Abstract
To delay evolution of pest resistance to transgenic crops producing insecticidal proteins from Bacillus thuringiensis (Bt), the “pyramid” strategy uses plants that produce two or more toxins that kill the same pest. In the United States, this strategy has been adopted widely, with two-toxin Bt cotton replacing one-toxin Bt cotton. Although two-toxin plants are likely to be more durable than one-toxin plants, the extent of this advantage depends on several conditions. One key assumption favoring success of two-toxin plants is that they kill insects selected for resistance to one toxin, which is called “redundant killing.” Here we tested this assumption for a major pest, Helicoverpa zea, on transgenic cotton producing Bt toxins Cry1Ac and Cry2Ab. Selection with Cry1Ac increased survival on two-toxin cotton, which contradicts the assumption. The concentration of Cry1Ac and Cry2Ab declined during the growing season, which would tend to exacerbate this problem. Furthermore, analysis of results from 21 selection experiments with eight species of lepidopteran pests indicates that some cross-resistance typically occurs between Cry1A and Cry2A toxins. Incorporation of empirical data into simulation models shows that the observed deviations from ideal conditions could greatly reduce the benefits of the pyramid strategy for pests like H. zea, which have inherently low susceptibility to Bt toxins and have been exposed extensively to one of the toxins in the pyramid before two-toxin plants are adopted. For such pests, the pyramid strategy could be improved by incorporating empirical data on deviations from ideal assumptions about redundant killing and cross-resistance.
Keywords: genetically modified, sustainability
Corn and cotton engineered to produce insecticidal proteins from Bacillus thuringiensis (Bt) have provided several benefits, including reduced insecticide use, regional pest suppression, protection of natural enemies, and increased or less variable yields (1–3). Evolution of resistance by pests, however, is the most serious threat to the continued efficacy of Bt crops. Significant increases in the frequency of alleles conferring resistance to Bt toxins produced by transgenic crops have been reported in some populations of at least seven target species (4–12). Analyses of monitoring data and field experiments suggest that refuges of host plants that do not produce Bt toxins and grow near Bt crops can reduce the risk of resistance (4, 5, 13). Such refuges of non–Bt host plants delay resistance by enabling survival of susceptible pests that can mate with resistant pests surviving on Bt crops.
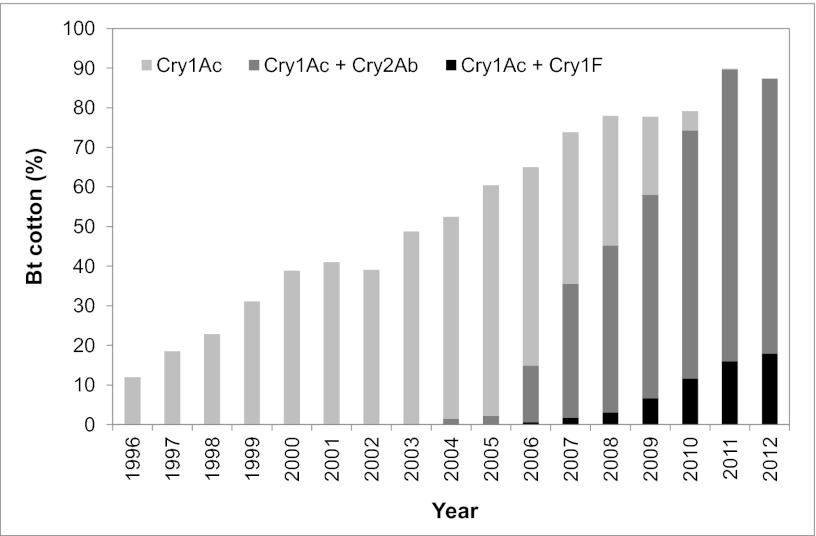
The first generation of Bt cotton, which was grown on a large scale starting in 1996 in the United States, produced only one Bt toxin, Cry1Ac. This Cry1Ac cotton was progressively and completely replaced in the United States from 2003 to 2011 by “pyramided” plants that produce two Bt toxins, either Cry1Ac and Cry2Ab or Cry1Ac and Cry1F (Fig. 1 and Fig. S1). This replacement was spurred by the idea that the evolution of resistance would be delayed substantially by two-toxin crops relative to one-toxin crops (1). A central assumption of the two-toxin pyramid strategy is that insects resistant to one toxin will be killed by the other toxin, which is called “redundant killing” (14, 15). With complete redundant killing and recessive resistance, only the insects homozygous for resistance to both toxins have high survival on a two-toxin cultivar. Such doubly resistant individuals are expected to be rare in populations that have not been exposed previously to either toxin.
Fig. 1.
Percentage of total hectares of upland cotton planted to Bt cotton from 1996 to 2012 in the United States (U.S. Department of Agriculture–Agricultural Marketing Service, 1996–2012 crops). The non–Bt cotton percentage is 100% minus the total height of each bar.
Several factors could reduce redundant killing and decrease the effectiveness of the pyramid strategy for pests such as Helicoverpa zea. Redundant killing is reduced when some susceptible pests survive exposure to the toxins produced by a pyramid (14). Survival of susceptible H. zea larvae on two-toxin cotton can reach 5% during the growing season (5, 16–18), which could reduce the efficacy of the pyramid strategy. Furthermore, as Bt cotton plants age, toxin concentrations decline, which could increase survival of pests that have inherently low susceptibility to Bt toxins (19, 20). Redundant killing could also be undermined if selection for resistance to one of the toxins causes cross-resistance to the other toxin (5, 14, 19, 21). Cry1Ac and Cry2Ab have been considered a good combination for pyramided Bt crops because they have low amino acid homology and bind to different target sites in the larval midgut (22, 23). However, in field-derived strains of H. zea and Helicoverpa armigera, responses to Cry1Ac and Cry2Ab were genetically correlated, indicating potential cross-resistance (5, 24–27). Although redundant killing is critical for the success of the pyramid strategy, little is known about factors affecting redundant killing in H. zea and other pests with low susceptibility to Bt toxins. For example, based on their modeling results, Onstad and Meinke (28) called for empirical evaluation of pyramids to develop resistance management plans.
Here we examined the previously untested assumption of redundant killing in H. zea on cotton producing Cry1Ac and Cry2Ab. We found that a strain selected for resistance to Cry1Ac had increased survival on two-toxin cotton relative to its unselected parent strain, which contradicts the assumption of redundant killing. We also found evidence of cross-resistance between Cry1A and Cry2A toxins from an analysis of data from 21 selection experiments including the results reported here. Incorporation of empirical data into simulation models shows that the observed deviations from ideal conditions could greatly reduce the benefits of the pyramid strategy for H. zea.
Results
Effects of Laboratory Selection with Cry1Ac on Susceptibility to Bt Toxins.
