Abstract
Glutamate receptor ion channels are membrane proteins that mediate excitatory synaptic transmission in the central nervous system of vertebrates. Insight into molecular mechanisms underlying glutamate receptor gating is limited by lack of structural information for receptors trapped in different conformational states. Here, we report the use of single-particle cryoelectron tomography to determine the structures, at ∼21 Å resolution, of full-length GluK2 kainate receptors trapped in antagonist-bound resting and agonist-bound desensitized states. The resting state, stabilized by the competitive antagonist LY466195, closely resembles the crystal structure of the AMPA receptor GluA2, with well-resolved proximal and distal subunits exhibiting cross-over between the twofold symmetric amino terminal domain and a twofold symmetric ligand binding domain (LBD) dimer of dimers assembly. In the desensitized state, the LBD undergoes a major rearrangement, resulting in a separation of the four subunits by ∼25 Å. However, the amino terminal domain, transmembrane, and cytoplasmic regions of the receptor have similar conformations in the resting and desensitized states. The LBD rearrangement was not anticipated in prior models based on crystal structures for soluble LBD dimer assemblies, and we speculate that subunit separation allows a better match to the fourfold symmetric ion channel domain. From fits of the amino terminal domain and LBD domains into the density map of the desensitized state we have derived a structural model for differences in quaternary conformation between the resting and desensitized states.
Keywords: cryoelectron microscopy, ion channel gating
Glutamate receptor ion channels (iGluRs) are major mediators of excitatory synaptic transmission in the central nervous system. Encoded by 18 genes in humans, each of the three major iGluR subtypes, named AMPA, kainate and NMDA receptors, has unique developmental and spatial expression profiles, with diverse kinetics and ion permeability (1). In their major functional role, these membrane proteins use the binding energy of neurotransmitters to open a cation selective pore; the resulting rapid influx of sodium and calcium ions leads to action potential generation and signaling through neuronal networks. On a longer time scale, regulation of iGluR synaptic targeting plays a key role in learning and storage of memories (2).
Understanding the structural basis by which iGluRs are gated by neurotransmitters is of fundamental interest to deciphering their diverse functional roles. Arranged like beads on a string, iGluR subunits contain four distinct domains, for which the extracellular amino terminal (ATD) and ligand binding (LBD) domains can be genetically isolated, expressed as soluble proteins, and crystallized as dimers (3). These domains are linked by short polypeptide linkers to the ion channel and intracellular carboxyl terminus. The 3.6-Å resolution X-ray structure of an engineered AMPA receptor construct (GluA2cryst Protein Data Bank ID code 3KG2), trapped in the closed state in complex with a competitive antagonist, revealed twofold symmetry in the extracellular domains and fourfold symmetry in the ion channel transmembrane region (4). Dimer pairs in the extracellular ATD and LBD layers of the receptor exchange subunits, resulting in a novel crossover between the proximal and distal subunit pairs in the LBD (4). Cysteine mutant cross-linking experiments suggest that this subunit exchange is preserved in kainate and NMDA receptors (5–8), but currently structural information for these iGluR subtypes is limited to the isolated ATDs and LBDs (3, 9).
A striking feature of iGluR signaling, particularly prominent in AMPA and kainate receptors, is a rapid onset of desensitization that shuts the ion channel within milliseconds after glutamate binds (10, 11). GluA2 gene targeting experiments reveal that disruption of AMPA receptor desensitization is embryonically lethal, whereas heterozygous animals show severe neurological dysfunction, indicating that desensitization plays a major role in iGluR function (12). Much has been learned about the regulation of desensitization by allosteric modulators (13–16), and by studying the effects of mutants in the ligand binding domain (13, 17–19), but our understanding of the underlying structural rearrangements remains limited. In part, this is due to the technical challenge of purifying and crystallizing iGluRs, which seem to be particularly difficult membrane protein targets owing to their unique domain organization and intrinsic conformational flexibility (20–22). Some insights into iGluR structure have come from negative-stain electron microscopy of native AMPA receptors purified from rat brain synaptosomes (23, 24) and recombinant AMPA receptors expressed in insect cells (25, 26). These studies suggest that AMPA receptors may adopt multiple conformations, some with large ATD movements, perhaps consistent with results from cysteine mutant cross-linking and single-molecule FRET experiments (20, 21). However, as noted previously for native iGluR preparations for which auxiliary subunits were removed by detergent solubilization, it is equally likely that deformations induced by negative-staining procedures may have been the primary cause of the different receptor shapes observed (24). Similar issues arise in interpreting the multiple molecular envelopes observed for AMPA receptor images obtained in the presence of glutamate (23), which could reflect either genuine differences in conformation of multiple desensitized states (27) or artifacts arising from negative-staining procedures and subsequent image analysis.
