Abstract
Objective
Minor elevations in C-reactive Protein (CRP, > 3 mg/L) are a nonspecific marker of systemic inflammation and predict the future onset of cardiovascular disease. This report examines the association between marital status and CRP levels after accounting for a range of relevant of demographic, subjective and objective health indicators, and psychological variables.
Methods
Data from the National Social Life, Health, and Aging Project (NSHAP), a population-based study of community-dwelling older adults in the United States, were used to study CRP elevations. Home-based interviews were conducted with the entire NSHAP sample, a subset of whom provided whole blood samples for the CRP analyses. The final sample consisted of 1,715 participants (838 men) with an average age of 69.51 years. Multiple and logistic regression analyses were conducted using CRP as a continuous and dichotomous outcome variable.
Results
Across the entire NSHAP sample, married men evidenced the lowest levels of CRP. After adjusting for the competing predictors, marriage remained a unique protective factor against elevated CRP for men (OR = .56, 95% CI: 39-.79). The absolute risk reduction (for being classified in the high-risk CRP group) associated with being a married man was roughly equivalent to that observed for adults who were normotensive, non-smokers, and those with a normal body mass index.
Conclusions
Remaining married in late adulthood affords men unique robust protection against elevated levels of CRP. The findings are discussed in terms of the pathways linking marital status and health outcomes among older adults.
Keywords: Marital status, C-reactive protein, inflammation, risk reduction, aging
The past two decades have witnessed a surge of research dedicated to understanding the association between close relationships and health outcomes. House and colleagues (1) summarized evidence from several studies indicating that adults who report high levels of social integration live longer than people who report low levels of integration. This seminal work spurred several lines of inquiry, and the available evidence indicates that high quality social relationships have salubrious associations with cardiovascular, neuroendocrine, and immune system functioning (2-6). Conversely, loneliness, isolation, and social exclusion are risk factors for poor health (7-9). For many adults, marriage constitutes the most important relational context in which they live, and both marital quality and marital status are linked to important health outcomes (2, 10-12). Whereas a fair amount of research studies the mechanisms linking marital quality and health (13), must less is known about precisely how widowhood or divorce increase risk for poor outcomes. In the present report, I address this limitation by investigating the associations among C-reactive protein (CRP; an immune parameter that binds to damaged cells, is involved in initiating phagocytosis, and rises dramatically during periods of acute inflammation), marital status, and self-reported psychological distress in a nationally representative sample of older adults.
One of the primary limitations for understanding how marital status is associated with health is that relatively few studies investigate biomarkers that have calibrated associations with morbidity and mortality. A number of studies have examined cortisol and immune responses following both divorce (14-16) and bereavement (17-19). In general, the results of these studies indicate that becoming divorced or widowed is associated with catabolic neuroendocrine and immune responses, and the magnitude of the is typically associated with the degree of subjective distress observed within the divorced or bereaved group. For example, in a series of important studies, Kiecolt-Glaser and colleagues (14, 15) investigated the association between beliefs about one's divorce, depressive symptomology, and immune functioning. Greater levels of nonacceptance (of marital termination) two years after divorce were associated with prolonged depression and compromised immune functioning.
Process-level research of this nature is important and can be extended by investigating biomarkers that are calibrated in clinically meaningful ways. Blood pressure (BP) and CD4 helper T cells are two examples of biological parameters that are associated with clinically meaningful outcomes.1 Systolic BP over 140 mm/Hg greatly increases risk for adverse cardiovascular and cereberovascular events; similarly, declines in CD4 forecast the worsening of HIV-related disease processes. Both of these outcomes have been studied in the context of loss events (17, 20). Similar to these measures, CRP represents an ideal biomarker for the further investigation of marital status and health. Over 50 population-based studies have documented that minor elevations in baseline CRP levels (typically > 2.5 – 3 mg/L) are associated with the prospective emergence of cardiovascular disease (CVD) and cerebrovascular events (21). Although minor elevations in CRP levels among healthy adults cannot be deemed causal in the cascade from atherogenesis to end-point CVD (22, 23), CRP levels predict numerous disease processes, and elevations in this biomarker may reflect the underlying tissue damage or distressed cells that are associated with all-cause morbidity and mortality (24). CRP levels covary with traditional indicators of medical risk, including smoking, obesity, hypertension, and insulin resistance (25-27), and recent evidence indicates that tracking CRP levels in clinical practice can augment the utility of statin regimens (28) and play a role in the secondary prevention of stroke (29).
As an acute phase protein, CRP is an essential component of innate immunity and is produced by the liver in response to proinflammatory cytokines (e.g., Interleukin-6, Tumor Necrosis Factor-Alpha), which are signaling molecules that stimulate immune cell proliferation and differentiation in response to injury and inflection (30). Because CRP levels change in response of proinflammatory cytokines, it represents a useful, downstream marker of systemic inflammation. A growing body of evidence from both animal and human studies indicates that CRP levels are associated with negative psychological states (31), and that systemic inflammation can induce passive withdrawal from the environment (32). The most robust findings in the human literature indicate that higher levels of CRP are associated with depressed mood (33-37) and greater psychological stress (38, 39), although there are failures to replicate these effects (40, 41).
One explanation for the association between CRP levels and mood disruptions is that inflammatory responses can lead to what is referred to as sickness behavior or an acute-phase response (33), which is one of the body's main mechanisms for fighting invading microorganisms or defending against physical trauma and tissue damage (42). Social withdrawal, cognitive alterations and depressed mood, disturbed sleep, general weakness, and heightened endocrine activity are all characteristic of both sickness and human loss reactions (32, 43). With respect to grief, the malaise, depression, lethargy, and apathy that typically follow loss may thus be reinterpreted in terms of the functional value of sickness (44-46). These findings suggest that there is substantial reason to believe that CRP levels would vary as a function of marital status and, in particular, as a function of adults’ psychological distress following a separation or loss experience. From this perspective, psychological distress may explain the association between marital status and CRP levels and be a putative mediator for further inquiry in prospective investigations.
