Abstract
Sphingomyelin synthase 1 (SMS1) catalyzes the conversion of ceramide to sphingomyelin. Here, we found that SMS1 null mice showed lipodystrophic phenotype. Mutant mice showed up-regulation of plasma triglyceride concentrations accompanied by reduction of white adipose tissue (WAT) as they aged. Lipoprotein lipase (LPL) activity was severely reduced in mutant mice. In vivo analysis indicated that fatty acid uptake in WAT but not in liver decreased in SMS1 null compared to wild-type mice. In vitro analysis using cultured cell revealed that SMS1 depletion reduced fatty acid uptake. Proteins extracted from WAT of mutant mice were severely modified by oxidative stress, and up-regulation of mRNAs related to apoptosis, redox adjustment, mitochondrial stress response and mitochondrial biogenesis was observed. ATP content of WAT was reduced in SMS1 null mice. Blue native gel analysis indicated that accumulation of mitochondrial respiratory chain complexes was reduced. These results suggest that WAT of SMS1 null mice is severely damaged by oxidative stress and barely functional. Indeed, mutant mice treated with the anti-oxidant N-acetyl cysteine (NAC) showed partial recovery of lipodystrophic phenotypes together with normalized plasma triglyceride concentrations. Altogether, our data suggest that SMS1 is crucial to control oxidative stress in order to maintain WAT function.
Introduction
Sphingolipids play vital roles in stabilizing membrane structure and in cell-cell recognition [1]–[5]. Some sphingolipid intermediates also act as signaling molecules in angiogenesis, cell growth, differentiation, and apoptosis [6]–[11]. One intermediate, ceramide, is a key metabolite in both anabolic and catabolic pathways, where it mediates cell differentiation, stress responses and apoptosis [6], [12], [13]. Although perturbed ceramide homeostasis reportedly accompanies many diseases [14], [15], the crucial role of ceramide in pathological states has not been fully characterized.
The initial step of sphingolipid synthesis is formation of ceramide from serine and fatty acyl-CoA in the endoplasmic reticulum (ER) [16], [17]. Synthesized ceramide is then transported to the Golgi complex by the ceramide transfer protein CERT [18], [19], where ceramide is converted to sphingomyelin by sphingomyelin synthase 1 (SMS1) [20]. Sphingomyelin is further transferred to the plasma membrane by exocytic vesicles and converted back to ceramide by sphingomyelin synthase 2 (SMS2) [17]. Thus, SMS1 has a central role in controlling sphingolipid homeostasis.
Recent genetic analysis of ceramide trafficking reveals that ceramide functions in numerous essential activities. CERT mutant mice exhibit embryonic lethality due to mitochondrial degeneration [21]. CERT null flies also exhibit a higher oxidative stress response and a shortened lifespan [22]. SMS2 mutant mice exhibit an attenuated inflammatory response in macrophages [23], decreased atherosclerosis [24] and resistance to high fat diet-induced obesity [25]. Analyses of SMS1 activity undertaken in cultured cells indicate that SMS1 has an important function in lymphoid cell proliferation [20]. Membrane sphingomyelin levels regulated by SMS1 and SMS2 activity are reportedly important for Fas translocation into lipid rafts, which promotes Fas-mediated apoptosis [26]. SMS1 suppression results in enhanced ceramide production and apoptosis after photodamage [27]. To investigate SMS1 function in vivo, we recently generated SMS1 knockout (SMS1-KO) mice and found that SMS1 is required to regulate generation of reactive oxygen species (ROS) and for normal mitochondrial function and insulin secretion in pancreatic β-cells [28]. In addition, SMS1-KO mice exhibited loss of epididymal white adipose tissue (WAT) mass [28]. However, the pathological consequences of that loss were not characterized.
Here, we analyzed the pathogenesis of lipodystrophic phenotypes observed in SMS1-KO mice. We report that mutant mice exhibit systemic loss of fat tissue mass. Epididymal WAT (epiWAT) mass was reduced in an age-dependent manner, accompanied by a reduction in adipose cell size. Plasma triglyceride concentrations in mutant mice increased and lipoprotein lipase (LPL) activity and fatty acid uptake activity were reduced in mutant WAT. In vitro analysis using cultured cells also showed reduction of fatty acid uptake by SMS1 depletion. Immunoblot analysis indicated that SMS1-KO WAT proteins were significantly modified by oxidative stress. Mutant mouse WAT showed up-regulation of mRNAs related to apoptosis, redox adjustment, mitochondrial stress response, and mitochondrial biogenesis. Reduced accumulation of mitochondrial respiratory chain complexes and lower ATP content were observed in WAT of SMS1 null mice. Treatment of mutant mice with the anti-oxidant N-acetyl cysteine (NAC) partially rescued these phenotypes and normalized plasma triglyceride concentrations. These data suggest that SMS1 controls oxidative stress and maintains WAT function.
Materials and Methods
Materials and Reagents
All reagents were purchased from Sigma-Aldrich (St. Louis, Missouri, USA) or Wako (Osaka, Japan), unless otherwise stated.
Animal Studies
All experiments were performed using F3 generation mice. Animals were housed in a temperature-controlled room with a 12 h-light/dark cycle. Food and water were available ad libitum unless noted. Mice were fed a normal diet (CE-2; CLEA, Japan). NAC (40 mM) was postnatally administered in drinking water. All experimental protocols were approved by the Ethics Review Committee for Animal Experimentation of Kumamoto University.
Metabolic Measurements
Mouse adiposity was examined by CT scanning (LaTheta; Aloka, Mitaka, Japan) as described elsewhere [29]. Plasma lipoproteins were analyzed using an HPLC system at Skylight Biotech (Akita, Japan), according to a previously described procedure [30].
Measurement of LPL Activity
LPL activity was measured using a Total Lipase Test kit (Progen Biotechnik, Heidelberg, Germany) as previously described [31]. Briefly, tissues were homogenized in Krebs-Ringer buffer (10 mM HEPES-KOH, pH 7.4, 120 mM NaCl, 4.7 mM KCl, 2.2 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.4 mM glucose), and heparin (Ajinomoto, Tokyo, Japan) was added to a final concentration of 100 U/ml. After 45 min-incubation at 37°C, homogenates were centrifuged, and the aqueous phase was recovered and assayed. LPL activity was normalized to total protein concentration.
In Vivo Analysis of Palmitate Uptake
Mice were deprived of food for 4 h and injected intraperitoneally with 0.02 µmol/kg [3H]palmitic acid bound to fatty acid-free BSA. After the indicated times, mice were sacrificed, and tissues were isolated and washed in PBS three times. Radioactivity in the tissues was measured by liquid scintillation counting and normalized to total protein concentration.
Palmitate Incorporation Assay
Mouse embryonic fibroblasts (MEFs) isolated from wild-type and SMS1-deficient embryos were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum at 37°C in an atmosphere of 5% CO2 and 95% air. For the assay, MEFs were pre-incubated in Krebs-Ringer buffer for 1 h, and then 0.05 µM [3H]palmitic acid bound to fatty acid-free BSA was added. After 10 min, cells were washed three times in the same buffer containing 200 µM phloretin. Cells were then lysed in water containing 0.1% SDS and the incorporated radioactive fatty acids were detected by liquid scintillation counting.