Here, we have used single-particle cryoelectron tomography to obtain 3D structures of full-length GluK2 kainate receptors. Compared with the GluA2 AMPA receptor, the desensitized state of GluK2 is at least 100-fold more stable, based on measurements of the rate of recovery from desensitization (11, 18), and thus potentially a more stable target for structural analysis. Detergent-solubilized GluK2 tetramers were plunge-frozen in liquid ethane cooled by liquid nitrogen to form a thin film of vitrified material. Starting with a series of tilted images from the plunge-frozen specimen, we converted these to tomograms representing 3D images of ice-embedded receptors. By extracting, classifying, and 3D-averaging subvolumes for individual receptors, we were able to obtain density maps at ∼21 Å resolution in both antagonist-bound resting and agonist-bound desensitized states. By interpreting the density maps using the crystal structure of GluA2, we derive a structural mechanism for the dramatic differences in quaternary structure between resting and desensitized states.
Results
Stabilization of GluK2 Resting and Desensitized States.
Based on baculovirus expression of kainate receptors in Sf9 insect cells, we identified the rat GluK2 subtype as a promising candidate for structural studies. With the goal of establishing a highly populated desensitized state suitable for structural analysis we used the agonist 2S,4R-4-methylglutamate, which binds to GluK2 with 100-fold higher affinity than glutamate (28). Likewise, to stabilize an antagonist-bound resting state we introduced four-point mutations, A487T, A658S, N690S, and F704L, based on the GluK1 LBD crystal structure (29) to create a binding site for the GluK1 selective competitive antagonist LY466195, Kd 38 nM. Using tryptophan fluorescence size-exclusion chromatography (30), we established that GluK2 preparations at concentrations of 1–2 mg/mL are monodisperse in both the resting and desensitized states when stored at 4 °C in a buffer containing 2 mM n-dodecyl-β-D-maltopyranoside (DDM) and 0.3 mM cholesterol hemisuccinate (Fig. 1A and Fig. S1).
Fig. 1.
GluK2 preparations used for structural analysis. (A) Tryptophan fluorescence size-exclusion chromatograms for GluK2 reveals elution as monodisperse tetramers in the apo state, and in the presence of either 1 mM 2S,4R-4-methylglutamate or 1 mM LY466195; the inset shows a Coomassie blue-stained SDS polyacrylamide gel during purification by immobilized metal affinity chromatography (IMAC), thrombin digestion (Thr), and size-exclusion chromatography (SEC), with bands at the expected molecular weight (MW) of 128.4, 99.5, and 28.9 kDa for the GluK2–EGFP fusion protein and the cleaved products. (B and C) Slices from tomograms obtained using plunge-frozen GluK2 specimens in the presence of LY466195 (B) or 2S,4R-4-methylglutamate (C). The red circles highlight individual GluK2 complexes. (Scale bar, 50 nm.)
Cryoelectron Tomography of GluK2 in Resting and Desensitized States.
Images from frozen-hydrated GluK2 deposited on holey carbon electron microscopic grids reveal protein complexes dispersed in a thin film of vitreous ice present above the carbon substrate and excluded from holes in the carbon film. Tomographic analyses established that proteins were not directly in contact with the carbon film. We therefore used cryoelectron tomography combined with subvolume averaging to determine 3D receptor structures because the use of tomography (instead of conventional single-particle 2D imaging) allows computational removal of the background contributed by the carbon film. Slices through tomograms of frozen-hydrated GluK2 specimens show the presence of discrete receptor assemblies (Fig. S2). Expanded views of a small region of the tomogram show that images from GluK2 in antagonist-bound (Fig. 1B) and desensitized (Fig. 1C) states show discrete molecular assemblies with dimensions of ∼10 nm, consistent with the expected dimensions of individual iGluR tetramers. Subvolumes corresponding to individual receptors identified in the tomograms were classified and averaged in 3D to obtain density maps for GluK2 in the two states, both at resolutions of ∼21 Å (Fig. S3). Visualization of density maps for GluK2 complexes trapped in resting and desensitized states as isosurface representations (Fig. 2 A and B) as well as views through different regions of the density map (Fig. 2 C and D) show that the two states display similar quaternary conformations in the ATD, the transmembrane region, and in the cytoplasmic domain. However, there are striking differences in the LBD, which is organized as two distinct subdomains with twofold symmetry in the resting state (Fig. 2C), whereas in the desensitized state the LBD separates into four subdomains (Fig. 2D). This separation is visible both in the surface representation of the maps (Fig. 2 A and B) and in cross-sectional views across the length of the LBD (lower rows in Fig. 2 C and D).
Fig. 2.
Density maps for GluK2 resting and desensitized states. (A and B) Isosurface representations in the resting (A) and desensitized (B) states obtained by cryoelectron tomography with subvolume averaging. (C and D) Absolute density representations clearly reveal differences between the two conformations; in each panel, the images in the upper row show four projections of the front view of the maps shown in A and B, spaced by successive rotations around the long axis by 15°; the bottom rows show five sections through the maps perpendicular to the long axis, at the locations indicated by the red arrows between A and B; the numbered arrows correspond to the sections shown in C and D, with approximate locations as follows: (1) middle of the transmembrane region, (2) membrane-proximal region on the extracellular side, (3) lower lobe of the ligand-binding domain (LBD), (4) upper lobe of the LBD, and (5) central region of the ATD.
Molecular Interpretation of the Resting-State Density Map.
