Abstract
Introduction
Altered thyroid status exerts a major effect on the heart. Individuals with hypo- or hyperthyroidism showed various changes in electrocardiograms. However, little is known about how variations in thyroid hormone levels within the normal range affect electrical activities of the heart in the general population.
Methods and Results
We conducted a cross-sectional analysis of 5,990 men and women from the Third National Health and Nutrition Examination Survey. Serum total T4 was measured by immunoassay and TSH was measured by chemiluminescent assay. We categorized T4 and TSH into 7 groups with cut-offs at the 5th, 20th, 40th, 60th, 80th, and 95th percentiles of the weighted population distribution. Electrocardiographic parameters were measured from the standard 12-lead electrocardiogram. We found a positive linear association between serum total T4 level and heart rate in men, and a U-shape association between T4 and PR interval in men and women. TSH level was positively associated with QRS interval in men, while a U-shape association between TSH and QRS was observed in women. No clear graded association between thyroid hormones and corrected QT or JT was found, except that men in the highest category of T4 levels appeared to have longer corrected QT and JT, and men in the lowest category of T4 appeared to have shorter corrected QT and JT.
Conclusions
Variation in thyroid hormone levels in the general population, even within the normal range, was associated with various ECG changes.
Introduction
Thyroid hormones can exert electrophysiologic and inotropic effects on the heart and may modulate the risk of developing atherosclerosis [1]–[4]. Patients with overt hypothyroidism show several electrocardiographic (ECG) changes including sinus bradycardia, low amplitude QRS complexes, QT interval prolongation, and alterations in T wave morphology, while patients with hyperthyroidism can develop atrial arrhythmias such as sinus tachycardia, atrial flutter and atrial fibrillation [5]–[8]. Notably, both prolonged and shortened QT intervals have been reported in hyperthyroidism [9]–[13].
While ECG abnormalities in patients with hypo- and hyperthyroidism are well established, relatively little is known about how variations in thyroid hormone levels within the normal range affect the electrical activity of the heart in the general population [11]–[12]. This study aimed to evaluate the association of total thyroxine (T4) and thyrotropin (TSH) levels with ECG parameters (heart rate, PR interval, QRS duration, QT interval, and JT interval) in a representative sample of the general US population.
Methods
Study population
We used data from the Third National Health and Nutrition Examination Survey (NHANES III), a cross-sectional study conducted between 1988 and 1994 that used a multistage stratified clustered probability design to select a representative sample of the civilian non-institutionalized US population. Thyroid hormones were measured in participants 12 years of age and older while 12-lead electrocardiograms (ECGs) were performed on participants 40 years of age and older. Therefore, the present study was restricted to participants 40 years of age and older (N = 8,561) who were eligible for both thyroid function tests and ECG measurements. We excluded 272 participants with missing ECG parameters, 728 participants with missing T4 or TSH, 525 participants with QRS ≥120 ms, and 603 participants with self-report goiter or thyroid disease, or on thyroid medications. In addition, 443 participants with overt or subclinical thyroid disease based on TSH levels were also excluded (413 with TSH>4.5 mU/L, 30 with TSH<0.1 mU/L) [14]. The final analysis was based on 5,990 participants (2,995 men and 2,995 women).
Data collection
NHANES III included a standardized questionnaire administered in the home by a trained interviewer and a detailed physical examination at a mobile examination center. Demographic characteristics, smoking status, alcohol consumption, medical history, and medication use were assessed during the interview. QT-prolonging medications were defined according to the Arizona Center for Education and Research on Therapeutics database [15]. This database, funded by the Agency for Healthcare Research and Quality (AHRQ), has summarized a list of all the known QT-prolonging drugs. Height and weight were measured and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured three times during the in-home interview and three additional times during the at the mobile exam center. Laboratory test results included total cholesterol, HDL cholesterol, serum creatinine, and plasma glucose. Diabetes was defined as a fasting plasma glucose ≥126 mg/dL, a nonfasting plasma glucose ≥200 mg/dL, and/or self-report diabetes with concurrent use of oral hypoglycemic agents or insulin. Estimated glomerular filtration rate (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) Study formula, re-expressed for standardized serum creatinine [16].
Measurement of thyroid hormones
Serum was frozen (−20°C) and shipped on dry ice to the Endocrine Services Laboratory, University of Southern California, (Los Angeles, CA) for analysis of T4 and TSH. T4 was measured using an immunoassay (Roche Molecular Biochemicals, Indianapolis, IN) with a reference (normal) range of 57.9 to 169.9 nmol/L. TSH was measured using a chemiluminescence immunometric assay (Nichols Institute Diagnostics, San Juan Capistrano, CA) with a reference (normal) range of 0.39 to 4.6 mU/L.
