The adaptive immune systems of vertebrates provide remarkable examples of evolutionary innovation. This is most evident in the unusual mechanisms that jawed vertebrates have invented to create and deploy T-cell receptor and Ig diversity. In a series of papers published over the past decade (detailed below), the variable lymphocyte receptors of the jawless vertebrates (VLRs) (Fig. 1A) have emerged as an equally powerful example of evolutionary novelty. This story of parallel solutions to the challenge of nonself recognition is made all of the more compelling when one considers the wider system in which these receptors function. Although the V(D)J system of mammals and the VLR system of the agnathans are structurally unrelated, their diversity is expressed and selected in the context of lymphocytic cells that at some level share homology. This similarity offers enormous opportunity for understanding how novel immune mechanisms evolve and are incorporated into a background of more ancient cell regulatory networks. In PNAS, Das et al. (1) present another advance in the understanding of the VLR-based adaptive immune system. Using the lamprey genome sequence (2) and cDNA sequences, the authors characterize the generation of diversity in the third lamprey VLR locus (VLRC) and establish yet another variation on how immune specificity is created.
Fig. 1.
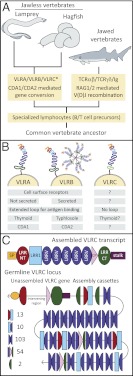
(A) Two parallel systems of adaptive immune receptors have evolved in the jawed and jawless vertebrates. Whether all three VLRs are present in hagfish awaits further genomic analysis (*). (B) The three types of VLR have unique characteristics and are expressed in distinct lymphocyte populations. (C) VLRC is assembled within the genome using a series of LRR-encoding cassettes. The structure of the proteins encoded by mature VLR transcripts and an abstraction of the VLRC genomic locus are shown. The number of each of the cassette types is indicated. CP, connecting peptide; LRR1, a short first LRR; LRRCT, C-terminal cap; LRRNT, LRR antigen binding region composed of an N-terminal cap; LRRV, variable LRRs.
The lamprey genome encodes three VLRs (VLRA, VLRB, and VLRC), each with distinct properties (3) (Fig. 1B). VLRB was first isolated in an expressed sequence tag survey targeting genes from activated lymphocyte-like cells (4). Hundreds of diversified transcripts were identified that encode leucine-rich repeat (LRR) proteins and are, very unexpectedly, transcribed from a single locus. Further characterization revealed that the incomplete germ-line VLR genes are surrounded by an extended array of several hundred LRR encoding cassettes (454 for VLRB) that somatically contribute to the mature gene (4, 5), (Fig. 1C). The overall organizational characteristics of VLRB apply also to VLRA and VLRC (1, 5, 6). The extant agnathans are monophyletic and comprise two lineages: lampreys and hagfish (7). Two VLR paralogs have been identified in the hagfishes: a clear VLRB ortholog and a locus that is related to lamprey VLRA and VLRC, although the orthology is unresolved (6, 8). It remains unknown whether a third VLR locus exists in the hagfish.
Proteins derived from the three VLR genes share a common structure: a signal peptide, an LRR antigen binding region composed of an N-terminal cap, a short first LRR, several variable LRRs, a connecting peptide, and a C-terminal cap (LRRCT), followed by a threonine/proline rich stalk region (Fig. 1C). VLRB proteins are expressed as glycosylphosphatidylinositol (GPI)-anchored cell surface receptors or secreted as multimeric antibodies (9). VLRA proteins are found only as GPI-linked cell-surface receptors (10). Crystal structures of both the VLRA and VLRB proteins show that the β-sheets of the LRR region form a concave antigen binding surface that is augmented by contacts with a distinctive loop structure formed by part of the LRRCT (11). The VLRs add to the growing list of LRR proteins in immunity and demonstrate the flexibility of this motif (12).