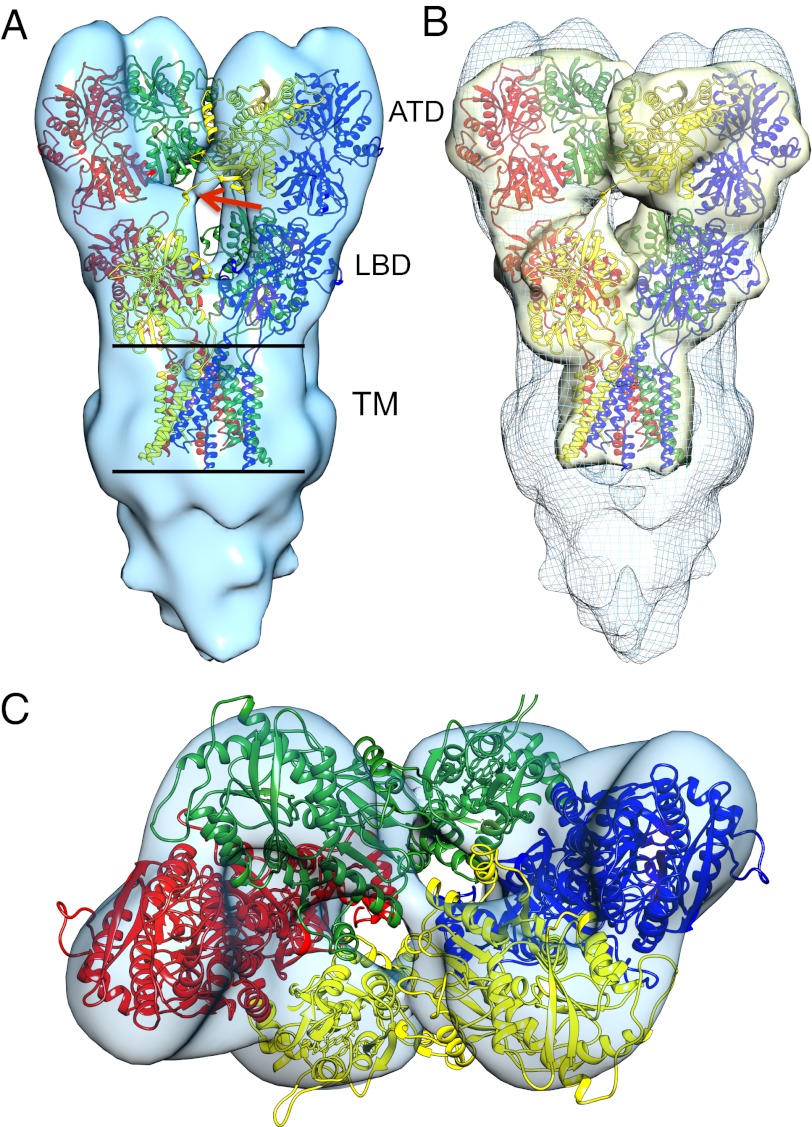
To obtain a molecular interpretation of the structural changes involved in desensitization, we first fitted coordinates for GluA2cryst into the density map of GluK2 in the resting state. Side (Fig. 3A) and top (Fig. 3C) views show general agreement of the structure derived here using cryoelectron tomography with the GluA2 crystal structure (Fig. 3B and Movie S1), including clear definition of the “dimer-of-dimers” assembly for the ATD and LBD layers. The map is slightly elongated in the direction of the long axis, suggestive of preferential orientation and the effect of the missing wedge in limited-angle tomography. Determination of the angles assigned to the individual volumes confirms the orientational bias, with molecules largely oriented “up” or “down” with respect to the plane of the carbon film (Fig. S4). There is a wide band of density visible in the transmembrane region of both resting and desensitized states, which we assign to the contribution of the detergent-cholesterol micelle surrounding the hydrophobic portions of the receptor. There is also an extra region of density in the cytoplasmic region, which is likely to have multiple origins: the 50-residue GluK2 cytoplasmic region, not present in the construct used for crystallography, additional detergent molecules associated with the two Cys-linked palmitoyl groups in the cytoplasmic domain, and possible contribution from the carbon substrate. An important feature of the map is the visualization of the cross-over between the ATD and LBD (Fig. 3A), first identified in the crystal structure of the GluA2 tetramer (Fig. 3B). The junction at which the cross-over occurs corresponds exactly to the site of the partially disordered linker region where GluA2cryst carries a six-amino-acid deletion as well as removal of two glycosylation sites, compared with the full-length receptor.
Fig. 3.
Molecular architecture of GluK2 complexed to LY466195 (resting state) fit with GluA2cryst (3KG2) coordinates. (A) Top and (C) side views illustrating the locations of the ATD and LBD relative to the lipid bilayer, and the cross-over between the ATD and LBD; the red arrow shows where six amino acids and two glycosylation sites were deleted in GluA2cryst (4). (B) Density map obtained by filtering coordinates for GluA2cryst to a resolution of 21 Å, superimposed on the GluK2 resting-state map.
Molecular Model for the Desensitized State.
