improves OLT function
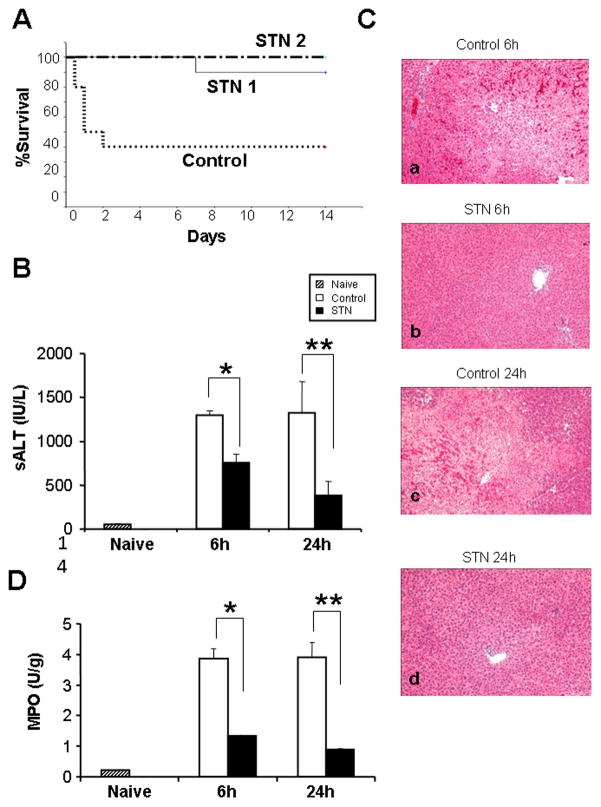
To assess the effects of STN against liver IRI, we used SD rat model of 30h hepatic cold ischemia followed by syngeneic OLT. As shown in Fig. 1A, STN treatment of liver donors and OLT recipients (Gr. I) or of OLT recipients alone (Gr. II) prolonged animal survival, as 9 out of 10 rats in Gr. I, and 6 out of 6 rats in Gr. II survived >14 days. In contrast, only 4 out of 10 control OLT recipients remained alive at day 14 (p<0.01). In parallel, we serially analyzed the hepatocellular function in OLT recipients (Fig. 1B). STN decreased sALT levels (IU/L), as compared with controls ([6h]: 758±92 vs. 1296±56; p<0.0005, [24h]: 385±156 vs. 1322±359; p<0.005). These data correlated with Suzuki’s histological grading of hepatic IRI, as STN treatment resulted in minimal sinusoidal congestion/vacuolization without necrosis in OLTs (Fig. 1C; 6h: 1.17±0.41; 24h: 0.83±0.41). In contrast, control OLTs revealed moderate-severe edema and extensive hepatocellular necrosis (Fig. 1C; 6h: 3.5±0.55; p<0.005; 24h: 3.17±0.75, p<0.005). The MPO assay, reflecting intrahepatic neutrophil activity (U/g), was markedly suppressed in STN group, compared with controls (Fig. 1D; 6h: 1.33±0.03 vs. 3.85±0.33, p<0.01; 24h: 0.89±0.03 vs. 3.90±0.5, p<0.05).
Figure 1. STN prolongs rat OLT survival, improves hepatocellular function, and ameliorates liver IRI.
(A) OLT survival. Donor SD rat livers were stored at 4 °C in UW solution for 30h prior to transplantation to syngeneic rats. Treatment with STN of liver donors (day -1) plus OLT recipients (day 0–3); or OLT recipients only (day 0–3), prolonged animal survival to 90% (9/10) and 100% (6/6), respectively. Forty percent of PBS-treated controls (4/10) remained alive at day 14 post-OLT (p<0.01).
(B) Hepatocellular function in OLT recipients. STN treatment decreased sALT levels, compared with controls. *p<0.0005, **p<0.005; n=3–4/group. Mean±SD are shown.