ECG parameters
Standard 12-lead resting ECG recordings were performed using a Marquette MAC 12 electrocardiograph (Marquette Medical Systems, Inc., Milwaukee, WI) with signals sampled at 250 Hz per channel. A representative P-QRS-T cycle was then derived by selective averaging using the Dalhousie ECG Analysis Program [17]. The Dalhousie measurement matrix contained several ECG measures of different parts of the ECG waveform, including resting heart rate, PR, QRS, and QT intervals. The JT interval was calculated as QT interval minus QRS interval.
The PR, QT, and JT intervals were adjusted for heart rate, race, and age using the residual method. These adjusted metrics (PRrra, QTrra, and JTrra) were used for subsequent analyses. More specifically, we first regressed the PR, QT, or JT interval, respectively, as dependent variables, on RR interval, race, and age. Adjustment for RR-interval duration was performed using restricted quadratic splines with knots at the 5th, 50th, and 95th percentiles of the overall study population to allow a non-linear relationship of the RR with the PR, QT, or JT intervals. We then obtained model residuals for each participant and centered the residuals at the mean value of the PR, QT, or JT intervals of the study population to facilitate the interpretation of the findings. Residuals are uncorrelated with the regressors (i.e., RR interval, race, and age) and thus adjusted for them. The residuals represent the component of the PR, QT and JT intervals that are not explained by the regressors.
Statistical analysis
The sampling weights for the ECG component of the survey were used to account for the complex design of NHANES III [18]. All analyses were conducted in men and women separately. To assess the dose-response relationship between thyroid hormone levels and ECG parameters, we categorized the distributions of T4 and TSH into 7 categories with cut-offs at the 5th, 20th, 40th, 60th, 80th, and 95th percentiles of the weighted population distribution.
We used multivariable linear regression models to estimate adjusted differences and 95% confidence intervals (CIs) for ECG parameters associated with each category of T4 and TSH compared with the middle category. The basic models were adjusted for age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, and other), and RR-interval splines (except for the models for heart rate). The full models were further adjusted for BMI, smoking (current, former, and never), alcohol consumption (<12, ≥12 drinks in the past year), systolic blood pressure, blood pressure lowing medication, total and HDL cholesterol, diabetes, history of myocardial infarction, history of congestive heart failure, use of QT-prolonging medications, and creatinine-based eGFR. Finally, we further included T4 and TSH simultaneously in the fully adjusted models. Tests for linear trend were computed by including a variable with the median values of T4 or TSH in each category in the linear regression models. Tests for quadratic trend were computed by using quadratic contrast coefficients.
For sensitivity analyses, we repeated all analyses after excluding participants with conditions that may affect ECG parameters (myocardial infarction, heart failure, use of QT-prolonging medications) or diabetes (final sample size 4,424). All analyses were conducted using SUDAAN (version 10.0.1; Research Triangle Institute, Research Triangle Park, NC).
Results
The average age of study participants was 54.8 years in men, and 55.8 years in women (Table 1). Average T4 and TSH levels were 107.0 nmol/L and 1.7 mU/L, respectively, in men, and 112.9 nmol/L and 1.9 mU/L, respectively, in women. The cut-offs for T4 and TSH categories were shown in Table 2.
Table 1. Baseline characteristics of study participants.
| Characteristic | Men (n = 2,995) | Women (n = 2,995) |
| Age (years) | 54.8 (11.6) | 55.8 (12.4) |
| Race | ||
| Non-Hispanic white | 81.2 | 78.4 |
| Non-Hispanic black | 8.3 | 10.1 |
| Mexican-American | 3.8 | 3.5 |
| Systolic blood pressure (mmHg) | 128.8 (16.4) | 127.2 (19.2) |
| Total cholestrol (mg/dL) | 212.3 (39.0) | 220.8 (43.5) |
| HDL (mg/dL) | 45.0 (14.0) | 56.1 (16.0) |
| BMI (kg/m2) | 27.3 (4.5) | 27.1 (6.2) |
| Estimated-GFR (mL/min/1.73 m2) | 86.1 (18.9) | 84.6 (25.7) |
| Diabetes | 9.1 | 7.5 |
| Myocardial infarction | 6.1 | 2.6 |
| Congestive heart failure | 2.6 | 1.8 |
| Smoking | ||
| Current | 27.9 | 20.8 |
| Former | 44.8 | 24.8 |
| Never | 27.3 | 54.4 |
| Consume alcohol | 59.1 | 38.3 |
| Use QT prolonging medication | 9.6 | 12.3 |
| Thyroxin (T4, nmol/L) | 107.0 (24.7) | 112.9 (26.8) |
| Thyrotropin (TSH, mU/L) | 1.7 (0.9) | 1.9 (0.9) |
| ECG parameters | ||
| Heart rate (beat/min) | 66.7 (11.3) | 69.2 (11.0) |
| PRrra (ms) * | 165.1 (26.4) | 159.3 (25.3) |
| QRS (ms) | 99.6 (9.3) | 93.5 (9.2) |
| QTrra (ms) * | 403.1 (18.4) | 407.5 (18.7) |
| JTrra (ms) * | 303.5 (18.1) | 314.0 (18.3) |
Values are means (SD) or percentages unless otherwise noted.