Moderators of Interest
The literature on marital status and health indicates that men tend to fare worse than women when relationships end. Said differently, the protective effect of marriage on health appears strong for men than women (2). Divorced men evidence faster times to death than women (47, 48), and the same effects are observed following conjugal bereavement (12). A variety of explanations are offered for this effect, most of which center on the idea that women cope with stress through social affiliation (49) and that for many married men, their primary source of social support is their wife (50). When relationships end, men lose their primary stress buffer, which is can lead to risky health behaviors (51), all of which contribute to worsened health outcomes. A recent laboratory study conducted by Sbarra and colleagues (20) found that men who found thinking about their divorce experience difficult evidenced substantial increases in systolic BP during a divorce-related laboratory task, whereas women, even those who were distressed by thinking about their divorce, evidenced no changes in BP across the task. Based on the body of evidence in this area, I hypothesize that unmarried men will evidence the highest levels of CRP relative to married men and both married and unmarried women.
When considering the association between marital status and CRP, I argued above that psychological distress may serve as a potential mediator of this effect. It is equally possible that psychological distress moderates the association between marital status and CRP, with the highest levels of CRP observed among unmarried adults who report high levels of distress. Moreover, if changes in marital status are particularly difficult for men, it would be reasonable to expect a three-way interaction revealing that unmarried men who report high levels of psychological distress also will evidence the highest levels of CRP.
The Present Study
The present report investigates the association between marital status and CRP using data from the National Social Life, Health, and Aging Project (NSHAP), which is a cross-sectional, population-based study of the social life and health of community-dwelling older adults in the United States. The main hypothesis of the study is that unmarried men will show significantly higher CRP levels than married men or women. In addition, two exploratory analyses are conducted to determine if the association between marital status and CRP is (a) explained by self-reported psychological distress, and/or (b) moderated by self-reported psychological distress. Because the determination of statistical mediation requires temporal ordering of variables (52) and the NSHAP is a cross-sectional study, the first set of exploratory analyses may be viewed as determining the relative contribution of distal (marital status) and more proximal (psychological distress) variables to understanding variability in CRP levels. Should these analyses point toward an explanatory role for psychological distress, future prospective studies can formally evaluate mediating mechanisms.
Method
Participants and Procedures
NSHAP is a nationally-representative probability sample of adults aged 57 – 85 years. In-home interviews were conducted in English and Spanish by the National Opinion Research Center (NORC) between July, 2005 and March, 2006 and yielded a total of 3,005 respondent (1,455 men and 1,550 women). The primary demographic and health characteristics of the sample are displayed in Table 1. Additional information on the NSHAP sample is available in a separate report (53). In addition to face-to-face interviews and a leave-behind questionnaire covering self-reported health and medical history, medication use, marital history, perceived stress, mood disturbances, and loneliness, a panel of biomarkers was collected including weight, waist circumference, height, blood pressure, and blood spots. Whole blood spot collection was randomized to 83% of the entire NSHAP sample; of the 2,048 specimens collected, acceptable CRP values were available for 1,939 participants (931 men and 1008 women).
Table 1.
NSHAP demographic characteristics
| Continuous Variables | Mean (Standard Deviation) |
| Age (n = 1715) | 69.55 (7.8) |
| BMI (kg/m2) | 28.94 (5.74) |
| Mean systolic blood pressure | 136.06(20.31) |
| Mean household income | $53,071 ($86,197) |
| Total number of medicinesa | .18 (.44) |
| Total number of medical conditionsb | .86 (.76) |
| Categorical Variables | Number (Percent of Sample) |
| Sex | |
| Men | 838 (48.7) |
| Women | 877 (51.3) |
| Marital Status | |
| Married | 1085 (63.3) |
| Separated | 29 (1.7) |
| Divorced | 195 (11.4) |
| Widowed | 406 (23.7) |
| Education | |
| Less than high school | 370 (21.6) |
| High school degree or equivalent | 461 (26.9) |
| Vocational school/some college | 516 (30.1) |
| Bachelors degree or more | 368 (21.5) |
| Ethnicity | |
| Caucasian | 1281 (74.7) |
| African American | 218 (12.7) |
| Hispanic | 173 (10.1) |
| Other | 36 (2.1) |
| Self-rated physical health | |
| Poor to fair | 433 (25.3) |
| Good | 515(30.0) |
| Very good to excellent | 761 (44.3) |
| Current smoker | |
| Yes | 248 (14.5) |
| No | 1467 (85.5) |
Note.
Represents a summary scale based on participants’ reports of currently taking combination antihyperlipidemic medication, current cholesterol absorption inhibitor medication, estrogen replacement, progestin replacement, and non-steroidal anti-inflammatory drugs (score ranges from 0 – 5)
computed as a summary scale based on the presence/absence of a history of heart attack(s), a diagnosis of diabetes, a diagnosis of arthritis, and a diagnosis of hepatitis (score ranged from 0- 4).
