Abstract
Bacteria within arthropods can be identified using culture-independent methods. This unit describes protocols for surface sterilization of arthropods, DNA extraction of whole bodies and tissues, touchdown PCR amplification using 16S rDNA general bacteria primers and profiling the bacterial community using denaturing gradient gel electrophoresis.
Keywords: arthropod, bacteria, community, sterilization, 16S rDNA, DGGE
INTRODUCTION
Arthropods generally harbor complex microbial communities. The majority of bacteria present are not merely transient passengers but involved in key physiological processes and have multifaceted symbiotic associations with their host (Ishikawa 2003). Recognition of the importance of bacterial symbionts to host physiology has lead to a wealth of studies that seek to examine the bacteria within arthropods (Moran 2001; Dillon and Dillon 2004; Kikuchi 2009). These studies examine general community dynamics and have significantly expanded the knowledge of bacteria types associated with arthropods. In recent years there has been an increase in availability of culture-independent molecular tools (Shi, Syrenne et al. 2010), which have overcome the limiting factors associated with culture-based methods. Using gene-specific PCR, molecular fingerprinting and FISH straining, bacteria can be detected within insects that had previously been uncultivable, opening new investigations into symbiotic associations within arthropods.
This unit describes the application of one of the available culture-independent methods for analyzing the microbial community within arthropods, denaturing gradient gel electrophoresis (DGGE). Culture-independent methods are ideal for identifying bacteria within arthropods, as only a subset of bacteria are likely to be cultivable using existing techniques (Dillon and Dillon 2004). Procedures are included for DNA isolation from arthropods and their tissues and amplification of DNA with 16S rDNA general bacteria primers. Profiling of the bacterial community is performed using DGGE and a BioRad D-Code Universal Mutation Detection System. DGGE is an electrophoretic separation method based on differences in melting temperature of double stranded DNA fragments and has been used successfully to evaluate microbial diversity within several different arthropods (Reeson, Jankovic et al. 2003; Schabereiter-Gurtner, Lubitz et al. 2003; Hui, Wei et al. 2006; Zahner, Lucarotti et al. 2008; Zhang and Jackson2008; Zouache, Raharimalala et al. 2011). Electrophoresis takes place in a vertically placed polyacrylamide gel with a gradient of denaturants and separates PCR products based on nucleotide content rather than size. Changes as small as one base pair may be detected. After amplification of rDNA using 16S general bacterial primers, the DGGE method allows separation of the mixed PCR product, providing a molecular fingerprint of the microbial community within the arthropod or tissue. Due to its broad application, relative ease of methods and quick turnover time from sample to results, DGGE is a suitable method for initial screens of the bacterial community within arthropod samples.
BASIC PROTOCOL 1 Isolation of bacterial DNA from arthropod tissue samples
Bacterial DNA can be isolated from many different types of arthropod tissues, such as eggs, individual organs and whole bodies. Environmental bacteria can reside on the surface of the arthropod, so sterilization is required if the focus is solely the bacteria that reside within the arthropod. Here a method is described for surface sterilization prior to either dissection or whole body DNA extractions. During dissection, care should be taken not to contaminate the sample with external bacteria. Only sterile dissecting solutions and tools should be used and tools need to be sterilized between samples. Once bodies have been sterilized or organs collected, an extraction kit can be used for isolation of bacterial DNA. Good results have been obtained using the Qiagen DNeasy Blood and Tissue kit. However, any standard kit should suffice. To increase bacterial DNA yield, it is recommended to pool arthropod samples, provided that will not alter the results of the experiment.
Materials
1.5 ml microcentrifuge tubes, autoclaved
Sterile pipet tips and pipetting aid
70% ethanol
Molecular grade water, autoclaved
Forceps and dissecting tools, sterile
Glass microscope slides, sterilized
PBS (see recipe), autoclaved
1 mm glass beads, autoclaved
Homogenization Buffer (see recipe)
Bead Beater
Qiagen DNeasy Blood and Tissue kit
Pestles
Surface sterilization of whole arthropods or eggs
-
1
Place arthropod to be extracted in a 1.5 ml microcentrifuge tube.
-
2
Rinse tissue with 200 μl of 70% ethanol by pipetting up and down for 2 minutes.
-
3
Let sample sit in 70% ethanol for 5 minutes.
-
4
Remove and discard ethanol.