Quantitative RT-PCR
Total RNA isolated from WAT was extracted with TRIzol reagent (Invitrogen, Carlsbad, California, USA), and DNase-treated RNA was reverse transcribed with a PrimeScript RT reagent Kit (Takara Bio, Osaka, Japan), following the manufacturer's protocol. PCR products were analyzed using a Thermal Cycler Dice Real Time system (Takara Bio), and transcript abundance was normalized to that of β-actin mRNA. PCR oligonucleotides and gene abbreviations are listed in Table S1.
Sphingolipid Extraction and LC/ESI-MS Analysis
Total lipids in WAT were extracted by Bligh and Dyer's method [32] and analyzed using an LC/ESI-MS system composed of a quadrapole/time of flight hybrid mass spectrometer (Q-TOF micro) and an ACQUITY UPLC (Waters Corporation, Milford, Massachusetts, USA) as described previously [28], [33]. MS data processing was applied using Mass++ software (http://masspp.jp/) to detect each chromatogram peak with quantitative accuracy. The arbitrary units were respectively calculated by the peak area ratio of sphingomyelin, ceramide, or GM3 molecular species to each internal standard (sphingomyelin/d18:1-12:0, ceramide/d18:1-12:0, GM3/d18:1-14:0).
Immunoblot Analysis
Isolated WAT or liver was homogenized in PBS containing 1% Triton X-100 supplemented with protease inhibitors. After centrifugation at 10,000× g for 5 min, the aqueous phase was recovered for the following immunoblot analysis. Total proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and analyzed using ECL Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, England) as described previously [34]. Immunoblotting was performed with anti-Hsc70 antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA) or anti-4-hydroxy-2-nonenal (4-HNE) antibody (R&D Systems, Minneapolis, Minnesota, USA). When protein carbonylation was detected, total protein was separated by SDS-PAGE and transferred to PVDF membrane. The membrane was treated with 100% methanol, and then treated with TBS buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl) containing 20% methanol. After equilibration in 2 M HCl, the membrane was incubated with 2,4-dinitrophenylhydrazone (DNPH) solution. After washing 5 times in 2 M HCl, the membrane was equilibrated in TBS buffer. The membrane was subjected to immunoblot analysis with anti-2,4-dinitrophenyl (DNP) antibody (SHIMA Laboratories, Tokyo, Japan).
Immunohistochemical Analysis
To stain carbonylated proteins, WAT isolated from mice was fixed in a solution of 60% methanol/30% chloroform/10% acetic acid and embedded in paraffin. Specimens were randomly cut into sections. Sections were deparaffinized through 3 changes of xylene and then rehydrated through a series of graded ethanols (100%, 100%, 100%, 90%, 80%, 70%). After washing in 0.6 M HCl, sections were incubated with DNPH solution for 30 min, followed by washing in 0.6 M HCl. Sections were further washed through a series of graded alcohols (80% ethanol, 100% ethanol, 50% ethanol containing 50% ethyl acetate, 80% ethanol) and then equilibrated in water. After quenching in 1% H2O2, sections were treated with 10% normal goat serum for blocking, followed by incubation with anti-DNP antibody and secondary antibodies conjugated with horseradish peroxidase. Protein carbonylation was detected by 3,3′-diaminobenzidine (DAB) staining.
Measurement of Caspase-3 Activity
Caspase-3 activity was measured by using caspase-3 assay kit (BioVision, Milpitas, California, USA). The assay was performed according to the manufacturer's instructions. In brief, the chromophore p-nitroaniline (pNA) after cleavage from the substrate DEVD-pNA was spectrophotometrically detected.
Isolation of Mitochondria from WAT and Blue Native PAGE (BN-PAGE) Analysis
Mitochondria were isolated by the method as described previously [28], [34]. WAT were isolated and homogenized in mitochondria isolation buffer (3 mM HEPES-KOH, pH 7.5, 210 mM mannitol, 70 mM sucrose, 0.2 mM EGTA). The homogenate was centrifuged at 500× g to remove lipid, nuclei and unbroken cells. After removing debris through nylon filter (100 µm mesh, Clontech), the recovered aqueous phase was further centrifuged at 10,000× g to obtain mitochondrial pellet. The pellet was suspended in the extraction buffer containing 2% digitonin. BN-PAGE analysis was performed by the method as shown previously [35].
When immunoblot analysis was performed, proteins in the gel were transferred to PVDF membrane. After 100% methanol treatment, the membrane was washed with water, and then subjected to immunoblot analysis with anti-NDUFA9 antibody (Invitrogen), anti-ATP5A1 antibody (Invitrogen), or anti-Tom40 antibody [34].
Measurement of Mitochondrial Respiratory Chain Activity
The gel slices obtained by BN-PAGE was used to detect mitochondrial respiratory chain activity as described [36]. Complex IV activity (cytochrome oxidase activity) was examined by incubating gel slices in the reaction buffer IV (50 mM sodium phosphate buffer (pH 7.4), 1 mg/ml DAB, 24 units/ml catalase, 1 mg/ml cytochrome c, 0.22 M sucrose). Color development was preserved in fixing buffer (50% methanol, 10% acetic acid), and the gel was stored in 10% acetic acid. Complex V activity (ATPase activity) was assessed by incubating gel slices in the reaction buffer V (35 mM Tris, 270 mM glycine, 14 mM MgSO4, 0.2% Pb(NO3)2, and 8 mM ATP). After overnight incubation, the color-developed gel was washed and stored in water. The remaining gel slice was stained with Coomassie Brilliant blue (CBB).
Statistical Analysis
Data were analyzed using Student's t-test and reported as means ± SEM, unless otherwise stated.