Measures
High-sensitivity CRP (mg/L), the primary outcome measure in this report, was derived from the dried blood spots taken during the home interview. During the interview, whole blood was collected via a finger-stick and disposable lancet, then applied to filter paper for transport and storage. The blood spot assays were conducted at the Laboratory for Human Biology Research at Northwestern University according to the procedures described by McDade and colleagues (54, 55). The correlation between blood spot CRP and serum-derived CRP is high (Pearson r = .96), suggesting whole blood spot methods demonstrates CRP performance characteristics similar to those observed in standard venipuncture techniques (56). CRP levels assayed from the dried blood were converted to a serum equivalent (55). Because CRP is highly skewed in the general population (24), the current values were log-transformed (log-CRP) to normalize the distribution for use as a continuous outcome measure. Following standard recommendations for assessing cardiovascular risk when interpreting CRP in public health analyses (23), CRP also was studied as a group variable; adults were classified into low- (CRP <=3.0 mg/L) and high-risk (CRP > 3.0 mg/L) groups. Because serum CRP >10 mg/L can reflect acute infection, adults scoring in this range (n = 224) were excluded from the analyses.
The primary predictor of interest, marital status, was assessed during the home interview and dichotomized into two groups: currently married (n = 1,085) and previously married (which consisted of separated, divorced, and widowed adults, n = 630). Participants who reported never marrying constituted a small percentage of the total sample and were excluded from the present analyses. Covariates, or competing predictors (57), included demographic variables, subjective and objective health measures, and assessments of three psychological states. Demographic variables included participants’ age, sex, ethnicity, education, and mean household income. Subjective health variables included self-rated health (on a 5-point scale from poor to excellent), number of physician diagnosed medical problems (computed as a summary scale based on the presence/absence of a history of heart attack(s), a diagnosis of diabetes, a diagnosis of arthritis, and a diagnosis of hepatitis; the summary scale was treated as a continuous index that ranged from 0 to 4 diseases), the total number of medications taken (computed as a summary scale based on participants’ reports of currently taking combination antihyperlipidemic medication, current cholesterol absorption inhibitor medication, estrogen replacement, progestin replacement, and non-steroidal anti-inflammatory drugs), and current smoker status (yes/no). Objective health data included body mass index (BMI, kg/m2) and mean systolic blood pressure (calculated across three resting assessments). Psychological distress was assessed via three self-report measures, including the 11-item Center for Epidemiological Studies Depression scale (CES-D, assessing symptoms of major depressive disorder, 58), a four-item perceived stress scale (PSS, assessing perceptions of coping with life stress, 59), and a three-item loneliness scale (assessing perceptions of lack of companionship, isolation, and social exclusion, 60). Because the three measures are highly correlated, scores on these scales were standardized, and a single self-report psychological distress index was computed for the present analyses (α = .72).
All respondents provided written informed consent. The protocol was approved by the institutional review boards of the University of Chicago, NORC (National Opinion Research Center), and the University of Arizona.
Data Analysis
Orthogonal planned contrasts were computed to test the hypothesis that unmarried men would evidence the highest CRP levels. The values of the contrast variables are displayed in Table 2. As shown, C1 compares unmarried men to all other participants, C2 compares unmarried men to women, and C3 compares married and unmarried women. Multiple regression analyses (61) were conducted with log-CRP as the outcome of interest. The first models, adjusted only for participants’ age, tested the planned contrasts and conducted the exploratory analyses that considered the psychological distress variable as a potential mediator and moderator. The next series of models included the demographic and health covariates of interest to determine if the unadjusted effects were retained after accounting for competing predictors. Finally, logistic regression (62) was used to model the likelihood of being classified as having elevated CRP levels as a function of marital status. For the classification analyses, results are presented as odds ratios (OR) with 95% confidence intervals (CI); where applicable, the absolute risk reduction for being classified as having elevated CRP levels is reported as a function of participants’ marital status. Analyses were performed using SPSS version 16.0 (63).
Table 2.
Mean CRP levels and planned contrast variables by participant sex and marital status
| Group (Sample Size) | Mean CRP Level- mg/L (Standard Deviation) | Contrast 1 (C1) | Contrast 2 (C2) | Contrast 3 (C3) |
|---|---|---|---|---|
| Married Men (662) | 2.16 (2.04) | 1/4 | -2/3 | 0 |
| Unmarried Men (423) | 2.72 (2.49) | -3/4 | 0 | 0 |
| Married Women (176) | 2.61 (2.28) | 1/4 | 1/3 | -1/2 |
| Unmarried Women (454) | 2.79 (2.34) | 1/4 | 1/3 | 1/2 |
Note. Statistical comparisons were computed using log-transformed CRP. C1 compares unmarried men to all other participants, C2 compares married men to women, and C3 compares married and unmarried women.
