Abstract
Osteoporosis is a significant health concern for the elderly; conjugated linoleic acid (CLA) has been shown to improve overall bone mass when calcium is included as a co-supplement. However, potential effects of CLA and calcium on bone mass during a period of bone loss have not been reported. The purpose of this study was to determine how dietary calcium modulates the effects of conjugated linoleic acid (CLA) in preventing bone loss, using an ovariectomised mouse model. CLA supplementation significantly prevented ovariectomy-associated weight and fat mass gain, compared to non-supplemented controls. CLA significantly increased bone markers without major changes in bone mineral composition in the femur compared to respective controls. CLA treatment increased serum parathyroid hormone (PTH) significantly (p = 0.0172), while serum 1,25-dihydroxyvitamin D3 concentration was not changed by CLA. Meanwhile, CLA significantly reduced femur tartrate resistant acid phosphatase (TRAP) activity, suggesting potential reduction of osteoclastogenesis. The data suggest that CLA, along with dietary calcium, has great potential to be used to prevent bone loss and weight gain associated with menopause.
Keywords: CLA, calcium, conjugated linoleic acid, mouse, bone, ovariectomy
Introduction
Osteoporosis is characterised by a decrease in bone mass and bone microstructure leading to increased bone fragility (Ammann & Rizzoli, 2003). It is a slowly progressing disease, which may develop over 20 or more years and presents a major health concern, particularly for the elderly. Approximately 10 million people in the US were estimated to suffer from osteoporosis in 2002, and 44 million or 55% of people 50 years of age and older are at risk for developing this silent disease (National Osteoporosis Foundation). The major complication of osteoporosis is an increased risk of fracture, negatively affecting the quality of life as well as increasing mortality due to complications. Particularly, postmenopausal osteoporosis is a heterogeneous disorder, which begins after natural or surgical menopause (National Institutes of Health Consensus Conference). That oestrogen deficiency after menopause plays a major role in postmenopausal bone loss is strongly supported by the higher prevalence of osteoporosis in women than in men (Nilas & Christiansen, 1987, Riggs & Melton, 1983). Ovariectomised animals are widely used as a model for postmenopausal osteoporosis (Barlet, Coxam, Davicco & Gaumet, 1994).
It is generally recommended that prevention may be the best option for avoiding osteoporosis, since the current clinical treatment options for osteoporosis have had either limited success or adverse effects (Komi et al., 2006). Among the prevention strategies for osteoporosis, the main emphasis has been placed on increasing dietary calcium intake. However, calcium by itself has limited efficacy on prevention of osteoporosis. Thus, any dietary component that can improve calcium’s effect on bone mass may potentially improve bone health significantly. One dietary bioactive component that has drawn significant attention over the last two decades for its effect on body fat reduction is conjugated linoleic acid (CLA). CLA is a group of geometric and positional isomers of linoleic acid, which was originally identified as an anticancer component found in ground beef (Dilzer & Park, 2012, Park & Pariza, 2007). Since then, CLA has shown additional biological functions, such as improving adverse effects of immune stimulation, promoting growth of young animals, reducing severity of atherosclerosis, modulating body fat, and most importantly, improving bone mass (Dilzer & Park, 2012, Park & Pariza, 2007, Park, Albright, Liu, Storkson, Cook & Pariza, 1997). However, reported effects of CLA on bone mass in animals and humans have been inconsistent (Dilzer & Park, 2012, Kelly & Cashman, 2004, Park & Pariza, 2007, Park, 2009, Park, Terk & Park, 2011, Rahman, Halade, Williams & Fernandes, 2011, Tsuzuki, Kawakami, Suzuki, Abe, Nakagawa & Miyazawa, 2005, Watkins, Li, Lippman, Reinwald & Seifert, 2004, Weiler, Fitzpatrick & Fitzpatrick-Wong, 2008). Previously it was suggested that the inconsistent responses of bone mass to CLA have been in part due to the interaction between CLA and calcium (Park, Pariza & Park, 2008, Park, Terk & Park, 2011). However, potential benefits of co-supplementation of CLA and calcium during a bone loss period have not been reported. Thus the purpose of this study was to determine the role of dietary calcium on CLA’s effects on bone parameters using an ovariectomised mouse model.
Materials and Methods
Materials
CLA was provided by Natural Lipids Ltd. AS (Hovdebygda, Norway). The purity of CLA was 80.7% CLA (37.8% cis-9, trans-11, 37.6% trans-10, cis-12 and 5.3% other isomers), 13.7% oleic acid, 3.2% stearic acid, 0.4% palmitic acid and 0.2% linoleic acid. Diet and ingredients were purchased from Harlan Laboratories (Madison, WI).
Animals and Diets
Ninety 6-month-old female ICR mice were purchased from Charles River Laboratories Inc. (Boston, MA). The Institutional Animal Care and Use Committee of the University of Massachusetts, Amherst, approved all animal procedures. Animals were housed in individual wire-bottomed cages in a windowless room with a 12-h light-dark cycle. Animals were fed semi-purified powdered diet (TD04460, Harlan Teklad, Madison, WI) using ‘vitamin-free’ tested casein to avoid naturally-occurring CLA. The composition of the 0.5% calcium diet was as follows (ingredient, g/kg): casein, “vitamin-free” tested, 194; corn starch, 373.6; soybean oil, 100; maltodextrin, 132; sucrose, 87.5; cellulose, 50; mineral mix, AIN-93M-MX without calcium (TD94049), 35; vitamin mix, AIN-93-VX (TD94047), 10; L-cystine, 3; choline bitartrate, 2.5; calcium carbonate 12.4; and TBHQ (antioxidant), 0.02. For 1.0% calcium diet, 24.9 g/kg calcium carbonate with 361.1g/kg corn starch was used. For CLA-containing diet, 0.5% soybean oil was substituted for CLA. Diets were kept at −20 °C until use. Diet and water were provided ad libitum. Fresh diet was provided three times a week. To eliminate calcium from drinking water, distilled water was provided throughout the experimental period. After a one-week acclimation period, mice were randomly separated into 6 treatment groups of 15 mice: 4 ovariectomised groups with two levels of calcium (0.5%, and 1%) with or without 0.5% CLA and two groups of sham-operated animals (with or without CLA). We used two groups for sham-operated animals with 1% calcium level as comparison, since 0.5% calcium and CLA did not improve bone mass in previous reports (Park, Pariza & Park, 2008, Park, Terk & Park, 2011). Body weight and food intake were monitored weekly. Wronski, Cintron and Dann (1988) reported that bone loss occurs as early as 2-weeks following ovariectomy and linear loss can be observed up to 100 days post-surgery in rodents. In addition, bone formation significantly declines starting 30 days post-surgery, thus we started our treatment diet 30 days post-surgery and continued it for 4 weeks. This would ensure enough time for animals to recover from surgery as well as observing potential benefits of CLA and calcium on preventing bone loss during a significant bone loss period.