(C) Representative liver histology at 6h (a, b) and 24h (c, d) post-OLT. (a & c) control OLTs with severe liver damage and hepatocellular necrosis (Suzuki’s score: 6h = 3.5±0.55; 24h = 3.17±0.75). (b & d) – STN-treated OLTs with well-preserved tissue architecture (Suzuki’s score: 6h = 1.17±0.41; 24h = 0.83±0.41). H&E staining; magnification ×100, n=3–4/group.
(D) Neutrophil activity in OLTs. Suppressed MPO activity in STN group, compared with controls. *p<0.01, **p<0.05. n=3–4/group. Mean±SD are shown.
STN reduces T cell and macrophage traffic to OLTs
We performed immunohistochemical staining of CD3 and CD68 cells that migrated to OLTs. As shown in Fig. 2A, STN treatment decreased (p<0.0001) the number of CD3+ T cells/HPF in OLTs, compared with controls (6h: 8.6±1.71 vs. 19.6±3.5; 24h: 5.1±1.2 vs. 14.5±3.72). Similarly, the number of CD68+ macrophages/HPF in OLTs was reduced (p<0.0001) after STN treatment, compared with controls (Fig. 2B; 6 h: 3.3±1.16 vs. 9.3±2.26; 24 h: 1.9±0.57 vs. 6.8±1.75).
Figure 2. Immunohistochemical staining for CD3 and CD68 in rat OLTs.
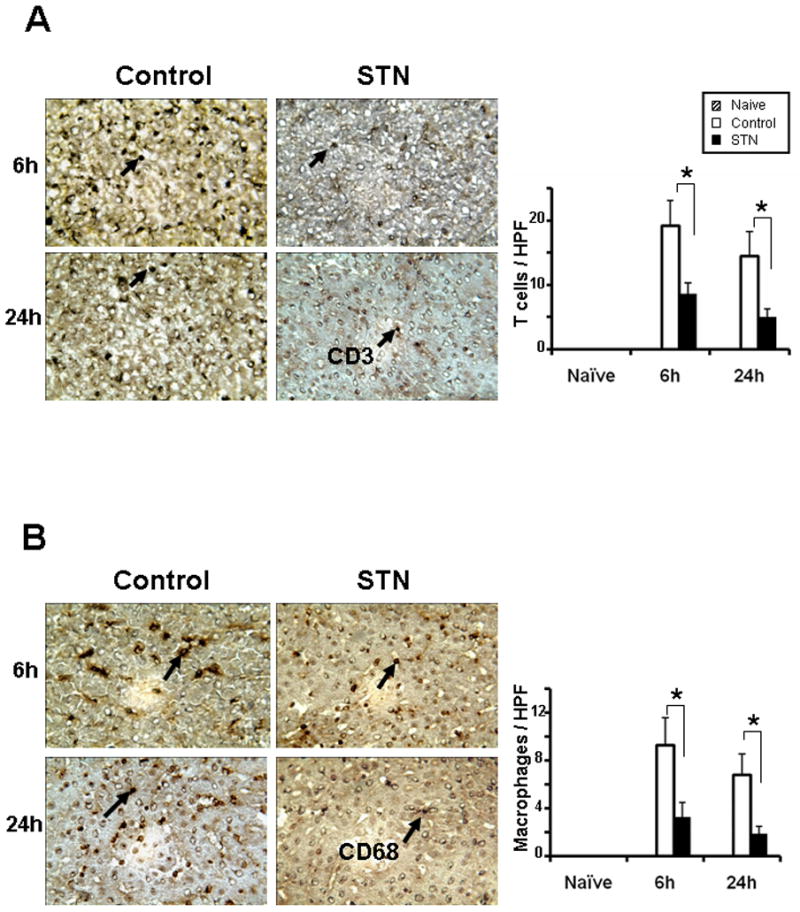
Left panels: Representative OLTs stained for CD3 (A) and CD68 (B) expression (magnification ×400).
Right panels: Cell quantification/HPF. Decreased T cell and macrophage sequestration in OLTs after STN, compared with controls. *p<0.0001, n=3–4/group.