We evaluated susceptibility to Bt toxins of three strains of H. zea: a susceptible laboratory strain (LAB-S), a field-derived strain from Georgia that was exposed to Bt toxins only in the field (GA), and a resistant strain (GA-R) that was derived from the GA strain and selected in the laboratory with Cry1Ac in diet for nine generations. Comparison between the susceptible strain and the GA strain suggests that exposure to Bt crops in the field had selected for some resistance to Cry1Ac and Cry2Ab in the founders of the GA strain. For the field-derived GA strain relative to LAB-S, the concentration of toxin killing 50% (LC50) was 55-fold higher for Cry1Ac and 14-fold higher for Cry2Ab (Table 1).
Table 1.
Responses of H. zea to Cry1Ac and Cry2Ab toxins incorporated in diet
| 95% Fiducial limits |
|||||||
| Toxin | Strain | N | LC50 (µg⋅ml−1) | Lower | Upper | Slope | RR |
| Cry1Ac | LAB-S | 336 | 0.42 | 0.08 | 0.80 | 1.1 | 1.0 |
| GA | 560 | 23 | 14 | 44 | 1.0 | 55 | |
| GA-R | 560 | 230 | 140 | 480 | 0.9 | 560 | |
| Cry2Ab | LAB-S | 336 | 2.2 | 1.6 | 2.8 | 2.2 | 1.0 |
| GA | 336 | 31 | 21 | 54 | 1.5 | 14 | |
| GA-R | 336 | 62 | 30 | 340 | 0.9 | 28 | |
LAB-S, susceptible LAB-S; GA, field-derived strain from Georgia; GA-R, resistant strain derived from the GA strain and selected with Cry1Ac in the laboratory; N, number of larvae tested; RR, resistance ratio, the LC50 of a strain divided by the LC50 of the susceptible LAB-S strain.
Selection with Cry1Ac increased resistance to Cry1Ac and caused strong cross-resistance to the closely related toxin Cry1Ab, but not to the more distantly related toxin Cry2Ab. After nine generations of laboratory selection of GA-R, the LC50 of Cry1Ac for GA-R was 10 times higher than for GA and 560 times higher than for LAB-S (Table 1). However, based on the conservative criterion of nonoverlap of 95% fiducial limits (FLs), selection with Cry1Ac did not significantly increase the LC50 of Cry2Ab (in µg toxin per ml diet) for GA-R (62) relative to GA (31) (Table 1). By contrast, selection with Cry1Ac caused a statistically significant, 15-fold increase in the LC50 of Cry1Ab for GA-R (940) relative to GA (63) (Table S1).
Survival from Neonate to Adult on Bt and non–Bt Cotton.
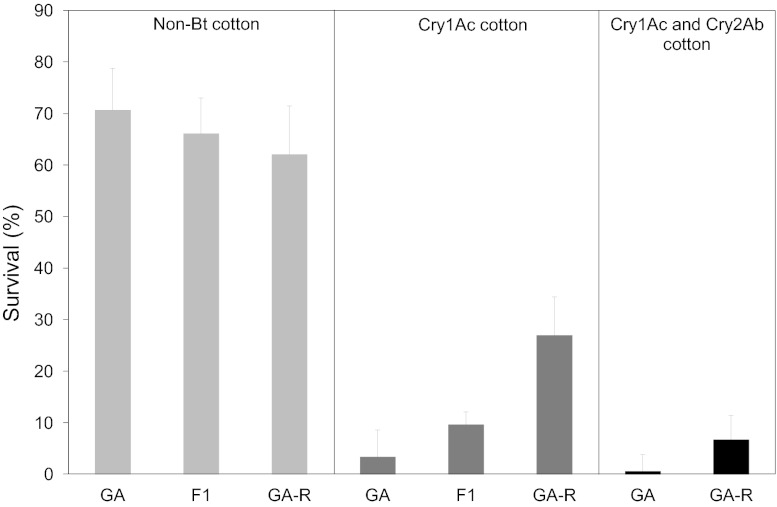
We next determined if selection for resistance to Cry1Ac affected survival on Bt plants. Laboratory selection with Cry1Ac increased survival of GA-R relative to GA on Bt cotton producing only Cry1Ac or both Cry1Ac and Cry2Ab (Fig. 2). The odds of survival for GA-R relative to GA were 11 times higher on Cry1Ac cotton (χ2 = 31.5, df = 2, P < 0.001) and 13 times higher on two-toxin cotton (χ2 = 11.3, df = 1, P < 0.001) (Fig. 2). Furthermore, inheritance of resistance on Cry1Ac cotton was not completely recessive. Survival on Cry1Ac cotton was three times higher for the F1 progeny of GA and GA-R relative to GA (χ2 = 4.86, df = 2, P = 0.028) (Fig. 2). The value of h, which varies from 0 for recessive resistance to 1 for dominant resistance, was 0.25.
Fig. 2.
Survival (+ 95% CI) from neonate to adult of H. zea from a field-derived strain (GA), a resistant strain (GA-R), and their F1 progeny reared on plant material from non–Bt cotton and Bt cotton producing Cry1Ac or both Cry1Ac and Cry2Ab.
Survival on non–Bt cotton did not differ between GA (71%) and GA-R (62%) (χ2 = 0.97, df = 1, P = 0.33). Thus, we did not detect a significant fitness cost affecting this trait (Fig. 2). Survival of GA-R was higher on non–Bt cotton than on either Cry1Ac cotton (χ2 = 19.8, df = 1, P < 0.001) or two-toxin cotton (χ2 = 71.3, df = 1, P < 0.001), indicating incomplete resistance (I = 0.43 on Cry1Ac cotton and 0.11 on two-toxin cotton).
Cross-Resistance Between Cry1A and Cry2A Toxins.
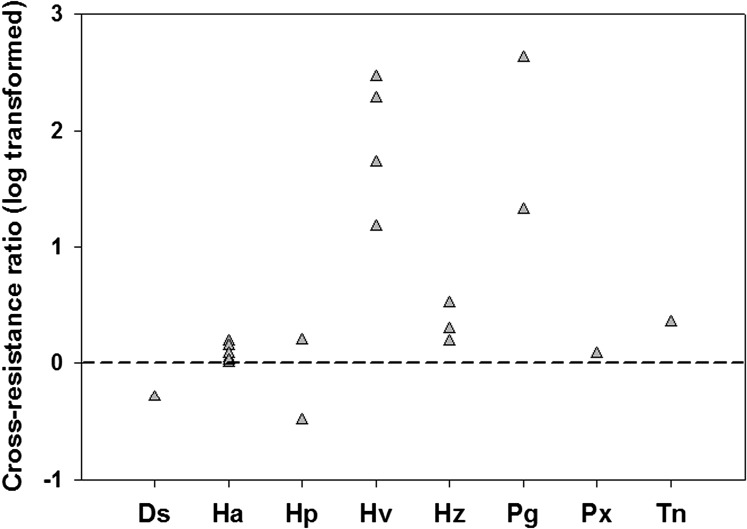
To test the widely held assumption that cross-resistance does not occur between Cry1A and Cry2A toxins, we analyzed results of 21 experiments in which strains of eight major lepidopteran pests had been selected for resistance to a Cry1A toxin and subsequently evaluated for cross-resistance to Cry2A, or vice versa. In 19 of 21 experiments, selection with one toxin decreased susceptibility to the other toxin (Fig. 3 and Table S2). The overall pattern in the 21 experiments considered together indicates significant cross-resistance between Cry1A and Cry2A toxins (signed-rank test, P = 0.0002). Analysis of each selection experiment separately detected significant cross-resistance in seven of 21 cases. In these seven cases, selection with one toxin caused an average 140-fold increase in the LC50 of the other toxin (range = 3- to 420-fold; Table S2). In the remaining 14 cases, significant cross-resistance was not detected when each experiment was analyzed individually, but selection with one toxin decreased susceptibility to the other toxin in 12 of 14 cases, which refutes the null hypothesis of no cross-resistance for these 14 cases (signed-rank test, P = 0.044). In these 14 cases, selection with one toxin caused an average increase of 1.3-fold in the LC50 or IC50 (concentration causing 50% inhibition of growth) of the other toxin (range = 0.32- to 2.2-fold; Table S2).
