Abstract
Children with an anxious temperament (AT) are at risk for developing psychiatric disorders along the internalizing spectrum, including anxiety and depression. Like these disorders, AT is a multidimensional phenotype and children with extreme anxiety show varying mixtures of physiological, behavioral, and other symptoms. Using a well-validated juvenile monkey model of AT, we addressed the degree to which this phenotypic heterogeneity reflects fundamental differences or similarities in the underlying neurobiology. The rhesus macaque is optimal for studying AT because children and young monkeys express the anxious phenotype in similar ways and have similar neurobiology. Fluorodeoxyglucose (FDG)-positron emission tomography (FDG-PET) in 238 freely behaving monkeys identified brain regions where metabolism predicted variation in three dimensions of the AT phenotype: hypothalamic-pituitary-adrenal (HPA) activity, freezing behavior, and expressive vocalizations. We distinguished brain regions that predicted all three dimensions of the phenotype from those that selectively predicted a single dimension. Elevated activity in the central nucleus of the amygdala and the anterior hippocampus was consistently found across individuals with different presentations of AT. In contrast, elevated activity in the lateral anterior hippocampus was selective to individuals with high levels of HPA activity, and decreased activity in the motor cortex (M1) was selective to those with high levels of freezing behavior. Furthermore, activity in these phenotype-selective regions mediated relations between amygdala metabolism and different expressions of anxiety. These findings provide a framework for understanding the mechanisms that lead to heterogeneity in the clinical presentation of internalizing disorders and set the stage for developing improved interventions.
Keywords: affective neuroscience, behavioral inhibition, developmental psychopathology, emotion, functional neuroimaging
There is substantial heterogeneity in the clinical presentation of anxiety disorders, both within and across diagnostic categories. Anxiety often emerges early in development and, here too, there is considerable variation in presentation. Clinically relevant anxiety is often accompanied and preceded by an anxious temperament (AT). AT is a trait-like phenotype that is evident early in life, stable over time, associated with increased amygdala reactivity to novelty and potential threat, and expressed similarly in children and young nonhuman primates (1–6). Extreme dispositional anxiety and behavioral inhibition in childhood is a well-established risk factor for the internalizing spectrum of psychiatric disorders, including anxiety and major depression (5, 7, 8). These disorders are highly prevalent and associated with substantial morbidity and mortality (9, 10). Like the internalizing disorders, childhood AT is a complex, multidimensional phenotype and children with extreme AT show varying mixtures of peripheral physiological, behavioral, and other kinds of anxiety-related symptoms (5, 11, 12). This diversity manifests as weak covariation among these features (2, 13, 14). From the perspective of diagnosis and treatment, an important unresolved question is the degree to which heterogeneity in anxious individuals’ symptoms reflects fundamental differences or similarities in the underlying neurobiology. To address this question, we used a well-validated nonhuman primate model of early-life AT in combination with high-resolution 18fluorodeoxyglucose (FDG)-positron emission tomography (FDG-PET) (2, 15).
Young rhesus macaques are ideal for understanding the neurobiology of dispositional anxiety in human children. Reflecting the two species recent evolutionary divergence, the brains of monkeys and humans are genetically, anatomically, and functionally similar (16–18). Homologous neurobiological substrates endow monkeys and humans with a shared repertoire of complex cognitive and socio-emotional behaviors (18). In particular, juvenile monkeys and young children express anxiety in similar ways, and in both species there are considerable individual differences in the presentation of the anxious phenotype. In monkeys, the AT phenotype can be elicited using the No-Eye Contact (NEC) condition of the Human Intruder Paradigm (15). During the NEC challenge, a human “intruder” enters the test room and presents his or her profile to the monkey while avoiding direct eye contact (15), similar to procedures used for assessing dispositional anxiety and behavioral inhibition in children (19). Using this challenge, individual differences in three fundamental dimensions of the anxious phenotype were assessed: hypothalamic-pituitary-adrenal (HPA) activity (increased plasma cortisol), behavior (increased freezing), and expressive communication (reductions in spontaneous vocalizations). All three dimensions show robust changes in response to the NEC challenge (15), paralleling observations made in dispositionally anxious and shy children (5).