. PRrra, QTrra, JTrra: RR-interval, race-, and age corrected PR, QT, and JT intervals.
Table 2. Percentiles and cut-offs of T4 and TSH distribution.
| Percentiles (%) | Cut-offs | Men (n = 2,995) | Women (n = 2,995) |
| T4 (nmol/L) | |||
| 1. [0, 5) | [0,70.2) | 142 | 114 |
| 2. [5, 20) | [70.2, 89.6) | 490 | 359 |
| 3. [20, 40) | [89.6, 103.2) | 680 | 543 |
| 4. [40, 60) | [103.2, 114.7) | 605 | 628 |
| 5. [60, 80) | [114.7, 129.1) | 589 | 631 |
| 6. [80, 95) | [129.1, 150.9) | 389 | 509 |
| 7. ≥95 | ≥ 150.9 | 100 | 211 |
| TSH (mU/L) | |||
| 1. [0, 5) | [0.1, 0.6) | 195 | 156 |
| 2. [5, 20) | [0.6, 1.0) | 445 | 384 |
| 3. [20, 40) | [1.0, 1.4) | 611 | 583 |
| 4. [40, 60) | [1.4, 1.8) | 640 | 622 |
| 5. [60, 80) | [1.8, 2.4) | 558 | 568 |
| 6. [80, 95) | [2.4, 3.6) | 435 | 518 |
| 7. ≥95 | [3.6, 4.5] | 111 | 164 |
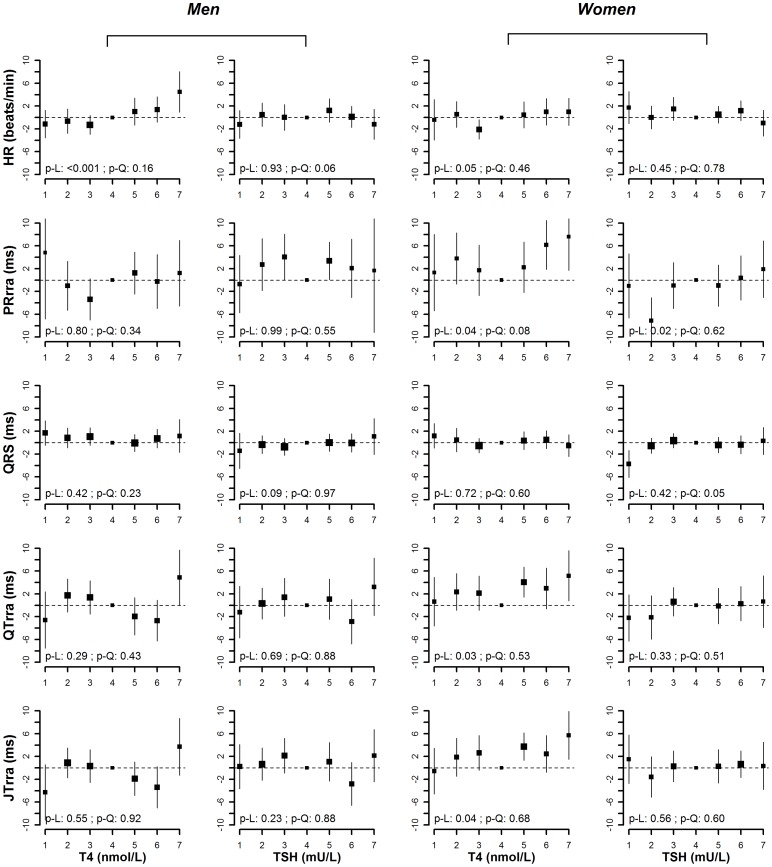
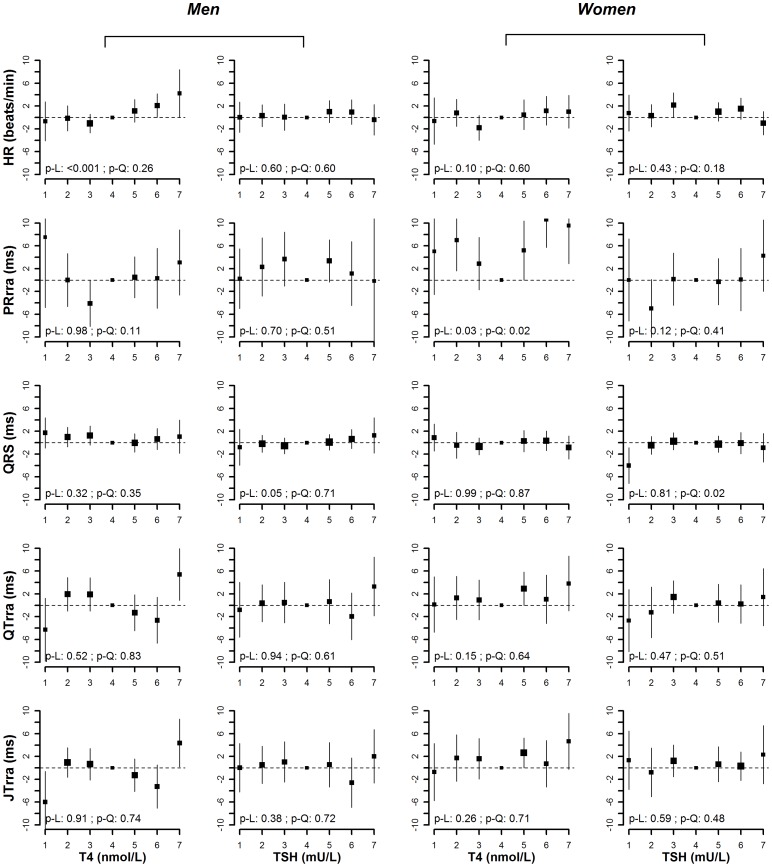
After adjusting for age, race/ethnicity and RR-interval, T4 levels were positively associated with heart rate in men and women (p-value for linear trend <0.001 in men and 0.05 in women) (Figure 1). In the fully adjusted model, the positive association between T4 and heart rate was still evident in men (p-value for linear trend <0.001), but not in women (p-value for linear trend = 0.10) (Figure 2). The average difference in heart rate comparing the highest vs. the lowest category of T4 was 4.9 beat/min (95% CI 0.6 to 9.2) in men and 1.7 beat/min (−2.7 to 6.0) in women.