Fig. 3.
Cross-resistance between Cry1A and Cry2A in 21 selection experiments. Insect strains were selected for resistance to a Cry1A toxin and subsequently evaluated for cross-resistance to Cry2A, or vice versa. The CRR is the LC50 (or IC50) of the toxin not used for selection (e.g., Cry2Ab) for the strain selected with the other toxin (e.g., Cry1Ac) divided by the LC50 (or IC50) of the toxin not used for selection (e.g., Cry2Ab) for an unselected, control strain. The expected value of log (CRR) is 0 if cross-resistance is absent and >0 if cross-resistance occurs. Nineteen of the 21 ratios were >0, indicating significant cross-resistance between Cry1A and Cry2A toxins. Insect strains were from the following species: Diatracea saccharalis (Ds), H. armigera (Ha), Helicoverpa punctigera (Hp), Heliothis virescens (Hv), H. zea (Hz), Pectinophora gossypiella (Pg), Plutella xylostella (Pg), and Trichoplusia ni (Tn).
Concentration of Cry1Ac and Cry2Ab Toxins in Terminal Leaves.
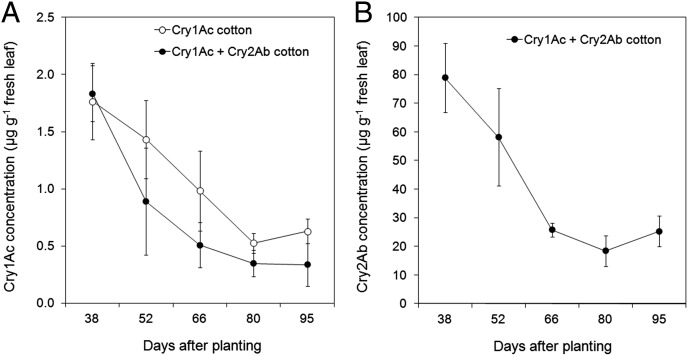
We next determined if the concentrations of Bt toxins varied between cultivars or due to plant aging to see how this might impact redundant killing in pyramid plants. The concentration of Cry1Ac in terminal leaves decreased significantly during the growing season in both one- and two-toxin cotton (F = 58.8, df = 4, P < 0.001) (Fig. 4A). In addition, the concentration of Cry1Ac was generally higher in one- than two-toxin cotton (F = 17.5, df = 1, P < 0.001). Seasonal changes in Cry1Ac concentration differed between the cultivars (cultivar × date interaction, F = 2.71, df = 4, P = 0.040). The concentration of Cry1Ac was significantly lower in two- than one-toxin cotton 52 and 66 d after planting (DAP) (linear contrasts, P < 0.004), but did not differ significantly between the cultivars at 28, 80, and 95 DAP (linear contrasts, P > 0.05). The Cry2Ab concentration also declined seasonally in terminal leaves in two-toxin cotton (F = 45.9, df = 4, P < 0.001) (Fig. 4B).
Fig. 4.
Concentration (±95% CI) of (A) Cry1Ac and (B) Cry2Ab in fresh terminal leaves of cotton producing only Cry1Ac (DP 448 B) and cotton producing Cry1Ac and Cry2Ab (DP 164 B2RF). Thirty-eight, 52, 66, 80, and 95 DAP correspond to presquaring, squaring, early fruiting (first flower), fruiting, and late fruiting stages of cotton, respectively. Least squares means toxin concentration and associated 95% CI for each cultivar and date were obtained from ANOVA.
Simulation Results.
The two-toxin pyramid strategy is expected to be most effective for delaying resistance when alleles conferring resistance to each toxin are rare, inheritance of resistance to each toxin is recessive, redundant killing is complete, and sufficient refuges are present (5, 14, 19). We used simulation modeling to evaluate the potential effects of deviations observed here from the ideal conditions of complete redundant killing and completely recessive resistance. The modeling results show that with a 10% refuge of non–Bt cotton, evolution of resistance to two-toxin Bt cotton was greatly accelerated by either a lack of complete redundant killing or by nonrecessive resistance (Fig. 5). As expected, resistance generally evolved slower with either lower initial resistance allele frequency or larger refuges (Figs. 5 and 6).
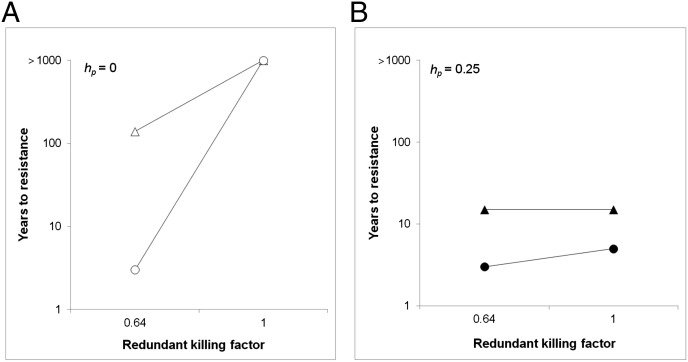
Fig. 5.
Simulated evolution of resistance to two-toxin cotton: effects of redundant killing, dominance, and initial frequency. Resistance to toxins one and two was conferred by alleles r1 and r2 at independent loci, respectively. The initial r2 allele frequency was 0.001. The initial r1 allele frequency was either 0.001 (triangles) or 0.1 (circles). The time to resistance was the number of years until ≥25% of the population could survive on Bt cotton in the third generation of each year. Analogous to the parameter h representing dominance of resistance to one toxin conferred by a single locus, we define hp as dominance of resistance to two-toxin plants conferred by two loci. We also define the RKF, which varies from 0 for no redundant killing to 1 for complete redundant killing (see SI Materials and Methods for additional details). (A) With recessive resistance (hp = 0), the time for resistance to evolve was >1,000 y with complete redundant killing (RKF = 1) and an initial r1 allele frequency of either 0.001 or 0.1. By contrast, when redundant killing was not complete (RKF = 0.64), recessive resistance evolved in 139 y with an initial r1 allele frequency of 0.001 and in 3 y with an initial r1 allele frequency of 0.1. (B) With partially recessive resistance (hp = 0.25) and initial r1 allele frequency = 0.001, resistance evolved in 15 y either with or without complete redundant killing. With hp = 0.25 and initial r1 allele frequency = 0.1, resistance evolved in 5 y with complete redundant killing and in 3 y without complete redundant killing.