Ovariectomy
The details of the operating method have been published elsewhere (Murray, 1936). Successful ovariectomy in the animals was confirmed by measuring uterine weight at sacrifice (Kelly & Cashman, 2004). Ovariectomised animals had significantly smaller uteruses compared to sham-operated animals (0.0723 ± 0.0224 g for ovariectomized animals vs. 0.6173 ± 0.0498 g, mean ± S.E. for sham-operated animals, p < 0.0001) with no difference in uterine weights between ovariectomised or sham-operated groups. No significant differences were observed by CLA or calcium supplementation for the uterine weights.
Sacrifice
Animals were sacrificed by CO2 asphyxiation after 4 hour of fasting. Blood samples were collected by cardiac puncture and the serum was separated by centrifugation. Serum samples from two animals were pooled to get enough serum for further analysis of parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D3. Serum parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D3 were measured using commercially available kits (ALPCO Diagnostics, Salem, NH). Tissues (liver, peri-uterine adipose tissue, mesenteric adipose tissue, retroperitoneal adipose tissue, spleen, lung, heart, and kidneys) were weighed and checked for any gross abnormalities. Femurs and tibias were used for tests described below.
Bone Analyses
For physical and biomechanical properties, peripheral Quantitative Computed Tomography (pQCT) scans (XCT SA Plus, Stratec Medizintechnik, Pforzheim, Germany) were performed ex vivo on mouse tibial bones. Initial scout scans were utilised to identify the total bone length and distal end plate of the tibia followed by high-density scans (single axial slices of 0.5 mm thickness, voxel size 0.1 mm). For image analyses and calculation of the bone indices, the manufacturer’s software package (version 6.0B) was used. Cortical bone properties were assessed at both the 10% and 50% sites using a threshold of 800 mg/cm3. For mechanical strength assessment we used the Polar Strength Strain Index (SSI polar) to analyse torsional resistance of the mid-shaft tibia. SSI polar was calculated using the following calculation: SSI polar = [Polar moment of inertia of the total bone area (mm4) / max distance to the centre (mm)].
Bone mineral contents of calcium, phosphorus, and magnesium were measured from the femur using inductively-coupled plasma optical emission spectrometry (ICP-OES, Varian, Palo Alto, CA). Alkaline phosphatase activity (ALP) and tartrate resistant acid phosphatase (TRAP) activity were measured in the femur using methods previously described (Mizutani, Sugiyama, Kuno, Matsunaga & Tsukagoshi, 2001) from protein homogenates of the proximal epiphysis. The ALP and TRAP activity was expressed as production of nmol of p-nitrophenol formed per mg of protein per minute. The standard curve was generated with p-nitrophenol, concentration between 0 to 103 μmol/mL. Protein concentrations were measured by protein assay kit (Bio-Rad Laboratories, Hercules, CA) with bovine serum albumin (Sigma-Aldrich, St. Louis, MO) as the standard.
Statistical analyses
Data were subjected to analysis using the Statistics Analysis System 9.2 (SAS Users Guide: Statistics, SAS Institute, Cary, NC). The design in our experiment was balanced-incomplete factorial designs with three factors: two types of mice (OVX and Sham), two levels of calcium (0.5% and 1.0%) and two levels of CLA (without and with). The response variables were measured using six treatment groups among the eight treatment groups. That is, two treatment groups, the sham-operated groups with 0.5% dietary calcium (with or without CLA) were not considered. This is because previous observations showed that there was no benefit of CLA in those animals and it was not possible to operate on 8 groups of 120 animals on the same day, due to limited resources. We first carried out a one-way analysis of variance (ANOVA) to examine the difference across the six treatment groups for each response variable (at each time for data in Figure 1). However, the main goal of this paper was to determine the effect of dietary calcium and CLA on prevention of bone loss for an ovariectomised mouse model. Thus, we performed a two-way ANOVA to test the two factors (CLA and calcium levels) and their interaction for ovariectomised animals only in each response variable. These were reported separately in each figure and table. Significance was determined at p < 0.05.
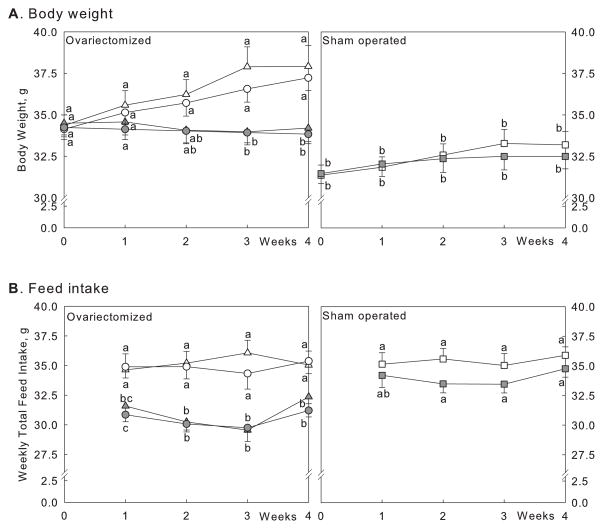
Figure 1.
Body weight (A) and food intake (B) after treatment with CLA and calcium. Controls (open symbols), CLA-fed animals (grey symbols), sham-operated animals (squares), ovariectomised animals: 1% dietary calcium (triangles), and 0.5% dietary calcium (circles). Numbers are mean ± SE (n = 15). Means with different letters indicate significant difference at p < 0.05 at each time point, analysed by one-way ANOVA.
Results
There was a significant difference in body weights between ovariectomised and sham-operated animals among controls with 1% calcium diets at each time point (Fig. 1A). No differences of body weights were observed between two dietary calcium levels (0.5% vs. 1%) in ovariectomised mice. Among ovariectomised animals, there were significant effects of CLA on body weight starting at week 2 (p = 0.0211 at week 2 and p < 0.0004 at weeks 3 & 4, respectively, from 2-way ANOVA). There was no difference in body weight in sham-operated animals (Fig. 1A). No significant interactions between calcium and CLA were observed on body weight during any time point among ovariectomised animals.
There were no differences in food intake between ovariectomised and sham-operated animals among controls with 1% calcium diets when compared at each time point (Fig. 1B). No significant effects of calcium levels on food intake were observed in ovariectomised animals. There were significant effects of CLA on food intake at all time points among ovariectomised animals (p < 0.001 for all); CLA supplementation reduced food intake in ovariectomised animals at both calcium levels. No significant interactions between calcium and CLA were observed at any time points among ovariectomised animals.