Results
SMS1-KO Mice Exhibit a Lipodystrophic Phenotype
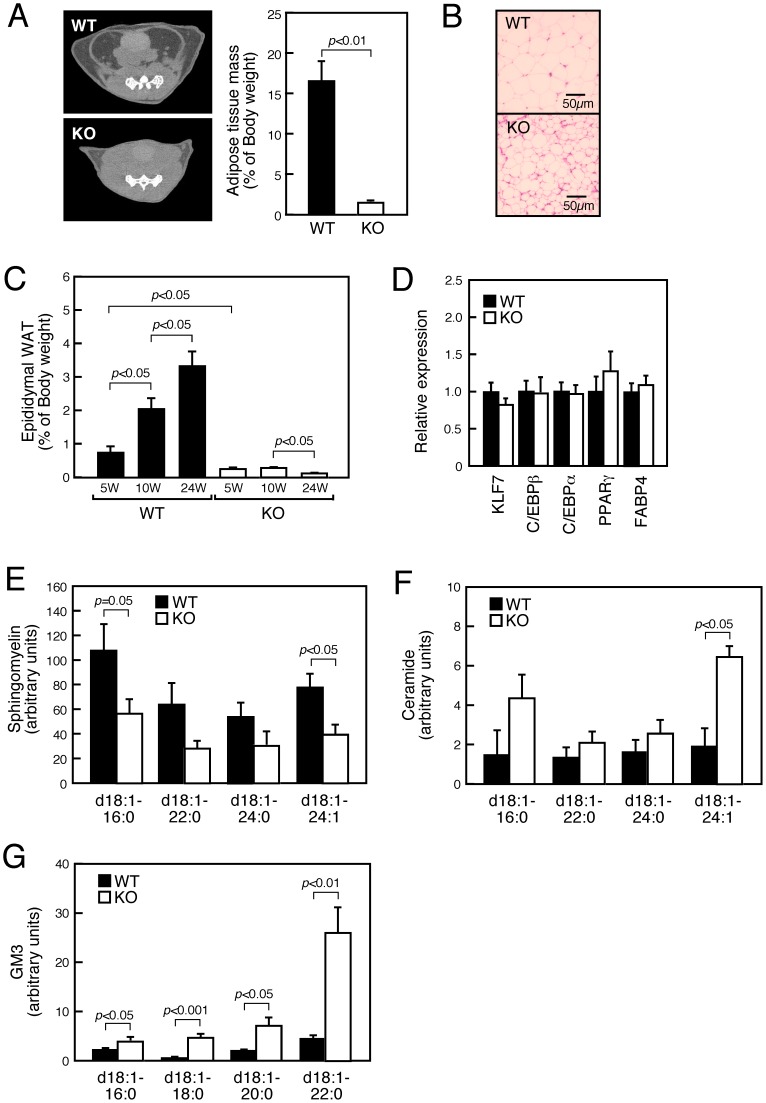
Previously, we reported that SMS1-KO mice appeared lean and showed decreased epiWAT mass [28]. Here we performed CT image analysis and observed that adipose tissue mass in SMS1-KO mice was severely reduced relative to that of wild-type mice (Fig. 1A). Histochemical analysis of epiWAT revealed that the size of adipose cells of SMS1-KO mice was severely reduced relative to controls, suggestive of a lipodystrophic phenotype (Fig. 1B). Indeed, the weight of SMS1-KO epiWAT decreased with advancing age (Fig. 1C).
Figure 1. SMS1-KO mice exhibit a lipodystrophic phenotype.
(A) Representative CT images of the lower abdomen (Left panel) of 20-week-old WT (n = 6) and KO (n = 4) mice and analysis of body fat mass (Right panel). (B) Sections of epiWAT from 20-week-old mice were stained with hematoxylin-eosin. (C) Age-dependent alteration of epiWAT size was observed in 5- (WT, n = 5; KO, n = 5), 10- (WT, n = 10; KO, n = 10) and 24-week-old (WT, n = 5; KO, n = 4) mice. (D) mRNA expression levels of genes encoding preadipocyte and mature adipocyte markers in WAT. Individual measurements were normalized to β–actin expression, and the wild-type group average was set to 1. n = 8 samples per group. (E–G) Levels of sphingomyelin (E), ceramide (F) and GM3 (G) species in isolated WAT of 10-week-old mice was analyzed by LC/ESI-MS. n = 6 samples per group.
Because insulin is a potent adipogenic hormone [37], [38], and based on our previous finding that insulin induction by glucose is decreased in SMS1-KO mice [28], we initially asked whether adipocyte differentiation in SMS1-KO WAT was perturbed. However, we did not observe overt changes in mRNA expression of the preadipocyte markers Krüppel-like factor 7 (KLF7) and C/EBPβ or of markers of mature adipocytes (C/EBPα, PPARγ and FABP4) [39], [40] (Fig. 1D, Table S1). These observations suggest that adipocyte differentiation proceeds normally in SMS1-KO mice.
Since SMS1 catalyzes ceramide conversion to sphingomyelin, an alternative possibility is that sphingolipid homeostasis is altered in WAT of SMS1-KO mice. To test this hypothesis we examined sphingolipid composition of SMS1-KO WAT (Fig. 1E–G) by LC/ESI-MS analysis and found that levels of sphingomyelin species were reduced, while levels of ceramide and monosialodihexosylganglioside (GM3) species increased. These findings support the idea that sphingolipid metabolism is disturbed in SMS1-KO WAT.
LPL Activity and Fatty Acid Uptake Are Reduced in WAT of SMS1-KO Mice
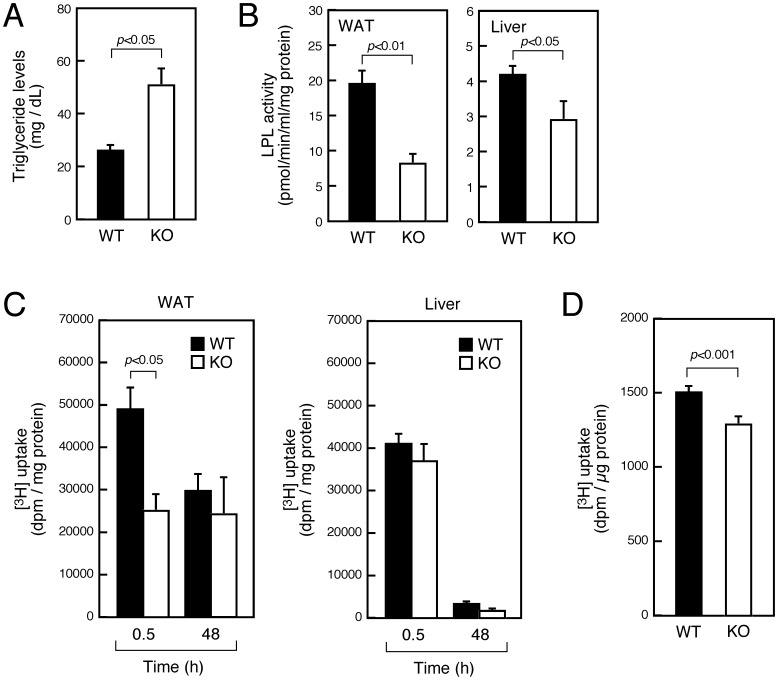
We next assessed triglyceride levels in blood plasma. Those concentrations were much higher in SMS1-KO compared to wild-type mice (Fig. 2A), suggesting that metabolic functions of liver and/or WAT are perturbed.
Figure 2. LPL activity and fatty acid uptake are reduced in WAT of SMS1-KO mice.
(A) Shown are triglyceride levels in blood plasma of 14-week-old mice (WT, n = 3; KO, n = 3). (B) LPL activity was assayed in WAT and liver of 20-week-old mice (WT, n = 6; KO, n = 5). (C) [3H]palmitic acid was intraperitoneally injected to 4 h-starved mice. After 0.5 h (WT, n = 8; KO, n = 5) or 48 h (WT, n = 7; KO, n = 3), tissues were isolated and radioactivity in tissues was measured. (D) Assessment of [3H]palmitic acid uptake into MEFs isolated from wild-type (WT, n = 30) and SMS1-deficient (KO, n = 30) embryos.