Figure 1. Age, race/ethnicity, RR-interval adjusted means (95% CI) of heart rate, PRrra interval, QRS duration, QTrra and JTrra interval by categories of T4 and TSH (p-L denotes p-value for linear trend, and p-Q denotes p-value for quadratic trend).
Figure 2. Multivariate adjusted means (95% CI) of heart rate, PRrra interval, QRS duration, QTrra and JTrra interval by categories of T4 and TSH (p-L denotes p-value for linear trend, and p-Q denotes p-value for quadratic trend).
Models were adjusted for age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, and other), RR-interval splines (except for the models of heart rate), BMI, smoking (current, former, and never), alcohol consumption (<12, ≥12 drinks in the past year), systolic blood pressure, blood pressure lowing medication, total and HDL cholesterol, diabetes, history of myocardial infarction, history of congestive heart failure, use of QT-prolonging medications, and creatinine-based eGFR.
We found a U-shaped association between T4 levels and PRrra interval in women (p-value for quadratic trend 0.08 in the basic model and 0.02 in the fully adjusted model). In the fully adjusted model, the average difference in PRrra interval comparing the lowest vs. the middle category of T4 was 5.0 ms (−2.5 to 12.6), and the average difference comparing the highest vs. the middle category was 9.6 ms (2.9 to 16.2). There was a similar U-shaped pattern between T4 levels and PRrra in men, but the p-values for quadratic trend did not reach statistical significance (p-value for quadratic trend 0.34 in the basic model and 0.11 in the fully adjusted model).