Results
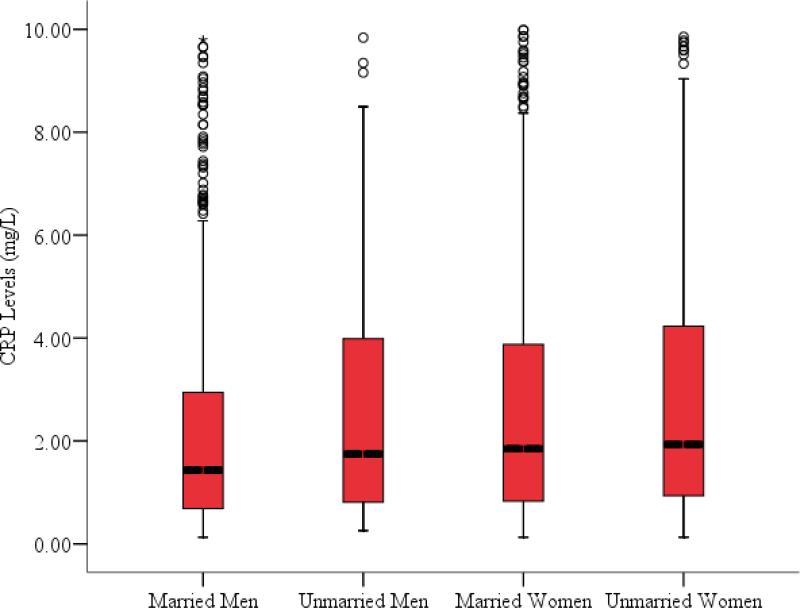
Figure 1 displays the distributions of CRP across the four marital status groups and Table 2 displays the means and standard deviations for each group. An analysis of variance (ANOVA) conducted on the log-transformed scores was significant, F(3,1711) = 9.45, p < .001, and post-hoc comparisons using Tukey's honestly significant different (HSD) test revealed that married men evidenced significantly lower CRP levels than married or unmarried women, and a trend (p = .07) toward lower levels than unmarried men. The upper panel of Table 3 presents the association between participants’ age, the three planned contrasts for marital status and log-CRP. Contrast 1 was not significantly associated with log-CRP, indicating that unmarried men do not evidence greater CRP levels than married men or women. Contrast 2 was significant, indicating that married men evidenced lower CRP levels than both married and unmarried women. Contrast 3 was not a significant predictor of log-CRP. When added to the model, and after accounting for participants’ age and the marital status contrasts, the psychological distress variable was uniquely associated with log-CPR, t(1714)= 2.12, p = .03, but the interaction between C1 and psychological distress was non-significant, t(1714)= -.02, p = .98. Participants’ reporting higher levels of psychological distress evidenced higher levels of CRP, but psychological distress does not moderate the association between marital status and CRP.
Figure 1.
Boxplot illustrating the distribution of CRP (mg/L) levels by participant sex and marital status. Boxes represent the distribution of CRP levels between the 25th and 75th CPR quartiles within each group; the median CRP value within group is indicated by the darkened horizontal line within each box. Error bars represent values the distribution of values 150% above and below the interquartile range (IQR); individual data points falling above the error bar are plotted in the figure.
Table 3.
Results of multiple regression analyses predicting log-transformed CRP levels
| Model 1 | B (95% CI) | SE | t | p |
|---|---|---|---|---|
| Age | -.003 (-.006, -.001) | -.001 | -2.44 | .02 |
| C1 (unmarried men vs. all others) | -.02 (-.08, .04) | -.03 | -.56 | .58 |
| C2 (married men vs. women) | .11 (.07, .15) | .02 | 5.31 | < .001 |
| C3 (married women vs. unmarried women) | .05 (-.004, .10) | .03 | 1.81 | .07 |
| Model 2 | β | t | p | |
|---|---|---|---|---|
| Age | -.003 (-.006, -.001) | .001 | -2.44 | .02 |
| C4 (married men vs. all others) | .10 (.06, .14) | .02 | 4.99 | < .001 |
| C5 (married women vs. unmarried men and women) | .03 (-.03, .08) | .03 | 1.06 | .29 |
| C6 (unmarried men vs. unmarried women) | .04 (-.03, .11) | .04 | 1.24 | .21 |
Note. CI = Confidence Interval. SE = Standard error. Inclusion of the three orthogonal contrasts in each model account for all of the variance in CRP levels associated with participant sex and marital status.
Given that the most robust effect observed in the first model was for C2 (the contrast comparing married men to women), a new set of contrasts was computed to determine if married men evidenced lower levels of CRP relative to all other participants. Thus, Contrast 4 (C4) compared married men to all other participants, Contrast 5 (C5) compared married women to unmarried men and unmarried women, and Contrast 6 (C6) compared unmarried men to unmarried women. The lower panel of Table 3 presents the association between participants’ age, the second set of marital status contrasts, and log-CRP. Relative to all other participants, married men evidenced the lowest CRP levels. As with the first set of contrasts, psychological distress was a significant positive predictor of CRP levels, t(1714)= 2.01, p = .04, but the interaction between C5 and psychological distress was non-significant, t(1714)= -1.59, p = .11. The association between C5 and log-CRP remained significant once psychological distress was entered into the model, t(1714)= 4.33, p < .001. Rather than explaining the association between marital status and CRP, it appears (in the unadjusted models) that psychological distress and marital status are unique correlates of CRP levels.
The next set of analyses sought to determine if the observed effects remained significant after accounting for variance associated with demographic and health variables of interest. The leftmost panels of Table 4 display the findings for the augmented log-CRP model. Contrast 5, comparing married men to all other participants, remained a unique predictor of CRP with married men evidencing the lowest level. Once the competing predictors of interest were entered into the model, the psychological distress composite was no longer associated with log-CRP. Exploratory analyses revealed that the association between psychological distress and log-CRP was fully explained by self-reported health; psychological distress and self-reported health were negatively correlated. r = -.32, p < .001, and the regression analyses indicated that the residualized health variable was a stronger predictor of CRP than the residualized psychological distress variable.
Table 4.