LPL plays a critical role in triglyceride homeostasis by catalyzing hydrolysis of triglyceride from plasma lipoproteins [31]. LPL activity in both WAT and liver (Fig. 2B) was significantly reduced in SMS1-KO relative to wild-type mice, although the reduction was more severe in WAT than in liver. We next determined whether fatty acid uptake is altered in WAT and liver of SMS1-KO mice in vivo by assessing radioactivity levels in these tissues after intraperitoneal injection of radiolabeled palmitic acid. After 30 min, wild-type mice showed high radioactivity in both WAT and liver. By contrast, SMS1-KO mice showed relatively lower radioactivity in WAT after 30 min, although radioactivity in liver was comparable to that in wild-type mice. By 48 h, radioactivity in wild-type and SMS1-KO liver was almost completely absent, whereas a larger portion of radioactivity remained in WAT of both genotypes (Fig. 2C). We also undertook assays to evaluate fatty acid uptake in vitro and observed a slight but significant reduction of palmitate uptake in SMS1-deficient MEFs (Fig. 2D). Overall, these results suggest that incorporation of palmitate into WAT rather than liver of SMS1-KO mice is disturbed due to deficient fatty acid uptake function.
SMS1-KO WAT Is Severely Damaged by Oxidative Stress
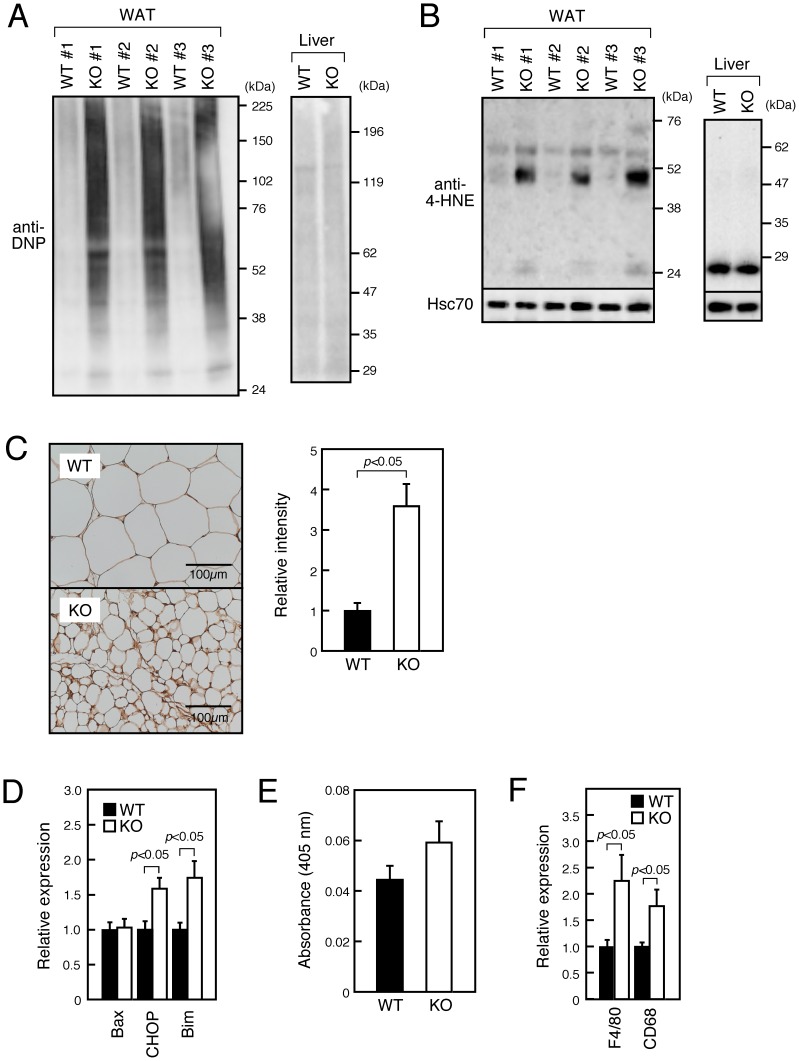
Previously we observed that islet cells in SMS1-KO mice were chronically damaged by oxidative stress [28]. Therefore, we asked whether oxidative stress also damaged WAT of mutant mice. Immunoblot analysis using anti-2,4-dinitrophenyl (DNP) antibody, which recognizes carbonylated proteins produced by oxidative modification [41], indicated that levels of ROS-modified proteins in SMS1-KO WAT were significantly greater than those seen in wild-type tissue (Fig. 3A). On the other hand, carbonylated proteins were not increased in the liver of SMS1-KO mice. Immunoblot analysis using the anti-4-hydroxy-2-nonenal (4-HNE) antibody, which recognizes ROS-modified proteins [42], also indicated that ROS-modified proteins were significantly increased in SMS1-KO WAT but not in the liver (Fig. 3B). Immunohistochemical analysis with an anti-DNP antibody confirmed that proteins in SMS1-KO WAT were highly modified by ROS (Fig. 3C). Interestingly, mRNA analysis showed that expression of CHOP and Bim, mRNAs encoding apoptotic factors, was increased in SMS1-KO WAT (Fig. 3D, Table S1), although we failed to observe significant increment of caspase-3 (a downstream apoptotic factor) activity (Fig. 3E). We also observed increased expression of macrophage markers (F4/80 and CD68) in SMS1-KO WAT, probably suggesting enhanced invasion of macrophage (Fig. 3F).
Figure 3. SMS1-KO WAT is severely damaged by oxidative stress.
(A) Proteins were extracted from epiWAT and liver of 10-week-old wild-type (WT, n = 3) or SMS1-KO (KO, n = 3) mice and carbonylated proteins were detected by immunoblot analysis using anti-DNP antibody. (B) Samples in (A) were subjected to immunoblot analysis using anti-4-HNE antibody to detect protein modification by ROS. Hsc70 staining served as a standard. (C) Carbonylated proteins in epiWAT of 20-week-old mice were immunohistochemically detected using an anti-DNP antibody (Left panel), and DNP signal intensity in the plasma membrane area was quantified (WT, n = 3; KO, n = 3) (Right panel). (D) mRNAs were extracted from WAT of 10-week-old mice. mRNA expression levels of genes encoding apoptotic factors were assessed by quantitative RT-PCR and normalized to β–actin expression. The wild-type group average was set to 1. n = 6–9 samples per group. (E) Activity of caspase-3 was spectrophotometrically assessed. n = 6 samples per group. (F) mRNA expression levels of genes encoding macrophage-related factors were assessed by quantitative RT-PCR. n = 10–12 samples per group.