There was a marginally positive linear association between TSH levels and QRS interval in men (p-value for linear trend 0.09 in the basic model and 0.05 in the fully adjusted model), but a U-shaped association in women (p-value for quadratic trend 0.05 in the basic model and 0.02 in the fully adjusted model). In the fully adjusted model, the average difference in QRS duration comparing the highest vs. the lowest category of TSH in men was 2.1 ms (−2.1 to 6.2). In women, the average difference in QRS duration comparing the lowest vs. the middle category of TSH was −4.0 ms (−7.2 to −0.9) and the average difference comparing the highest vs. the middle category was −0.9 ms (−3.4 to 1.6).
In models adjusted for age, race/ethnicity and RR-interval, T4 levels were positively associated with QTrra and JTrra intervals in women (p-value for linear trend = 0.03 for QTrra, and 0.04 for JTrra), but the associations became non-significant in the fully adjusted models (p-value for linear trend 0.15 for QTrra, and 0.26 for JTrra). In men, the association between T4 levels with QTrra or JTrra intervals was not significant, although men in the highest category of T4 appeared to have longer QTrra and JTrra intervals and men in the lowest category of T4 appeared to have shorter QTrra and JTrra intervals compard to the rest of the groups. In men, the average difference in QTrra and JTrra intervals comparing the lowest vs. the middle category of T4 in the fully adjusted models were −4.3 ms (−9.8 to 1.3) and −6.0 ms (−11.4 to −0.6), respectively, while the average difference in QTrra and JTrra intervals comparing the highest vs. the middle category of T4 were 5.4 ms (0.8 to 10.0) and 4.4 ms (0.2 to 8.6).
Finally, including T4 and TSH in the same model (Appendix S1) and exclusion of individuals with diabetes, myocardial infarction, heart failure, or taking QT-prolonging medications (Appendix S2) resulted in similar findings.
Discussion
This analysis of NHANES III men and women with thyroid hormone levels within the normal range showed a positive linear association between serum T4 levels and heart rate in men and a U-shaped association between T4 levels and PR interval in men and women. In addition, TSH levels were marginally positively associated with QRS interval in men, but showed a U-shaped association with QRS interval in women. There was no consistent association between thyroid hormone levels and QT or JT intervals, except that men in the highest category of T4 levels appeared to have longer QT and JT intervals and men in the lowest category of T4 levels appeared to have shorter QT and JT intervals compared to the middle group.
Tachycardia and bradycardia are well known signs of hyper- and hypothyroidism, respectively [9], [13], [19]–[24]. Experimental data suggested that the faster heart rate in hyperthyroidism was partly due to the regulatory effect of thyroid hormones on sodium pump density and enhancement of Na+ and K+ currents [25]. Thyroid hormones can increase heart rate by increasing sinus node automaticity, decreasing the action potential duration and the refraction period of the atrial myocardium as well as the atrioventricular nodal refraction period [25]. In addition, increased sympathetic and decreased vagal modulation in hyperthyroidism could also increase the heart rate [26].
Less is known, however, about how thyroid hormone levels within the normal range affect heart rate in the general population. The Study of Health in Pomerania (1,748 men and 1,862 women in northeast Germany) seems to be the only previous population-based study that has investigated this question. In this study, there was no significant association between TSH levels and heart rate [12]. We found, however, that T4 was positively related to heart rate in men, but not in women. This gender difference has not been described previously in the literature and warrants confirmation in other population-based studies.
The effect of thyroid hormones on the QT interval duration is controversial. A majority of animal and clinical studies have reported a prolonged heart rate-corrected QT interval (QTc) in hypothyroidism [22], [27]–[32]. The underlying mechanism may be decreasing IKs, or an increased sympathetic influence on the autonomic cardiovascular system (i.e., elevated catecholamine levels, particularly norepinephrine release from sympathetic nerves) as shown in some studies [9], [31], [33]. In contrast, while several animal models have reported a shortened repolarization time in the hyperthyroid state [27], [34], small studies in patients with hyperthyroidism have reported prolonged QTc intervals [9]–[10], [13]. A study comparing 16 hyperthyroid patients with Graves' disease with matched healthy controls found significant prolongations in the 24-h average QTc in hyperthyroid patients, with positive correlations of free T3 and free T4 with QTc [9]. Another study reported significant increases in QTc interval in 32 patients with subclinical hyperthyroidism compared to healthy controls, but QTc was not correlated with free T4 or TSH levels [10]. In addition, QTc intervals significantly decreased in 47 patients with hyperthyroidism after achievement of euthyroid state, however this may be mainly driven by the changes in heart rate [13].