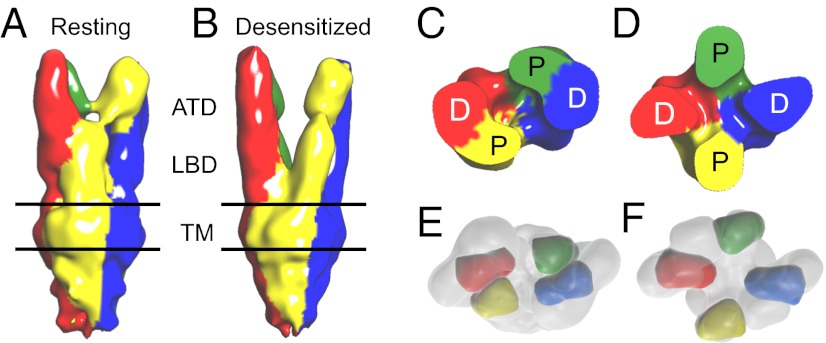
The most notable difference between density maps of the resting and desensitized states is in the arrangement of the LBD, which separates into four distinct domains in the latter conformation. The extent of the change in structure of the LBD can be appreciated by comparing the fit of GluA2cryst coordinates to the LBD in the resting state (Fig. 4A) and desensitized-state (Fig. 4B) density maps. The four subunits, which are organized as a pair of dimers in the resting-state map, separate into four distinct monomeric domains in the desensitized-state map due to a conformational change that involves large shifts of ∼25 Å in the LBD layer. By contrast, the density maps reveal only minimal changes in the ATD layer, with good fits of ATD dimer assemblies in both states. Density maps rendered to show each of the four chains that form the GluK2 tetrameric assembly reveal that proximal subunits in the LBD layer (colored green and yellow) undergo a substantial counterclockwise rotation in the desensitized state, whereas the distal subunits (colored red and blue) rotate clockwise, but to a much smaller extent (Fig. 5). However, although the overall displacement of the LBD in each subunit is clearly visible, we cannot resolve the change in cleft closure that occurs in response to binding of agonist compared with the antagonist bound state (29, 31); likewise, the orientation of the ligand binding site in individual LBD monomers cannot be determined with certainty at the present resolution. Nevertheless, the fits provide an impression of dramatic changes in the LBD layer that occur when binding of glutamate triggers desensitization and show how the overall conformational change between the two states might be considered to resemble the winding and unwinding of the bundle of four monomers that constitute the tetrameric receptor assembly (Movie S2).
Fig. 4.
Separation of LBD protomers in the GluK2 desensitized state. Slices through the LBD (Upper) and ATD (Lower) regions in resting- (A) and desensitized-state (B) density maps shown as isosurface representations, with fits of 3KG2 coordinates. There is a large rearrangement in the LBD layer on desensitization, which results in separation of the four GluK2 protomers with only a small rotation of the ATD layer. Fits were obtained using automated procedures, except for the LBD desensitized state, which was performed manually.
Fig. 5.
Quaternary structures of resting and desensitized states. (A and B) Density maps rendered to show GluK2 subunits in the tetrameric assembly. The desensitized state displays a clear separation of LBD protomers with a small twist of ATD dimers compared with the resting state. (C and D). Sections through the LBD, illustrating the dimer-of-dimers assembly in the resting state and domain separation in the desensitized state, accompanied by large rotations of the proximal (P), but not distal (D), subunit pairs. (E and F), Top view through the density maps of the resting (E) and desensitized (F) states where the LBD regions are approximated with molecular envelopes at 20 Å resolution to illustrate the conformational change. In the resting state, the entire LBD tetramer was fit automatically using UCSF Chimera, whereas for the desensitized state the four monomers were placed manually in the map in the absence of strong structural constraints to produce reliable automated fits.
Differences Between AMPA and Kainate Receptor Structures.
Although the gating mechanisms and structures of AMPA and kainate receptors are likely to be similar, we observed subtle differences in the relative locations of the ATD and LBD in the GluK2 resting-state map compared with the resting-state GluA2cryst tetramer. Compared with the fit of the entire receptor (see Fig. S5A), fitting only the ATD segment results in a poorer fit at the LBD region (see Fig. S5B) because of an anticlockwise rotation of the LBD. Similarly, fitting only the LBD segment results in a poorer fit at the ATD region because of a clockwise rotation of the ATD (see Fig. S5C). The separation between the Cα coordinates of residues Gly-389 and Leu-390 (which flank the deleted set of six residues) ranges between 10–15 Å when the ATD and LBD are fit separately, consistent with the likely ATD–LBD linker length in a partially extended, unstructured conformation. The extent of mismatch in the fits of the ATD and LBD regions is thus in opposite directions when GluA2cryst is used for docking, suggesting that the deletion of the six amino acids in the linker region between the ATD and LBD constrains the engineered construct by introducing rotational strain. Our results suggest that when the full-length linker is present, the domains “untwist” into a more relaxed and less strained conformation, although it is also possible that the GluA2 and GluK2 resting-state conformations are intrinsically different.