-
5
Rinse the sample for a second time with 200 μl of 70% ethanol by pipetting up and down for 2 minutes.
-
6
Remove 70% ethanol and discard.
-
7
Rinse the tissue sample with 200 μl molecular grade water by pipetting up and down for 1 minute.
-
8
Remove water and discard.
-
9
For DNA extraction from whole bodies and eggs, continue to step 13.
Dissection of arthropod tissues
-
10
If the focus is arthropod organs and tissues, make sure that all tools are sterilized prior to dissection to prevent contamination.
-
11
Place the surface sterilized arthropod on a microscope slide and add 5 μl of sterile PBS prior to dissection.
Use a new slide and sterile tools for each arthropod
-
12
Once organs and tissues are collected, place directly into 100 μl of homogenization buffer in a 1.5 ml microcentrifuge tube. Add a single 1 mm glass bead and continue to step 14.
DNA extraction of arthropod samples
-
13
Add 100 μl of homogenization buffer and a single 1 mm glass bead to the tissue sample.
-
14
Beat for 1.5 minutes in a bead beater.
If the sample is not completely homogenized, beat for longer. For example, 3 minutes will pulverize a pool of 30 mosquito eggs.Alternatively, if no bead beater is available or if complete pulverization of the arthropod is not the goal, the arthropod can be homogenized by hand in homogenization buffer using a pestle. -
15
Extract DNA from the sample using Qiagen DNeasy Blood and tissue kit following the manufacturer’s instructions for extracting animal tissues using a spin column.
-
16
Store DNA sample at −20°C or if using immediately place at 4°C.
ALTERNATE PROTOCOL 1 Arthropod homogenization
It is difficult to homogenize hard-bodied arthropods, such as ticks, by hand. If no bead beater is available, hard-bodied arthropods can be frozen in liquid nitrogen prior to homogenization.
Materials
Liquid nitrogen
Homogenization of arthropod whole bodies
Continued from step 9
Place hard-bodied arthropod in a1.5 ml microcentrifuge tube.
-
Keeping the lid open, place tube in liquid nitrogen for 1 minute.
Keeping the lid open prevents the tube from freezing shut. Use pestle to crush arthropod against the walls of the microcentrifuge tube.
Add 100 μl of homogenization buffer.
Continue to step 15.
BASIC PROTOCOL 2 Using PCR to amplify bacterial DNA from arthropods
This protocol describes PCR amplification of the DNA template extracted in Basic Protocol 1. A PCR reaction mix that includes general 16S rDNA bacteria-specific primers is used to amplify bacterial DNA from the arthropod whole body or tissue samples. The 5′ end of the forward primer contains a 40 base-pair GC-clamp, which is important for downstream DGGE applications in Basic Protocol 3. The thermal cycler program involves touchdown PCR, which begins with a high annealing temperature that gradually decreases. This increases both specificity and yield of the final product.
Materials
Molecular grade H2O, autoclaved
10X PCR Buffer (MgCl2 included)
20 μM forward primer
20 μM reverse primer
10 mM dNTPs
Taq DNA polymerase (5U/μl)
Template DNA
0.5 ml thin-walled PCR tubes
Automated Thermal Cycler
1.5% agarose gel (see recipe)
0.5X TBE Buffer (see recipe)
Gel Red Nucleic Acid Stain (10,000x in DMSO)
1 Kb DNA ladder
PCR amplification of bacterial 16S DNA for DGGE analysis
-
Prepare reaction mix for PCR.
Each 25 μl reaction should contain:
19.8 μl Molecular grade H2O
2.5 μl 10X PCR Buffer
0.5 μl 10 mM dNTPS
0.5 μl 20 μM forward primer
0.5 μl 20 μM reverse primer
0.2 μl Taq polymerase (5U/μl)
1.0 μl of DNA template (Added in step 2)
Forward primer: 5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GCC TACGGGAGGCAGCAG-3′
Reverse primer: 5′-ATT ACC GCG GCT GCT GG-3′
Note: The GC-clamp on the 5′ end of the forward primer stabilizes the product as it is electrophoresed through the polyacrylamide gel (Basic Protocol 3), yielding clear bands.
-
Aliquot PCR reaction mix into 0.5 ml thin-walled PCR tubes and add 1 μl DNA template to each tube.