A key advantage of the juvenile monkey AT model is that it permits concurrent measures of neural activity and naturalistic responses to an ethologically relevant potential threat, an opportunity not afforded by research in children. Here, FDG-PET was used to quantify brain metabolic activity in 238 freely behaving juvenile monkeys. FDG-PET, which provides a measure of regional brain metabolism integrated over the entire 30-min NEC challenge, is ideally suited for assessing sustained, trait-like neural responses (1).
Using these measures, we identified brain regions where metabolism predicts variation in one or more of the three AT dimensions. To understand the degree to which heterogeneity in the presentation of AT reflects invariant or distinct neural mechanisms, we distinguished “common” and “selective” substrates. Common neural substrates are those shared by individuals with varying expressions of anxiety; that is, a core set of brain regions where metabolism predicts variation in all three dimensions of the AT phenotype (HPA activity, freezing behavior, and expressive communication). Based on imaging and lesion studies in monkeys and humans (4, 6, 20–22), we hypothesized that the central nucleus of the amygdala (Ce) would be among the regions identified as shared substrates. Selective neural substrates are those specifically engaged by individuals with high levels of a particular dimension; that is, regions where metabolism predicts variation in only one of the AT dimensions. Mechanistic studies in rodents suggest that the Ce can orchestrate a broad spectrum of responses to threat via projections to response-specific targets (23, 24). Therefore, we further hypothesized that the association between common substrates (e.g., Ce metabolism) and particular dimensions of the anxious phenotype would be mediated by the appropriate phenotype-selective substrate. Distinguishing common from selective substrates, and clarifying the nature of their relationships with one another, is important for understanding how phenotypic variation in AT early in development gives rise to heterogeneity in the clinical presentation of internalizing disorders later in life, for facilitating the development of improved therapeutic interventions, and for understanding emotional traits.
Results
Heterogeneity and Continuity in the Presentation of the AT Phenotype.
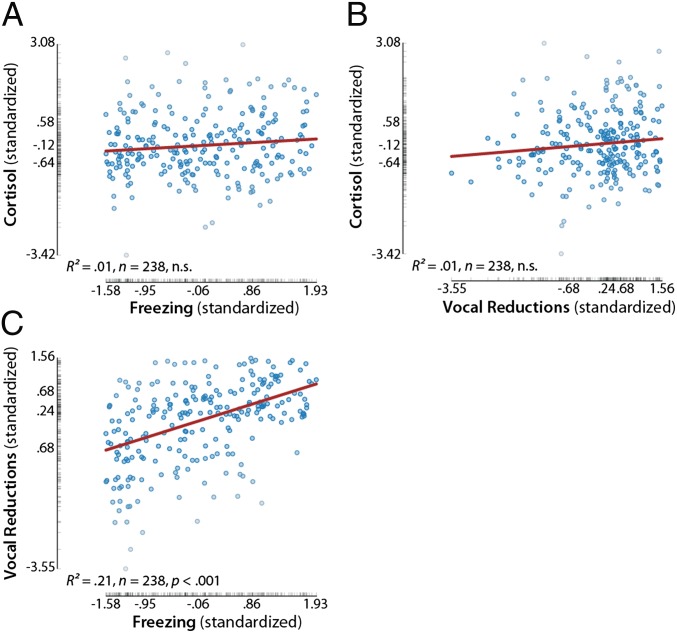
Like other emotional traits, individual differences in the profile of anxiety-related symptoms manifests as weak covariation among anxiety-related measures (2, 13, 14). Consistent with this, in the present sample a series of robust regressions revealed that variation in cortisol was largely independent of freezing and vocal reductions, R2 < 0.02, P > 0.06. As shown in Fig. 1, some individuals are characterized by extreme HPA activity and little freezing and vocal reductions, some show the opposite presentation, and yet others show intermediate presentations. Regression analyses also showed that, although individuals who froze more emitted fewer vocalizations, the two measures were far from redundant, R2 = 0.23, P < 0.001. These results underscore the substantial heterogeneity in the presentation of AT. Furthermore, all three dimensions of the anxious phenotype were reliable (SI Methods) and continuously distributed (Fig. 1). This continuity suggests that AT, like the internalizing disorders (11, 12), represents a spectrum of closely related phenotypes rather than a mixture of categorically distinct subgroups.