Oxidative Stress Response, the Mitochondrial Stress Response and Mitochondrial Biogenesis Are Enhanced in SMS1-KO WAT
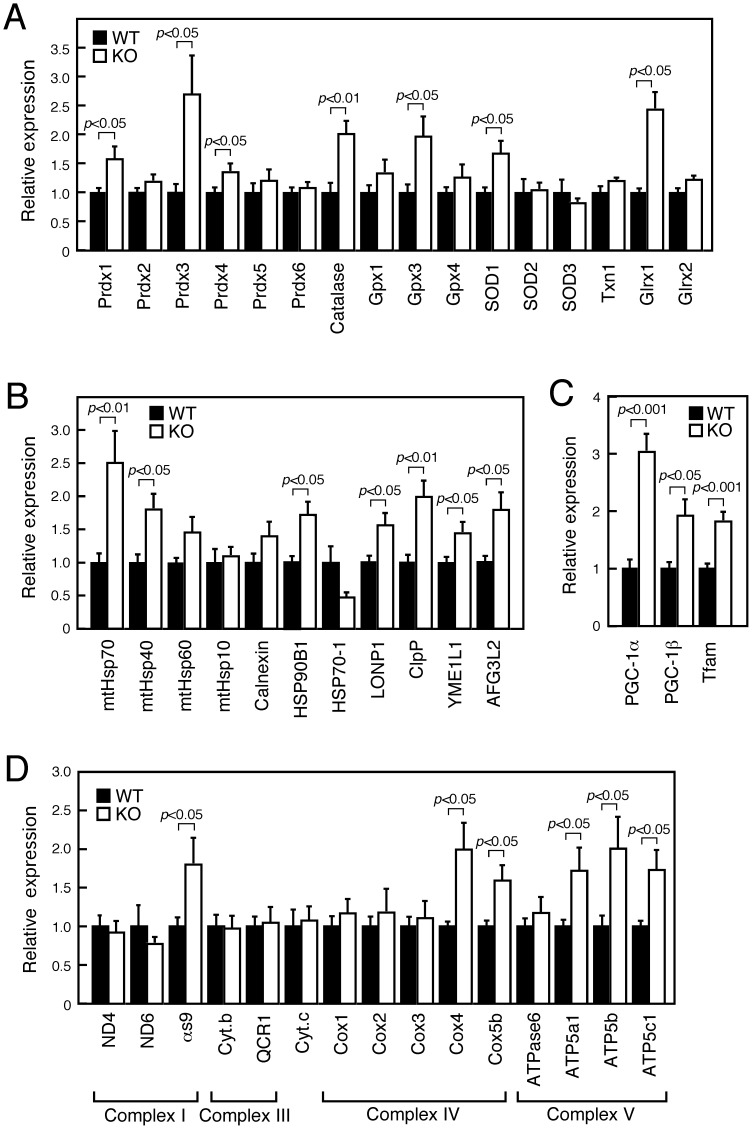
To confirm whether oxidative stress occurs in SMS1-KO WAT, we examined expression of mRNAs encoding ROS detoxification enzymes. In SMS1-KO WAT, expression of some isoforms of peroxiredoxin (Prdx), which reduces hydrogen peroxide and alkyl hydroperoxides, was increased (Fig. 4A, Table S1). Expression of catalase, which catalyzes decomposition of hydrogen peroxide, also increased. Other ROS detoxification enzymes, such as glutathione peroxidase (Gpx), superoxide dismutase (SOD) and glutaredoxin (Glrx), also showed increased expression. These data indicate that the oxidative stress response is enhanced in SMS1-KO WAT.
Figure 4. The oxidative stress response, mitochondrial stress response and mitochondrial biogenesis are activated in SMS1-KO WAT.
(A–D) WAT of 10-week-old mice was analyzed for mRNA expression levels of genes encoding ROS detoxification enzymes (A), mitochondrial stress-related factors (B), mitochondrial biogenesis-related factors (C) and mitochondrial respiration complex factors (D). Values were normalized to β–actin expression. The wild-type group average was set to 1. n = 6–8 samples per group.
Next we examined expression of mRNAs encoding mitochondrial stress response-related factors. Most of these, including mitochondrial chaperones (mtHsp70, mtHsp40) and mitochondrial proteases (LONP1, ClpP, YME1L1, AFG3L2), were up-regulated in SMS1-KO WAT (Fig. 4B, Table S1). On the other hand, mRNA expression of calnexin, an ER chaperone, or HSP70-1, a cytosolic chaperone, was not affected. We also observed increases in mRNAs encoding factors relevant to mitochondrial biogenesis, such as PPARγ coactivatorsPGC-1α and PGC-1β) and mitochondrial transcription factor A (Tfam) in SMS1-KO WAT relative to wild-type tissues (Fig. 4C, Table S1). To confirm these findings, we assessed expression of components of the mitochondrial electron transport complex and ATP synthase (Fig. 4D, Table S1). Some of the former, such as αs9 (NDUFA9) and cytochrome c oxidase subunits IV (Cox4) and Vb (Cox5b), were up-regulated in SMS1-KO WAT relative to control tissue, as were components of the latter, such as ATP synthase, H+-transporting, mitochondrial F1 complex, α subunit 1 (ATP5a1), β polypeptide (ATP5b) and γ polypeptide 1 (ATP5c1).
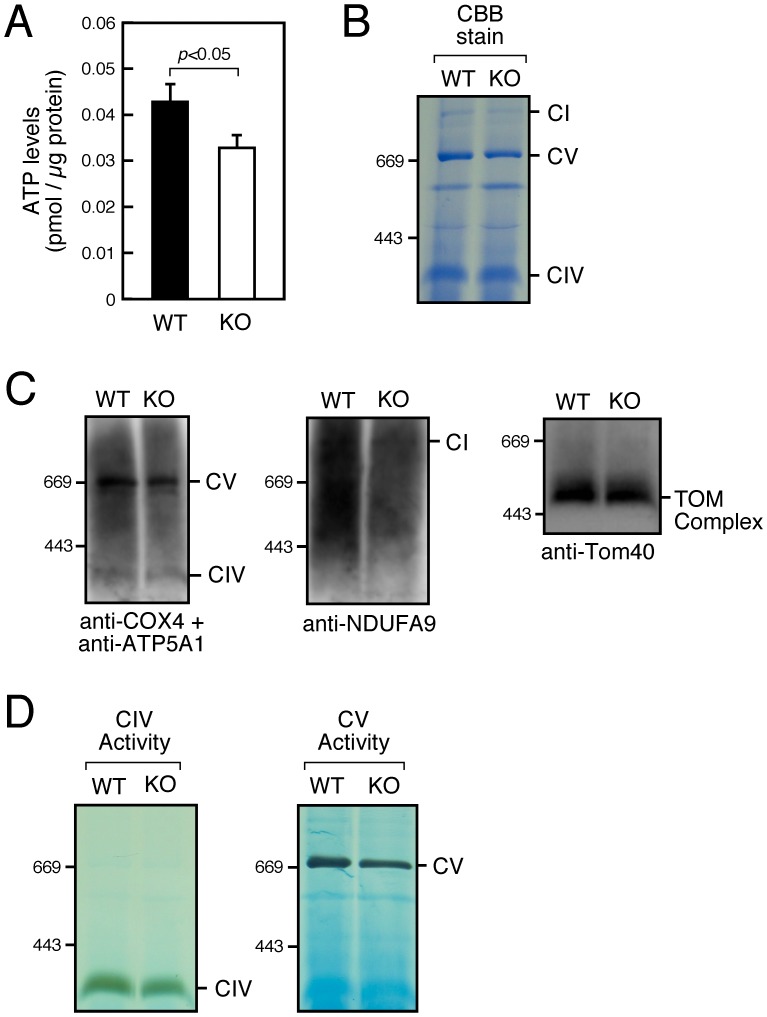
Mitochondrial Function Is Disrupted in SMS1-KO WAT
To examine mitochondrial function of SMS1-KO WAT, we first examined ATP level in WAT. We found that the ATP content of SMS1-KO WAT was slightly but significantly decreased compared to levels seen in wild-type mice (Fig. 5A). CBB-stain and immunoblot analysis of BN-PAGE indicated that the amounts of respiration complex I and V were reduced in the mitochondria of SMS1-KO WAT, whereas that of complex IV was not (Fig. 5B and 5C). The amount of translocase of the outer membrane (TOM complex) was not changed (Fig. 5C). These results suggest that accumulations of complex I and V are reduced in the mitochondria of SMS1-KO WAT, probably due to their instability. We further examined mitochondrial respiration complex activity in BN-PAGE gel (Fig. 5D). The activity of complex V was reduced in the mitochondria of SMS1-KO WAT, whereas that of complex IV was not. Together, these results suggest that the functions of respiration complexes in the mitochondria of SMS1-KO WAT are disturbed.