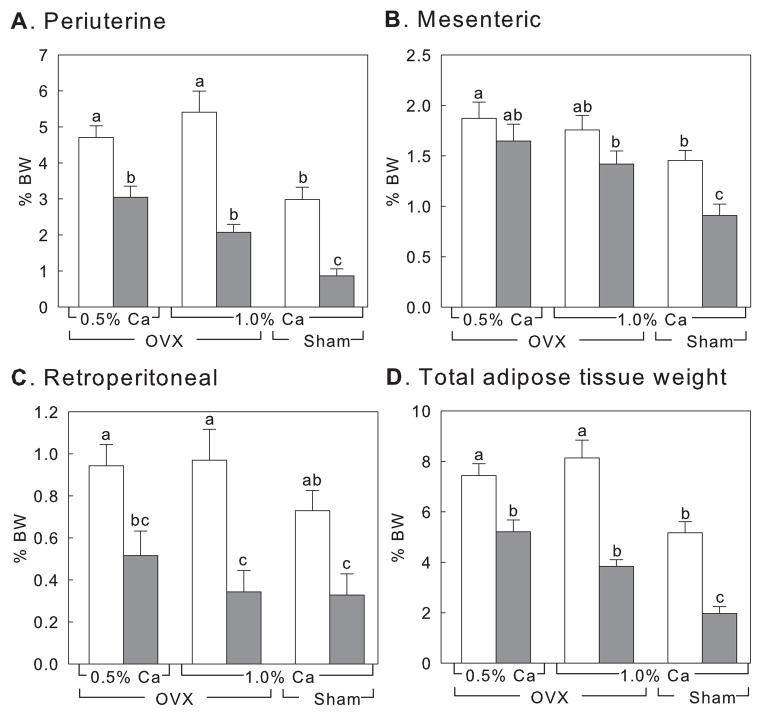
Ovariectomy caused significant increase in fat deposition in animals compared to sham-operated animals when controls at 1% calcium were compared (Fig. 2). There were no differences in adipose mass due to dietary calcium regardless of whether or not the animals received CLA. In contrast, CLA supplementation significantly reduced adipose tissue masses in ovariectomised animals (Fig. 2, p < 0.0001 except mesenteric adipose tissue). There was evidence of significant interaction between CLA and dietary calcium in periuterine and total adipose tissue weight (p = 0.0336 and p = 0.0414, respectively), which suggests the potential contribution of calcium to CLA’s effect on fat reduction after ovariectomy.
Figure 2.
Effect on adipose tissue weight after 4-weeks supplementaion with CLA and calcium. Controls (white bars) and CLA-fed animals (grey bars). OVX, ovariectomised animals; Sham, sham-operated animals. Numbers are mean ± SE (n = 15). Means with different letters indicate significant difference at p < 0.05, analysed by one-way ANOVA.
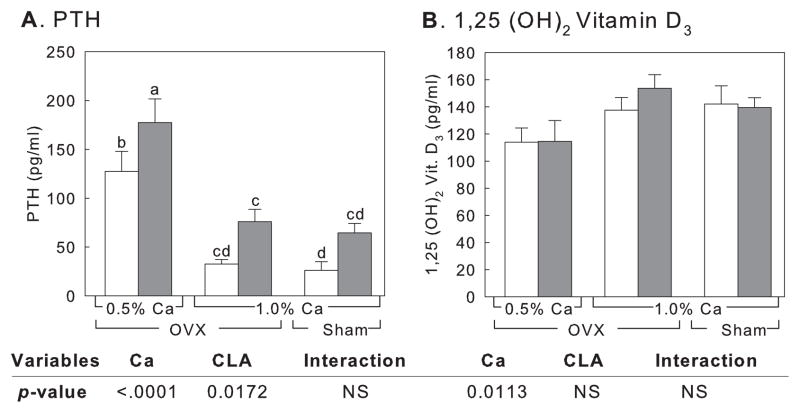
There were no differences in serum PTH and 1,25-dihydroxyvitamin D3 between ovariectomized and sham-operated animals when controls of 1% calcium group were compared (Fig. 3A & 3B). Among ovariectomized mice, significant decreases in serum PTH and increases in serum 1,25-dihydroxyvitamin D3 were observed following calcium supplementation (p < 0.0001 for PTH and p = 0.0113 for 1,25-dihydroxyvitamin D3, respectively) (Fig. 3A and 3B). CLA treatment increased serum PTH significantly (p = 0.0172), while serum 1,25-dihydroxyvitamin D3 concentration was not changed. No interactions were found for calcium and CLA for PTH and 1,25-dihydroxyvitamin D3.
Figure 3.
Effect on PTH (A) and 1,25-dihydroxyvitamin D3 (B) levels in serum after treatment with CLA and different calcium level. Controls (white bars) and CLA-fed animals (grey bars). OVX, ovariectomised animals; Sham, sham-operated animals. Numbers are mean ± SE (n = 6–7). NS, not significant. Means with different letters indicate significant difference at p < 0.05, analysed by one-way ANOVA. p-values from 2-way ANOVA are from ovariectomised groups only.
Results from bone parameters measured using pQCT are shown in Table 1. As expected, ovariectomised controls with 1% calcium had significant differences in all parameters except cortical thickness at the epiphyseal region (distal end of the tibia, 10% region of interest) and periosteal circumference at the diaphyseal region (50% region of interest) compared to sham-operated animals (1% calcium). Among sham-operated animals, CLA supplementation significantly increased total bone content, total bone density, cortical bone content and periosteal circumference at the epiphyseal region, while significant increases were observed for total bone content, cortical bone content, and SSI polar at the diaphyseal region. In ovariectomised animals, dietary calcium results in increased total bone density, cortical bone density, and cortical attenuation at the epiphyseal region, and significant increases in total bone content and total bone density at the diaphyseal region. CLA supplementation had significant effects on total bone density at the epiphyseal region and significant increase in cortical bone content, total bone content, cortical thickness, and SSI polar at the diaphyseal region in these animals. No interactions of CLA and calcium supplementation were observed; however, p-values for SSI polar at the epiphyseal region and total bone densities at the diaphyseal region approached significance in ovariectomised animals (p = 0.07 and 0.09, respectively).
Table 1.