Fig. 1.
Heterogeneity and continuity in the presentation of AT. Pairwise partial correlations revealed that cortisol was largely independent of freezing and vocal reductions (A and B). Freezing and vocal reductions were correlated but far from redundant (C). All three dimensions were continuously distributed, suggesting that AT represents a multidimensional spectrum of phenotypes, rather than a mixture of distinct subgroups. Axis labels indicate the minimum, maximum, and interquartile range.
Heterogeneity in the Phenotypic Presentation of AT Reflects both Common and Selective Substrates.
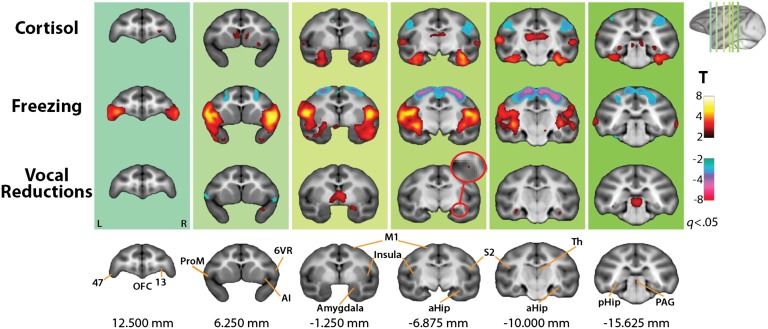
Because HPA activity, freezing behavior, and vocal reductions are continuously distributed (Fig. 1), we adopted a dimensional approach to identifying the neural circuitry underlying different expressions of anxiety. Specifically, we performed a series of voxelwise partial correlations between metabolism and each AT dimension while accounting for variation in the other two (using robust regression techniques; see SI Methods). This process revealed a number of regions where metabolism predicted the unique variance in one or more dimensions of the phenotype, including the orbitofrontal cortex (OFC; areas 13 and 47), anterior insula (AI), primary motor cortex (M1), amygdala, hippocampus, and periaqueductal gray (PAG) (Fig. 2 and Tables S1–S3).
Fig. 2.
Brain regions where metabolic activity significantly predicted one AT dimension independent of variation in the other two (FDR q < 0.05). Red circular inset shows magnified view. Coordinates indicate millimeter from the anterior commissure. aHip, anterior hippocampus; AI, anterior insula; L, left; M1, primary motor cortex (area 4); OFC, orbitofrontal cortex (architectonic areas 13 and 47); PAG, periaqueductal gray; pHip, posterior hippocampus; R, right; Th, thalamus.