Discussion
Prior negative-stain EM analysis of AMPA receptor structures revealed substantial heterogeneity in quaternary conformation that has been variously attributed to the use of different preparations, ligands, and to the conformational freedom intrinsic to the multidomain structure of iGluRs (23–26, 32). Notably, the GluA2 resting-state crystal structure solved in a complex with the high-affinity antagonist ZK200775 revealed a Y-shaped, twofold symmetric ATD dimer-of-dimers assembly that differed substantially from that reported by earlier EM analyses. For native AMPA receptors in ligand-free solutions, negative-stain EM images were interpreted to reveal an asymmetric structure, in which the ATD dimer pairs were tilted to one side. In the presence of 1 mM glutamate a heterogeneous population of highly asymmetric structures was reported (23). Notably, even in the nominal absence of glutamate, ∼40% of the reconstructed EM image population in ligand-free solutions was reported to display asymmetric shapes similar to the desensitized state. By contrast, images from a negative-stain EM analysis of recombinant unliganded AMPA receptors were interpreted to reveal a strikingly different O-shaped structure with twofold symmetry, but with a shape different from that of the GluA2 crystal structure (25, 26).
In the present study we purified full-length kainate receptors with native glycosylation. Membranes were solubilized in a DDM–cholesterol mixture that thermostabilizes G protein-coupled receptors and other membrane proteins (33, 34), possibly due to a sterol-mediated ordering effect on the DDM hydrocarbon tail, such that the central core of the resulting micelle resembles a membrane-like bilayer (34). Our goal was to obtain uniform populations of receptors trapped in either resting or desensitized states. This was facilitated by the use of high-affinity ligands, and in addition, by the 100-fold greater stability of the desensitized state for GluK2 versus GluA2. The resulting proteins were imaged using cryoelectron tomography of vitrified receptor preparations. The use of vitrified specimens allows preservation of the structural integrity of protein complexes and avoids artifacts caused by staining and drying at room temperature. The use of geometric constraints provided by cryoelectron tomography enables the determination of 3D molecular volumes from individual receptors, avoiding some of the shortcomings of approaches that use only 2D projection images for generation of initial models used in subsequent map refinement. Such errors include ambiguity of orientation assignment, handedness determination, and model bias that can result during refinement steps carried out with 2D projection images. In contrast, starting with 3D tomograms of ice-embedded molecules, the application of routines for reference-free alignment provides a powerful method for de novo structure determination that does not rely on the availability of initial models or on making assumptions about symmetry. Compared with 2D projection images, the augmented information contained in 3D volumes minimizes errors in angular assignments because alignment is more robust when done in three dimensions. Moreover, our methods for averaging and classification of subvolumes enable the successful separation of closely related conformations that are present simultaneously within the sample (35). This is demonstrated in Fig. S6, which shows that whereas 1 mM concentrations of LY466195 and 2S,4R-4-methylglutamate produce high occupancy of the resting and desensitized states, respectively, the classification methods are good enough to reliably resolve the presence of ∼10% of desensitized-state molecular volumes computationally mixed with 90% of the antagonist-bound state.
Resting-State Conformation.
The similar cDNA sequences for AMPA and kainate receptors, coupled with crystallographic analysis that reveals nearly identical structures for their isolated ATD and LBD dimer assemblies (3, 9, 36), and functional studies that reveal conserved assembly and gating mechanisms (13, 18, 37–39), suggests that GluA2 and GluK2 should have similar structures. Remarkably, the overall conformation of GluK2 in the resting state is nearly identical to the crystal structure of GluA2, with an upright position of the ATD dimers, as opposed to the tilted ATD assemblies reported in previous negative-stain EM work on native AMPA receptors. This upright conformation of GluK2 contrasts with the proposal that shortening the ATD–LBD linker in the GluA2cryst construct reduces structural complexity and conformational variation in the ATD region. Because the GluK2 structure was solved by single-particle cryoelectron tomography, using vitrified material, it seems likely that the GluA2cryst structure was not substantially perturbed by the crystal lattice, and that GluA2 and GluK2 antagonist complexes adopt similar low-energy conformations. By contrast, the resting-state structure described here differs substantially from those reported in prior EM studies on AMPA receptors.
A rigid body docking of GluA2cryst coordinates into the resting-state EM map yields a qualitatively accurate fit in both the ATD and LBD domains, but the fit can be improved if the ATD and LBD are fit independently, leading to a clockwise rotation of the ATD relative to its position in the crystal structure (Fig. S5). Although we cannot exclude small differences in the resting-state structures of GluA2 and GluK2, the full-length linker sequence in GluK2 might allow the LBD to “untwist” into its native, unconstrained conformation compared with the GluA2cryst structure that has a six-amino-acid deletion in the LBD–ATD linker. The twist of the ATD is ∼5–10° larger in the desensitized state, and it is noteworthy that the GluA2cryst construct exhibits increased desensitization compared with wild-type GluA2, indicating that the linker deletion does subtly affect receptor structure and function, but not in ways previously anticipated (32).
Insights into the Mechanism of Desensitization.