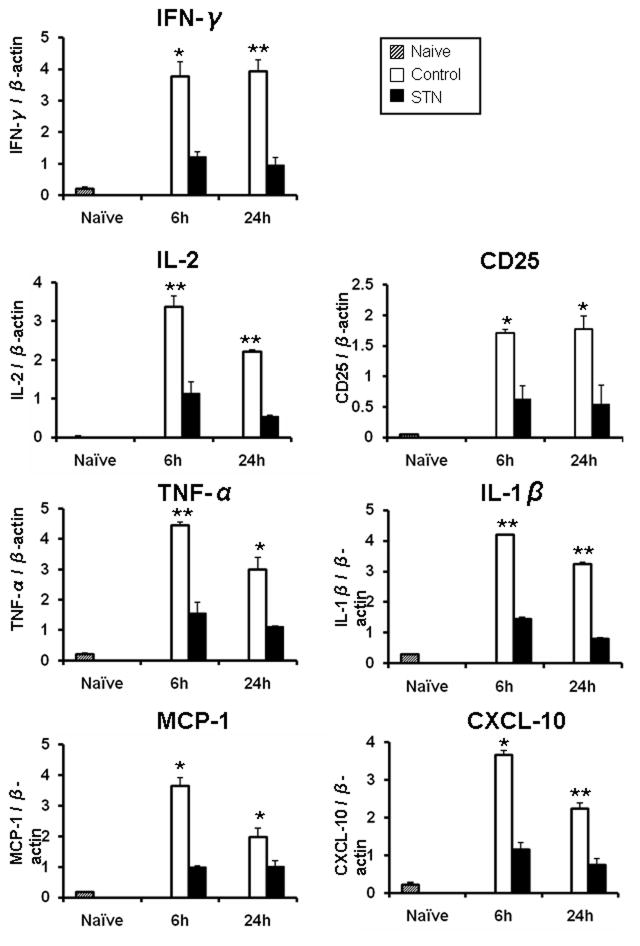
STN reduces T cell and innate cytokine/chemokine programs in OLTs
We used qRT-PCR to analyze OLT expression of T cell cytokines (IFN-γ, IL-2), IL-2 receptor α-chain (CD25), and innate inflammatory mediators (TNF-α, IL-1β, CXCL-10, MCP-1). As shown in Fig. 3, STN decreased hepatic expression of mRNA levels coding for IFN-γ (6h/24h: p<0.05/p<0.005), IL-2 (6h/24h: p<0.005/p<0.005), CD25 (6h/24h: p<0.05/p<0.05), TNF-α (6h/24h: p<0.005/p<0.05), IL-1β (6h/24h: p<0.005/p<0.005), MCP-1 (6h/24h: p<0.05/p<0.05), and CXCL-10 (6h/24h: p<0.05/p<0.005), compared with controls.
Figure 3. Quantitative RT-PCR-assisted detection of CD25; cytokines (IFN-γ, IL-2, TNF-α, IL-1β); and chemokines (CXCL-10, MCP-1) in OLTs. Data were normalized to β-actin gene expression. *p<0.05, **p<0.005, n=3–4/group. Mean±SD are shown.