Summary of covariate adjusted multiple and logistic regression models predicting CRP levels
| Variable | B (95% CI)a | SE | t | p | Odds Ratio (95% CI)b | p |
|---|---|---|---|---|---|---|
| Age | .001 (-.02, .04) | .002 | .67 | .50 | 1.00 (.98, 1.03) | .47 |
| Ethnicity | .02 (-.03, .08) | .03 | .81 | .41 | .80 (.55, 1.14) | .24 |
| Education | -.03 (-.06, -.01) | -.08 | -2.62 | .009 | 1.98 (1.12, 3.31) | .009 |
| Household income | .00 (.00, .00) | .01 | -.82 | .41 | 1.00 (1.00, 1.00) | .69 |
| Self-rated physical health | -.04 (-.06, -.01) | -.09 | -3.03 | .002 | .71 (.60, .84) | < .001 |
| Smoker status | .13 (.07, .20) | .12 | 3.99 | < .001 | .54 (.36, .82) | .004 |
| BMI (kg/m2) | .02 (.02, .03) | .31 | 10.67 | < .001 | 1.10 (1.07, 1.13) | < .001 |
| Mean systolic blood pressure | .00 (.00, .01) | .05 | 1.99 | .05 | 1.00 (1.01, 1.02) | .01 |
| Total diseases | -.02 (-.05, .01) | -.04 | -1.27 | .20 | .95 (.76, 1.19) | .66 |
| Total medications | .05 (.00, .11) | .06 | 1.96 | .05 | 1.17 (.82, 1.68) | .37 |
| Psychological distress | -.00 (-.03, .03) | .02 | -.28 | .78 | .93 (.76, 1.14) | .48 |
| C4 (married men vs. all others) | .09 (.05, .15) | .12 | 3.69 | < .001 | .56 (.39, .79) | .001 |
| C5 (married women vs. unmarried men and women) | -.04 (-.10, .03) | -.03 | 1.06 | .30 | .71 (.48, 1.08) | .11 |
| C6 (unmarried men vs. unmarried women) | .02 (-.05, .10) | .02 | .57 | .56 | .90 (.54, 1.51) | .70 |
Note.
Model predicting continuous, log-transformed CRP levels.
Model predicting CRP group membership; partcipants were classified into low- (CRP <=3.0 mg/L) and high-risk (CRP > 3.0 mg/L) groups. CI = Confidence Interval.
SE = Standard error. Ethnicity grouping compares Caucasians to all other participants; Education grouping compares those with less than a high school degree to all other participants. Due to missing data across the different variables, the sample size for this analysis was 1,115 participants.
A final series of analyses, presented in the rightmost panels of Table 4, were conducted using the CRP grouping variable. Relative to all other participants, married men were 44% less likely to be classified in the high risk CRP group than all other participants. As with the continuous CRP variable, psychological distress did not predict group membership once the other variables were entered into the model. The unadjusted absolute risk (AR) for being classified in the high-risk CRP group for married men was 18.73/100, whereas the AR for women and unmarried men was 27.7/100, yielding an absolute risk reduction (ARR) for elevated CRP among married men of 8.97%.2 Table 5 displays the ARR associated with being a married male relative to the ARR associated with being a non-smoker, normotensive (< 140 mm/Hg), and of normal BMI (< 25 kg/m2). As shown, the raw ARR percentages across these four groups are roughly equivalent.
Table 5.
Absolute risk reduction associated with being a married man, a non-smoker, having normotensive systolic blood pressure (< 140 mm/Hg), and having BMI within normal limits (< 25 kg/m2)
| Variable | Absolute Risk Reduction (ARR) |
|---|---|
| Married men | 10.34% |
| Non-smokers | 7.94% |
| Normotensive SBP | 3.42% |
| Normal BMI | 10.34% |
Note. SBP = Systolic blood pressure; BMI = Body Mass Index. ARR for being a married male was calculated by subtracting the absolute risk observed in this group (number of married men classified evidencing high-risk CRP levels/all married men) from the absolute risk observed among all other participants (number of women and unmarried men evidencing high-risk CRP levels/total number of women and unmarried men).
Discussion
Based on literature indicating that men suffer more adverse health consequences than women when marital relationships end, it was hypothesized that unmarried men in the NSHAP sample would evidence the highest CRP levels. The results provided no support for this hypothesis. However, the initial set of planned contrasts indicated that married men had significantly lower CRP levels than women, and a series of follow-up analyses indicated that married men had lower CRP levels relative to all other participants. Using a well-validated approach to classifying people into high- and low-risk CRP groups (23), married men were 44% less likely to be classified in the high risk group relative to all other participants. There was no evidence that the association between marital status and CRP could be explained by participants’ self-reported psychological distress, nor did psychological distress moderate the effects of interest.
Over 50 population-based studies have documented that minor elevations in baseline CRP levels (typically > 2.5 – 3 mg/L) are associated with the prospective emergence of CVD and cerebrovascular events (21). Older married men are protected against minor elevations in CRP that have proven clinically meaningful in a large number of prior studies. This effect held after adjusting for a range of demographic characteristics, subjective and objective health measures, and self-reported psychological distress. Importantly, as shown in Figure 2, the magnitude of ARR (for being classified in the high risk CRP group) among married men is roughly equivalent to that of being a non-smoker, nomotensive, and having a BMI within a normal range, suggesting that the protection afforded men by marriage is as robust a correlate of CRP as these objective-- and widely studied-- health indices. It is important to recognize, however, that the NSHAP sample is a mean age of almost 70 years, which is far higher than the life expectancy for a current smoker (64). Thus, the ARR statistics must be qualified by noting that the marital protection effect for men approximates that of being a non-smoker, nomotensive, and having a BMI within a normal range among adults who live into their late 60s and early 70s. Because men's average life expectancy (75.2 years) is roughly five years lower than women's (80.4 years) (65) the results also must be understood in relation to the normative death rate. In this respect, men in the NSHAP are closer to their average life expectancy than the women in the sample and women are more likely to be widowed than men.