Figure 5. Mitochondrial function is disrupted in SMS1-KO WAT.
(A) ATP content in WAT was measured (WT, n = 10; KO, n = 10). (B and C) Formation of respiratory complexes in WAT mitochondria was analyzed by BN-PAGE, followed by Coomassie blue staining (B) and immunoblot analysis (C). The complexes I, IV and V were detected by using anti-αs9 (NDUFA9), anti-COX4 and anti-ATP5A1 antibodies, respectively. TOM complex was detected by using anti-Tom40 antibody. (D) Activity of complexes IV and V was assessed (see MATERIALS AND METHODS). CI, complex I; CIV, complex IV; CV, complex V.
Hyperlipidemia and Lipodystrophy in SMS1-KO Mice Are Rescued by Anti-oxidant Treatment
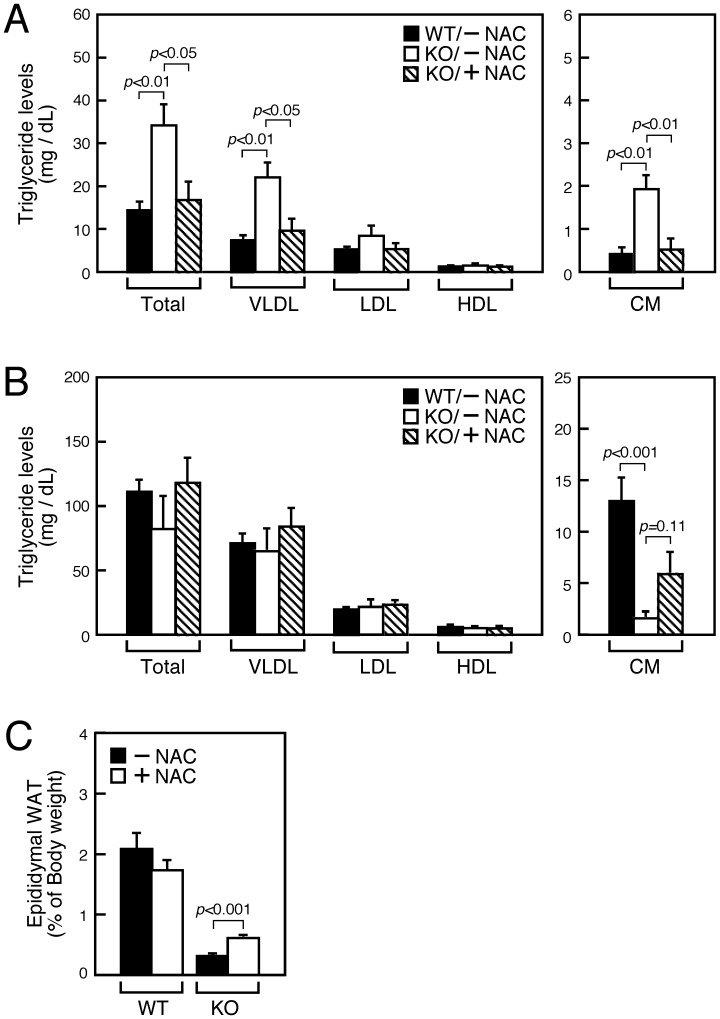
Our results support the idea that WAT cells of SMS1-KO mice are functionally damaged by increased oxidative stress. Indeed, triglyceride levels were greatly increased in blood plasma of SMS1-KO compared to wild-type mice, indicative of hyperlipidemia (Fig. 6A, also see Fig. 2A). In particular, triglyceride levels of chylomicron (CM) and very-low-density lipoprotein (VLDL), which normally function in muscle or are stored in adipocytes [43], were greatly increased in blood plasma, suggesting that lipid storage function of adipose cells is compromised in SMS1-KO mice (Fig. 6A).
Figure 6. NAC treatment antagonizes WAT deficits seen in SMS1-KO mice.
Mice supplied normal (−NAC) or NAC-containing (+NAC) drinking water were analyzed as follows. (A) Blood plasma isolated from 4 h-fasted mice (20-week-old) was subjected to lipoprotein analysis (WT/−NAC, n = 5; KO/−NAC, n = 4; KO/+NAC, n = 6). CM, chylomicron; VLDL, very-low-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein. (B) Ten-week-old mice supplied normal (−NAC) or NAC-containing (+NAC) drinking water were subjected to 30 h-fasting. Blood plasma was collected and subjected to lipoprotein analysis. n = 9–10 mice per group. (C) Measurement of epiWAT size of 10-week-old mice. n = 10 samples per group.
To further examine whether oxidative stress mediates these phenotypes, we supplied the anti-oxidant N-acetyl cysteine (NAC) in the drinking water of mutant mice and analyzed potential changes in blood plasma triglyceride levels (Fig. 6A). NAC-treated SMS1-KO mice showed robust decreases in blood triglyceride levels, almost to levels comparable to wild-type mice.
Finally, we subjected mice to fasting for 30 hours and assessed triglyceride levels in blood plasma (Fig. 6B). Those levels increased in fasting wild-type mice and in SMS1-KO mice supplied normal drinking water. However, CM levels in fasting SMS1-KO mice were significantly decreased compared to wild-type mice. By contrast, SMS1-KO mice supplied with NAC-containing drinking water exhibited partially recovered CM levels. The volume of SMS1-KO epiWAT was also partially rescued by NAC-treatment (Fig. 6C). Altogether, these results indicate that increased oxidative stress underlies a failure of lipid storage function in adipocytes of SMS1-KO mice.