Limited data from population-based studies were also inconsistent. The Study of Health in Pomerania showed a positive association between TSH levels and QTc intervals [12]. Another study of 939 elderly men and women in Netherlands without clinical or subclinical hypothyroidism found no significant association between TSH and QTc, but they reported a positive linear association between free T4 and QTc duration in men but not in women [11]. Our analysis of NHANES data suggested no clear graded association between thyroid hormones and corrected QT or JT intervals. However, we found that men in the highest category of T4 levels appeared to have longer corrected QT and JT intervals compared to the middle group, and men in the lowest category of T4 appeared to have shorter corrected QT and JT intervals. These observations were still evident after including T4 and TSH simultaneously in the model. The different findings may partly explained by the differences in population characteristics, choices of QT correction formula, or selection of cut-points for T4 and TSH. Future studies are necessary to validate our findings in other population-based samples.
Finally, very few studies have evaluated the association of thyroid hormone levels with other electrocardiographic parameters such as PR interval or QRS duration. Experimental studies in hypothyroid dogs have reported both no changes in PR or QRS duration [35], and prolonged P wave and QRS duration compared to euthyroid dogs [32]. In humans, a study of 18 patients with hypothyroidism found no significant changes in the PR and QRS duration after L-thyroxine treatment [29], and a study of 25 patients with hyperthyroidism reported no difference in PR interval compared to healthy controls [20]. Our results showed a U-shaped relationship between T4 and PR interval in both men and women. We also found a linear association between TSH levels and QRS durations in men and a U-shaped association in women. Again, additional studies are needed to confirm these observations.
Several limitations of this study need to be considered. Firstly, the cross-sectional design limited our ability to make statements regarding the causality of the relation between thyroid hormone levels and ECG parameters. Secondly, we only had available measurements of total T4, but not of free T4 or T3 measurements in NHANES III. T3 is much more potent than T4, and we might expect stronger associations if we would have used T3 in the analysis. Furthermore, most of the circulating thyroid hormone in the blood is bound to transport proteins and only a very small fraction is free (unbound) and biologically active. Therefore, the measurement of total T4 levels may not represent the actual level of free (active) T4. Finally, thyroid hormones and ECG measurements were performed only at a single time at baseline, which may result in non-differential measurement error as there might be substantial within person variability. Repeated measurements may be needed to reduce measurement error and better characterize the association between thyroid status and ECG changes in future studies.
Conclusion
In conclusion, data from NHANES III suggested that variation in thyroid hormone levels within the normality range in the general population was associated with various ECG changes, including a linear association between serum total T4 level and heart rate in men, a U-shaped association between T4 and PR interval in men and women, a linear association between TSH and QRS duration in men, and a U-shaped association between TSH and QRS in women. In addition, men in the highest category of T4 had longer QT and JT intervals, although there was no clear graded association between thyroid hormone levels and QT or JT intervals. Further research is needed to confirm these findings and to elucidate the underlying mechanisms, especially those related to gender differences in the effect of thyroid hormones on ECG parameters.
Supporting Information
Multivariate adjusted means (95% CI) of heart rate, PRrra interval, QRS duration, QTrra and JTrra interval by categories of T4 and TSH (p-L denotes p-value for linear trend, and p-Q denotes p-value for quadratic trend). Models were adjusted for age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, and other), RR-interval splines (except for the models of heart rate), BMI, smoking (current, former, and never), alcohol consumption (<12, ≥12 drinks in the past year), systolic blood pressure, blood pressure lowing medication, total and HDL cholesterol, diabetes, history of myocardial infarction, history of congestive heart failure, use of QT-prolonging medications, creatinine-based eGFR, and T4 (in the models for TSH), and TSH (in the models for T4).
(TIF)
Multivariate adjusted means (95% CI) of heart rate, PRrra interval, QRS duration, QTrra and JTrra interval by categories of T4 and TSH, excluding participant with diabetes, myocardial infarction, heart failure, or taking QT-prolonging medications (p-L denotes p-value for linear trend, and p-Q denotes p-value for quadratic trend). Models were adjusted for for age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, and other), RR-interval splines (except for the models of heart rate), BMI, smoking (current, former, and never), alcohol consumption (<12, ≥12 drinks in the past year), systolic blood pressure, blood pressure lowing medication, total and HDL cholesterol, diabetes, history of myocardial infarction, history of congestive heart failure, use of QT-prolonging medications, and creatinine-based eGFR.