If low product yields are observed after PCR, template can be increased to 2 μl. -
Amplify bacterial DNA using the following program for thermal cycler:
94°C for 1 min
3 cycles (94°C 15 s, 61°C for 15 s and 72°C for 1 min)
3 cycles (94°C for 15 s, 58°C for 15 s and 72°C for 1 min)
28 cycles (94°C for 15 s, 55°C for 15 s and 72°C for 1 min)
72°C for 6 min
-
Pour a 1.5% agarose gel stained with Gel Red.
Gel Red is a non-carcinogenic alternative to ethidium bromide. Ethidium bromide may also be used for staining if Gel Red is unavailable. Allow agarose gel at least 1 hour to solidify.
-
Confirm amplification and size of PCR product by electrophoresing a small amount with a 1 Kb DNA ladder on the 1.5% agarose gel submerged in 0.5X TBE buffer.
Products should be approximately 150 base pairs in length. Store PCR product at −20°C or if using immediately place at 4°C.
BASIC PROTOCOL 3 DGGE analysis of PCR products using a BioRad D-Code System
The PCR performed in Basic Protocol 2 utilizes general 16S rDNA bacterial primers, resulting in product that is a mix of different bacterial DNA. DGGE allows separation of the mixed product, providing a fingerprint of the microbial community within the arthropod or tissue. This protocol describes the set up of the BioRad D-Code System for DGGE, which includes assembling the device, pouring the gradient gel, loading samples and analysis of gel results. Assembly is exactly as discussed in the standard operating procedures manual for the system. However, an alternate method is utilized to pour the gradient gel. The peristaltic pump/gradient mixer method listed in this protocol is user-friendly and creates replicable gradient gels, which allows for more accurate comparisons between gels run at different times. The gradient gel in this protocol has a cap that contains no denaturing solution, ensuring that denaturation of the double stranded PCR product starts at the base of the well, not when the product is in the well. DGGE bands are identified using a method involving excision of bands, PCR reamplification and sequencing. Sequencing can be outsourced to a university facility and results can be edited using standard bioinformatics software and identified using the NCBI BLASTn algorithm.
CAUTION: Unpolymerized polyacrylamide is a neurotoxin. Follow all appropriate biosafety requirements relevant to the use of this reagent, including personal protection and waste disposal.
CAUTION: UV light burns, so care should be taken to limit exposure. Make sure all exposed skin (wrists, neck) is covered. Wear goggles AND a UV face plate. Turn on the light only when necessary (cutting bands). Turn off when not in use.
Materials
BioRad D-Code Universal Mutation Detection System
Variable Flow Mini-Peristaltic Pump, Fisher Scientific, Model 3386-medium flow
Gradient Mixer, 100 mL
Magnetic stir plate
Silastic Laboratory Tubing, Dow Corning, 1.57 mm I.D. × 2.18 mm O.D.
Pipet tips and pipetting aid
Gel-Loading Tips, Fisherbrand Premium Multiflex, 0.5–200 ul
Magnetic stir bar
-
10% polyacrylamide denaturing solution (see recipes)
Low concentration -40%
High concentration -70%
10% ammonium persulfate (see recipe)
TEMED
ddH2O
Paper towels or card stock
10 ml syringe, no sharp
0% polyacrylamide denaturing solution (see recipe)
1X TAE Run buffer (see recipe)
GC-clamped PCR product from Basic Protocol 2
Loading dye
Staining container
Aluminum foil
Gel Red Staining Solution (see recipe)
Clinical Rotator, Fisher Scientific
UV Transilluminator
Micro-spatulas, metal, sterilized with flame
0.5 ml microcentrifuge tubes
Molecular grade H2O
1.5% agarose gel (see recipe)
Geneious Pro Bioinformatics software
Assemble the perpendicular gradient gel sandwich
-
1
Using 16 cm × 16 cm plates with 1 mm spacers, assemble the perpendicular gradient gel sandwich.
This step is listed in detail in the standard operating procedures manual included with the BioRad D-Code System.
Pouring the gradient gel
This is a different method than the one listed in the BioRad D-Code standard operating procedures manual.
-
2
Set up peristaltic pump, gradient mixer and magnetic stir plate on bench top. Gradient mixer should be placed on magnetic stir plate.
-
3
Close the knobs on the gradient mixer prior to use.
Knobs will face straight up when closed. -
4
Place end of peristaltic pump tubing between plates.