Effect of CLA and calcium supplementation on tibial bone parameters1
| Bone parameters | OVX | OVX | OVX | OVX | Sham | Sham | p-value2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 0.5% Ca | 0.5% Ca | 1.0% Ca | 1.0% Ca | 1.0% Ca | 1.0% Ca | |||||
|
| ||||||||||
| - | +CLA | - | +CLA | - | +CLA | Pooled SEM | Calcium | CLA | Interaction | |
| ROI3 10% | ||||||||||
| Total bone content*, mg/mm | 1.69c | 1.68c | 1.67c | 1.79c | 2.02b | 2.32a | 0.06 | NS3 | NS | NS |
| Total bone density, mg/mm3 | 397d | 410cd | 416cd | 437c | 522b | 567a | 12.8 | 0.011 | 0.049 | NS |
| Cortical bone content, mg/mm | 1.26d | 1.28d | 1.31cd | 1.43c | 1.66b | 1.86a | 0.05 | NS | NS | NS |
| Cortical bone density, mg/mm3 | 803bc | 801c | 828bc | 838b | 896a | 906a | 13.0 | 0.004 | NS | NS |
| Cortical bone attenuation, cm−1 | 1.21bc | 1.21c | 1.24bc | 1.25b | 1.32a | 1.34a | 0.02 | 0.004 | NS | NS |
| Cortical thickness, mm | 0.19 | 0.21 | 0.24 | 0.30 | 0.30 | 0.29 | 0.05 | NS | NS | NS |
| Periosteal circumference, mm | 7.53a | 7.37ab | 7.22ab | 7.43a | 7.04b | 7.41a | 0.12 | NS | NS | NS |
| SSI polar4 | 0.34b | 0.32b | 0.33b | 0.35b | 0.38a | 0.41a | 0.01 | NS | NS | NS (0.07) |
|
| ||||||||||
| ROI 50% | ||||||||||
| Total bone content, mg/mm | 1.40c | 1.45c | 1.45c | 1.59b | 1.57b | 1.71a | 0.03 | 0.008 | 0.008 | NS |
| Total bone density, mg/mm3 | 762c | 763c | 774c | 815b | 863a | 834ab | 11.7 | 0.011 | NS (0.08) | NS (0.09) |
| Cortical bone content, mg/mm | 1.29d | 1.36cd | 1.33d | 1.44bc | 1.46b | 1.59a | 0.03 | NS (0.08) | 0.014 | NS |
| Cortical bone density, mg/mm3 | 1207c | 1206c | 1207c | 1219bc | 1237ab | 1241a | 7.00 | NS | NS | NS |
| Cortical bone attenuation, cm−1 | 1.70c | 1.70c | 1.70c | 1.71bc | 1.74ab | 1.74a | 0.01 | NS | NS | NS |
| Cortical thickness, mm | 0.27c | 0.29bc | 0.28c | 0.30b | 0.32a | 0.33a | 0.01 | NS | 0.014 | NS |
| Periosteal circumference, mm | 4.58 | 4.65 | 4.18 | 4.69 | 4.47 | 4.57 | 0.19 | NS | NS | NS |
| SSI polar | 0.17bc | 0.18bc | 0.17c | 0.18b | 0.18bc | 0.20a | 0.01 | NS | 0.035 | NS |
Means with different superscripts at each variable indicate significant difference at p < 0.05 (n = 11), analysed by one-way ANOVA.
p-values from 2-way ANOVA are from ovariectomised groups only.
ROI, Region of Interest; NS, Not significant.
Polar moment of inertia of the total bone area. Initial scout scans were utilised at a scan speed of 30 mm/sec to identify the total bone length and distal end plate of the tibia. Subsequently, high-density scans of the tibia (single axial slices of 0.5 mm thickness, voxel size 0.1 mm, measured diameter 90 mm) were taken at a translation speed of 10 mm/s at 10% and 50% of the approximated segment length proximal to the subchondral endplate of the distal tibia. For image analyses and calculation of the various bone indices, the manufacturer’s software package (version 6.0B) was used. A threshold algorithm (contour mode 31) was used to separate bone from the soft tissue background using a <169 mg/cm3 threshold. Cortical bone properties were assessed at both the 10% and 50% sites using a threshold of 800 mg/cm3.
We further measured the dry, ash and organic weights, and the major minerals, calcium, phosphorus and magnesium in the femur (Table 2). The function of these elements is to maintain bone formation through involvement in crystallisation of bone micro-chemical structures, directly and indirectly (Seeman, 2003). The dry weight, ash, organic weight, and phosphorus contents from the femur were significantly higher in sham-operated over ovariectomised animals when controls with 1% calcium groups were compared (Table 2). Among sham-operated animals, CLA significantly increased femur dry weight and organic weights over control. For ovariectomised animals, dietary calcium significantly increased femur dry weight, total ash, organic weight, calcium, and phosphorus content, while CLA significantly increased dry and organic weights of femurs without changing ash, calcium, or magnesium content (Table 2). No interactions between calcium and CLA were observed except total dry weight of the femur (p < 0.001) in these animals. However, no significant differences caused by calcium or CLA in ash, calcium, phosphorus, or magnesium as percentage (percent per dry weight) were observed. This suggests that CLA supplementation increases bone mass without adversely effecting bone formation, especially calcium deposition, following ovariectomy.
Table 2.
Effects of CLA and calcium on bone mineral content in the femur1
| Parameters | OVX | OVX | OVX | OVX | Sham | Sham | P-value2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 0.5% Ca | 0.5% Ca | 1.0% Ca | 1.0% Ca | 1.0% Ca | 1.0% Ca | |||||
|
| ||||||||||
| - | +CLA | - | +CLA | - | +CLA | Pooled SEM | Calcium | CLA | Interaction | |
| mg | ||||||||||
| Dry weight | 38.8f | 39.6e | 42.5d | 43.8c | 45.8b | 47.0a | 0.66 | <0.001 | <0.001 | <0.001 |
| Ash | 23.6b | 24.0b | 24.8b | 25.3ab | 27.8a | 27.9a | 0.45 | 0.0063 | NS3 | NS |
| Organic weight4 | 15.3f | 15.7e | 17.7d | 18.5b | 18.1c | 19.1a | 0.13 | <0.001 | <0.001 | NS |
| Ca | 15.6c | 15.8c | 16.4bc | 16.7abc | 18.2ab | 18.4a | 0.29 | 0.0094 | NS | NS |
| P | 7.82c | 7.94c | 8.28c | 8.47ab | 9.35a | 9.33a | 0.15 | 0.0031 | NS | NS |
| Mg | 0.17 | 0.18 | 0.19 | 0.19 | 0.21 | 0.21 | 0.01 | <0.001 | NS | NS |
|
| ||||||||||
| % | ||||||||||
| Ash | 60.8 | 60.4 | 58.4 | 57.8 | 60.6 | 59.5 | 0.05 | NS | NS | NS |
| Ca | 66.0 | 66.0 | 65.9 | 65.8 | 65.6 | 65.8 | 0.05 | NS | NS | NS |
| P | 33.2 | 33.3 | 33.4 | 33.5 | 33.67 | 33.4 | 0.01 | NS | NS | NS |
| Mg | 0.76 | 0.74 | 0.77 | 0.77 | 0.77 | 0.76 | 0.45 | NS | NS | NS |
Means with different superscripts at each variable indicate significant difference at p < 0.05 (n = 3–4), analysed by one-way ANOVA.
p-values from 2-way ANOVA are from ovariectomised groups only.
NS, Not significant.
Calculated by subtracting dry weight from ash weight.