During somatic assembly of the mature VLR gene, LRR cassettes are incorporated stepwise into the germ-line intervening region from either the 5′ or 3′ direction. Similar to the V(D)J-mediated diversification systems, in most lymphocyte-like cells it appears that only a single allele is assembled (5). In rare cases where two alleles are assembled, one allele typically encodes a nonfunctional receptor (13). Various studies analyzing germ-line VLR loci and partially rearranged intermediates have identified short stretches of homologous sequence that potentially guide assembly in a gene conversion-like process (14). Maturation of VLR genes is associated with the expression of two AID/ABOBEC-like cytidine deaminases, CDA1 and CDA2, which are candidates as mediators of the assembly process (5). Notably, CDA1 is exclusively expressed in VLRA+ cells and CDA2 in VLRB+ cells (10). Unlike V(D)J recombination in jawed vertebrate Ig and T-cell receptor genes, VLR assembly has not been associated with any form of template-independent junctional diversity (14). In all, this powerful diversification mechanism can generate an estimated repertoire of 1014 antigen receptors (15).
Studies using specific antibodies have demonstrated that VLRA and VLRB are expressed on distinct lymphocyte populations. Furthermore, these cell types bear similarities in terms of gene expression and behavior to jawed vertebrate T and B cells, respectively (10). This is a remarkable finding, given the independent origins of the diversifying immune receptor systems in the jawed and jawless vertebrates, and may suggest that the evolution of the antigen receptors occurred within the context of specialized lymphocyte subtypes, rather than the receptors driving the evolution of the cells (10, 16).
Lampreys and hagfish lack recognizable immune organs such as thymus, spleen, and bone marrow, but several tissues have been described in which lymphocyte-like cells abound (17). The VLRA loci likely mature in the recently described thymoids, patches of thymus-like tissue located at the tips of the gill filaments. Cells in these regions coexpress the thymic epithelial transcription factor FoxN1, as well as lymphocytic CDA1 and VLRA. Genomic DNA isolated from the thymoids contains nonfunctionally assembled VLRA loci compared with that from circulating VLRA+ lymphocytes, where these are not found, suggesting that VLRA assembly takes place at this site (18). In contrast, CDA2 and VLRB expression are observed in the typhlosole (gut-associated lymphoid-like tissue). The lack of spatial overlap in CDA1 and CDA2 expression suggests that maturation of different VLR types is restricted to separate compartments.
Das et al. analyze the organization of the VLRC locus from the genome sequence of Petromyzon marinus and compare this sequence to a set of VLRC cDNA sequences collected from larval leukocytes (1). VLRC was first described from the Japanese lamprey as an additional locus that was distinct from the VLRA and VLRB paralogs (6). Phylogenetic analysis indicates that VLRC is more closely related to VLRA than VLRB. Mature VLRC transcripts are assembled and expressed in a unique population of lymphocyte-like cells that is negative for both VLRA and VLRB. Das et al. identify similarities to the extended VLRA and VLRB loci but also several differences that suggest a distinct function for this receptor. The genome sequence contains 182 VLRC cassettes, which can be classified into five types based on their placement within the mature VLRC transcript (Fig. 1C). Unlike VLRA and VLRB, each of these cassettes is out of phase with the VLRC protein elements (i.e., each module in the mature protein is a chimera of two genomic cassettes). As in VLRA and VLRB, short nucleotide sequence identities are present to guide VLRC assembly. Although no template-independent insertions or deletions were observed at the cassette junctions, the
Das et al. present another advance in the understanding of the VLR-based adaptive immune system.
exact boundary from individual cassettes can vary based on the position of the double-strand break. This mechanism may further increase the potential diversity of the antigen receptor repertoire. The authors have also isolated partially rearranged VLRC sequences from thymoid tissue genomic DNA, suggesting that—like the VLRA genes—VLRC is assembled there. Finally, unlike the VLRA and VLRB proteins, VLRC lacks the antigen binding LRRCT protrusion. The effects of this deletion on the antigen binding site remain unknown but it does indicate a clear difference with other paralogs.