A gel loading tip with the point cut off works well for this step. -
5
Place magnetic stir bar in right well.
-
6
Using a pipet, add 11.5 ml of each denaturing solution to the gradient mixing block wells. Put lower (40%) concentration in left well and higher (70%) concentration in right well.
Good separation for several different arthropods has been achieved with a 10% polyacrylamide gel with a 40% to 70% denaturing gradient. If this protocol does not yield good separation results, try changing the denaturing gradient using the guide in the reagents and solutions section. -
7
Add 81 μl 10% ammonium persulfate and 4.5 μl TEMED to the denaturing solution in each well and mix.
Steps 8–15 need to be completed relatively quickly (within 30 minutes) before the denaturing solutions begin to polymerize. Storing the polyacrylamide denaturing solutions at 4°C and removing them from this temperature just prior to step 6 will slow down this process. -
8
Open left valve of gradient mixer by turning to the left.
Clear any bubbles that form in the tube that connects the wells by lightly tapping corner of gradient mixer once on bench top. -
9
Turn on magnetic stir plate.
-
10
Open right valve by turning to the right.
-
11
Turn on peristaltic pump (speed = 0). Gel will begin to pour.
Be sure the polyacrylamide flows constantly without stopping, but not too fast that it splashes. -
12
Once gel is finished pouring, add 25 ml of ddH2O to each gradient mixer well. Run ddH2O through tubing to clean out polyacrylamide. Collect water in a waste container.
The waste will contain unpolymerized polyacrylamide and should be handled with caution and disposed of properly. -
13
After about half of the water has been pulled through the tubing, pour a layer of ddH2O onto the top of the gel
Use a very slow speed and be careful not disturb the gradient. If the gel “bounces,” the speed is too fast. -
14
Allow polyacrylamide gel to polymerize for 1 hour.
-
15
Clean tubing by adding ddH2O to gradient mixer wells and choosing the purge setting on peristaltic pump. Collect water in waste container for proper disposal.
Pouring the cap gel
-
16
Once the gel has polymerized, remove the water layer by pouring and blotting with paper towels or card stock
The card stock can be pushed between plates to absorb all H2O. DO NOT touch the gel with the blotting card stock. -
17
Attach the gel sandwich to the core prior to pouring the cap gel solution using standard manufacturer’s instructions.
-
18
Make solution for cap gel. For 1 gel, add 40 μl 10% ammonium persulfate solution and 2.5 μl TEMED to 5 ml of 0% denaturing solution.
-
19
Pull the cap gel solution into a 10 ml syringe without a sharp, place the syringe tip against the glass and push the 0% denaturing solution into the space over the gradient gel. Fill to halfway.
-
20
Place the comb slowly into the gel.
If bubbles format the base of the comb wells, remove the comb and slowly insert again. -
21
Fill the remaining space with cap gel solution. Be careful not to overflow the space. Allow 2 hours for polymerization.
Do not let the gel sit for longer than 6 hours or it will begin to dry. -
22
Mix 1xTAE Run Buffer in electrophoresis tank.
-
23
Put temperature control module on the electrophoresis tank, turn on and begin heating running buffer to 56°C. Ramp rate should be at the highest setting. This step will take about 2 hours.
-
24
Once buffer is 56°C and cap gel is fully polymerized, turn off and remove temperature control module and place core with attached gel assemblies into the electrophoresis tank.
-
25
Pour 350 ml of running buffer into the upper chamber.
-
26
Carefully remove comb.
Occasionally the wells collapse in on themselves during this step. If this happens, a thin piece of wire can be inserted between the plates and used to straighten out the wells. -
27
Clean unpolymerized polyacrylamide from wells by pipetting up and down with gel loading tip.
-
28
Add 5 ul loading dye to PCR product to be run on gel.
-
29
Load all PCR product into wells and a standard if available (see Support Protocol)
-
30
Replace temperature control module on tank, turn on and bring temperature back up to 56°C. This will take about 10 to 15 minutes.
-
31
Once temperature returns to 56°C, turn on voltage (70 volts) and run gel for 17 hours.
-
32
Turn off and remove temperature control module from tank. Carefully remove the core from the tank and the gel assembly from the core.
-
33
Carefully remove gel from gel assembly and place into a staining container.
-
34
Dye with Gel Red solution. Cover the pan with aluminum foil. Stain for 40 minutes under constant agitation in a clinical rotator.