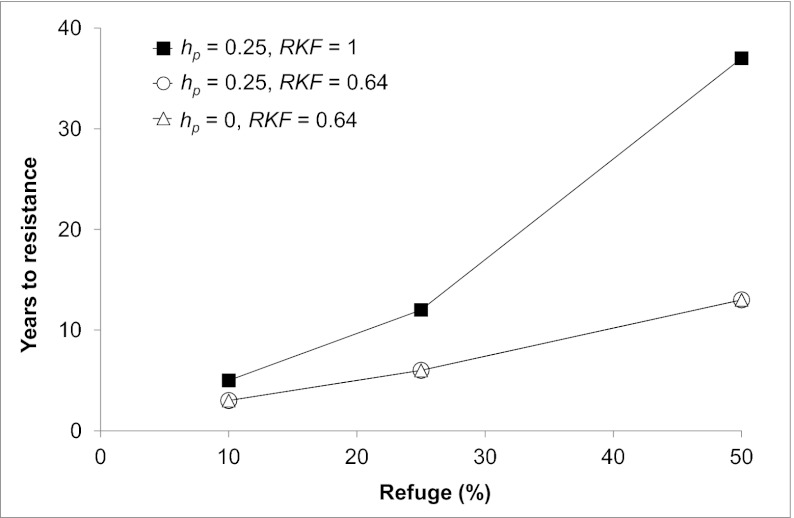
Fig. 6.
Simulated effects of refuge percentage, dominance, and redundant killing on evolution of resistance to two-toxin Bt cotton. The RKF was either 1, which represents the ideal condition of complete redundant killing, or 0.64, which reflects the higher survival on two-toxin Bt cotton for individuals selected for resistance to one toxin (Cry1Ac) relative to susceptible individuals, based on empirical data for survival of the GA-R strain relative to the GA strain (Fig. 2). Table S3 gives fitness values for each genotype under each set of conditions simulated.
Discussion
Redundant killing, which occurs when each toxin produced by a two-toxin Bt cultivar kills all insects resistant to the other toxin, is essential for optimal success of the pyramid strategy (14, 15, 20, 28). Here we found that this assumption did not apply, because laboratory selection for resistance to Cry1Ac of a field-derived strain of H. zea (GA) significantly increased survival of larvae from the GA-R strain on both one- and two-toxin Bt cotton. Furthermore, our analysis of 21 selection experiments with eight species of lepidopteran pests shows pervasive cross-resistance between Cry1A and Cry2A toxins. When susceptible insects can survive on a Bt crop, even alleles with small effects on resistance can increase survival and contribute to the evolution of resistance (19). Accordingly, in pests with low susceptibility to Cry1A and Cry2A toxins, cross-resistance between Cry1A and Cry2A will generally hasten evolution of resistance. In accord with results from other simulation models (14, 15, 20, 28), the modeling results presented here (Figs. 5 and 6) indicate that resistance to a two-toxin pyramid evolves faster when each toxin of a pyramid does not kill all individuals resistant to the other toxin, as seen with H. zea (Fig. 2). The joint effects on evolution of resistance to pyramids of both cross-resistance and a lack of complete redundant killing have received limited attention previously. Our results imply that, to advance resistance management for pyramids, this issue needs more attention.
Increased survival of GA-R on two-toxin cotton likely occurred because the concentration of Cry2Ab was not sufficient to kill individuals resistant to Cry1Ac. Survival of GA-R was 6.7% on two-toxin cotton and 27% on one-toxin cotton, yielding 25% survival on two-toxin cotton relative to one-toxin cotton (6.7%/27%), which translates to 75% mortality on two-toxin cotton relative to one-toxin cotton. In the SP15 strain of H. armigera selected for homozygous resistance to Cry2Ab, but not selected with Cry1Ac, survival on cotton producing both toxins increased as the growing season progressed (29). The Bt concentration of plants was not measured in this case, but a seasonal decline in the concentration of Cry1Ac likely reduced levels of redundant killing (19). In the results reported here, a seasonal decline in the concentration of Cry2Ab may have reduced redundant killing of H. zea. This conclusion is based on the assumption that seasonal declines in the concentration of Bt toxins measured in leaves paralleled declines in bolls and squares on which larvae were also fed.
Another factor that may have reduced redundant killing on two-toxin cotton is weak cross-resistance between Cry1Ac and Cry2Ab. After selection with Cry1Ac, the LC50 of Cry2Ab for the GA-R strain was about double relative to its parent strain GA, and this difference was not significant based on the overlap between 95% FLs (Table 1). However, the criterion of nonoverlap of 95% FLs is statistically conservative (30) and we cannot exclude the possibility of weak cross-resistance. Moreover, weak cross-resistance is consistent with the results from our analysis of 21 selection experiments, and the significant positive genetic correlations between resistance to Cry1Ac and Cry2Ab found in field-derived strains of H. zea and H. armigera (5, 26).
Resistance of H. zea was not completely recessive to Cry1Ac cotton here (h = 0.25) or to Cry1Ac in diet as reported previously (h = 0.83) (4, 31). As shown here and in other studies using simulation models, nonrecessive resistance can accelerate the evolution of resistance to two-toxin cotton (14, 15, 20). Fitness costs, which occur when fitness on non–Bt host plants is lower for Bt–resistant than susceptible insects, can slow evolution of resistance, particularly to two-toxin plants (19, 32). Although we did not detect a significant fitness cost associated with resistance to Cry1Ac, we might have underestimated cost because we compared the resistant GA-R strain with its parent strain GA, which apparently contained some resistance alleles. We did detect incomplete resistance, indicated by the lower survival of the GA-R strain on either Cry1Ac cotton or two-toxin cotton relative to non–Bt cotton (Fig. 2). The incomplete resistance detected, which can help to delay resistance (33, 34), was incorporated in our simulations (Table S3).
The results in our study are mainly similar to previous results in terms of the relative toxicity of Cry1Ac and Cry2Ab to H. zea and the concentrations of these toxins in Bt cotton plants (SI Materials and Methods). However, unlike our results showing similar seasonal declines of about threefold in Cry1Ac and Cry2Ab in DP 164 B2RF, previous studies with other cultivars found that the seasonal decline in toxin concentration was generally less for Cry2Ab (35–37) than Cry1Ac (38–42). In any case, seasonal declines in the concentration of Bt toxins could be an important factor affecting redundant killing because there is extensive variation among cotton cultivars in patterns of production of Bt toxins (43).