A decade ago the search for the origins of vertebrate adaptive immunity had, in some respects, come to a dead end. All of the essential molecular components of the system as it had been defined in mammals and birds, including IgH, IgL, TCR-α, -β, -γ, and -δ, as well as both MHCI and II, had been identified in even the most distant jawed vertebrates, the chondrichthyans (sharks, rays, and chimaeras) (19, 20). In contrast, no clear orthologs of these were present in the extant jawless vertebrates (the lampreys and hagfish) or in any invertebrates. It seemed that the ultimate origins of the adaptive immune system may have been lost to time. The discovery of the VLRs, however, has revolutionized our understanding of the evolution of immunity in ways that were completely unanticipated by those of us working on these problems. Of course many intriguing questions about the VLR-based adaptive immune system remain. What roles are the VLRC+ lymphocytes playing within the lamprey immune system? Do the VLRC receptors remain bound to the cell surface or do they exist in secreted forms? Is there any analog of antigen processing and presentation? Do the VLRs undergo any form of somatic hypermutation or secondary diversification? And finally, if the cellular selection system predates these diversifying receptors, how ancient is this genetic circuitry? If the past 10 years of advancements in this field are any indication, answers to these questions are likely to be more exciting than anything we now imagine.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6043.
References
- 1.Das S, et al. Organization of lamprey variable lymphocyte receptor C locus and repertoire development. Proc Natl Acad Sci USA. 2013;110:6043–6048. doi: 10.1073/pnas.1302500110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JJ, et al. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat Genet. 2013 doi: 10.1038/ng.2568. 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm T, et al. VLR-based adaptive immunity. Annu Rev Immunol. 2012;30:203–220. doi: 10.1146/annurev-immunol-020711-075038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pancer Z, et al. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430(6996):174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 5.Rogozin IB, et al. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8(6):647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 6.Kasamatsu J, et al. Identification of a third variable lymphocyte receptor in the lamprey. Proc Natl Acad Sci USA. 2010;107(32):14304–14308. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimberg AM, Cowper-Sal-lari R, Sémon M, Donoghue PCJ, Peterson KJ. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc Natl Acad Sci USA. 2010;107(45):19379–19383. doi: 10.1073/pnas.1010350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pancer Z, et al. Variable lymphocyte receptors in hagfish. Proc Natl Acad Sci USA. 2005;102(26):9224–9229. doi: 10.1073/pnas.0503792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrin BR, et al. Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci USA. 2008;105(6):2040–2045. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo P, et al. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459(7248):796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velikovsky CA, et al. Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nat Struct Mol Biol. 2009;16(7):725–730. doi: 10.1038/nsmb.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley KM, Rast JP. Dynamic evolution of toll-like receptor multigene families in echinoderms. Front Immunol. 2012;3:136. doi: 10.3389/fimmu.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishishita N, et al. Regulation of antigen-receptor gene assembly in hagfish. EMBO Rep. 2010;11(2):126–132. doi: 10.1038/embor.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagawa F, et al. Antigen-receptor genes of the agnathan lamprey are assembled by a process involving copy choice. Nat Immunol. 2007;8(2):206–213. doi: 10.1038/ni1419. [DOI] [PubMed] [Google Scholar]
- 15.Alder MN, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9(3):319–327. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 16.Hsu E. The invention of lymphocytes. Curr Opin Immunol. 2011;23(2):156–162. doi: 10.1016/j.coi.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amemiya CT, Saha NR, Zapata A. Evolution and development of immunological structures in the lamprey. Curr Opin Immunol. 2007;19(5):535–541. doi: 10.1016/j.coi.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajoghli B, et al. A thymus candidate in lampreys. Nature. 2011;470(7332):90–94. doi: 10.1038/nature09655. [DOI] [PubMed] [Google Scholar]
- 19.Litman GW, Rast JP, Fugmann SD. The origins of vertebrate adaptive immunity. Nat Rev Immunol. 2010;10(8):543–553. doi: 10.1038/nri2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: Genetic events and selective pressures. Nat Rev Genet. 2010;11(1):47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]