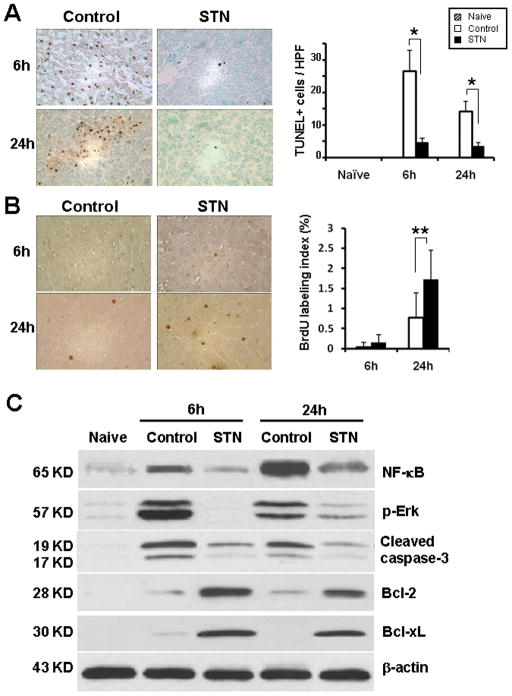
STN promotes anti-apoptotic function and depresses NF-κB/ERK signaling in OLTs
To determine whether STN affected apoptosis pathway, we performed TUNEL staining in OLTs. The number of TUNEL+ cells/HPF decreased (p<0.0001) in STN-treated OLTs, compared with controls (Fig. 4A; 6h: 4.8±1.14 vs. 26.6±6.42; 24h: 3.5±1.08 vs. 14.1±3.11). We assessed the expression of anti-apoptotic and pro-apoptotic gene products by Western blots. The relative expression levels in absorbance units (AU) were analyzed by densitometry. Figure 4C shows that although STN depressed expression of cleaved caspase-3 (6h: 0.2–0.5 vs. 1.7–1.9 in controls; 24h: 0.1–0.2 vs. 1.2–1.4 in controls), it enhanced Bcl-2/Bcl-xl (6h: 2.4–2.6 and 2.2–2.4 vs. 0.05–0.2; 24h: 1.5–1.7 and 1.8–2.0 vs. 0.05–0.3). Moreover, unlike in controls, the expression of NF-κB/ERK in OLTs decreased markedly after STN (6h: 0.2–0.3 and 0.01–0.05 vs. 1.4–1.6 and 3.2–3.4 in controls; 24h: 0.9–1.1 and 0.1–0.3 vs. 3.6–3.8 and 1.6–1.8 in controls).
Figure 4. STN ameliorates apoptosis, depresses NF-κB/ERK signaling and promotes anti-apoptotic function.
(A) TUNEL-assisted detection of apoptosis in OLTs. Left panel: Representative staining of apoptotic positive cells (magnification ×400). Right panel: Quantification of apoptotic cells /HPF in STN-treated vs. control OLTs. *p<0.0001, n=3–4/group.
(B) Immunohistochemical staining of liver cell proliferation using the BrdU method. Brown-stained nuclei represent actively proliferating hepatocytes. Representative of n=2/group.
(C) Western blot-assisted analysis of phospho-Erk, NF-κB, cleaved caspase-3, Bcl-2, and Bcl-xl in OLTs. β-actin was used as an internal control. Representative of n=3–4/group.
STN increases hepatocyte proliferation in OLTs
We performed BrdU staining in untreated and STN treated OLTs. As shown in Figure 4B, no differences were noted in BrdU labeling index (<0.2%) between both transplant groups at 6h post-OLT. However, by 24h, the index in STN group significantly increased as compared with control OLTs (1.73±0.72% and 0.77±0.62%, respectively; p<0.01).
STN depresses T cell activation and modulates T cell macrophage cross talk in vitro
We next investigated the effects of STN in well-controlled in vitro culture systems that mimic liver IRI (21). Addition of STN into ConA-stimulated T cells, consistently depressed Western blot-detected activation of PKCθ/IκBα, compared with controls (AU; Fig. 5A; 24h: 0.5–0.7/1.0–1.2 vs. 2.0–2.2/2.1–2.3; 48h: 1.0–1.2/1.0–1.2 vs. 2.6–2.8/2.1–2.3; 72h: 0.4–0.6/0.3–0.5 vs. 1.8–2.0/1.9–2.2). Furthermore, STN significantly reduced (p<0.005) ConA-induced mRNA levels coding for IL-2 (Fig. 5B) and the production (pg/ml) of IFN-γ by spleen T cells, compared with controls (Fig. 5C). Interestingly, in a macrophage - T cell co-culture (24–72h), STN decreased ConA-induced production (pg/ml) of TNF-α and IL-1β, the key macrophage-derived mediators of liver IRI (Fig. 5D/E). In contrast, although STN depressed TNF-α/IL-1β in spleen T-cell cultures, it did not affect macrophage cultures devoid of T cells.
Figure 5. STN depresses T cell activation in rat in vitro T cell cultures.