The GluK2 desensitized-state structure we present here is strikingly different from those observed in prior EM studies on AMPA receptors. In contrast to previously reported AMPA receptor type-II desensitized-state EM structures that imply large ATD dimer movements (23), ATD dimers in the GluK2 desensitized state show little difference in conformation compared with the resting state. Large changes in the ATD are difficult to reconcile with the short length of the linkers connecting the ATD and LBD and the subunit cross-over observed in the GluA2 crystal structure. Instead, we find that large conformational rearrangements occur in the LBD, in excellent agreement with prior biochemical, structural, and electrophysiological studies that show that AMPA and kainate receptors share conserved gating mechanisms, and that desensitization involves rupture of LBD dimer assemblies in which intermolecular contacts are mediated exclusively by the upper lobes of the LBD (13, 17, 18, 39, 40). Prior mechanistic studies on AMPA and kainate receptor desensitization did not consider how the four subunits are arranged in a tetrameric assembly in an intact receptor. Instead, they focused on a model in which the lower lobes of two subunits in a LBD dimer pair approach each other, such that Cys mutants introduced in the LBD lower lobes inhibit receptor activation by forming disulfide bonds that restrict movement of the cross-linked LBD assembly (40). As noted previously, it is unlikely that the isolated LBD crystal structures of the G725C and S729C mutants that inhibit receptor activity are representative of the native desensitized state (40), underscoring the need to obtain a structure of the desensitized state of the entire receptor.
In the GluK2 desensitized-state structure reported here, the LBDs no longer form dimer assemblies but emerge instead as four separate protomers, whereas the ATD dimers remain essentially intact. Although as noted above, a dimer-centric view of iGluR activation and desensitization has dominated our thinking so far, biochemical studies reveal that AMPA and kainate receptor LBDs interact very weakly, with monomer–dimer and dimer–tetramer Kds in the 10 mM and higher range (13, 39). Such weak interactions provide little driving force for maintenance of LBD dimer assembly in the desensitized state, raising the possibility that the fourfold symmetry of the ion channel domain is imposed on the LBD, thus forcing a separation of the four protomers at this layer of the receptor assembly. By contrast, although monomer–dimer Kds for ATD dimer assemblies are in the low nanomolar range (38, 41, 42), and thus ATD dimer dissociation is unlikely during desensitization, the ATD dimer pairs also interact weakly, and thus are free to move relative to each other, within limits imposed by the ATD–LBD linkers.
Our analysis does not reveal how the peptide linkers connecting the lipid-embedded ion channel with the LBD, and the LBD with the ATD mediate the change in symmetry of the resting and desensitized states. Nor can we establish whether the resting and desensitized states of the ion channel segments differ subtly in conformation, as might be expected given the large change in the LBD reported here. The existence of multiple desensitized states for native AMPA receptors, with a 25-fold difference in stability, has been proposed based on analysis of rates of recovery from brief and prolonged applications of agonist (27). Due to the dimer-of-dimers assembly of the iGluR LBD, it is possible that there is an intermediate state in which one dimer pair “desensitizes” while the other remains in the glutamate-bound active conformation; it is thus plausible that the desensitized-state model proposed on the basis of Cys mutant cross-linking experiments represents such an intermediate, whereas the structure reported here represents the final desensitized state. Addressing these issues using a combination of cryo-EM analyses at higher resolution and crystallographic studies of the desensitized state(s) of receptors in this family remains a challenge for ongoing and future studies.
Materials and Methods
Protein Purification, Cryoelectron Tomography, and Subvolume Averaging.
Methods used for purification of full-length GluK2 receptors are described in detail in SI Materials and Methods. Frozen-hydrated GluK2 samples were prepared for cryoelectron microscopy on Quantifoil multi-A grids in a humidity- and temperature-controlled environment (95% relative humidity and 293 K, respectively; Vitrobot Mark IV from FEI Company). The grids were imaged in an FEI Company Titan Krios electron microscope at an accelerating voltage of 80 kV, pixel size of 1.7 Å at the specimen plane, and nominal underfocus of 2.0 μm. A series of 61 tilted images (± 60°, 2° intervals, approximately two electrons per square angstrom per image) was recorded using a 4K × 4K CCD (Gatan Inc.) and automatically aligned using the program RAPTOR (43). Three-dimensional volumes of the field of view were calculated using weighted back projection (44), and 2,500 molecular volumes were selected from each dataset and were classified (Fig. S7), aligned, and averaged using missing-wedge-aware methods as described (45, 46). We first carried out subvolume averaging with no symmetry imposed, identified the presence of twofold symmetry, and used it in subsequent iterations and refinement. At no point did we use fourfold symmetrization, and the pseudofourfold symmetry in the LBD in the desensitized state was verified to be independent of the imposition of even twofold symmetry (Fig. S8). The final structures presented in Fig. 2 are from 522 and 841 subvolumes for the antagonist-bound and desensitized conformations; these represent the subset of molecular volumes with the highest signal-to-noise ratios from the initial set.