Relative to women, men suffer more adverse health consequences when marriage ends, either through partner death or divorce (12, 48). Evidence suggests that women's health is more responsive to variations in marital quality than men, whereas men's health is more closely linked to marital status (2). The present findings qualify this association by demonstrating that the observed protective effects on CRP for married men are not due to lower levels of psychological distressed, indexed in the NSHAP sample as a combination of perceived stress, mood disturbance, or social isolation. Furthermore, the group differences in CRP cannot be attributed to self-rated health or the measured health behaviors (smoking status). Of course, numerous unmeasured variables exist that can explain these effects. Marriage may confer support effects for men that are not associated with three NSHAP measures of psychological distress, and these processes, in turn, may reduce CRP by enhancing positive and/or diminishing negative emotions. When considering these points, a key conceptual distinction is whether the observed effects constitute a protective effect (for married men) or a risk effect (for all other participants). Statistically, protection and risk are the opposite sides of the same coin; showing that married men are 44% less likely (OR = .56) to be classified in the high risk category is equivalent to saying that unmarried men and women are 44% more likely (OR = 1.44) than married men to be classified in the high risk CRP grouping. Because the means of all groups are in the normal range of CRP functioning, and married men evidence significantly lower levels of CRP than all other participants, it is reasonable to describe the observed CRP grouping effect as one of protection for married men rather than risk for all other participants.
The search for underlying mechanisms linking social relationships to objective health outcomes typically considers three related pathways (66): social selection (whereby variables associated with poor health-- e.g., hostility-- also increase the likelihood for relationship instability and distress), health behaviors (whereby relationships have a positive effect on health decisions), and psychological stress (whereby the acute and chronic stress associated with disharmonious or lost relationships leads to greater physiological reactivity). The present findings indicate that, for men, marital status may have a direct effect on CRP levels that remains significant after accounting for a variety of measures of health behavior and putative psychological stress. A fourth pathway of interest, relatively new to the literature and denoted as social baseline theory (67), may help explain why the lowest levels of CRP are observed among married men. According to the theory, social bonds represent a default state for mitigating against threats in the environment and for dealing with the acute challenges of daily life; from this perspective, mammals are best equipped to regulate environmental threats in the context of an intact social bond by sharing resources and distributing risk across the dyad or group (rather than managing environmental demands at the level of the individual). It is widely recognized that married women shoulder many more of the burdens for family life than married men (68) and show greater and more sustain physiological responses to relational conflict (2). A key aspect of the social baseline theory is that uneven load sharing is metabolically costly and, in inequitable and high conflict situations, can result in negative physical outcomes. Gendered divisions of family life may provide married men a unique context in which they are protected against the vicissitudes of daily life relative to their unmarried counterparts or to women in general.
Despite the fact that this is one of the first reports to examine the association between marital status and CRP levels, the relative limitations of the work should be noted. First, the extent to which the observed effects generalize to all married couples remains unknown. The NSHAP is a population-based study of older adults, and the average participant was almost 70 years old (with a range from 57 to 89 years). If replicated across samples with a greater age range, the results suggest that marital status should be considered among a group of important risk and protective factors associated with clinically meaningful elevations in CRP. Second, the cross-sectional design of NSHAP precludes are more detailed analysis of the ways in which changes in marital status are associated with changes in CRP levels; demonstrating that CRP levels are sensitive to changes in social context would provide a more informative series of analyses than those conducted here. An NSHAP follow-up assessment is planned, and it will be possible to conduct these types of analyses in the future.
Conclusion
Using data from the population-based NSHAP, this report investigated the role of marital status in predicting clinically meaningful elevations in CRP among older adults. After accounting for a range of relevant demographic, subjective and objective health, and psychological variables, the findings revealed that married men demonstrated the lowest CRP levels. Being married in older adulthood affords men an ARR (for being classified among the high-risk CRP group) that is almost three times greater than having normotensive blood pressure and roughly equivalent to being a non-smoker and having a healthy BMI, three widely studied biomarkers that have clear public health relevance. One of the unique aspects of the present findings is that marital status represents a true protective factor for older men. None of the four groups had mean CRP levels greater that 2.5 – 3 mg/L, and it is therefore inaccurate to describe being unmarried men or female as risk factors for elevated CRP; rather, the results underscore the protective benefits from elevated CRP afforded to married men.
Acknowledgments
The author wishes to thank Thomas McDade, Ph.D. for helpful comments on an earlier version of this manuscript. NSHAP was supported by grants from the National Institutes of Health, including the National Institute on Aging, the Office of Research on Women's Health, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (5R01 AG021487), and by the National Opinion Research Center (NORC). The NORC was responsible for the interview data collection. All other aspects of study design and data collection were conducted independent of the funding sources. For the present analyses, the publically available version of the NSHAP data was used: Waite LJ, Laumann EO, Levinson W, et al. National Social Life, Health, and Aging Project (NSHAP) [computer file]. ICPSR20541-v3.: Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2008-12-08. doi:10.3886/ICPSR20541, 2008. The author of this paper had access to all publically available data, and was responsible for all data analysis and report writing. While writing this manuscript the author was supported in part by grants from the National Institute of Aging (AG#028454) and the National Institute of Mental Health (MH#074637).
Abbreviations
- AR
Absolute risk
- ARR
Absolute risk reduction
- BMI
Body mass index
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- NSHAP
National Social Life and Aging Project
Footnotes
This point does not diminish the importance of studying neuroendocrine markers such as cortisol. An enormous body of animal and human literature demonstrates that hypothalamic-pituitary-adrenal (HPA) axis dysregulation leading to enhanced or suppressed cortisol responding that can compromise health (69, 70), and that neuroendocrine responses covary in expectable ways with psychological stress (39). However, there is no commonly accepted metric for determining when cortisol levels are clinically elevated. This represents a limitation for understanding group differences in health outcomes; divorced and bereaved adults may evidence significantly higher levels of cortisol responding than married adults, but there is no way of determining if the levels of both groups are within a normative range of responding.