-
35
Carefully remove gel from staining pan and fluoresce with a UV transilluminator.
Excision and identification of bands in gel
-
36
Excise bands from gel with sterilized metal spatulas and place into 0.5 ml microcentrifuge tubes.
Spatulas need to be flame sterilized. Be careful to limit exposure to UV light. -
37
Rinse excised band with 200 μl molecular grade H2O. Discard water.
-
38
Add 100 μl molecular grade H2O to the microcentrifuge tube.
-
39
Let sit overnight at 4°C. Use next day or store at minus;20°C.
-
40
Amplify 2 μl band supernatant using the same PCR conditions as listed in Basic Protocol 2.
-
41
Confirm amplification by electrophoresing PCR product on a 1.5% agarose gel.
-
42
Sequence product using forward primer without the GC clamp.
Primer sequence: 5′-CCT ACG GGA GGC AGC AG-3′
-
43
Edit, identify and analyze sequence data using bioinformatics software or sequence analysis program.
Geneious Pro and NCBI BLASTn are recommended as they are user-friendly and yield consistent quality results.
SUPPORT PROTOCOL Creation of a ladder from bacteria bands
To facilitate comparisons between samples that are run on multiple gels and identify common bacteria types within arthropod samples, a ladder can be created. This is not a size ladder, but rather one created from amplification of specific bacteria types. Once the common bands in the arthropod sample have been identified, they can be reamplified with PCR, pooled together and the ladder can be used as a marker for comparisons between gels.
Materials
See Basic Protocol 3.
Follow methods listed in Basic Protocol 3.
-
Determine which bands on the resulting gel would be most useful as a ladder.
For example, using common bands would reduce the need to sequence the bands every run. It is also best to not choose bands that are very close together, as they would be difficult to differentiate. Use the supernatant from basic protocol 3 step 40 as template for PCR as in Basic Protocol 2.
Confirm amplification by electrophoresing a small amount of PCR product on a 1.5% agarose gel.
Combine PCR product from multiple samples. This can be aliquoted and frozen at 20°C.
For each DGGE run, 10 μl should be placed alongside the PCR product that is being analyzed.
REAGENTS AND SOLUTIONS
Use deionized distilled water in all recipes and protocol steps.
Phosphate buffered saline buffer (PBS)
8 g NaCl
0.2 g KCl
1.44 g Na2HPO4
0.24 g KH2PO4
Add H2O to 1000 ml. Adjust pH to 7.4
Autoclave and store at room temperature.
Homogenization buffer
0.6 g Tris base
0.19 g EDTA
1.46 g NaCl
Add H2O to 500 ml.
Autoclave and store at room temperature.
0.5x TBE Buffer
Make 5x Concentrated Stock Solution:
54g Tris base
27.5 g Boric acid
20 ml 0.5 M EDTA (pH 8.0)
Add H2O to 1000 ml
Autoclave and store at room temperature.
To create 0.5xworking stock solution:
Add 100 ml 5xTBE Buffer to 900 ml of H2O
1.5% Agarose gel (100 ml)
1.5 g agarose
100 ml 0.5x TBE buffer
Heat to boiling to allow agarose to melt.
Cool to 70°C.
Add 10 μl of Gel Red
Pour gel into cast
Allow to solidify for 1 hour before use.
50x TAE Buffer
242 g Tris base
57.1 ml glacial acetic acid
100 ml 0.5 M EDTA
Add H2O to 1 liter. Adjust pH to 8.5.
Autoclave and store at room temperature.
Polyacrylamide denaturing solutions
The denaturing solutions in this unit contain 10% polyacrylamide, which is ideal for separation of PCR products that are 100 to 300 base pairs long. Components of the denaturing solutions include:
-
40% Acrylamide/Bis (37.5:1)
Light sensitive, cover bottle in aluminum foil and store at 4°C.
50X TAE Buffer
-
Formamide
Light sensitive, cover bottle in aluminum foil and store at 4°C.
Urea
Glycerol
H2O
The denaturing gradients are created with varying degrees of urea and formamide. This protocol utilizes a 10% polyacrylamide gel with a low concentration of 40% and a high concentration of 70%. However, any percent denaturing solution can be made using Table 1.
Table 1.