Conclusions
Previous experimental evidence on the pyramid strategy comes primarily from a model system with diamondback moth and noncommercial Bt broccoli plants producing Cry1Ac and Cry1C (44, 45). Although most of the optimal conditions for pyramids apply to this model system, they may not apply for some other pest-Bt crop combinations, particularly when pests have inherently low susceptibility to one or more of the toxins in the pyramid (19, 20, 28, 46). Here we found several deviations from optimal conditions for H. zea and Bt cotton producing Cry1Ac and Cry2Ab. Our results show that the commercially available two-toxin Bt cotton plants we tested did not cause complete redundant killing of H. zea [redundant killing factor (RKF) = 0.64]. Also, inheritance of resistance to Cry1Ac was not completely recessive (h = 0.25).
The deviations from ideal conditions we found with H. zea and two-toxin Bt cotton, which entail both lack of complete redundant killing and nonrecessive resistance, are likely to accelerate resistance relative to the conditions examined in previous modeling studies focusing primarily on complete or nearly complete redundant killing (RKF = 0.99–1), recessive inheritance (hp ≤ 0.05), or both (28, 32, 45, 47, 48) (see Table S3 for details).
Our simulation results under ideal conditions (hp = 0 and RKF = 1) correspond closely with the projected outcomes in simulations under the favorable assumptions examined previously. By contrast, the substantial deviations from ideal conditions based on empirical data from H. zea (hp = 0.25 and RKF = 0.64) yielded much faster evolution of resistance in simulations (Figs. 5 and 6). Moreover, H. zea populations were exposed extensively to Cry1Ac cotton before and after two-toxin plants were introduced (Fig. 1 and Fig. S1). Because of cross-resistance between Cry1Ac and Cry1Ab (Table S1), similar exposure to Cry1Ab corn is also problematic because it would tend to increase the frequency of resistance to Cry1Ac.
Field monitoring data show decreased susceptibility of H. zea populations to both Cry1Ac and Cry2Ab in some regions in the United States (4, 5). In light of these data and the deviations from optimal conditions summarized above, effective management of resistance in this case may require relatively large refuges of non–Bt host plants in conjunction with multiple control tactics as part of integrated pest management (4, 20, 46). Our results also suggest that management of resistance to Bt crop pyramids in pests with inherently low susceptibility to Bt toxins could be enhanced by addressing effects of cross-resistance, less than complete redundant killing, and seasonal declines in the concentration of Bt toxins.
Materials and Methods
Insect Strains, Rearing, and Selection.
We used three strains of H. zea: a susceptible LAB-S obtained from Benzon Research Inc (Carlisle, PA), a field-derived strain from Georgia that was exposed to Bt toxins only in the field (GA), and a resistant strain derived from the GA strain that we selected in the laboratory with Cry1Ac in diet for nine generations (GA-R) as described below.
We provided moths with cotton balls wetted with a 10% dilution of honey in water for feeding and cheesecloth for egg-laying. Eggs were harvested daily and larvae were reared on diet (Southland Products Inc.). Strains were maintained at 27 ± 1 °C, 60 ± 10% relative humidity (RH), and 14 light (L):10 dark (D).
The GA strain originated from 180 larvae collected in July 2008 from Cry1Ab corn (Zea mays L.) hybrid “DKC 6971” (MON810) near Tifton, Georgia, and was reared on diet without exposure to toxins. After two generations of laboratory rearing, we used a subset of insects from GA to start the GA-R strain. We selected GA-R for resistance to Cry1Ac during each of nine nonconsecutive generations by exposing at least 1,000 GA-R neonates to Cry1Ac in diet. In each selected generation, only larvae that reached third instar after 7 d of feeding on diet treated with Cry1Ac were transferred to non–Bt diet and reared to pupation to continue the strain. The concentration (µg of Cry1Ac mL−1 diet) increased progressively with 10–20 in selected generations 1–3 and 100–1,000 in selected generations 4–9.
Bt Toxins.
We used protoxin crystals with spores of Cry1Ab and Cry1Ac prepared by J. Sánchez as described previously (49) and Cry2Ab produced by a recombinant acrystalliferous strain of Bt ssp. kurstaki (HD73 cry–) that was transformed with the Cry2Ab gene from strain HD1 of Bt ssp. kurstaki (50). We used the protoxin preparations described above in all experiments, with one exception. For generations 4–9 of selection of GA-R, we needed higher toxin concentrations and we used MVP II (Dow AgroSciences) containing 20% Cry1Ac protoxin (51).
Diet Bioassays.
We used diet incorporation bioassays (52) to assess the responses of LAB-S, GA, and GA-R to Cry1Ac, Cry2Ab, and Cry1Ab. Diet bioassays were conducted simultaneously using generation 12 of GA-R and generation 14 of GA. Toxins were suspended in distilled water and incorporated into a bean-based diet (53) at 45–55 °C (1:5 volume of toxin solution to diet). We dispensed 0.5 mL of diet in each well of 128-cell bioassay trays (Bioserv) using a 25 mL Repeater Plus Pipettor (Eppendorf). For controls, distilled water was mixed with the diet. All assays included six to seven toxin concentrations ranging from 0 to 300 μg Cry1Ac or Cry1Ab mL−1 diet, and 0–50 μg Cry2Ab mL−1 diet.
After the diet was dry, one <24 h-old neonate was transferred onto the diet surface of each cell. Trays were then covered with plastic ventilated covers and incubated at 27 ± 1 °C, 60 ± 10% RH, and a photoperiod of 14L:10D. We replicated each combination of insect population and toxin concentration four to five times, with 16 neonates per replicate. Mortality was recorded after 7 d. We considered larvae dead if stimulation with a blunt needle did not elicit a coordinated response.
Survival on Bt Cotton and non–Bt Cotton Plant Material.
To evaluate survival on non–Bt, Cry1Ac and Cry1Ac + Cry2Ab cotton, neonates of GA, GA-R, and reciprocal crosses between these strains were fed on cotton in the laboratory. Reciprocal crosses produced F1 progeny designated as F1a and F1b. F1a was obtained by crossing GA-R males with GA females, and F1b was obtained by crossing GA-R females with GA males. Reciprocal mass crosses were conducted with a minimum of 30 mating pairs, and were only tested on non–Bt and Cry1Ac cotton. Bioassays with plants were conducted simultaneously using insects from generation 13 and 15 of GA-R and GA, respectively.
Survival on plant material was assessed in 2010, using field-grown cotton planted on June 5 on nutrient-rich heavy loam soil at the West Campus Agricultural Center of the University of Arizona. We used Bt cotton cultivars DP 448 B, which produces only Cry1Ac, and DP 164 B2RF, which produces Cry1Ac and Cry2Ab, and non–Bt cotton cultivar DP 5415 as a control. Plants were flood irrigated and did not receive any fertilizer, as we did not see evidence of nutrient deficiency in plants as the season progressed. Pest abundance was low throughout the growing season and no insecticides were required to protect plants.