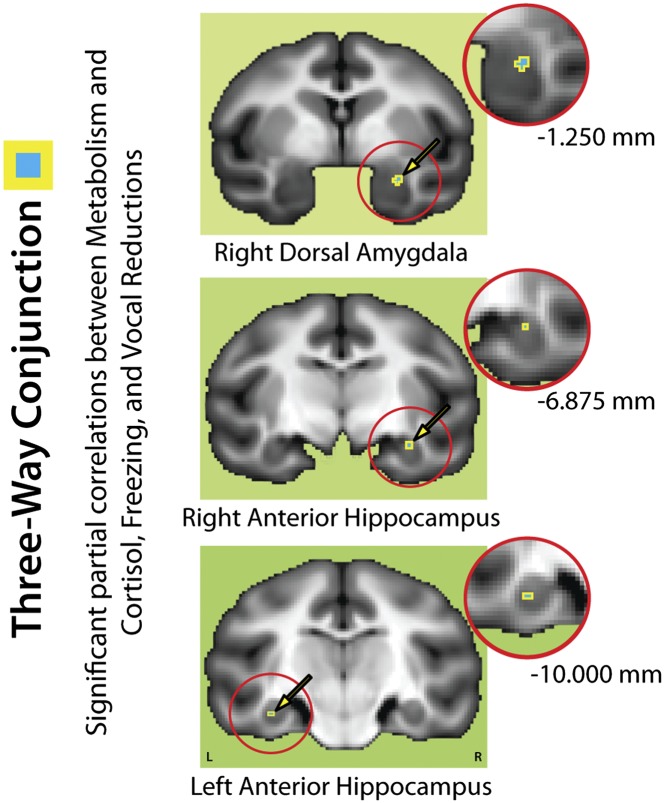
To distinguish common neural substrates, those shared by individuals with different expressions of the anxious phenotype, we used a three-way conjunction (i.e., logical AND) of the thresholded partial correlation maps shown in Fig. 2 to identify regions where metabolism predicted variation in HPA activity, freezing behavior, and vocal reductions [false-discovery rate (FDR) q < 0.05]. As shown in Fig. 3, this analysis revealed a cluster in the right dorsal amygdala and bilateral clusters in the hippocampi (Table S4) (hemispheric asymmetry analyses were not significant for the amygdala cluster; see SI Methods). Using a probabilistic chemoarchitectonic map (SI Methods), we localized the dorsal amygdala cluster to the lateral division of the central nucleus (CeL) (Fig. S1). The magnitude of the three partial correlations was similar in the amygdala and anterior hippocampus (P > 0.19), reinforcing the idea that these regions make similar contributions to the three dimensions of the anxious phenotype. Taken together, these results demonstrate that elevated metabolism in the CeL and anterior hippocampus is a consistent feature of individuals with divergent phenotypic expressions of anxiety. This implication reflects the fact that the partial correlations estimate the unique relationship between each phenotype dimension and metabolism at a fixed level of the other two. For example, the partial correlation for cortisol indicates that individuals with high levels of HPA activity (and intermediate levels of freezing behavior and vocalizations on average) show more activity in the CeL and anterior hippocampus when compared with individuals with low levels of HPA activity (and intermediate levels of freezing behavior and vocalizations on average). For illustrative purposes, these effects are shown in Fig. S2. [Note: The results of the conjunction analysis suggest, but do not demonstrate, that aggregating the three dimensions—cortisol, freezing, and vocalization reductions—into a composite would provide a more sensitive index of AT-related variation in metabolic activity. Confirmatory analyses demonstrated that this was indeed the case (SI Methods).]
Fig. 3.
Shared neural substrates: Amygdalar and hippocampal metabolism predicts all three dimensions of the AT phenotype. Figure depicts the three-way minimum conjunction (logical AND) of the significant partial correlations shown in Fig. 2. Regions shown in cyan/gold predicted the unique variance in all three dimensions of the AT phenotype. Circular insets show magnified views. For other conventions, see Fig. 2.
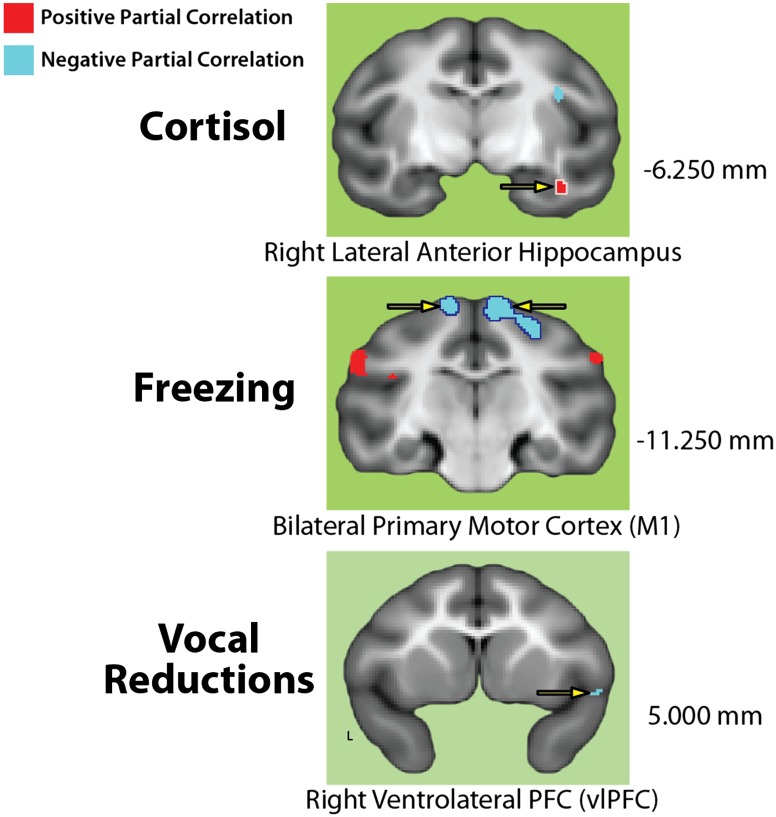