Components of denaturing solutions for 10% polyacrylamide gel
| Components | 0% | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 100% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 40% Acrylamide/Bis | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| 50x TAE | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Formamide | - | 2.0 | 4.0 | 6.0 | 8.0 | 10.0 | 12.0 | 14.0 | 16.0 | 18.0 | 20.0 |
| Urea | - | 2.1 | 4.2 | 6.3 | 8.4 | 10.5 | 12.6 | 14.7 | 16.8 | 18.9 | 21.0 |
| Glycerol | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
|
|
|||||||||||
| ddH2O | To 50 ml | ||||||||||
Mix all components together in a 50 ml conical tube, wrap in aluminum foil and store at 4°C. Solutions can be stored for 1 month.
If using the solution immediately, it needs to be chilled to 4°C to prevent polymerization mid-pour.
At higher concentrations, it is difficult to dissolve all the urea. Placing the solution in a heated water bath with occasional agitation can decrease time to dissolution.
10% Ammonium persulfate solution
Add 0.1 g of ammonium persulfate to 1 ml ddH2O
Mix thoroughly
This can be made in bulk, aliquoted and stored at minus;20°C for 3 months
1x TAE run buffer
Add 140 ml 50x TAE Buffer to 6860 ml ddH2O.
This may be saved and used for a second time.
50 ml may be used for the Gel Red Solution (see below).
Gel Red solution
Mix 50 ml 1x TAE Buffer and 10 μl Gel Red Nucleic Acid Stain (10,000x in DMSO).
Solution is light sensitive. Store in sealed container such as 50 mL conical tube wrapped in aluminum foil until use.
COMMENTARY
Background Information
The complex microbial community within arthropods has the potential to become a resource for the management of arthropod-related problems (Crotti, Balloi et al. 2012). Studying individuals of the bacterial community may determine how bacteria affect host physiology and is important in identifying possible candidates for these control applications. Initial studies aimed at cataloguing the bacterial community in its entirety focused on culture-dependent techniques and may have presented a biased description of the microbiota present within arthropods. The media used had been developed for medical studies and did not take into account different pH and nutrients that may be available to the bacteria in the midgut (Dillon and Dillon 2004). In addition, insects are known to harbor bacterial symbionts in specialized organs (Dale and Moran 2006; Kikuchi 2009), and may have different growth requirement from those present in the midgut, making multiple cultivation methods necessary to identify all members of the bacterial community.
There are several culture-independent approaches that have been used to investigate the array of microbes within arthropods(Shi, Syrenne et al. 2010). The majority of these target amplification of 16S rDNA using PCR, which allows for general identification of the microbial community as a whole. The mixed PCR products resulting from these amplifications can be separated using a multitude of methods, such as denaturing gradient gel electrophoresis (DGGE), temperature gradient gel electrophoresis (TGGE), restriction fragment length polymorphism (RFLP), single strand conformation polymorphism (SSCP) and random amplified polymorphic DNA (RAPD) (Shi, Syrenne et al. 2010). Of these techniques, DGGE is the most commonly used in studies that focus on identifying the microbial community within arthropods.
DGGE was originally developed to identify point mutations in human molecular genetics but has since been adapted for evaluating microbial diversity in a number of different environments, including arthropods (Muyzer, Dewaal et al. 1993; Muyzer and Smalla 1998). Using DGGE, the entire microbial community can be profiled on a single gel simply by using PCR and electrophoresing the product through a vertical denaturing gradient gel that separates the partial amplicons based on nucleotide sequence composition (Muyzer, Dewaal et al. 1993). After band excision and reamplification, sequence information can be acquired from genetic databases. DGGE has several advantages over other molecular techniques that separate mixed PCR products, such as cloning. DGGE allows direct analysis of DNA in environmental samples without requiring cell isolation or culture. Bacterial community structures from a relatively large number of samples can be directly compared using molecular fingerprint profiles present on the gel. This allows for community level knowledge, rather than individual. In addition, band intensities can convey useful information about the relative proportions of different genotypes in a community, provided that PCR bias is taken into account.