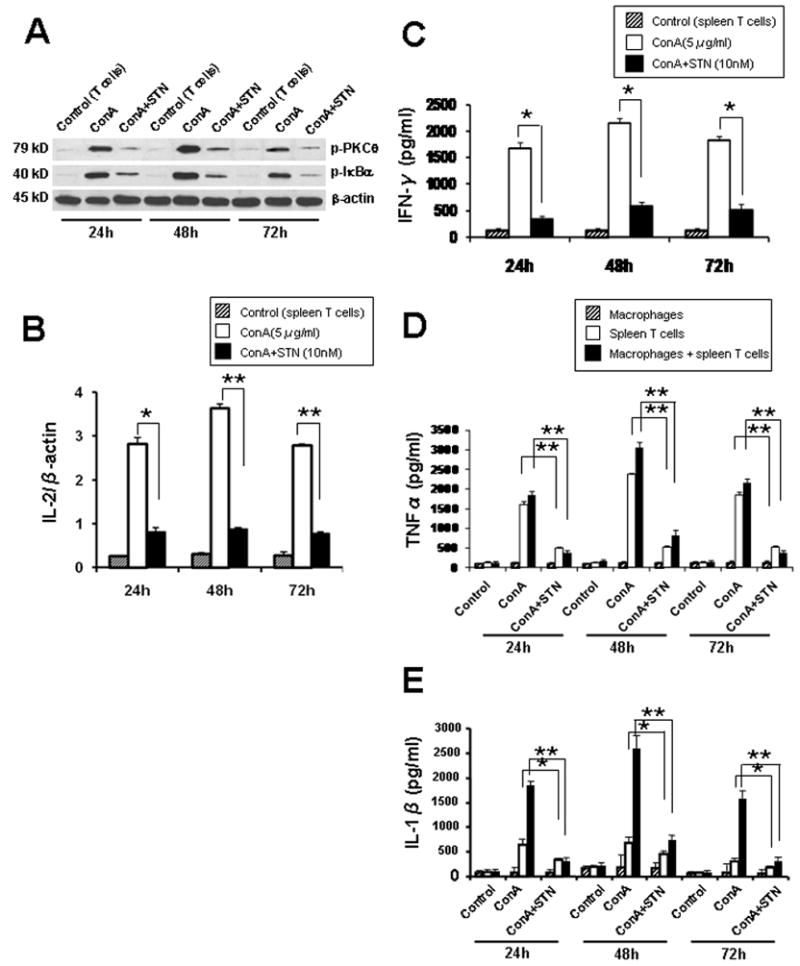
(A) Western blot-assisted phospho-PKCθ and phospho-IκBα expression in ConA-stimulated T cells. Representative of n=3.
(B) Quantitative RT-PCR-assisted detection of IL-2 in Con A-stimulated T cells. *p<0.005, **p<0.001, n=3/group.
(C) IFN-γ production in Con A-stimulated T cells. *p<0.005, n=3/group.
(D–E) TNF-α and IL-1β production in Con A-stimulated T cell - macrophages co-cultures. STN suppressed TNF-α/IL-1β levels. *p<0.05, **p<0.005, n=3/group. No effect of STN on TNF-α/IL-1β in macrophage cultures devoid of T cells.
Discussion
This is the first study to investigate the cytoprotective function and putative mechanisms by which STN, a novel targeted pan-PKC inhibitor, may reduce the impact of organ IRI in a clinically relevant rat model of extended (30h) liver cold ischemia followed by OLT. Our results show that an oral STN regimen, which effectively blocked PKC signaling, prolonged OLT survival (>90% vs. 40% in controls), decreased sALT levels, and depressed local T cell, macrophage and neutrophil OLT sequestration. Moreover, STN depressed the expression of pathogenic T cell/innate pro-inflammatory mediators, reduced hepatocellular apoptosis, and inhibited cleaved caspase-3, and NF-κB/ERK activation, yet upregulated anti-apoptotic Bcl-2/Bcl-xL in OLTs. Our in vitro studies confirmed the efficacy of STN to prevent T cell activation in ConA-stimulated spleen T cell cultures, and most interestingly, to interrupt T cell – macrophage cross talk in the co-culture system. Collectively, our findings document the previously unrecognized STN potential to mitigate organ damage due to prolonged cold ischemia in transplant recipients.