We started the insect feeding experiment 79 days after planting (DAP), when cotton was bearing bolls. For the first 7 d, larvae were placed individually in 30 mL clear plastic cups (ProPak@) that contained a 2-cm-diameter leaf disk from the terminal on a 5 mL mixture of 2% agar (2 g of agar per 100 ml of water) and 0.1% sorbic acid for moisture and microbial control (29). Cups were covered with a clear plastic lid and put in a growth chamber at 27 ± 1 °C, 60 ± 10% RH, and photoperiod 14L:10D. We used 60–240 neonates for each combination of cotton type and insect type (GA, GA-R, and F1 progeny).
After 7 d or earlier if larvae had molted to third instar in less than 7 d, larvae were transferred individually to 470 mL clear plastic cups (Fabri-Kal) ventilated with a 5-cm-diameter mesh-covered hole in the lid. These older larvae were offered plant terminals bearing small bolls, leaves, and squares. Plant stems were inserted in the lid of a 30 mL cup of water placed at the bottom of the container, and the containers disposed in a growth chamber maintained at 27 ± 1 °C, 60 ± 10% RH, with a photoperiod of 14:10 (L:D). Plant material and water were renewed as needed (generally twice a week) until the insects pupated within the container. Survival was recorded every 4 d.
Analysis of Cross-Resistance.
We reviewed experiments in which insect strains had been selected for resistance to a Cry1A toxin and subsequently evaluated for cross-resistance to Cry2A, or vice versa. For each selection experiment and toxin not used for selection, the LC50 or IC50 (i.e., toxin concentration inhibiting 50% of growth) of the strain selected for resistance was divided by the LC50 or IC50 of the unselected control strain. The expected value of this cross-resistance ratio (CRR) is 1 if cross-resistance is absent and >1 if cross-resistance is present. However, the logarithm of the CCR was used in statistical analyses to improve linearity and normality of this variable (54). The expected value of log CRR is 0 if cross-resistance is absent and >0 if cross-resistance is present.
Toxin Concentration in Plants.
The concentrations of Cry1Ac and Cry2Ab in cotton leaves were analyzed with ELISA (see SI Materials and Methods for additional details).
Population Genetics Model.
To simulate the evolution of H. zea resistance to two-toxin cotton, we used a deterministic model with two loci. Locus one affected responses to Cry1Ac and locus 2 affected responses to Cry2Ab. Each locus had two alleles: r1 and r2 conferring resistance and s1 and s2 susceptibility to Cry1Ac and Cry2Ab, respectively. We assumed initial gametic equilibrium (15). The time to resistance was the number of years until ≥25% of the population could survive on Bt cotton in the third generation of each year (see SI Materials and Methods for additional details).
Data Analysis.
We used probit analysis to estimate the toxin concentration causing 50% mortality (LC50), its 95% FLs, and slope of the concentration–mortality line (55). Log linear models were fit with WINDL 2.0 (56). LC50 values were considered significantly different if their 95% FL did not overlap. We estimated the resistance ratio as the LC50 of a strain divided by the LC50 of the susceptible LAB-S strain. Maternal effects and sex linkage affecting resistance to Cry1Ac cotton were evaluated by comparing survival of insects from the reciprocal crosses with a Pearson’s χ2 test. Survival on Cry1Ac cotton did not differ significantly between the F1 progeny from reciprocal crosses (GA females × GA-R males and vice versa; χ2= 0.83, df = 1, P = 0.36), indicating autosomal inheritance. Accordingly, we pooled data from the two reciprocal crosses for subsequent analyses. We compared survival of strains GA, GA-R, and their hybrid progeny (F1) reared on fresh plant material of non–Bt, Cry1Ac, and two-toxin cotton using logistic regression for binary data. These analyses compared survival to adulthood of the strains separately for each cultivar. Dominance (h) was evaluated from the corrected survival of the F1 progeny relative to that of parental strains GA and GA-R (57). Values of h range from 0 (completely recessive resistance) to 1 (completely dominant resistance).
Fitness costs associated with resistance to Bt toxins occur if Bt–resistant individuals have lower fitness than Bt–susceptible individuals on non–Bt plants (58). Potential fitness costs associated with Cry1Ac resistance were evaluated by comparing survival of GA, F1, and GA-R on non–Bt cotton with logistic regression. Incomplete resistance occurs when resistant individuals have lower survival on a Bt crop than on a non–Bt crop (19, 58). Incomplete resistance was assessed by comparing survival of GA on Cry1Ac or two-toxin cotton with survival on non–Bt cotton with a Pearson’s χ2 test. Levels of incomplete resistance (I) were calculated by dividing survival of GA-R on Cry1Ac cotton (or two-toxin cotton) by survival of GA-R on non–Bt cotton.
The change in Cry1Ac concentration in leaves across the growing season was assessed using a two-way ANOVA including the effects of cultivar (Cry1Ac or two-toxin cotton), time (treated as a categorical variable), and the interaction between these factors. Linear contrasts were used to compare the concentration of Cry1Ac between cultivars on each date. The seasonal change in Cry2Ab concentration in leaves was assessed using a one-way ANOVA.
The log-response ratio of the CRR was not normally distributed when all data (Shapiro–Wilk test, P = 0.0009) or experiments not detecting significant cross-resistance (P = 0.026) were considered. In both cases, we used a one-tailed Wilcoxon signed-rank test to test the hypothesis that the average log-response ratio was greater than 0 (54). All statistical analyses were performed in JMP 9.0 (SAS Institute).
Supplementary Material
Acknowledgments
We thank M. Hill, R. A. Garcia, C. M. Jones, and A. Mazza for technical assistance. We thank J. Sánchez from the group of A. Bravo and M. Soberón (Universidad Nacional Autónoma de México, Cuernavaca) for providing Cry1Ab and Cry1Ac. We thank Mark Sisterson and David Crowder for providing comments on this manuscript. This study was supported by U.S. Department of Agriculture (USDA) National Research Initiative Competitive Grants Program Project 2007-02227 and USDA Biotechnology Risk Assessment Grant Award 2011-33522-30729.
Footnotes
Conflict of interest statement: B.E.T. received support for research that is not related to this publication from the following sources: Cotton Foundation, Cotton Inc., National Cotton Council, Monsanto, and Dow AgroSciences. He is also a coauthor of a patent on engineering modified Bt toxins to counter pest resistance, which is related to research described by Tabashnik et al. (2011, Nature Biotechnology 29: 1128-1131).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216719110/-/DCSupplemental.