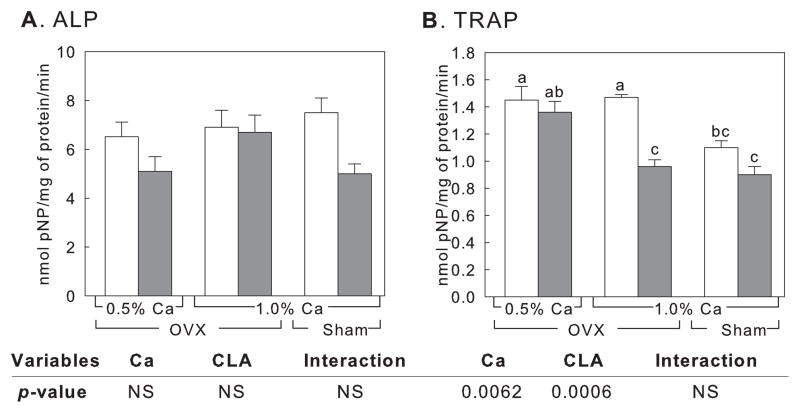
The activities of alkaline phosphatase (ALP) and tartrate resistant acid phosphatase (TRAP), markers of bone resorption, were measured from the femur (Figure 4). No significant differences of alkaline phosphatase activities were observed in any of the treatment groups (Figure 4A). No differences in TRAP activities were observed in sham-operated animals. TRAP activities were significantly decreased by CLA and calcium (p-values were 0.0006 for CLA and 0.0062 for calcium, respectively), without any significant interaction of calcium and CLA in ovariectomised animals (Figure 4).
Figure 4.
Effect on ALP (A) and TRAP (B) activities in the femur after treatment with CLA and different calcium levels. Controls (white bars) and CLA-fed animals (grey bars). OVX, ovariectomised animals; Sham, sham-operated animals. Numbers are mean ± SE (n = 5). NS, not significant. Means with different letters indicate significant difference at p < 0.05, analysed by one-way ANOVA. p-values from 2-way ANOVA are from ovariectomised groups only.
Discussion
Although there were inconsistent reports regarding CLA and bone mass, we and others have previously reported that co-supplementation of calcium and CLA improve bone mass as measured by total body ash and bone ash contents (Dilzer & Park, 2012, Kelly & Cashman, 2004, Park & Pariza, 2007, Park, 2009, Park, Terk & Park, 2011, Rahman, Halade, Williams & Fernandes, 2011, Tsuzuki, Kawakami, Suzuki, Abe, Nakagawa & Miyazawa, 2005, Watkins, Li, Lippman, Reinwald & Seifert, 2004, Weiler, Fitzpatrick & Fitzpatrick-Wong, 2008). Current results confirm these previous findings of interaction between dietary CLA and calcium on improving total bone content as well as total density (sham-operated animals in Table 1). Current results further extend our previous findings that CLA treatment improved bone strength without adversely influencing other bone parameters and bone mineral contents during a bone loss period. Moreover, CLA significantly reduced TRAP activities, which implies improvement in bone mass by controlling bone resorption. In addition, CLA influences PTH levels, which impacts calcium homeostasis, while no effects of CLA on 1,25-dihydroxyvitamin D3 were observed. This is the first report of CLA on PTH and 1,25-dihydroxyvitamin D3 in an ovariectomised animal model. Lastly, the results in this report showed independent effects of CLA on body weight and body fat, while high dietary calcium may potentiate the effects of CLA on body fat reduction.
It needs to be pointed out that the CLA preparation used in this study is a mixture of primarily cis-9, trans-11 and trans-10, cis-12 CLA isomers. The predominant CLA isomer in nature is the cis-9, trans-11, approximately 80–95% in food sources (Dilzer & Park, 2012, Park & Pariza, 2007). However, when CLA is prepared by chemical processes from linoleic acid, along with 50% of the cis-9, trans-11 isomer, the trans-10, cis-12 CLA is present at a level of approximately 50%. This preparation is referred to as ‘50:50’ CLA mixture, which most CLA studies have used previously (Park & Pariza, 2007). Previous studies suggested that the interaction of these two CLA isomers are important for CLA’s overall bioactivity, including an independent role of the trans-10, cis-12 CLA isomer on body fat reduction (Park, Storkson, Albright, Liu & Pariza, 1999). Based on these reports, even though we used CLA mixed isomer, we infer any effects of CLA on body fat reduction in the current study would be the result of the trans-10, cis-12 CLA isomer, including reduction of weight gain associated with ovariectomy (Kanaya & Chen, 2010, Park, Storkson, Albright, Liu & Pariza, 1999). However, we cannot rule out the potential effects of the cis-9, trans-11 CLA isomer on other factors, such as the effects of CLA on controlling PTH, TRAP, and/or other bone markers (Kelly & Cashman, 2004, Watkins, Li, Lippman, Reinwald & Seifert, 2004, Weiler, Fitzpatrick & Fitzpatrick-Wong, 2008).
Alternatively, it is possible that the cis-9, trans-11 isomer may work as an antagonist to the trans-10, cis-12 CLA isomer as to its role on insulin sensitivity (Dilzer & Park, 2012, Park & Pariza, 2007). Further study is needed to confirm the potential role of these two isomers on bone parameters.
Overall bone mass is the result of the balance between bone formation by osteoblasts and bone resorption by osteoclasts (Seeman, 2003). Osteoblasts originate from bone marrow mesenchymal stem cells, which may also differentiate into adipocytes or chondrocytes. Osteoclasts, however, originate from macrophage lineage hematopoietic stem cells (Kronenberg, 2003, Pittenger et al., 1999). Since osteoblasts and adipocytes share the same cell origin, a significant reciprocal role of bone marrow adipocytes on overall bone mass has been suggested. Thus, it can be inferred that the contribution of CLA to adipocyte metabolism may be directly linked to its role in bone metabolism (Kim, Park, Lee & Park, 2012, Platt & El-Sohemy, 2009b). In fact, CLA has previously been reported to reduce bone marrow adiposity along with improved bone mineral density (Halade, Rahman, Williams & Fernandes, 2011, Rahman, Halade, Williams & Fernandes, 2011). This is further supported by reports that peroxisome-proliferator activated receptor-γ (PPARγ), the key regulator of adipogenesis, plays a significant inhibitory effect on osteoblastogenesis during mesenchymal stem cell differentiation (Akune et al., 2004, Jeon et al., 2003, Rzonca, Suva, Gaddy, Montague & Lecka-Czernik, 2004, Takada, Suzawa, Matsumoto & Kato, 2007). Since inhibition of PPARγ by CLA has been suggested as a potential key molecular mechanism, CLA may improve osteoblastogenesis by indirect control of PPARγ (Brown et al., 2003, Granlund, Juvet, Pedersen & Nebb, 2003, McNeel & Mersmann, 2003, Pariza, 2004). This is supported by reports that CLA improves osteoblastogenesis and reduces adipogenesis by PPARγ-mediated mechanism from bone marrow mesenchymal stem cells (Kim, Park, Lee & Park, 2012, Platt & El-Sohemy, 2009b). This also supports the concept that the effects of CLA on improving bone mass are related to its effect on adipocyte metabolism.