The PKC family, consisting of at least ten distinct serine threonine protein kinases, plays a central role in the adaptive immunity (16). Based on the phenotype and biochemical profiles, PKCα/θ isoforms were found to be selectively expressed and to exert non-redundant functions in CD3+ T lymphocytes. PKCθ, the only isotype recruited to the immunological synapse upon T cell engagement, controls the development of Th2/Th17 cells, and by regulating NF-κB/AP-1 during TCR/CD28 co-stimulation, it is critical in the inflammation response. On the other hand, PKCα is essential for IFNγ expression and Th1 immunity. Here, we show that STN, a specific inhibitor of early T cell activation, which effectively inhibits primarily α, β, and θ PKC isoforms (1), ameliorated liver transplant damage due to cold IRI by preventing early local T cell sequestration and activation in OLTs. Our findings are in agreement with the efficacy of STN to mitigate post-transplant preservation injury in rat renal transplants (Chaykovska, et al. TTS abstract, Vancouver 2010). Some PKC isoforms (e.g., delta) regulate cardiac IRI by inducing myocardial apoptosis/oncosis during reperfusion (17). Thus, it is plausible STN may exert its protective effects by inhibiting one or more PKC isoforms of liver parenchymal cells. Moreover, based on the data from other organ IRI systems (18, 19), PKC-deficiency should be equally or even more protective as its exogenous blockade. However, an obvious technical limitation of liver transplantation in mice needs to be taken into account.
Key advantages of STN are its oral formulation, and favorable pharmacokinetics, with peak blood concentrations at 2–3h, and half-life of about 6h in humans (8). In our “proof of concept” rat study, two short-term STN regimens of donor/recipient or recipient only treatment (30mg/kg b.i.d) resulted in >90% animal survival (vs. 40% in controls). The hepatocellular function and liver histology confirmed the efficacy of STN to prevent organ damage in this stringent rat model of extended (30h) liver cold ischemia followed by syngeneic OLT.
The early phase of liver IRI at 1–6h of reperfusion in mouse or rat models is characterized by increased T lymphocyte and Kupffer cell influx/activation, whereas the later phase, at 18–24h, involves local neutrophil sequestration (10). Our findings of diminished liver CD3 T cell infiltration/activation after STN, evidenced by OLT histology and PCR-assisted detection of cytokine programs is consistent with the idea that T cell activation is required for IR-induced liver inflammation. In addition, activated Kupffer cells produce panels of pro-inflammatory TNF-α and IL-1 that promote NF-κB activation, MCP-1, and CXCL-10, thereby further enhancing local recruitment of activated T cells at the inflammation site. Thus, STN-mediated inhibition of PKC and its downstream transcription factor NF-κB confer cytoprotection in IR-induced inflammatory response.
Neutrophils are important liver IRI mediators that contribute to local inflammation, resulting in the hepatocellular damage (10). STN treatment decreased otherwise consistently increased neutrophil activation in control OLTs. The reduced MPO activity levels may be secondary to the inhibition of T cells, resulting in less inflammatory cytokines and chemokines in OLTs of the treated animals. Moreover, the known STN-mediated impairment of β2-integrin mediated adhesion (1) may also depress hepatic neutrophil sequestration. The latter, however, can be addressed in the mouse rather than rat liver IRI model.
The ultimate fate of ischemic OLTs depends on the balance between local cell apoptosis and proliferation (10). Extracellular signal-regulated protein kinases (ERKs) of the mitogen-activated protein kinase (MAPKs) superfamily, are involved in cell survival mechanism, whereas inactivation of ERK reduces tissue damage by inhibiting local apoptosis (20). Though the mechanism of ERK-mediated apoptosis remains unclear, increasing TNF-α production and caspase-3 activity may trigger ERK-mediated apoptosis (21). STN downregulated ERK phosphorylation and cleaved caspase-3, with simultaneous enhancement of anti-apoptotic Bcl-2/Bcl-xL. This data was supported by decreased TUNEL-assisted hepatocellular apoptosis in the treated OLTs, consistent with the ability of TCL1 oncoprotein to impair ERK and inhibit activation-induced cell death (AICD) in the tumor cell line (22). As a sign of early OLT healing, STN-mediated protection against liver cell apoptosis was accompanied by enhanced hepatic cell proliferation by 24 of transplantation. As our findings may reflect a reduced pro-inflammatory activation in the tissue subsequent to local T cell inhibition, further studies are needed to elucidate exact mechanisms of STN cytoprotection.