To distinguish selective neural substrates, regions that are specifically engaged by individuals with high levels of a particular AT dimension, we used a two-way conjunction to identify regions where metabolism: (i) predicted one of the three AT dimensions after controlling for variation in the other two (FDR q < 0.05) and (ii) explained more variance in that dimension compared with the other two (assessed using a voxelwise test of the difference in correlations; FDR q < 0.05). That is, we distinguished regions where one of the three partial correlations shown in Fig. 2 was both significant and significantly stronger than the other two. This process revealed a number of regions (Tables S1–S3). In particular, as shown in Fig. 4, individuals characterized by high levels of HPA activity were distinguished by elevated metabolic activity in the lateral anterior hippocampus, whereas those who presented with high levels of freezing behavior showed attenuated activity in the M1, and those with low levels of vocal communication showed increased activity in the ventrolateral prefrontal cortex (vlPFC). The lateral anterior hippocampal cluster that was selective to cortisol was located several millimeters from the nearest cluster that predicted all three AT dimensions (Fig. 3 and Table S4). These results clarify the neural systems underlying heterogeneity in the expression of anxiety.
Fig. 4.
Selective substrates: Regions where one AT dimension predicted significant variance in brain metabolism (partial correlation: FDR q < 0.05) and explained more variance than the other two dimensions (difference in correlations: FDR q < 0.05), identified using a two-way conjunction (logical AND). Conventions are described in Fig. 2.
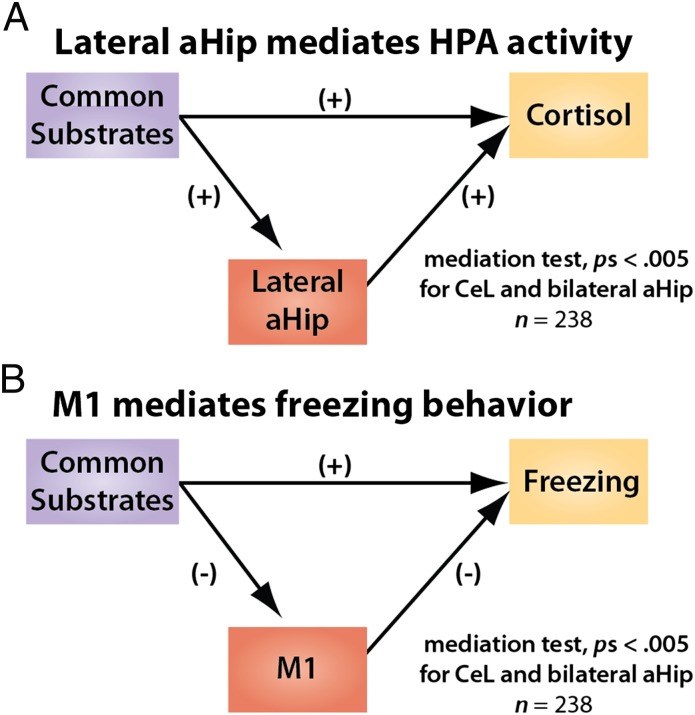
We identified the CeL and anterior hippocampus as shared substrates, regions where metabolism predicts all three dimensions of the anxious phenotype. To assess whether those brain-phenotype relationships are explained by activity in phenotype-selective regions, we used a series of multivariate mediation models (SI Methods) to test whether the partial correlation between the shared substrates (Fig. 3) and a particular dimension of the AT phenotype depends on the relevant selective substrate (Fig. 4) (e.g., CeL → M1 → freezing). As shown in Fig. 5 and detailed in Table S5, this process demonstrated that relations between the shared substrates and HPA activity were mediated by activity in the cortisol-selective anterior hippocampal region, P < 0.005 (uncorrected). Similarly, relations between the shared substrates and freezing behavior were mediated by activity in M1, P < 0.005 (uncorrected). Significant mediation was not found for vocal reductions. To assess the specificity of the cortisol and freezing results, we recomputed the mediation models after reversing the candidate mediating regions (e.g., CeL → lateral anterior hippocampus → freezing). None of these control models was significant, indicating a double dissociation (Table S5). Specifically, metabolism in the lateral anterior hippocampus explains relations between the shared substrates and HPA activity, but not freezing behavior, whereas M1 metabolism explains relations between the shared substrates and freezing, but not HPA activity. Collectively, these results clarify how shared substrates can influence multiple dimensions of the anxious phenotype. In particular, these results indicate that relations between the core set of shared substrates, such as the CeL, and the endocrine and behavioral dimensions of the anxious phenotype strongly depend upon regions that are dimension-specific.