Disadvantages with DGGE, as with any PCR-based technique, are the inherent bias associated differential cell lysis during DNA extraction and preferential amplification during PCR (Suzuki and Giovannoni 1996; Polz and Cavanaugh 1998). The absence of a DGGE band may not indicate the absence of the bacterium from the sample. This issue can be avoided by combining PCR-DGGE and culture-dependent techniques to provide a more complete profile of the microbial community within insects (Lindh and Lehane 2011; Zouache, Raharimalala et al. 2011). Even with this disadvantage, DGGE remains an excellent tool for the initial characterization of bacterial communities within arthropods as evidenced by its widespread use in the literature (Reeson, Jankovic et al. 2003; Schabereiter-Gurtner, Lubitz et al. 2003; Hui, Wei et al. 2006; Fall, Hamelin et al. 2007; Behar, Yuval et al. 2008; Mrazek, Strosova et al. 2008; Zahner, Lucarotti et al. 2008; Dillon, Webster et al. 2010).
Critical Parameters and Troubleshooting
Premature polymerization
If polyacrylamide gel solutions are polymerizing mid-poor and plugging tubing, it could be due to temperature of the solution and timing. Once TEMED and ammonium persulfate are added to the denaturing gel solutions, the polymerization process begins. Have all materials that will be used in this step set up prior to addition of the polymerizing reagents and work quickly to ensure the gel does not polymerize mid-pour. In addition, polyacrylamide gels polymerize faster at room temperature. Care should be taken to store the denaturing gel solutions at 4°C prior to pouring. If solutions are left at room temperature for too long, polymerization will occur within 20 minutes of adding TEMED and ammonium persulfate.
Poor polyacrylamide gel polymerization
If polyacrylamide gels failed to polymerize, replacing the TEMED and making fresh ammonium persulfate may fix this problem.
Poor band separation
If bands are not separating as well as expected, a more stringent denaturing gradient may alleviate this issue. See Table 1 for components of other denaturing solutions.
Anticipated Results
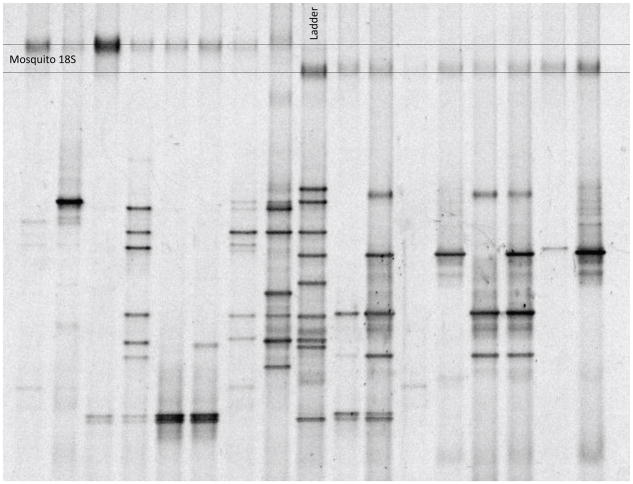
Proper use of sterilization and DGGE methods should produce a gel with clean discernable bands that are easily excised (Figure 1). Amplification of the bands using PCR, sequencing and BLASTn should yield identities for a majority of bands. In some cases, 18S rDNA is also amplified with the general 16S rDNA primers.
Figure 1.
DGGE profile of 16S rDNA gene fragments amplified by PCR from mosquitoes with corresponding ladder. Each column represents one mosquito sample. Each band represents a different bacterial identity.
Time Considerations
Surface sterilization is a relatively quick method, provided a small number of arthropods are being processed. Processing large numbers of arthropods or dissecting tissues can increase this handling time. DNA extraction has an overnight incubation period. This can be decreased to 3 hours if DNA yield is not dependent of time of incubation period. The touchdown PCR program takes about 1.5 hours.
From start to finish, pouring the gradient gel to full polymerization takes about 4 hours. Polymerization of the polyacrylamide gel usually occurs within 1 hour, provided that the solutions are kept at 4°C. This step can be performed simultaneously with PCR. Once the PCR products are loaded onto the gel, the DGGE runs for 17 hours. This can be run overnight. Staining the polyacrylamide gel takes 40 minutes. Illumination and cutting bands should not take more than 30 minute, as exposure to UV light needs to be limited. Bands are placed at 4°C overnight before reamplification. The entire process takes about 3 days from start to finish.
Acknowledgments
This research was supported by a grant from the National Institutes of Health [AI-067434].