Alternatively, CLA influences bone mass by reducing osteoclastogenesis, as seen in our results as reduced TRAP expression in the femur, following CLA-supplementation. TRAP is a metalloenzyme that is highly expressed in osteoclasts (Minkin, 1982), and generates highly destructive oxygen species that can destroy bone structure (Halleen et al., 1999). This enzyme is a well-known marker for bone resorption (Minkin, 1982). Platt and El-Sohemy (2009a) demonstrated that the cis-9, trans-11 CLA isomer inhibited TRAP activity in CD4+ monocytes in vitro, while the trans-10, cis-12 CLA isomer increased ALP activity in human mesenchymal stem cells. Others reported reduced markers of osteoclastogenesis from serum and urine after CLA supplementation (Rahman, Bhattacharya & Fernandes, 2006, Rahman, Halade, Williams & Fernandes, 2011). Based on these reports, it is important to consider that CLA is not only involved in improved bone formation by enhancing osteoblastogenesis but also in reducing bone resorption by inhibiting osteoclastogenesis. It is important to point out that Brownbill, Petrosian, and Ilich (2005) reported beneficial effects of CLA on bone mass in post-menopausal women with naturally-occurring CLA, which is primarily the cis-9, trans-11 isomer. Others reported that the trans-10, cis-12 CLA plays a significant role in osteoclastogenesis (Rahman, Bhattacharya & Fernandes, 2006, Rahman, Halade, Williams & Fernandes, 2011). Since it is not clear which is the active CLA in this regard in the current study, further studies using both CLA isomers are needed to confirm the role of the specific CLA isomers on osteoclastogenesis and osteoblastogenesis in vivo, or in humans.
Weiler et al.(2004) previously reported a potential role of CLA on PTH reduction in male rats. However, in the follow-up study, they concluded that this was specific to male rats, as CLA, particularly the cis-9, trans-11, did not influence PTH levels in females (Weiler, Fitzpatrick & Fitzpatrick-Wong, 2008). The major difference between Weiler et al. (2008) and our current experiment is that our observations were made in ovariectomised mice. Thus it is possible that CLA may influence PTH levels differently in this model. It is not clear how CLA modulates PTH in the current study, however, it has been suggested that PTH release may be regulated by prostaglandin E2 (PGE2) and CLA may influence PGE2 (Brown & Swartz, 1985, Li, Barnes, Butz, Bjorling & Cook, 2005, Li, Butz, Dong, Park, Pariza & Cook, 2006, Miller, Park, Pariza & Cook, 1994, Stachowska et al., 2009). Increased PTH by CLA may have contributed to CLA’s effects on improvement of overall bone mass, as PTH is reported to assist the recruitment of osteoblasts in bone remodelling as well as enhancing mesenchymal proliferation during bone repair (Dobnig & Turner, 1995, Kakar et al., 2007, Nakazawa, Nakajima, Shiomi, Moriya, Einhorn & Yamazaki, 2005). With higher dietary calcium it would be expected to observe decreased serum 1,25-dihydroxyvitamin D3 levels (Martini & Wood, 2002). Although there were no statistical differences between individual group comparisons, we observed a significant effect of calcium in ovariectomised animals, which was different from what we would have expected. It is not clear why we observed this difference, thus warranting further investigation.
Current results of increased PTH without influencing 1,25-dihydroxyvitamin D3 following CLA supplementation eventually contribute to the improved calcium absorption. In fact, others have shown that calcium absorption is potentiated by CLA both in vivo and in vitro, using the human colon adenocarcinoma Caco-2 cell line (Brownbill, Petrosian & Ilich, 2005, Jewell, Cusack & Cashman, 2005, Kelly, Cusack, Jewell & Cashman, 2003, Roche, Terres, Black, Gibney & Kelleher, 2001). This suggests that CLA not only influences bone formation and resorption, but also potentially influences calcium homeostasis. It is important to point out that both the trans-10, cis-12 and cis-9, trans-11 CLA isomers were involved in improving calcium absorption in these studies (Brownbill, Petrosian & Ilich, 2005, Jewell, Cusack & Cashman, 2005, Kelly, Cusack, Jewell & Cashman, 2003, Roche, Terres, Black, Gibney & Kelleher, 2001). Thus, this suggests a potential independent role of CLA on calcium absorption to improve overall bone health.
The role of calcium on adipogenesis has been previously suggested (Zemel, Shi, Greer, Dirienzo & Zemel, 2000, Zemel, 2001). However, we did not observe any independent effects of calcium on body fat in the current study, which is consistent with others (Gunther et al., 2006, Lin, Lyle, McCabe, McCabe, Weaver & Teegarden, 2000, Weaver et al., 2011). Our results imply a potential role for calcium on the effects of CLA on body fat reduction. In addition, it is important to point out that dietary calcium increased bone dry and organic weights along with increased total mineral content, while CLA only increased bone dry and organic weights without changes in mineral content. This suggests that the effects of calcium and CLA on bone formation may be independent, where CLA’s effect is primarily on improving organic matter during bone formation (Cowin, 2001). Overall these results suggest improved efficacy of CLA with calcium co-supplementation for body fat and bone mass control, particularly for those at risk on developing obesity and osteoporosis.
In summary, the results from this report are consistent with previous observations that CLA improves bone mass with calcium supplementation (Park, Terk & Park, 2011, Tsuzuki, Kawakami, Suzuki, Abe, Nakagawa & Miyazawa, 2005). In addition, this is the first report to demonstrate a beneficial effect of CLA and calcium co-supplementation on bone mass in an ovariectomised animal model, including effects of CLA on PTH and 1,25-dihydroxyvitamin D3. CLA and calcium supplementation significantly improved bone parameters as well as reducing markers of bone resorption. Our results also suggest that dietary calcium may potentiate CLA’s effect on body fat reduction. Thus CLA, along with dietary calcium, has great potential to be used to control obesity as well as osteoporosis, particularly associated with menopause. This can be further extended to those who are susceptible to bone loss, such as astronauts who are occupationally exposed to microgravity (Collet et al., 1997). Further clinical studies, such as studying the effects of co-supplementation of CLA and calcium on bone health as well as the role of weight bearing exercise, will be needed to extend the application of CLA in humans.
Highlights.
CLA and calcium significantly improved bone markers during bone loss period.
CLA reduced bone resorption during bone loss period.
CLA with calcium may be used to prevent bone loss associated with menopause.
CLA with calcium may be used to control weight gain following menopause.