Fig. 5.
Linking common and selective substrates: Brain-phenotype relations are selectively mediated. (A) Metabolism in the cortisol-selective region of lateral anterior hippocampus mediates relations between the common substrates (CeL and aHip) (Fig. 3) and HPA activity, P < 0.005 (uncorrected). (B) Metabolism in the freezing-selective region of M1 mediates relations between the common substrates and freezing behavior, P < 0.005 (uncorrected). The plus and minus symbols indicate the sign of the partial correlation (see Table S5).
Discussion
Like the internalizing disorders, there is marked variation in the presentation of AT during early development. Our observations provide compelling evidence that this heterogeneity reflects the joint contribution of common substrates, a core set of brain regions that are shared by individuals with different manifestations of extreme anxiety, and selective substrates, regions that are specifically associated with particular expressions. Consistent with prior work (2, 13, 14), the endocrine, behavioral, and communicative dimensions of the anxious phenotype were weakly correlated and continuously distributed, suggesting that AT represents a multidimensional spectrum of closely related phenotypes (Fig. 1). Using a dimensional analytic approach that circumvented the need to impose artificial categorical boundaries on the data, we identified a number of regions where metabolic activity predicts one or more dimensions of the AT phenotype (e.g., amygdala, hippocampus, PAG, AI, and OFC) (Fig. 2). We demonstrated that variation in each dimension of the phenotype—increased HPA activity, more freezing behavior, and fewer expressive vocalizations—was independently predicted by activity in the CeL and anterior hippocampus (Fig. 3). Elevated activity in this core set of brain regions was consistently found in individuals who displayed high levels of any of these dimensions (Fig. S2). We identified a second set of regions that specifically predict particular dimensions of the anxious phenotype, including the lateral anterior hippocampus and M1, and vlPFC (Fig. 4). Activity in these phenotype-selective regions distinguished individuals with high levels of HPA activity, freezing behavior, and vocal reductions, respectively. Finally, we demonstrated that these regions selectively mediate the association between the shared substrates, such as the CeL, and the endocrine and behavioral dimensions of the AT phenotype (Fig. 5). In sum, these observations suggest that variation in the expression of dispositional anxiety reflects the activity of a neurobiological system comprised of both shared and phenotype-selective components. As described below, these findings have mechanistic, translational, and theoretical implications.