In silico separation of different conformations in the sample was achieved with a variation of previously established procedures for classification of tomographic subvolumes (35, 45, 46) using a recently developed collaborative alignment-classification procedure (47) that is outlined briefly below. Our approach uses an iterative joint alignment classification technique, in which the classical similarity measure based on pairwise distances between two particles, or a particle and a class average, is replaced by a one-to-many collaborative similarity function measured between a particle and a group of particles. This collaborative alignment framework enables robust alignment of linearly correlated heterogeneous images. Mathematically, the collaborative similarity measure can be defined as follows: For n completely aligned and identical d-dimensional data elements (subvolumes), normalized, and ordered in a vector form, the matrix obtained by stacking all of the vectors columnwise is defined to be V = [v1, …, vn] should have a rank of 1. When the subvolumes are not perfectly aligned, the rank of V grows. Therefore, aligning all data vectors is equivalent to reducing the rank of V back to 1. The same argument works when the data are composed of m different shape classes, with m ≤ n. When the subvolumes are aligned, the rank of V is equal to m, which grows when the alignment is altered. This observation provides us with a collaborative reference frame for the alignment procedure (i.e., the optimal alignment parameters are those that minimize the rank of V). This collaborative scheme allows harnessing contributions of all particles collectively for alignment, as opposed to approaches that are based on pairwise comparisons.
Fitting Coordinates to the Density Map.
The resolutions achieved by cryoelectron tomography and subvolume averaging are typically in the range of 20–30 Å and in many cases are sufficient to unambiguously place X-ray coordinates of various components into the reconstructed density maps, which can then be used to derive the molecular architecture of the complex. AMPA and kainate receptor crystal structure coordinates (Protein Data Bank ID codes 3KG2, 3H6G, 2QS4, and 3G3F) were manually placed into the EM maps. A 20-Å simulated map of the coordinates was automatically fit based on cross-correlation about mean data values using the software package UCSF (University of California, San Francisco) Chimera (48), except in the case of the GluK2 glutamate-bound LBD monomer (3G3F). In this case, the resolution of the EM map was not sufficient to provide enough of a constraint to allow accurate automatic fitting. Instead, 3G3F was automatically fit as a dimer to the resting-state map, then separated into monomers that were manually translated and rotated to approximately match the desensitized-state map.
Supplementary Material
Acknowledgments
We thank Eric Gouaux and members of his laboratory for advice on membrane protein expression and purification, Dr. David Bleakman (Eli Lilly and Company) for the gift of LY466195, and Dr. Haifeng He for advice on electron microscopic data collection. This work was supported by funds from the Center for Cancer Research, National Cancer Institute, National Institutes of Health (NIH) (to S.S.) and from the intramural research program of the National Institute of Child Health and Human Development, NIH, Department of Health and Human Services (to M.L.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217549110/-/DCSupplemental.
References
- 1.Traynelis SF, et al. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 3.Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure. 2011;19(10):1370–1380. doi: 10.1016/j.str.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462(7274):745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das U, Kumar J, Mayer ML, Plested AJ. Domain organization and function in GluK2 subtype kainate receptors. Proc Natl Acad Sci USA. 2010;107(18):8463–8468. doi: 10.1073/pnas.1000838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438(7065):185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Gouaux E. Amino terminal domains of the NMDA receptor are organized as local heterodimers. PLoS ONE. 2011;6(4):e19180. doi: 10.1371/journal.pone.0019180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salussolia CL, Prodromou ML, Borker P, Wollmuth LP. Arrangement of subunits in functional NMDA receptors. J Neurosci. 2011;31(31):11295–11304. doi: 10.1523/JNEUROSCI.5612-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa H. Structure and function of glutamate receptor amino terminal domains. J Physiol. 2012;590(Pt 1):63–72. doi: 10.1113/jphysiol.2011.213850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otis T, Zhang S, Trussell LO. Direct measurement of AMPA receptor desensitization induced by glutamatergic synaptic transmission. J Neurosci. 1996;16(23):7496–7504. doi: 10.1523/JNEUROSCI.16-23-07496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone AL, Plested AJ. Coupled control of desensitization and gating by the ligand binding domain of glutamate receptors. Neuron. 2012;74(5):845–857. doi: 10.1016/j.neuron.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Christie LA, et al. AMPA receptor desensitization mutation results in severe developmental phenotypes and early postnatal lethality. Proc Natl Acad Sci USA. 2010;107(20):9412–9417. doi: 10.1073/pnas.0908206107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, et al. Mechanism of glutamate receptor desensitization. Nature. 2002;417(6886):245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 14.Jin R, et al. Mechanism of positive allosteric modulators acting on AMPA receptors. J Neurosci. 2005;25(39):9027–9036. doi: 10.1523/JNEUROSCI.2567-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plested AJ, Mayer ML. Structure and mechanism of kainate receptor modulation by anions. Neuron. 2007;53(6):829–841. doi: 10.1016/j.neuron.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Plested AJ, Vijayan R, Biggin PC, Mayer ML. Molecular basis of kainate receptor modulation by sodium. Neuron. 2008;58(5):720–735. doi: 10.1016/j.neuron.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horning MS, Mayer ML. Regulation of AMPA receptor gating by ligand binding core dimers. Neuron. 