Discussion
Previously, we generated SMS1-KO mice and found that they exhibit phenotypes suggestive of adipose tissue dysfunction [28]. Here, we confirmed and extended those findings. Histochemical analysis revealed that the adipose cell size was severely reduced in epiWAT of SMS1-KO mice, and epiWAT volume was reduced age-dependently, suggesting that mutant mice exhibit progressive lipodystrophy. No changes were observed in expression of factors required for adipogenesis, suggesting that adipocyte differentiation proceeds normally in WAT cells of knockout mice. However, analysis of sphingolipid composition revealed reduced levels of sphingomyelin species, while ceramide and GM3 species increased in SMS1-KO WAT. Therefore, we conclude that sphingolipid metabolism is perturbed in SMS1-KO WAT cells.
Hypertriglyceridemia is reportedly a common feature of inherited lipodystrophies [44], [45]. We observed increased triglyceride levels in blood plasma of knockout mice, while LPL activity in SMS1-KO liver and WAT was reduced. In vivo analysis confirmed that the primary defect of SMS1-KO mice is in fatty acid uptake into WAT rather than liver. The deficiency may be a common feature among many types of SMS1-KO cells, because SMS1-deficient MEF showed a slight but significant deficiency in fatty acid uptake.
We previously showed that increased ceramide species in pancreas islets of SMS1-KO mice perturbed mitochondrial function and increased ROS generation [28]. The present study confirms that WAT of mutant mice is damaged by oxidative stress promoted by increased ROS. Others have reported that increased levels of ceramide species and oxidative stress promote cell death [46]–[48]. In agreement, we observed increased expression of apoptosis-inducing factors in SMS1-KO WAT, further evidence that the function of WAT cells is severely compromised. In addition, increased expression of macrophage markers was observed in SMS1-KO WAT, showing enhanced invasion of macrophage. These observations may indicate that damaged and dead adipocytes by oxidative stress are rapidly removed by phagocytosis by invaded macrophage in SMS1-KO WAT.
It is noteworthy that expression of ROS detoxification enzymes and mitochondrial stress response-related factors increased in SMS1-KO WAT, including components of the mitochondrial respiratory complex and factors required for mitochondrial biogenesis. These observations suggest that a mitochondrial recovery mechanism previously characterized by others [49], [50] is activated in SMS1-KO WAT. Nonetheless, we observed decreased ATP production in SMS1-KO WAT. We also observed reduced accumulations of mitochondrial respiration complexes I and V in the mitochondria of SMS1-KO WAT. The reduction of complex V activity was also observed. The reduction of these complexes is likely reflective of the profound damage brought on by oxidative stress. Indeed, the mitochondrial respiratory complex and ATP synthase are reportedly highly susceptible to modification by ROS [51]–[53]. We suppose that disorder of sphingolipid homeostasis in SMS1-KO adipocytes may cause mitochondrial protein dysfunction, that lead to increased ROS production resulting in reduced ATP production. It would be possible that these damaged adipocytes have deficiency in secretion of LPL and uptake of fatty acid.
Our conclusion that SMS1 loss interferes with lipid storage function in WAT due to oxidative stress was confirmed by the observation that levels of CM and VLDL, which are normally taken up into adipocytes [43], were increased in blood plasma of SMS1-KO mice. Anti-oxidant treatment rescued these phenotypes, indicating that reduced oxidative stress improves lipid storage function in SMS1-KO adipocytes. Analysis of SMS1-KO mice under fasting conditions further confirmed these findings. Blood CM levels in SMS1-KO mice were significantly decreased, suggesting that triglycerides derived from CM in peripheral tissues are completely consumed as an energy source. Again, anti-oxidant treatment partially normalized CM levels and also increased epiWAT volume in SMS1-KO mice. Overall, these results indicate that increased oxidative stress underlies lipid storage failure in adipocytes of SMS1-KO mice.
In conclusion, we demonstrate that manipulation of sphingolipid flux in vivo and consequent ceramide accumulation in WAT cells leads to oxidative stress and defects in lipid storage function. Our approach identifies an essential role for SMS1 in adipocyte function and provides molecular insight into the role of the de novo sphingolipid biosynthetic pathway in regulating oxidative stress, observations highly relevant to metabolic disease. To date, it has also been reported that SMS1-KO mice exhibit hearing impairment [54] and T-cell dysfunction [55]. These observations would be also attributable to loss of fundamental function of the cells by disturbance of sphingolipid. Further study is necessary to clarify the common nature of these observations.
Supporting Information
Primer sequences used in quantitative RT-PCR.
(PDF)
Acknowledgments
We thank our colleagues for valuable suggestions and discussion. We also thank Mss. Rieko Shindo and Yasuko Indo for technical assistance.
Funding Statement
This work was supported by grants-in-aid 19790215 (to MY) and 23590340 (to MY), and 22116009 (to YO) from the Ministry of Education, Science, Technology, Sports and Culture of Japan, by a grant-in-aid 22659156 (to YO) from the Japan Society for the Promotion of Science, and by grants-in-aid from the Takeda Science Foundation (to MY) and Inamori Foundation (to MY). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Futerman AH, Hannun YA (2004) The complex life of simple sphingolipids. EMBO Rep 5: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, et al. (2006) Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758: 1864–1884. [DOI] [PubMed] [Google Scholar]
- 3. Simons K, Gerl MJ (2010) Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 11: 688–699. [DOI] [PubMed] [Google Scholar]
- 4. Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327: 46–50. [DOI] [PubMed] [Google Scholar]
- 5. Milhas D, Clarke CJ, Hannun YA (2010) Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett 584: 1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150. [DOI] [PubMed] [Google Scholar]
- 7. Zeidan YH, Hannun YA (2007) Translational aspects of sphingolipid metabolism. Trends Mol Med 13: 327–336. [DOI] [PubMed] [Google Scholar]
- 8. Alvarez SE, Milstien S, Spiegel S (2007) Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab 18: 300–307. [DOI] [PubMed] [Google Scholar]
- 9. van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wymann MP, Schneiter R (2008) Lipid signalling in disease. Nat Rev Mol Cell Biol 9: 162–176. [DOI] [PubMed] [Google Scholar]
- 11. Fyrst H, Saba JD (2010) An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol 6: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hannun YA, Obeid LM (2002) The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 277: 25847–25850. [DOI] [PubMed] [Google Scholar]
- 13. Mao C, Obeid LM (2008) Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta 1781: 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haughey NJ, Bandaru VV, Bae M, Mattson MP (2010) Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim Biophys Acta 1801: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park JH, Schuchman EH (2006) Acid ceramidase and human disease. Biochim Biophys Acta 1758: 2133–2138. [DOI] [PubMed] [Google Scholar]