References
- 1.National Research Council . The Impact of Genetically Engineered Crops on Farm Sustainability in the United States. Washington, DC: National Academies Press; 2010. [Google Scholar]
- 2.Tabashnik BE, et al. Suppressing resistance to Bt cotton with sterile insect releases. Nat Biotechnol. 2010;28(12):1304–1307. doi: 10.1038/nbt.1704. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Wu K, Jiang Y, Guo Y, Desneux N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature. 2012;487(7407):362–365. doi: 10.1038/nature11153. [DOI] [PubMed] [Google Scholar]
- 4.Tabashnik BE, Gassmann AJ, Crowder DW, Carrière Y. Insect resistance to Bt crops: Evidence versus theory. Nat Biotechnol. 2008;26(2):199–202. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- 5.Tabashnik BE, Van Rensburg JBJ, Carrière Y. Field-evolved insect resistance to Bt crops: Definition, theory, and data. J Econ Entomol. 2009;102(6):2011–2025. doi: 10.1603/029.102.0601. [DOI] [PubMed] [Google Scholar]
- 6.Van Rensburg JBJ. First report of field resistance by stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S Afr J Plant Soil. 2007;24(3):147–151. [Google Scholar]
- 7.Bagla P. India. Hardy cotton-munching pests are latest blow to GM crops. Science. 2010;327(5972):1439–1439. doi: 10.1126/science.327.5972.1439. [DOI] [PubMed] [Google Scholar]
- 8.Storer NP, et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol. 2010;103(4):1031–1038. doi: 10.1603/ec10040. [DOI] [PubMed] [Google Scholar]
- 9.Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW. Field-evolved resistance to Bt maize by western corn rootworm. PLoS ONE. 2011;6(7):e22629. doi: 10.1371/journal.pone.0022629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang HN, et al. Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS ONE. 2011;6(8):e22874. doi: 10.1371/journal.pone.0022874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan P, et al. Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS ONE. 2012;7(1):e29975. doi: 10.1371/journal.pone.0029975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downes S, Parker T, Mahon R. Incipient resistance of Helicoverpa punctigera to the Cry2Ab Bt toxin in Bollgard II cotton. PLoS ONE. 2010;5(9):e12567. doi: 10.1371/journal.pone.0012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrière Y, et al. Large-scale, spatially-explicit test of the refuge strategy for delaying insecticide resistance. Proc Natl Acad Sci USA. 2012;109(3):775–780. doi: 10.1073/pnas.1117851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roush RT. Two-toxin strategies for management of insecticidal transgenic crops: Can pyramiding succeed where pesticide mixtures have not? Philos Trans R Soc Lond Ser B. 1998;353(1376):1777–1786. [Google Scholar]
- 15.Gould F. Simulation models for predicting durability of insect-resitant germ plasm: A deterministic diploid, two-locus model. Environ Entomol. 1986;15(1):1–10. [Google Scholar]
- 16.Jackson RE, Bradley JR, Jr, Van Duyn JW, Gould F. Comparative production of Helicoverpa zea (Lepidoptera: Noctuidae) from transgenic cotton expressing either one or two Bacillus thuringiensis proteins with and without insecticide oversprays. J Econ Entomol. 2004;97(5):1719–1725. doi: 10.1603/0022-0493-97.5.1719. [DOI] [PubMed] [Google Scholar]
- 17.Storer NP. Resistance management rationale and strategy for Widestrike insect protection (Cry1F/Cry1Ac) in cotton. In: Richter DA, editor. Proceedings, 2005 Beltwide Cotton Conferences, 4-7 January 2005, New Orleans, LA. Memphis, TN: National Cotton Council of America; 2005. [Google Scholar]
- 18.Greenberg SM, Li YX, Liu TX. Effect of age of transgenic cotton on mortality of lepidopteran larvae. Southwest Entomologist. 2010;35(3):261–268. [Google Scholar]
- 19.Carrière Y, Crowder DW, Tabashnik BE. Evolutionary ecology of insect adaptation to Bt crops. Evol Appl. 2010;3(5-6):561–573. doi: 10.1111/j.1752-4571.2010.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brévault T, Achaleke J, Nibouche S, Carrière Y. Assessing the role of non-cotton refuges in delaying Helicoverpa armigera resistance to Bt cotton in West Africa. Evol Appl. 2012;5(1):53–65. doi: 10.1111/j.1752-4571.2011.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gould F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu Rev Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- 22.Caccia S, et al. Binding site alteration is responsible for field-isolated resistance to Bacillus thuringiensis Cry2A insecticidal proteins in two Helicoverpa species. PLoS ONE. 2010;5(4):e9975. doi: 10.1371/journal.pone.0009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández-Rodríguez CS, Van Vliet A, Bautsoens N, Van Rie J, Ferré J. Specific binding of Bacillus thuringiensis Cry2A insecticidal proteins to a common site in the midgut of Helicoverpa species. Appl Environ Microbiol. 2008;74(24):7654–7659. doi: 10.1128/AEM.01373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burd AD, Gould F, Bradley JR, Van Duyn JW, Moar WJ. Estimated frequency of nonrecessive Bt resistance genes in bollworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in eastern North Carolina. J Econ Entomol. 2003;96(1):137–142. doi: 10.1093/jee/96.1.137. [DOI] [PubMed] [Google Scholar]
- 25.Jackson RE, Bradley JR, Van Duyn JW. Performance of feral and Cry1Ac-selected Helicoverpa zea (Lepidoptera: Noctuidae) strains on transgenic cottons expressing one or two Bacillus thuringiensis ssp kurstaki proteins under greenhouse conditions. J Entomol Sci. 2004;39(1):46–55. [Google Scholar]
- 26.Gao YL, Wu KM, Gould F, Shen ZC. Cry2Ab tolerance response of Helicoverpa armigera (Lepidoptera: Noctuidae) populations from CrylAc cotton planting region. J Econ Entomol. 2009;102(3):1217–1223. doi: 10.1603/029.102.0347. [DOI] [PubMed] [Google Scholar]
- 27.Ali MI, Luttrell RG. Susceptibility of bollworm and tobacco budworm (Lepidoptera: Noctuidae) to Cry2Ab2 insecticidal protein. J Econ Entomol. 2007;100(3):921–931. doi: 10.1603/0022-0493(2007)100[921:sobatb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Onstad DW, Meinke LJ. Modeling evolution of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) to transgenic corn with two insecticidal traits. J Econ Entomol. 2010;103(3):849–860. doi: 10.1603/ec09199. [DOI] [PubMed] [Google Scholar]
- 29.Mahon RJ, Olsen KM. Limited survival of a Cry2Ab-resistant strain of Helicoverpa armigera (Lepidoptera: Noctuidae) on Bollgard II. J Econ Entomol. 2009;102(2):708–716. doi: 10.1603/029.102.0232. [DOI] [PubMed] [Google Scholar]