With respect to mechanism, our results show that the CeL and anterior hippocampus are consistently engaged by individuals with divergent presentations of anxiety (Fig. 3 and Fig. S2). This finding is in accord with evidence that the amygdala and anterior hippocampus show exaggerated activation to potentially threat-relevant cues in individuals with a variety of anxiety disorders or a childhood history of extreme AT (6, 21). Similarly, lesions of either region attenuate many signs of anxiety (20, 22, 23, 25). Interestingly, recent work in rodents suggests that the CeL plays a key role in gating the output of the amygdala (26, 27). In particular, the CeL is poised to modulate both acute fear and sustained anxiety via inhibitory projections to the two major output stations of the extended amygdala: the medial division of the Ce and the lateral division of the bed nucleus of the stria terminalis (26, 27).
Our results indicate that individual differences in the expression of anxiety reflect the proximal contribution of phenotype-selective regions. In particular, we observed a double dissociation: M1 mediated freezing behavior, but not cortisol, whereas the lateral anterior hippocampus showed the opposite profile. The selective role of the lateral anterior hippocampus in the endocrine dimension of dispositional anxiety is consistent with mechanistic evidence that the hippocampus regulates the HPA axis (28). This result may reflect the dense distribution of mineralocorticoid receptors in the primate hippocampus (29), which are involved in more trait-like or basal aspects of HPA activity (28). The involvement of M1 in the behavioral dimension of AT is consistent with its well-established role in voluntary action. We obtained more limited evidence that the vlPFC is selectively involved in the reduction of expressive coo vocalizations, consistent with work implicating ventral premotor areas in vocalizations and other orofacial behaviors (30).
Although noninvasive techniques, such as FDG-PET, cannot establish causation, our results are in accord with mechanistic research demonstrating that the Ce orchestrates many of the peripheral physiological, behavioral, and expressive dimensions of anxiety and that these effects are mediated by functional interactions with response-specific targets (22–24). Our results address the proximal substrates of individual differences in the presentation of anxiety. Individuals characterized by high levels of freezing, for example, are distinguished by attenuated activity in M1. However, the distal determinants of this heterogeneity remain unclear; it may reflect variation in the strength of functional connectivity between the CeL and anterior hippocampus and particular phenotype-specific regions. Another possibility is that it reflects individual differences in subpopulations of phenotype-specific neurons that are intermingled at a level beyond the resolution of conventional imaging techniques (31). Indeed, evidence for distinct subpopulations of freezing- and cardiovascular-specific neurons in the Ce has led some investigators to suggest the possibility of developing therapeutic interventions targeting disorder-specific or patient-specific differences in symptom profiles (31), consistent with earlier suggestions in the translational literature (32).
Between one-third and two-thirds of anxiety patients are treatment-resistant or refractory (33), underscoring the need to develop more efficacious interventions. The present results highlight the potential utility of broad-spectrum (i.e., multisymptom) approaches. In particular, our findings suggest that therapeutics aimed at molecular targets within the CeL and anterior hippocampus, particularly when administered early in life, could ameliorate a variety of maladaptive or excessive responses to potential threat. Over time, such responses likely promote more complex and chronic symptoms (e.g., avoidance, anticipatory worry) and neurobiological alterations (34, 35). This suggestion is reinforced by evidence that anxiety disorders with distinct presentations—including posttraumatic stress disorder, social anxiety disorder, specific phobias, and generalized anxiety disorder—are all characterized by elevated amygdala reactivity to aversive or potentially threatening stimuli (21, 36). Targeting upstream regions that are poised to regulate the CeL and anterior hippocampus (37), such as the OFC, represents another approach to broad-spectrum treatment. In this regard, cognitive-behavioral therapy, which is thought to be mediated by emotion regulatory processes implemented in the prefrontal cortex (38, 39), or cognitive-behavioral therapy combined with novel pharmacological interventions that increase neuroplasticity, may be particularly effective (40). Developing more effective early-life interventions is particularly important for minimizing the cumulative social and interpersonal damage associated with extreme anxiety and behavioral inhibition during early development (5).