(TIF)
Funding Statement
The present study was funded in part by grants from the National Center for Cardiovascular Research (CNIC Translational Cardiology grant 2008-03), the National Institutes of Health (grants ES015597 and HL091062), and the Donald W. Reynolds Cardiovascular Clinical Research Center at Johns Hopkins University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, et al. (2006) Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295: 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danzi S, Klein I (2002) Thyroid hormone-regulated cardiac gene expression and cardiovascular disease. Thyroid 12: 467–472. [DOI] [PubMed] [Google Scholar]
- 3. Toft AD, Boon NA (2000) Thyroid disease and the heart. Heart 84: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, et al. (2010) Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fadel BM, Ellahham S, Ringel MD, Lindsay J Jr, Wartofsky L, et al. (2000) Hyperthyroid heart disease. Clin Cardiol 23: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sawin CT (2002) Subclinical hyperthyroidism and atrial fibrillation. Thyroid 12: 501–503. [DOI] [PubMed] [Google Scholar]
- 7. Osborn LA, Skipper B, Arellano I, MacKerrow SD, Crawford MH (1999) Results of resting and ambulatory electrocardiograms in patients with hypothyroidism and after return to euthyroid status. Heart Dis 1: 8–11. [PubMed] [Google Scholar]
- 8. Tribulova N, Knezl V, Shainberg A, Seki S, Soukup T (2010) Thyroid hormones and cardiac arrhythmias. Vascul Pharmacol 52: 102–112. [DOI] [PubMed] [Google Scholar]
- 9. Colzani RM, Emdin M, Conforti F, Passino C, Scarlattini M, et al. (2001) Hyperthyroidism is associated with lengthening of ventricular repolarization. Clin Endocrinol (Oxf) 55: 27–32. [DOI] [PubMed] [Google Scholar]
- 10. Owecki M, Michalak A, Nikisch E, Sowinski J (2006) Prolonged ventricular repolarization measured by corrected QT interval (QTc) in subclinical hyperthyroidism. Horm Metab Res 38: 44–47. [DOI] [PubMed] [Google Scholar]
- 11. van Noord C, van der Deure WM, Sturkenboom MC, Straus SM, Hofman A, et al. (2008) High free thyroxine levels are associated with QTc prolongation in males. J Endocrinol 198: 253–260. [DOI] [PubMed] [Google Scholar]
- 12. Dorr M, Ruppert J, Robinson DM, Kors JA, Felix SB, et al. (2006) The relation of thyroid function and ventricular repolarization: decreased serum thyrotropin levels are associated with short rate-adjusted QT intervals. J Clin Endocrinol Metab 91: 4938–4942. [DOI] [PubMed] [Google Scholar]
- 13. Guntekin U, Gunes Y, Tuncer M, Simsek H, Gumrukcuoglu HA, et al. (2009) QTc dispersion in hyperthyroidism and its association with pulmonary hypertension. Pacing Clin Electrophysiol 32: 494–499. [DOI] [PubMed] [Google Scholar]
- 14. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, et al. (2002) Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87: 489–499. [DOI] [PubMed] [Google Scholar]
- 15.Drugs that Prolong the QT Interval and/or Induce Torsades de Pointes Ventricular Arrhythmia. Available: http://wwwazcertorg/medical-pros/drug-lists/drug-listscfm. Accessed 2009 Sept.
- 16.NCHS Note for Correction of Serum Creatinine for NHANES III, NHANES 1999-2000, 2001-2002 and 2003-2004. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/general_%20note_for_serum_creatinine.pdf. Accessed 2010 April.