AR is calculated as ratio of participants classified in the high risk CRP group relative to all participants in a given group. For example, 124 out of 662 married men evidenced CRP elevations in the high risk range, yielding an AR of 18.73%. ARR is the simple difference in AR between two target groups of interest (e.g., married men relative to unmarried men and women; non-smokers relative to smokers, etc.).
References
- 1.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 2.Kiecolt-Glaser J, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 3.Ryff CD, Singer BH. Emotions, social relationships, and health. Oxford University Press; New York: 2001. [Google Scholar]
- 4.Uchino BN. Social support and physical health: Understanding the health consequences of relationships. Yale University Press; 2004. [Google Scholar]
- 5.Waite LJ, Gallagher M. Why married people are happier; healthier; and better off financially. Broadway Books; New York: 2000. The Case for Marriage. [Google Scholar]
- 6.Cohen S, Syme LS. Social support and health. Academic Press; Orlando, FL: 1985. [Google Scholar]
- 7.Cacioppo JT, Hawkley LC, Crawford E, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: Potential mechanisms. Psychosomatic Medicine. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Seeman TE, Kaplan GA, Knudsen L, Cohen R, Guralnik J. Social network ties and mortality among the elderly in the Alameda County Study. American Journal of Epidemiology. 1987;126:714–23. doi: 10.1093/oxfordjournals.aje.a114711. [DOI] [PubMed] [Google Scholar]
- 9.Seeman TE, Singer BH, Ryff CD, Dienberg Love G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosomatic Medicine. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Hu YR, Goldman N. Mortality differentials by marital-status- An international comparison. Demography. 1990;27:233–250. [PubMed] [Google Scholar]
- 11.Sbarra DA, Nietert PJ. Divorce and death: Forty years of the Charleston Heart Study. Psychological Science. 2009;20:107–113. doi: 10.1111/j.1467-9280.2008.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–73. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- 13.Robles TF, Kiecolt-Glaser JK. The physiology of marriage: pathways to health. Physiol Behav. 2003;79:409–16. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- 14.Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, et al. Marital quality, marital disruption, and immune function. Psychosomatic Medicine. 1987;49:13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kiecolt-Glaser JK, Kennedy S, Malkoff S, Fisher L, et al. Marital discord and immunity in males. Psychosomatic Medicine. 1988;50:213–229. doi: 10.1097/00006842-198805000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Powell LH, Lovallo WR, Matthews KA, Meyer P, Midgley AR, Baum A, Stone AA, Underwood L, McCann JJ, Herro KJ, Ory MG. Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosomatic Medicine. 2002;64:502–509. doi: 10.1097/00006842-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, Kemeny ME, Taylor SE, Fahey JL. Cognitive processing, discovery of meaning, CD4 decline, and AIDS-related mortality among bereaved HIV-seropositive men. Journal of Consulting & Clinical Psychology. 1998;66:979–986. doi: 10.1037//0022-006x.66.6.979. [DOI] [PubMed] [Google Scholar]
- 18.Goodkin K, Feaster DJ, Tuttle RS, Blaney NT, Kumar M, Baum MK, Shapshak P, Fletcher MA. Bereavement is associated with time-dependent decrements in cellular immune functioning in asymptomatic Human Immunodeficieny Virus type 1-seropositive homosexual men. Clinical and Diagnostic Laboratory Immunology. 1996;3:109–118. doi: 10.1128/cdli.3.1.109-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin M, Daniels M, Risch SC, Bloom ET, Weiner H. Plasma cortisol and natural killer cell activity during bereavement. Biological Psychiatry. 1988;24:173–178. doi: 10.1016/0006-3223(88)90272-7. [DOI] [PubMed] [Google Scholar]
- 20.Sbarra DA, Law RW, Lee LA, Mason AE. Marital dissolution and blood pressure reactivity: Evidence for the specificity of emotional intrusion-hyperarousal and task-rated emotional difficulty. Psychosomatic Medicine. doi: 10.1097/PSY.0b013e3181a23eee. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridker PM. C-reactive protein: Eighty years from discovery to emergence as a major risk marker for cardiovascular disease. Clinical Chemistry. 2009;55:209–15. doi: 10.1373/clinchem.2008.119214. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd-Jones DM, Liu K, Tian L, Greenland P. Narrative review: Assessment of C-reactive protein in risk prediction for cardiovascular disease. Annals of Internal Medicine. 2006;145:35–42. doi: 10.7326/0003-4819-145-1-200607040-00129. [DOI] [PubMed] [Google Scholar]
- 23.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr., Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 24.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? American Journal of Medicine. 2006;119:166, e17–28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 25.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. Journal of the American Medical Association. 2006;295:1412–9. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 26.Rohde LE, Hennekens CH, Ridker PM. Survey of C-reactive protein and cardiovascular risk factors in apparently healthy men. Am J Cardiol. 1999;84:1018–22. doi: 10.1016/s0002-9149(99)00491-9. [DOI] [PubMed] [Google Scholar]
- 27.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Journal of the American Medical Association. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 29.Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, Emsley HC, Forconi S, Hopkins SJ, Masotti L, Muir KW, Paciucci A, Papa F, Roncacci S, Sander D, Sander K, Smith CJ, Stefanini A, Weber D. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–29. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 30.Ballou SP, Kushner I. C-reactive protein and the acute phase response. Advances in Internal Medicine. 1992;37:313–36. [PubMed] [Google Scholar]
- 31.Miller GE, Blackwell E. Turning Up the Heat: Inflammation as a Mechanism Linking Chronic Stress, Depression, and Heart Disease. Current Directions in Psychological Science. 2006;15:269–272. [Google Scholar]