To investigate how STN treatment may affect T cell - macrophage cross talk in triggering inflammation response, we used in vitro cell culture system that mimics, to a certain extent, liver IRI (14). First, consistent with our in vivo data, we confirmed that STN blocked ConA-stimulated T lymphocyte activation, evidenced by depressed phospho-PKCθ, and IL-2/IFN-γ levels. By using rat T cell – bone marrow-derived macrophage co-culture, we then found that STN significantly decreased production of TNF-α and IL-1β, the key macrophage-derived mediators of liver IRI. No significant changes in TNF-α/IL-1β were detected in STN-treated macrophage cultures devoid of T cells. Thus, our findings provide evidence for the critical role of STN upon T cell activation, to mitigate local inflammation and subsequent macrophage activation.
In conclusion, this study complements the ongoing clinical renal and liver transplantation trials, and is the first to document the previously unrecognized efficacy of STN, a targeted PKC inhibitor, to ameliorate IR organ damage, and to promote cytoprotection in a clinically relevant model of rat extended liver cold preservation followed by transplantation.
Table: Primer sequences for the amplification of IFN-γ, IL-2, CD25, TNF-α, IL-1β, CXCL-10, MCP-1 and β-actin.
Supplementary Material
Acknowledgments
This study was funded by Novartis and the Dumont-UCLA Research Foundation. JWKW was supported in part by NIH Grants DK 062357 and DK 083408.
Abbreviations
- IRI
ischemia/reperfusion injury
- MPO
myeloperoxidase
- OLT
orthotopic liver transplantation
- sALT
serum alanine aminotransferase
- ERK
extracellular signal-regulated protein kinase
- PKC
protein kinase C
- STN
Sotraustaurin
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose, as described by the American Journal of Transplantation.
References
- 1.Evenou JP, Wagner J, Zenke G, Brinkmann V, Wagner K, Kovarik J, et al. The potent protein kinase C-selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J Pharmacol Exp Ther. 2009;330(3):792–801. doi: 10.1124/jpet.109.153205. [DOI] [PubMed] [Google Scholar]
- 2.Matz M, Naik M, Mashreghi MF, Glander P, Neumayer HH, Budde K. Evaluation of the novel protein kinase C inhibitor sotrastaurin as immunosuppressive therapy after renal transplantation. Expert Opin Drug Metab Toxicol. 2011;7(1):103–113. doi: 10.1517/17425255.2011.540238. [DOI] [PubMed] [Google Scholar]
- 3.Weckbecker G, Pally C, Beerli C, Burkhart C, Wieczorek G, Metzler B, et al. Effects of the novel protein kinase C inhibitor AEB071 (Sotrastaurin) on rat cardiac allograft survival using single agent treatment or combination therapy with cyclosporine, everolimus or FTY720. Transpl Int. 2010;23(5):543–552. doi: 10.1111/j.1432-2277.2009.01015.x. [DOI] [PubMed] [Google Scholar]
- 4.Fang YH, Joo DJ, Lim BJ, Kim JY, Kim MS, Jeong HJ, et al. AEB-071 Versus Tacrolimus Monotherapy to Prevent Acute Cardiac Allograft Rejection in the Rat: A Preliminary Report. Transplant Proc. 2010;42(3):976–979. doi: 10.1016/j.transproceed.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Wagner J, Evenou JP, Zenke G, Brinkmann V, Pally C, Bigaud M, et al. The first-in-class oral protein kinase C inhibitor AEB071 prolongs renal allograft survival in nonhuman primates and suppresses lymphocyte proliferation at safe exposures in human proof-of-concept studies [abstract] Transplant. 2006;82(S3):86. [Google Scholar]