Acknowledgments
We thank Ms. Jayne M. Storkson for assistance with manuscript preparation. This work was supported by NIH 1R21AT004456, and by the US Army MRMC. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Army or the Department of Defense. Dr. Yeonhwa Park is one of the inventors of CLA use patents that are assigned to the Wisconsin Alumni Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. The Journal of Clinical Investigation. 2004;113(6):846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003;14(Suppl 3):S13–8. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- Barlet JP, Coxam V, Davicco MJ, Gaumet N. Animal models of post-menopausal osteoporosis. Reproduction, Nutrition, Development. 1994;34(3):221–236. [PubMed] [Google Scholar]
- Brown EM, Swartz SL. Production of prostaglandins by dispersed cells and fragments from bovine parathyroid glands. Prostaglandins. 1985;29(1):35–46. doi: 10.1016/0090-6980(85)90149-2. [DOI] [PubMed] [Google Scholar]
- Brown JM, Boysen MS, Jensen SS, Morrison RF, Storkson J, Lea-Currie R, Pariza M, Mandrup S, McIntosh MK. Isomer-specific regulation of metabolism and PPARgamma signaling by CLA in human preadipocytes. Journal of Lipid Research. 2003;44(7):1287–1300. doi: 10.1194/jlr.M300001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownbill RA, Petrosian M, Ilich JZ. Association between dietary conjugated linoleic acid and bone mineral density in postmenopausal women. Journal of the American College of Nutrition. 2005;24(3):177–181. doi: 10.1080/07315724.2005.10719463. [DOI] [PubMed] [Google Scholar]
- Collet P, Uebelhart D, Vico L, Moro L, Hartmann D, Roth M, Alexandre C. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20(6):547–551. doi: 10.1016/s8756-3282(97)00052-5. [DOI] [PubMed] [Google Scholar]
- Cowin SC. Bone mechanics handbook. Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- Dilzer A, Park Y. Implication of Conjugated Linoleic Acid (CLA) in Human Health. Critical Reviews in Food Science and Nutrition. 2012;52:488–513. doi: 10.1080/10408398.2010.501409. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136(8):3632–3638. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- Granlund L, Juvet LK, Pedersen JI, Nebb HI. Trans10, cis12-conjugated linoleic acid prevents triacylglycerol accumulation in adipocytes by acting as a PPARgamma modulator. Journal of Lipid Research. 2003;44(8):1441–1452. doi: 10.1194/jlr.M300120-JLR200. [DOI] [PubMed] [Google Scholar]
- Gunther CW, Legowski PA, Lyle RM, Weaver CM, McCabe LD, McCabe GP, Peacock M, Teegarden D. Parathyroid hormone is associated with decreased fat mass in young healthy women. International Journal of Obesity. 2006;30(1):94–99. doi: 10.1038/sj.ijo.0803066. [DOI] [PubMed] [Google Scholar]
- Halade GV, Rahman MM, Williams PJ, Fernandes G. Combination of conjugated linoleic acid with fish oil prevents age-associated bone marrow adiposity in C57Bl/6J mice. The Journal of Nutritional Biochemistry. 2011;22(5):459–469. doi: 10.1016/j.jnutbio.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleen JM, Raisanen S, Salo JJ, Reddy SV, Roodman GD, Hentunen TA, Lehenkari PP, Kaija H, Vihko P, Vaananen HK. Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. The Journal of Biological Chemistry. 1999;274(33):22907–22910. doi: 10.1074/jbc.274.33.22907. [DOI] [PubMed] [Google Scholar]
- Jeon MJ, Kim JA, Kwon SH, Kim SW, Park KS, Park SW, Kim SY, Shin CS. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. The Journal of Biological Chemistry. 2003;278(26):23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- Jewell C, Cusack S, Cashman KD. The effect of conjugated linoleic acid on transepithelial calcium transport and mediators of paracellular permeability in human intestinal-like Caco-2 cells. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2005;72(3):163–171. doi: 10.1016/j.plefa.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, Toben D, Jacobsen KA, Al-Sebaei MO, Song M, Trackman PC, Morgan EF, Gerstenfeld LC, Barnes GL. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. Journal of Bone and Mineral Research. 2007;22(12):1903–1912. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- Kanaya N, Chen S. Conjugated linoleic acid reduces body weight gain in ovariectomized female C57BL/6J mice. Nutrition Research (New York, NY) 2010;30(10):714–721. doi: 10.1016/j.nutres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly O, Cashman KD. The effect of conjugated linoleic acid on calcium absorption and bone metabolism and composition in adult ovariectomised rats. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2004;71(5):295–301. doi: 10.1016/j.plefa.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Kelly O, Cusack S, Jewell C, Cashman KD. The effect of polyunsaturated fatty acids, including conjugated linoleic acid, on calcium absorption and bone metabolism and composition in young growing rats. The British Journal of Nutrition. 2003;90(4):743–750. doi: 10.1079/bjn2003951. [DOI] [PubMed] [Google Scholar]
- Kim J, Park Y, Lee SH, Park Y. trans-10, cis-12 Conjugated linoleic acid promotes bone formation by inhibiting adipogenesis by peroxisome proliferator activated receptor- -dependent mechanisms and by directly enhancing osteoblastogenesis from bone marrow mesenchymal stem cells. The Journal of Nutritional Biochemistry. 2012 doi: 10.1016/j.jnutbio.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komi J, Lankinen KS, DeGregorio M, Heikkinen J, Saarikoski S, Tuppurainen M, Halonen K, Lammintausta R, Vaananen K, Ylikorkala O, Erkkola R. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. Journal of Bone and Mineral Metabolism. 2006;24(4):314–318. doi: 10.1007/s00774-006-0689-9. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Li G, Butz D, Dong B, Park Y, Pariza MW, Cook ME. Selective conjugated fatty acids inhibit guinea pig platelet aggregation. European Journal of Pharmacology. 2006;545(2–3):93–99. doi: 10.1016/j.ejphar.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Li G, Barnes D, Butz D, Bjorling D, Cook ME. 10t,12c-conjugated linoleic acid inhibits lipopolysaccharide-induced cyclooxygenase expression in vitro and in vivo. Journal of Lipid Research. 2005;46(10):2134–2142. doi: 10.1194/jlr.M500064-JLR200. [DOI] [PubMed] [Google Scholar]
- Lin YC, Lyle RM, McCabe LD, McCabe GP, Weaver CM, Teegarden D. Dairy calcium is related to changes in body composition during a two-year exercise intervention in young women. Journal of the American College of Nutrition. 2000;19(6):754–760. doi: 10.1080/07315724.2000.10718075. [DOI] [PubMed] [Google Scholar]
- Martini L, Wood RJ. Relative bioavailability of calcium-rich dietary sources in the elderly. The American Journal of Clinical Nutrition. 2002;76(6):1345–1350. doi: 10.1093/ajcn/76.6.1345. [DOI] [PubMed] [Google Scholar]
- McNeel RL, Mersmann HJ. Effects of isomers of conjugated linoleic acid on porcine adipocyte growth and differentiation. The Journal of Nutritional Biochemistry. 2003;14(5):266–274. doi: 10.1016/s0955-2863(03)00031-7. [DOI] [PubMed] [Google Scholar]
- Miller CC, Park Y, Pariza MW, Cook ME. Feeding conjugated linoleic acid to animals partially overcomes catabolic responses due to endotoxin injection. Biochemical and Biophysical Research Communications. 1994;198(3):1107–1112. doi: 10.1006/bbrc.1994.1157. [DOI] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcified Tissue International. 1982;34(3):285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Mizutani A, Sugiyama I, Kuno E, Matsunaga S, Tsukagoshi N. Expression of matrix metalloproteinases during ascorbate-induced differentiation of osteoblastic MC3T3-E1 cells. Journal of Bone and Mineral Research. 2001;16(11):2043–2049. doi: 10.1359/jbmr.2001.16.11.2043. [DOI] [PubMed] [Google Scholar]
- Murray WS. Some effects of ovariectomy during the period of declining reproductive powers in mice. The Journal of Experimental Medicine. 1936;63(6):893–900. doi: 10.1084/jem.63.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Nakajima A, Shiomi K, Moriya H, Einhorn TA, Yamazaki M. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1–34) on chondrogenesis in a model of experimental fracture healing. Bone. 2005;37(5):711–719. doi: 10.1016/j.bone.2005.06.013. [DOI] [PubMed] [Google Scholar]
- [Accessed 10.01.12];National Osteoporosis Foundation Fast Facts. 2005 URL http://www.nof.org/node/40.