- 32.Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 33.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–4. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 35.Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O, Duncko R, Drevets WC, Manji HK, Charney DS, Gold PW, Neumeister A. Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biological Psychiatry. 2007;62:309–13. doi: 10.1016/j.biopsych.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller AH, Manji HK. On redefining the role of the immune system in psychiatric disease. Biological Psychiatry. 2006;60:796–8. doi: 10.1016/j.biopsych.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosomatic Medicine. 2006;68:376–81. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 39.Segerstrom SC, Miller GE. Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glassman AH, Miller GE. Where there is depression, there is inflammation... sometimes! Biol Psychiatry. 2007;62:280–1. doi: 10.1016/j.biopsych.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 41.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain, Behavior, and Immunity. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 42.Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain, Behavior, and Immunity. 2002;16:622–635. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 43.Hofer MA. On the nature and consequences of early loss. Psychosomatic Medicine. 1996;58:570–581. doi: 10.1097/00006842-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Hennessy MB, Deak T, Schiml-Webb PA. Stress-induced sickness behaviors: An alternative hypothesis for responses during maternal separation. Developmental Psychobiology. 2001;39:76–83. doi: 10.1002/dev.1031. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy MB, Schiml-Webb PA, Miller EE, Maken DS, Bullinger KL, Deak T. Anti-inflammatory agents attenuate the passive responses of guinea pig pups: Evidence for stress-induced sickness behavior during maternal separation. Psychoneuroendocrinology. 2007;32:508–515. doi: 10.1016/j.psyneuen.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiml-Webb PA, Deak T, Greenlee TM, Maken D, Hennessy MB. Alpha-melanocyte stimulating hormone reduces putative stress-induced sickness behaviors in isolated guinea pig pups. Behavioural Brain Research. 2006;168:326–330. doi: 10.1016/j.bbr.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Ikeda A, Iso H, Toyoshima H, Fujino Y, Mizoue T, Yoshimura T, Inaba Y, Tamakoshi A. Marital status and mortality among Japanese men and women: the Japan Collaborative Cohort Study. BMC Public Health. 2007;7:73. doi: 10.1186/1471-2458-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: the national longitudinal mortality study. Ann Epidemiol. 2000;10:224–38. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 49.Taylor SE. Tend and Befriend: Biobehavioral bases of affiliation under stress. Current Directions in Psychological Science. 2006;15:273–277. [Google Scholar]
- 50.Phillipson C. Social relationships in later life: A review of the research literature. International Journal of Geriatric Psychiatry. 1997;12:505–512. doi: 10.1002/(sici)1099-1166(199705)12:5<505::aid-gps577>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 51.Umberson D. Gender, marital status and the social control of health behavior. Social Science & Medicine. 1992;34:907–917. doi: 10.1016/0277-9536(92)90259-s. [DOI] [PubMed] [Google Scholar]
- 52.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 53.Lindau ST, Schumm LP, Laumann EO, Levinson W, O'Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–74. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nallanathan B, Williams S, McDade T, Lindau ST. Dried Blood Spot Measurement of C-reactive protein in Wave I of the National Social Life, Health & Aging Project (NSHAP) NORC and the University of Chicago; 2008. [Google Scholar]
- 55.McDade TW, Burhop J, Dohnal J. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin Chem. 2004;50:652–4. doi: 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- 56.Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131:1631S–6S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- 57.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–8. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 58.Radloff LS. The CESD scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 59.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 60.Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A Short Scale for Measuring Loneliness in Large Surveys: Results From Two Population-Based Studies. Res Aging. 2004;26:655–672. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Lawrence Erlbaum Associates, Publishers; 2003. [Google Scholar]
- 62.Alan A. Categorical Data Analysis. John Wiley & Sons, Inc.; New York: 2003. [Google Scholar]
- 63.SPSS . SPSS Base 16.0 for Windows User's Guide. SPSS Inc.; Chicago IL: 2007. [Google Scholar]
- 64.Ferrucci L, Izmirlian G, Leveille S, Phillips CL, Corti MC, Brock DB, Guralnik JM. Smoking, physical activity, and active life expectancy. Am J Epidemiol. 1999;149:645–53. doi: 10.1093/oxfordjournals.aje.a009865. [DOI] [PubMed] [Google Scholar]
- 65.Arias E. National vital statistics reports. 9. Vol. 56. National Center for Health Statistics; Hyattsville, MD: 2007. United States life tables, 2004. [PubMed] [Google Scholar]
- 66.Cacioppo JT, Hawkley LC, Bernston GG. The anatomy of loneliness. Current Directions in Psychological Science. 2003;12:71–74. [Google Scholar]
- 67.Coan JA. Toward a Neuroscience of Attachment. In: Cassidy J, Shaver PR, editors. Handbook of attachment:Theory, research, and clinical applications. 2nd ed. Guildford Publications; New York: 2008. pp. 241–265. [Google Scholar]
- 68.Brines J. Economic Dependency, Gender, and the Division of Labor at Home. American Journal of Sociology. 100:1994. [Google Scholar]
- 69.Chrousos GP, McCarty R, Pacak K, Cizza G, Sternberg E, Gold PW, Kvetnansky R. Stress: Basic mechanisms and clinical implications, Annals of the New York Academy of Sciences. Vol. 771. New York Academy of Sciences; New York: 1995. [Google Scholar]
- 70.Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]