- 16. Menaldino DS, Bushnev A, Sun A, Liotta DC, Symolon H, et al. (2003) Sphingoid bases and de novo ceramide synthesis: enzymes involved, pharmacology and mechanisms of action. Pharmacol Res 47: 373–381. [DOI] [PubMed] [Google Scholar]
- 17. Tafesse FG, Ternes P, Holthuis JC (2006) The multigenic sphingomyelin synthase family. J Biol Chem 281: 29421–29425. [DOI] [PubMed] [Google Scholar]
- 18. Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, et al. (2003) Molecular machinery for non-vesicular trafficking of ceramide. Nature 426: 803–809. [DOI] [PubMed] [Google Scholar]
- 19. Hanada K, Kumagai K, Tomishige N, Yamaji T (2009) CERT-mediated trafficking of ceramide. Biochim Biophys Acta 1791: 684–691. [DOI] [PubMed] [Google Scholar]
- 20. Yamaoka S, Miyaji M, Kitano T, Umehara H, Okazaki T (2004) Expression cloning of a human cDNA restoring sphingomyelin synthesis and cell growth in sphingomyelin synthase-defective lymphoid cells. J Biol Chem 279: 18688–18693. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Rao RP, Kosakowska-Cholody T, Masood MA, Southon E, et al. (2009) Mitochondrial degeneration and not apoptosis is the primary cause of embryonic lethality in ceramide transfer protein mutant mice. J Cell Biol 184: 143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rao RP, Yuan C, Allegood JC, Rawat SS, Edwards MB, et al. (2007) Ceramide transfer protein function is essential for normal oxidative stress response and lifespan. Proc Natl Acad Sci USA 104: 11364–11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hailemariam TK, Huan C, Liu J, Li Z, Roman C, et al. (2008) Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscl Thromb Vasc Biol 28: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 24. Liu J, Huan C, Chakraborty M, Zhang H, Lu D, et al. (2009) Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ Res 105: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitsutake S, Zama K, Yokota H, Yoshida T, Tanaka M, et al. (2011) Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J Biol Chem 286: 28544–28555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyaji M, Jin ZX, Yamaoka S, Amakawa R, Fukuhara S, et al. (2005) Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis. J Exp Med 202: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Separovic D, Semaan L, Tarca AL, Awad Maitah MY, Hanada K, et al. (2008) Suppression of sphingomyelin synthase 1 by small interference RNA is associated with enhanced ceramide production and apoptosis after photodamage. Exp Cell Res 314: 1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yano M, Watanabe K, Yamamoto T, Ikeda K, Senokuchi T, et al. (2011) Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem 286: 3992–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oike Y, Akao M, Yasunaga K, Yamauchi T, Morisada T, et al. (2005) Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med 11: 400–408. [DOI] [PubMed] [Google Scholar]
- 30. Usui S, Hara Y, Hosaki S, Okazaki M (2002) A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J Lipid Res 43: 805–814. [PubMed] [Google Scholar]
- 31. Nunes AF, Saraiva MJ, Sousa MM (2006) Transthyretin knockouts are a new mouse model for increased neuropeptide Y. FASEB J 20: 166–168. [DOI] [PubMed] [Google Scholar]
- 32. Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 33. Ikeda K, Oike Y, Shimizu T, Taguchi R (2009) Global analysis of triacylglycerols including oxidized molecular species by reverse-phase high resolution LC/ESI-QTOF MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2639–2647. [DOI] [PubMed] [Google Scholar]
- 34. Yano M, Terada K, Gotoh T, Mori M (2007) In vitro analysis of Bcl-2 proteins in mitochondria and endoplasmic reticulum: similarities in anti-apoptotic functions and differences in regulation. Exp Cell Res 313: 3767–3778. [DOI] [PubMed] [Google Scholar]
- 35. Schägger H, Pfeiffer K (2000) Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19: 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dabbeni-Sala F, Di Santo S, Franceschini D, Skaper SD, Giusti P (2001) Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J 15: 164–170. [DOI] [PubMed] [Google Scholar]
- 37. Klemm DJ, Leitner JW, Watson P, Nesterova A, Reusch JE, et al. (2001) Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J Biol Chem 276: 28430–28435. [DOI] [PubMed] [Google Scholar]
- 38. Fajas L, Fruchart JC, Auwerx J (1998) Transcriptional control of adipogenesis. Curr Opin Cell Biol 10: 165–173. [DOI] [PubMed] [Google Scholar]
- 39. Lefterova MI, Lazar MA (2009) New developments in adipogenesis. Trends Endocrinol Metab 20: 107–114. [DOI] [PubMed] [Google Scholar]
- 40. White UA, Stephens JM (2009) Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol 318: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA (2008) Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem 283: 21837–21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128. [DOI] [PubMed] [Google Scholar]
- 43. Voshol PJ, Rensen PC, van Dijk KW, Romijn JA, Havekes LM (2009) Effect of plasma triglyceride metabolism on lipid storage in adipose tissue: studies using genetically engineered mouse models. Biochim Biophys Acta 1791: 479–485. [DOI] [PubMed] [Google Scholar]
- 44. Simha V, Garg A (2009) Inherited lipodystrophies and hypertriglyceridemia. Curr Opin Lipidol 20: 300–308. [DOI] [PubMed] [Google Scholar]
- 45. Garg A, Agarwal AK (2009) Lipodystrophies: disorders of adipose tissue biology. Biochim Biophys Acta 1791: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hannun YA (1996) Functions of ceramide in coordinating cellular responses to stress. Science 274: 1855–1859. [DOI] [PubMed] [Google Scholar]
- 47. Pettus BJ, Chalfant CE, Hannun YA (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585: 114–125. [DOI] [PubMed] [Google Scholar]
- 48. Richter C, Gogvadze V, Laffranchi R, Schlapbach R, Schweizer M, et al. (1995) Oxidants in mitochondria: from physiology to diseases. Biochim Biophys Acta 1271: 67–74. [DOI] [PubMed] [Google Scholar]
- 49. Baker BM, Haynes CM (2011) Mitochondrial protein quality control during biogenesis and aging. Trends Biochem Sci 36: 254–261. [DOI] [PubMed] [Google Scholar]
- 50. Aldridge JE, Horibe T, Hoogenraad NJ (2007) Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS One 2: e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J (2004) Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta 1688: 95–101. [DOI] [PubMed] [Google Scholar]
- 52. Singh NR, Rondeau P, Hoareau L, Bourdon E (2007) Identification of preferential protein targets for carbonylation in human mature adipocytes treated with native or glycated albumin. Free Radic Res 41: 1078–1088. [DOI] [PubMed] [Google Scholar]
- 53. Wang T, Si Y, Shirihai OS, Si H, Schultz V, et al. (2010) Respiration in adipocytes is inhibited by reactive oxygen species. Obesity (Silver Spring) 18: 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lu MH, Takemoto M, Watanabe K, Luo H, Nishimura M, et al. (2012) Deficiency of sphingomyelin synthase-1 but not sphingomyelin synthase-2 causes hearing impairments in mice. J Physiol 590: 4029–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dong L, Watanabe K, Itoh M, Huan CR, Tong XP, et al. (2012) CD4+ T-cell dysfunctions through the impaired lipid rafts ameliorate concanavalin A-induced hepatitis in sphingomyelin synthase 1-knockout mice. Int Immunol 24: 327–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences used in quantitative RT-PCR.
(PDF)