- 30.Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: What do they mean in terms of statistical significance? J Insect Sci. 2003;3:34. doi: 10.1093/jis/3.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burd AD, Bradley JR, Van Duyn JW, Gould F. Resistance of bollworm, Helicoverpa zea, to CryIA(c) toxin. In: Dugger CP, Richter DA, editors. Proceedings, 2000 Beltwide Cotton Conferences, 4-8 January 2000, San Antonio, TX. Memphis, TN: National Cotton Council of America; 2000. pp. 923–926. [Google Scholar]
- 32.Gould F, Cohen MB, Bentur JS, Kennedy GG, Van Duyn J. Impact of small fitness costs on pest adaptation to crop varieties with multiple toxins: A heuristic model. J Econ Entomol. 2006;99(6):2091–2099. doi: 10.1603/0022-0493-99.6.2091. [DOI] [PubMed] [Google Scholar]
- 33.Carrière Y, Tabashnik BE. Reversing insect adaptation to transgenic insecticidal plants. P Roy Soc B-Biol Sci. 2001;268(1475):1475–1480. doi: 10.1098/rspb.2001.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowder DW, Carrière Y. Comparing the refuge strategy for managing the evolution of insect resistance under different reproductive strategies. J Theor Biol. 2009;261(3):423–430. doi: 10.1016/j.jtbi.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Addison SJ, Rogers DJ. Potential impact of differential production of the Cry2Ab and Cry1Ac proteins in transgenic cotton in response to cold stress. J Econ Entomol. 2010;103(4):1206–1215. doi: 10.1603/ec09369. [DOI] [PubMed] [Google Scholar]
- 36.Adamczyk JJ, Jr, Adams LC, Hardee DD. Field efficacy and seasonal expression profiles for terminal leaves of single and double Bacillus thuringiensis toxin cotton genotypes. J Econ Entomol. 2001;94(6):1589–1593. doi: 10.1603/0022-0493-94.6.1589. [DOI] [PubMed] [Google Scholar]
- 37.Greenplate JT, et al. Partial characterization of cotton plants expressing two toxin proteins from Bacillus thuringiensis: Relative toxin contribution, toxin interaction, and resistance management. J Appl Entomol. 2003;127(6):340–347. [Google Scholar]
- 38.Adamczyk JJ, Sumerford DV. Potential factors impacting seasonlong expression of Cry1Ac in 13 commercial varieties of Bollgard cotton. J Insect Sci. 2001;1:13. [PMC free article] [PubMed] [Google Scholar]
- 39.Adamczyk JJ, Hardee DD, Adams LC, Sumerford DV. Correlating differences in larval survival and development of bollworm (Lepidoptera: Noctuidae) and fall armyworm (Lepidoptera: Noctuidae) to differential expression of Cry1A(c) delta-endotoxin in various plant parts among commercial cultivars of transgenic Bacillus thuringiensis cotton. J Econ Entomol. 2001;94(1):284–290. doi: 10.1603/0022-0493-94.1.284. [DOI] [PubMed] [Google Scholar]
- 40.Greenplate JT. Quantification of Bacillus thuringiensis insect control protein Cry1Ac over time in Bollgard cotton fruit and terminals. J Econ Entomol. 1999;92(6):1377–1383. [Google Scholar]
- 41.Olsen KM, Daly JC, Holt HE, Finnegan EJ. Season-long variation in expression of Cry1Ac gene and efficacy of Bacillus thuringiensis toxin in transgenic cotton against Helicoverpa armigera (Lepidoptera: Noctuidae) J Econ Entomol. 2005;98(3):1007–1017. doi: 10.1603/0022-0493-98.3.1007. [DOI] [PubMed] [Google Scholar]
- 42.Siebert MW, et al. Quantification of Cry1Ac and Cry1F Bacillus thuringiensis insecticidal proteins in selected transgenic cotton plant tissue types. J Econ Entomol. 2009;102(3):1301–1308. doi: 10.1603/029.102.0357. [DOI] [PubMed] [Google Scholar]
- 43.Showalter AM, Heuberger S, Tabashnik BE, Carrière Y. A primer for using transgenic insecticidal cotton in developing countries. J Insect Sci. 2009;9:22. doi: 10.1673/031.009.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao JZ, et al. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc Natl Acad Sci USA. 2005;102(24):8426–8430. doi: 10.1073/pnas.0409324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao JZ, et al. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat Biotechnol. 2003;21(12):1493–1497. doi: 10.1038/nbt907. [DOI] [PubMed] [Google Scholar]
- 46.Tabashnik BE, Gould F. Delaying corn rootworm resistance to Bt corn. J Econ Entomol. 2012;105(3):767–776. doi: 10.1603/ec12080. [DOI] [PubMed] [Google Scholar]
- 47.Ives AR, Glaum PR, Ziebarth NL, Andow DA. The evolution of resistance to two-toxin pyramid transgenic crops. Ecol Appl. 2011;21(2):503–515. doi: 10.1890/09-1869.1. [DOI] [PubMed] [Google Scholar]
- 48.Carrol MW, Head G, Caprio M. When and where a seed mix refuge makes sense for managing insect resitance to Bt plants. Crop Prot. 2012;38:74–79. [Google Scholar]
- 49.Gómez I, et al. Specific epitopes of domains II and III of Bacillus thuringiensis Cry1Ab toxin involved in the sequential interaction with cadherin and aminopeptidase-N receptors in Manduca sexta. J Biol Chem. 2006;281(45):34032–34039. doi: 10.1074/jbc.M604721200. [DOI] [PubMed] [Google Scholar]
- 50.Bah A, van Frankenhuyzen K, Brousseau R, Masson L. The Bacillus thuringiensis Cry1Aa toxin: Effects of trypsin and chymotrypsin site mutations on toxicity and stability. J Invertebr Pathol. 2004;85(2):120–127. doi: 10.1016/j.jip.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Tabashnik BE, et al. Inheritance of resistance to Bt toxin crylac in a field-derived strain of pink bollworm (Lepidoptera: Gelechiidae) J Econ Entomol. 2002;95(5):1018–1026. doi: 10.1603/0022-0493-95.5.1018. [DOI] [PubMed] [Google Scholar]
- 52.Ali MI, Luttrell RG, Young SY., 3rd Susceptibilities of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) populations to Cry1Ac insecticidal protein. J Econ Entomol. 2006;99(1):164–175. doi: 10.1093/jee/99.1.164. [DOI] [PubMed] [Google Scholar]
- 53.Burton RL. Mass Rearing the Corn Earworm in the Laboratory. 1969. (U.S. Department of Agriculture, New Orleans, LA) Vols 33–134. [Google Scholar]
- 54.Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80(4):1150–1156. [Google Scholar]
- 55.Finney DJ. Probit Analysis. 3rd Ed. Cambridge, UK: Cambridge Unive Press; 1971. [Google Scholar]
- 56.CIRAD . WINDL, Version 2.0. Montpellier, France: Centre de coopération Internationale en Recherche Agronomique pour le Développement; 1999. [Google Scholar]
- 57.Liu YB, Tabashnik BE. Inheritance of resistance to the Bacillus thuringiensis toxin Cry1C in the diamondback moth. Appl Environ Microbiol. 1997;63(6):2218–2223. doi: 10.1128/aem.63.6.2218-2223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gassmann AJ, Carrière Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis. Annu Rev Entomol. 2009;54:147–163. doi: 10.1146/annurev.ento.54.110807.090518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.