- 6.Skvara H, Dawid M, Kleyn E, Wolff B, Meingassner JG, Knight H, et al. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest. 2008;118(9):3151–3159. doi: 10.1172/JCI35636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budde K, Sommerer C, Becker T, Asderakis A, Pietruck F, Grinyo JM, et al. Sotrastaurin, a novel small molecule inhibiting protein kinase C: first clinical results in renal-transplant recipients. Am J Transplant. 2010;10(3):571–581. doi: 10.1111/j.1600-6143.2009.02980.x. [DOI] [PubMed] [Google Scholar]
- 8.Kovarik JM, Neuhaus P, Cillo U, Weber M, Stitah S, Gatlik E, et al. Sotrastaurin single-dose pharmacokinetics in de novo liver transplant recipients. Transpl Int. 2011;24(3):276–283. doi: 10.1111/j.1432-2277.2010.01196.x. [DOI] [PubMed] [Google Scholar]
- 9.Merani S, Edgar RL, Toso C, Emamaullee J, Thiesen A, Shapiro AM. AEB-071 has minimal impact on onset of autoimmune diabetes in NOD mice. Autoimmunity. 2009;42(3):242–248. doi: 10.1080/08916930802587950. [DOI] [PubMed] [Google Scholar]
- 10.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury -a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil R, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173(12):7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 12.Shen X, Wang Y, Gao F, Ren F, Busuttil RW, Kupiec-Weglinski JW, et al. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology. 2009;50(5):1537–1546. doi: 10.1002/hep.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen X, Reng F, Gao F, Uchida Y, Busuttil RW, Kupiec-Weglinski JW, et al. Alloimmune activation enhances innate tissue inflammation/injury in a mouse model of liver ischemia/reperfusion injury. Am J Transplant. 2010;10(8):1729–1737. doi: 10.1111/j.1600-6143.2010.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida Y, Ke B, Freitas MC, Ji H, Zhao D, Benjamin ER, et al. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51(4):1363–1372. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen XD, Ke B, Zhai Y, Tsuchihashi SI, Gao F, Duarte S, et al. Diannexin, a novel annexin V homodimer, protects rat liver transplants against cold ischemia-reperfusion injury. Am J Transplant. 2007;7(11):2463–2471. doi: 10.1111/j.1600-6143.2007.01967.x. [DOI] [PubMed] [Google Scholar]
- 16.Baier G. The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol Rev. 2003;192:64–79. doi: 10.1034/j.1600-065x.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 17.Sivaraman V, Hausenloy DJ, Kolvekar S, Hayward M, Yap J, Lawrence D, et al. The divergent roles of protein kinase C epsilon and delta in simulated ischaemia-reperfusion injury in human myocardium. J Mol Cell Cardiol. 2009;46(5):758–64. doi: 10.1016/j.yjmcc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Shimohata T, Zhao H, Hoon Sung J, Sun G, Mochly-Rosen D, Steinberg GK. Suppression of PKC activation after focal cerebral ischemia contributes to the protective effect of hypothermia. Journal of Cerebral Blood Flow & Metabolism. 2007;27:1463–1475. doi: 10.1038/sj.jcbfm.9600450. [DOI] [PubMed] [Google Scholar]
- 19.Kong L, Andrassy M, Chang JS, Huang C, Asai T, Szabolcs MJ, et al. PKC-beta modulates ischemia-reperfusion injury in the heart. Am J Physiol Heart Circ Physiol. 2008;294(4):H1862–70. doi: 10.1152/ajpheart.01346.2007. [DOI] [PubMed] [Google Scholar]
- 20.Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS, Jung JS, et al. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol. 2005;25(5):374–382. doi: 10.1002/jat.1081. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275(50):39435–39443. doi: 10.1074/jbc.M004583200. [DOI] [PubMed] [Google Scholar]
- 22.Despouy G, Joiner M, Le Toriellec E, Weil R, Stern MH. The TCL1 oncoprotein inhibits activation-induced cell death by impairing PKCtheta and ERK pathways. Blood. 2007;110(13):4406–4416. doi: 10.1182/blood-2006-11-059501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.