Literature Cited
- Behar A, Yuval B, et al. Community structure of the Mediterranean fruit fly microbiota: Seasonal and spatial sources of variation. Isr J Ecol Evol. 2008;54(2):181–191. [Google Scholar]
- Crotti E, Balloi A, et al. Microbial symbionts: a resource for the management of insect-related problems. Microbial Biotech. 2012;5(3):307–317. doi: 10.1111/j.1751-7915.2011.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C, Moran NA. Molecular interactions between bacterial symbionts and their hosts. Cell. 2006;126(3):453–465. doi: 10.1016/j.cell.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Dillon RJ, Dillon VM. The gut bacteria of insects: Nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Dillon RJ, Webster G, et al. Diversity of gut microbiota increases with aging and starvation in the desert locust. Anton Leeuw Int J G. 2010;97(1):69–77. doi: 10.1007/s10482-009-9389-5. [DOI] [PubMed] [Google Scholar]
- Fall S, Hamelin J, et al. Differences between bacterial communities in the gut of a soil-feeding termite (Cubitermes niokoloensis) and its mounds. Appl Environ Microb. 2007;73(16):5199–5208. doi: 10.1128/AEM.02616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui XA, Wei GF, et al. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera) Can J Microbiol. 2006;52(11):1085–1092. doi: 10.1139/w06-064. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Insect symbiosis: An introduction. In: Bourtzis K, Miller TA, editors. Insect symbiosis. Boca Raton, FL: CRC Press; 2003. pp. 1–22. [Google Scholar]
- Kikuchi Y. Endosymbiotic bacteria in insects: Their diversity and culturability. Microbes Environ. 2009;24(3):195–204. doi: 10.1264/jsme2.me09140s. [DOI] [PubMed] [Google Scholar]
- Lindh JM, Lehane MJ. The tsetse fly Glossina fuscipes fuscipes (Diptera: Glossina) harbours a surprising diversity of bacteria other than symbionts. Anton Leeuw Int J G. 2011;99(3):711–720. doi: 10.1007/s10482-010-9546-x. [DOI] [PubMed] [Google Scholar]
- Moran NA. Bacterial menageries inside insects. P Natl Acad Sci USA. 2001;98(4):1338–1340. doi: 10.1073/pnas.98.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek J, Strosova L, et al. Diversity of insect intestinal microflora. Folia Microbiol. 2008;53(3):229–233. doi: 10.1007/s12223-008-0032-z. [DOI] [PubMed] [Google Scholar]
- Muyzer G, Dewaal EC, et al. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59(3):695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G, Smalla K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Anton Leeuw Int J G. 1998;73(1):127–141. doi: 10.1023/a:1000669317571. [DOI] [PubMed] [Google Scholar]
- Polz MF, Cavanaugh CM. Bias in template-to-product ratios in multitemplate PCR. Appl Environ Microb. 1998;64(10):3724–3730. doi: 10.1128/aem.64.10.3724-3730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeson AF, Jankovic T, et al. Application of 16S rDNA-DGGE to examine the microbial ecology associated with a social wasp Vespula germanica. Insect Mol Biol. 2003;12(1):85–91. doi: 10.1046/j.1365-2583.2003.00390.x. [DOI] [PubMed] [Google Scholar]
- Schabereiter-Gurtner C, Lubitz W, et al. Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. J Microbiol Methods. 2003;52(2):251–260. doi: 10.1016/s0167-7012(02)00186-0. [DOI] [PubMed] [Google Scholar]
- Shi WB, Syrenne R, et al. Molecular approaches to study the insect gut symbiotic microbiota at the ‘omics’ age. Insect Sci. 2010;17(3):199–219. [Google Scholar]
- Suzuki MT, Giovannoni SJ. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microb. 1996;62(2):625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner V, Lucarotti CJ, et al. Application of 16S rDNA-DGGE and plate culture to characterization of bacterial communities associated with the sawfly, Acantholyda erythrocephala (Hymenoptera: Pamphiliidae) Curr Microbiol. 2008;57(6):564–569. doi: 10.1007/s00284-008-9243-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jackson TA. Autochthonous bacterial flora indicated by PCR-DGGE of 16S rRNA gene fragments from the alimentary tract of Costelytra zealandica (Coleoptera: Scarabaeidae) J Appl Microbiol. 2008;105(5):1277–1285. doi: 10.1111/j.1365-2672.2008.03867.x. [DOI] [PubMed] [Google Scholar]
- Zouache K, Raharimalala FN, et al. Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol Ecol. 2011;75(3):377–389. doi: 10.1111/j.1574-6941.2010.01012.x. [DOI] [PubMed] [Google Scholar]