- Nilas L, Christiansen C. Bone mass and its relationship to age and the menopause. The Journal of Clinical Endocrinology and Metabolism. 1987;65(4):697–702. doi: 10.1210/jcem-65-4-697. [DOI] [PubMed] [Google Scholar]
- Pariza MW. Perspective on the safety and effectiveness of conjugated linoleic acid. The American Journal of Clinical Nutrition. 2004;79(6 Suppl):1132S–1136S. doi: 10.1093/ajcn/79.6.1132S. [DOI] [PubMed] [Google Scholar]
- Park Y, Pariza MW, Park Y. Co-supplementation of dietary calcium and conjugated linoleic acid (CLA) improves bone mass in mice. Journal of Food Science. 2008;73:C556–C560. doi: 10.1111/j.1750-3841.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- Park Y, Pariza MW. Mechanisms of body fat modulation by conjugated linoleic acid (CLA) Food Research International. 2007;40:311–323. [Google Scholar]
- Park Y, Terk M, Park Y. Interaction between dietary conjugated linoleic acid and calcium supplementation affecting bone and fat mass. Journal of Bone and Mineral Metabolism. 2011;29:268–278. doi: 10.1007/s00774-010-0212-1. [DOI] [PubMed] [Google Scholar]
- Park Y. Conjugated linoleic acid (CLA): Good or bad trans fat? Journal of Food Composition and Aanalysis. 2009;22S:S4–S12. [Google Scholar]
- Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW. Evidence that the trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34(3):235–241. doi: 10.1007/s11745-999-0358-8. [DOI] [PubMed] [Google Scholar]
- Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32(8):853–858. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Platt I, El-Sohemy A. Effects of 9cis, 11trans and 10trans, 12cis CLA on osteoclast formation and activity from human CD14+ monocytes. Lipids in Health and Disease. 2009a;8:15. doi: 10.1186/1476-511X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ID, El-Sohemy A. Regulation of osteoblast and adipocyte differentiation from human mesenchymal stem cells by conjugated linoleic acid. The Journal of Nutritional Biochemistry. 2009b;20(12):956–964. doi: 10.1016/j.jnutbio.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Halade GV, Williams PJ, Fernandes G. t10c12-CLA maintains higher bone mineral density during aging by modulating osteoclastogenesis and bone marrow adiposity. Journal of Cellular Physiology. 2011;226(9):2406–2414. doi: 10.1002/jcp.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Bhattacharya A, Fernandes G. Conjugated linoleic acid inhibits osteoclast differentiation of RAW264.7 cells by modulating RANKL signaling. Journal of Lipid Research. 2006;47(8):1739–1748. doi: 10.1194/jlr.M600151-JLR200. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ., 3rd Evidence for two distinct syndromes of involutional osteoporosis. The American Journal of Medicine. 1983;75(6):899–901. doi: 10.1016/0002-9343(83)90860-4. [DOI] [PubMed] [Google Scholar]
- Roche HM, Terres AM, Black IB, Gibney MJ, Kelleher D. Fatty acids and epithelial permeability: effect of conjugated linoleic acid in Caco-2 cells. Gut. 2001;48(6):797–802. doi: 10.1136/gut.48.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E. Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2003;14(Suppl 3):S2–8. doi: 10.1007/s00198-002-1340-9. [DOI] [PubMed] [Google Scholar]
- Stachowska E, Dolegowska B, Dziedziejko V, Rybicka M, Kaczmarczyk M, Bober J, Rac M, Machalinski B, Chlubek D. Prostaglandin E2 (PGE2) and thromboxane A2 (TXA2) synthesis is regulated by conjugated linoleic acids (CLA) in human macrophages. Journal of Physiology and Pharmacology. 2009;60(1):77–85. [PubMed] [Google Scholar]
- Takada I, Suzawa M, Matsumoto K, Kato S. Suppression of PPAR transactivation switches cell fate of bone marrow stem cells from adipocytes into osteoblasts. Annals of the New York Academy of Sciences. 2007;1116:182–195. doi: 10.1196/annals.1402.034. [DOI] [PubMed] [Google Scholar]
- Tsuzuki T, Kawakami Y, Suzuki Y, Abe R, Nakagawa K, Miyazawa T. Intake of conjugated eicosapentaenoic acid suppresses lipid accumulation in liver and epididymal adipose tissue in rats. Lipids. 2005;40(11):1117–1123. doi: 10.1007/s11745-005-1475-0. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Li Y, Lippman HE, Reinwald S, Seifert MF. A test of Ockham’s razor: implications of conjugated linoleic acid in bone biology. The American Journal of Clinical Nutrition. 2004;79(6 Suppl):1175S–1185S. doi: 10.1093/ajcn/79.6.1175S. [DOI] [PubMed] [Google Scholar]
- Weaver CM, Campbell WW, Teegarden D, Craig BA, Martin BR, Singh R, Braun MM, Apolzan JW, Hannon TS, Schoeller DA, Dimeglio LA, Hickey Y, Peacock M. Calcium, dairy products, and energy balance in overweight adolescents: a controlled trial. The American Journal of Clinical Nutrition. 2011;94:1163–1170. doi: 10.3945/ajcn.110.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler H, Austin S, Fitzpatrick-Wong S, Nitschmann E, Bankovic-Calic N, Mollard R, Aukema H, Ogborn M. Conjugated linoleic acid reduces parathyroid hormone in health and in polycystic kidney disease in rats. The American Journal of Clinical Nutrition. 2004;79(6 Suppl):1186S–1189S. doi: 10.1093/ajcn/79.6.1186S. [DOI] [PubMed] [Google Scholar]
- Weiler HA, Fitzpatrick S, Fitzpatrick-Wong SC. Dietary conjugated linoleic acid in the cis-9, trans-11 isoform reduces parathyroid hormone in male, but not female, rats. The Journal of Nutritional Biochemistry. 2008;19(11):762–769. doi: 10.1016/j.jnutbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Cintron M, Dann LM. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcified Tissue International. 1988;43(3):179–183. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- Zemel MB. Calcium modulation of hypertension and obesity: mechanisms and implications. Journal of the American College of Nutrition. 2001;20(5 Suppl):428S–435S. doi: 10.1080/07315724.2001.10719180. discussion 440S–442S. [DOI] [PubMed] [Google Scholar]
- Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. The FASEB Journal. 2000;14(9):1132–1138. [PubMed] [Google Scholar]