2004;41(3):379–388. doi: 10.1016/s0896-6273(04)00018-2. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhry C, Weston MC, Schuck P, Rosenmund C, Mayer ML. Stability of ligand-binding domain dimer assembly controls kainate receptor desensitization. EMBO J. 2009;28(10):1518–1530. doi: 10.1038/emboj.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nayeem N, Mayans O, Green T. Conformational flexibility of the ligand-binding domain dimer in kainate receptor gating and desensitization. J Neurosci. 2011;31(8):2916–2924. doi: 10.1523/JNEUROSCI.4771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plested AJ, Mayer ML. AMPA receptor ligand binding domain mobility revealed by functional cross linking. J Neurosci. 2009;29(38):11912–11923. doi: 10.1523/JNEUROSCI.2971-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landes CF, Rambhadran A, Taylor JN, Salatan F, Jayaraman V. Structural landscape of isolated agonist-binding domains from single AMPA receptors. Nat Chem Biol. 2011;7(3):168–173. doi: 10.1038/nchembio.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau AY, Roux B. The free energy landscapes governing conformational changes in a glutamate receptor ligand-binding domain. Structure. 2007;15(10):1203–1214. doi: 10.1016/j.str.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433(7025):545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa T, Cheng Y, Sheng M, Walz T. Three-dimensional structure of an AMPA receptor without associated stargazin/TARP proteins. Biol Chem. 2006;387(2):179–187. doi: 10.1515/BC.2006.024. [DOI] [PubMed] [Google Scholar]
- 25.Tichelaar W, Safferling M, Keinänen K, Stark H, Madden DR. The three-dimensional structure of an ionotropic glutamate receptor reveals a dimer-of-dimers assembly. J Mol Biol. 2004;344(2):435–442. doi: 10.1016/j.jmb.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 26.Midgett CR, Gill A, Madden DR. Domain architecture of a calcium-permeable AMPA receptor in a ligand-free conformation. Front Mol Neurosci. 2012;4:56. doi: 10.3389/fnmol.2011.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: Evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991;6(5):785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- 28.Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: Molecular mechanisms underlying kainate receptor selectivity. Neuron. 2005;45(4):539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Alushin GM, Jane D, Mayer ML. Binding site and ligand flexibility revealed by high resolution crystal structures of GluK1 competitive antagonists. Neuropharmacology. 2011;60(1):126–134. doi: 10.1016/j.neuropharm.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawate T, Gouaux E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 2006;14(4):673–681. doi: 10.1016/j.str.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Mayer ML, Ghosal A, Dolman NP, Jane DE. Crystal structures of the kainate receptor GluR5 ligand binding core dimer with novel GluR5-selective antagonists. J Neurosci. 2006;26(11):2852–2861. doi: 10.1523/JNEUROSCI.0123-06.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa T. The biochemistry, ultrastructure, and subunit assembly mechanism of AMPA receptors. Mol Neurobiol. 2010;42(3):161–184. doi: 10.1007/s12035-010-8149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hattori M, Hibbs RE, Gouaux E. A fluorescence-detection size-exclusion chromatography-based thermostability assay for membrane protein precrystallization screening. Structure. 2012;20(8):1293–1299. doi: 10.1016/j.str.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson AA, et al. GPCR stabilization using the bicelle-like architecture of mixed sterol-detergent micelles. Methods. 2011;55(4):310–317. doi: 10.1016/j.ymeth.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank GA, et al. Computational separation of conformational heterogeneity using cryo-electron tomography and 3D sub-volume averaging. J Struct Biol. 2012;178(2):165–176. doi: 10.1016/j.jsb.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440(7083):456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 37.Kumar J, Schuck P, Mayer ML. Structure and assembly mechanism for heteromeric kainate receptors. Neuron. 2011;71(2):319–331. doi: 10.1016/j.neuron.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossmann M, et al. Subunit-selective N-terminal domain associations organize the formation of AMPA receptor heteromers. EMBO J. 2011;30(5):959–971. doi: 10.1038/emboj.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weston MC, Schuck P, Ghosal A, Rosenmund C, Mayer ML. Conformational restriction blocks glutamate receptor desensitization. Nat Struct Mol Biol. 2006;13(12):1120–1127. doi: 10.1038/nsmb1178. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127(1):85–97. doi: 10.1016/j.cell.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 41.Kumar J, Schuck P, Jin R, Mayer ML. The N-terminal domain of GluR6-subtype glutamate receptor ion channels. Nat Struct Mol Biol. 2009;16(6):631–638. doi: 10.1038/nsmb.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao H, et al. Analysis of high-affinity assembly for AMPA receptor amino-terminal domains. J Gen Physiol. 2012;139(5):371–388. doi: 10.1085/jgp.201210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amat F, et al. Markov random field based automatic image alignment for electron tomography. J Struct Biol. 2008;161(3):260–275. doi: 10.1016/j.jsb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116(1):71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 45.Harris A, et al. Trimeric HIV-1 glycoprotein gp140 immunogens and native HIV-1 envelope glycoproteins display the same closed and open quaternary molecular architectures. Proc Natl Acad Sci USA. 2011;108(28):11440–11445. doi: 10.1073/pnas.1101414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartesaghi A, et al. Classification and 3D averaging with missing wedge correction in biological electron tomography. J Struct Biol. 2008;162(3):436–450. doi: 10.1016/j.jsb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuybeda O, et al. A collaborative framework for 3D alignment and classification of heterogeneous subvolumes in cryo-electron tomography. J Struct Biol. 2013;181(2):116–127. doi: 10.1016/j.jsb.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettersen EF, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.