- 17. Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, et al. (1990) Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med 29: 362–374. [PubMed] [Google Scholar]
- 18. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1: 1–407. [PubMed] [Google Scholar]
- 19. McDevitt DG, Shanks RG, Hadden DR, Montgomery DA, Weaver JA (1968) The role of the thyroid in the control of heart-rate. Lancet 1: 998–1000. [DOI] [PubMed] [Google Scholar]
- 20. Dazai Y (1999) Left atrial systolic time interval in hyperthyroidism. Angiology 50: 591–598. [DOI] [PubMed] [Google Scholar]
- 21. Osman F, Franklyn JA, Daykin J, Chowdhary S, Holder RL, et al. (2004) Heart rate variability and turbulence in hyperthyroidism before, during, and after treatment. Am J Cardiol 94: 465–469. [DOI] [PubMed] [Google Scholar]
- 22. Sarma JS, Venkataraman K, Nicod P, Polikar R, Smith J, et al. (1990) Circadian rhythmicity of rate-normalized QT interval in hypothyroidism and its significance for development of class III antiarrhythmic agents. Am J Cardiol 66: 959–963. [DOI] [PubMed] [Google Scholar]
- 23. Kohno I, Iwasaki H, Okutani M, Mochizuki Y, Sano S, et al. (1998) Circadian blood pressure and heart rate profiles in normotensive patients with mild hyperthyroidism. Chronobiol Int 15: 337–347. [DOI] [PubMed] [Google Scholar]
- 24. Galloe AM, Rolff M, Nordin H, Ladefoged SD, Mogensen NB (1993) Cardiac performance and thyroid function. The correlation between systolic time intervals, heart rate and thyroid hormone levels. Dan Med Bull 40: 492–495. [PubMed] [Google Scholar]
- 25. Kahaly GJ, Dillmann WH (2005) Thyroid hormone action in the heart. Endocr Rev 26: 704–728. [DOI] [PubMed] [Google Scholar]
- 26. Chen JL, Chiu HW, Tseng YJ, Chu WC (2006) Hyperthyroidism is characterized by both increased sympathetic and decreased vagal modulation of heart rate: evidence from spectral analysis of heart rate variability. Clin Endocrinol (Oxf) 64: 611–616. [DOI] [PubMed] [Google Scholar]
- 27. Binah O, Arieli R, Beck R, Rosen MR, Palti Y (1987) Ventricular electrophysiological properties: is interspecies variability related to thyroid state? Am J Physiol 252: H1265–1274. [DOI] [PubMed] [Google Scholar]
- 28. Fazio S, Biondi B, Lupoli G, Cittadini A, Santomauro M, et al. (1992) Evaluation, by noninvasive methods, of the effects of acute loss of thyroid hormones on the heart. Angiology 43: 287–293. [DOI] [PubMed] [Google Scholar]
- 29. Kweon KH, Park BH, Cho CG (2007) The effects of L-thyroxine treatment on QT dispersion in primary hypothyroidism. J Korean Med Sci 22: 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Galetta F, Franzoni F, Fallahi P, Rossi M, Carpi A, et al. (2006) Heart rate variability and QT dispersion in patients with subclinical hypothyroidism. Biomed Pharmacother 60: 425–430. [DOI] [PubMed] [Google Scholar]
- 31. Bosch RF, Wang Z, Li GR, Nattel S (1999) Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 277: H211–220. [DOI] [PubMed] [Google Scholar]
- 32. Paslawska U, Noszczyk-Nowak A, Kungl K, Bioly K, Popiel J, et al. (2006) Thyroid hormones concentrations and ECG picture in the dog. Pol J Vet Sci 9: 253–257. [PubMed] [Google Scholar]
- 33. Cacciatori V, Gemma ML, Bellavere F, Castello R, De Gregori ME, et al. (2000) Power spectral analysis of heart rate in hypothyroidism. Eur J Endocrinol 143: 327–333. [DOI] [PubMed] [Google Scholar]
- 34. Sharp NA, Neel DS, Parsons RL (1985) Influence of thyroid hormone levels on the electrical and mechanical properties of rabbit papillary muscle. J Mol Cell Cardiol 17: 119–132. [DOI] [PubMed] [Google Scholar]
- 35. Venkatesh N, Lynch JJ, Uprichard AC, Kitzen JM, Singh BN, et al. (1991) Hypothyroidism renders protection against lethal ventricular arrhythmias in a conscious canine model of sudden death. J Cardiovasc Pharmacol 18: 703–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multivariate adjusted means (95% CI) of heart rate, PRrra interval, QRS duration, QTrra and JTrra interval by categories of T4 and TSH (p-L denotes p-value for linear trend, and p-Q denotes p-value for quadratic trend). Models were adjusted for age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, and other), RR-interval splines (except for the models of heart rate), BMI, smoking (current, former, and never), alcohol consumption (<12, ≥12 drinks in the past year), systolic blood pressure, blood pressure lowing medication, total and HDL cholesterol, diabetes, history of myocardial infarction, history of congestive heart failure, use of QT-prolonging medications, creatinine-based eGFR, and T4 (in the models for TSH), and TSH (in the models for T4).
(TIF)
Multivariate adjusted means (95% CI) of heart rate, PRrra interval, QRS duration, QTrra and JTrra interval by categories of T4 and TSH, excluding participant with diabetes, myocardial infarction, heart failure, or taking QT-prolonging medications (p-L denotes p-value for linear trend, and p-Q denotes p-value for quadratic trend). Models were adjusted for for age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, and other), RR-interval splines (except for the models of heart rate), BMI, smoking (current, former, and never), alcohol consumption (<12, ≥12 drinks in the past year), systolic blood pressure, blood pressure lowing medication, total and HDL cholesterol, diabetes, history of myocardial infarction, history of congestive heart failure, use of QT-prolonging medications, and creatinine-based eGFR.
(TIF)