These data also have implications for general theories of emotion. The peripheral physiological and behavioral features that are the hallmarks of emotion have traditionally been cast as tightly synchronized (41, 42). However, faced with growing evidence of weak response coupling, theorists have speculated that no single brain region could orchestrate all of these responses—that the alterations in the face, voice, body, and mental experience characteristic of emotional states and traits reflect the activity of segregated neural circuits (14, 43). This possibility is not addressed by prior imaging studies, which have typically measured only one or two concurrent responses or composite anxiety measures. Nor has it been directly addressed by lesion studies, which lack the statistical power required to investigate phenotypic heterogeneity. Therefore, the present observation that activity in the CeL and anterior hippocampus explains independent variation in endocrine, behavioral, and communicative responses to the NEC challenge provides unique grounds for rejecting strict claims of neural segregation. More generally, this finding indicates that a lack of strong covariation among different dimensions of anxiety does not preclude the existence of a response-independent substrate (44); individuals can vary in the strength and predominance of different anxiety dimensions, yet rely on the same core set of shared substrates.
In summary, using a well-validated nonhuman primate model of AT and high-resolution functional imaging, the present study demonstrates that striking diversity in the presentation of anxiety reflects the distinct contributions of both shared and phenotype-selective substrates. Individuals characterized by high levels of HPA activity, high levels of freezing behavior, or low levels of expressive vocalizations all exhibit elevated metabolic activity in the CeL and anterior hippocampus. These brain-phenotype associations were dependent upon a second set of regions, including the lateral anterior hippocampus and M1, which selectively mediate particular dimensions of the anxious phenotype. Importantly, these results were obtained using a relatively large unselected sample and robust analytic procedures, increasing the likelihood of replication. More broadly, these observations provide a framework for understanding the neurobiology of early-life anxiety and other emotional traits and set the stage for mechanistic studies aimed at identifying more effective interventions for the internalizing spectrum of disorders.
Methods
Subjects received FDG before testing. The AT phenotype was elicited by the presentation of a human intruder’s profile (30-min). An observer quantified freezing behavior and expressive vocalizations. Following testing, plasma was collected and subjects were scanned. Higher FDG-PET signals indicate greater metabolism during testing. Plasma cortisol was quantitated by radioimmunoassay. MRI and PET images were processed using standard methods and normalized to a stereotactic template (0.625 mm3). Robust regressions identified regions where activity predicted the unique variance in cortisol, freezing, and vocal reductions. Analyses controlled for nuisance variation in mean-centered age, sex, and voxelwise gray matter probability. Common and phenotype-selective substrates were identified using criteria described in the text. Mediation analyses used standard analytic techniques.
Supplementary Material
Acknowledgments
We thank E. Ahlers, A. Alexander, V. Balchen, R. Birn, B. Christian, A. Converse, L. Friedman, M. Jesson, E. Larson, K. Mayer, T. Oakes, P. Roseboom, N. Vack, H. Van Valkenberg, L. Williams, the staffs of the Harlow Center for Biological Psychology, HealthEmotions Research Institute, and Wisconsin National Primate Center, and two anonymous reviewers for their assistance. This work was supported by the National Institutes of Health (NIH) (Grants HD003352, HD008352, MH018931, MH046729, MH081884, MH084051, MH091550, OD011106, and RR000167), the HealthEmotions Research Institute, and Meriter Hospital.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214364110/-/DCSupplemental.
References
- 1.Fox AS, et al. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc Natl Acad Sci USA. 2012;109(44):18108–18113. doi: 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3(7):e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. Child Dev. 1991;62(5):1175–1183. [PubMed] [Google Scholar]
- 4.Oler JA, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466(7308):864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 6.Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders: A review of neuroimaging findings. Child Adolesc Psychiatr Clin N Am. 2012;21(3):501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am J Psychiatry. 2010;167(1):40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1066–1075.e1. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins PY, et al. Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health Grand challenges in global mental health. Nature. 2011;475(7354):27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutter M. Research review: Child psychiatric diagnosis and classification: Concepts, findings, challenges and potential. J Child Psychol Psychiatry. 2011;52(6):647–660. doi: 10.1111/j.1469-7610.2011.02367.x. [DOI] [PubMed] [Google Scholar]
- 12.Hyman SE. The diagnosis of mental disorders: The problem of reification. Annu Rev Clin Psychol. 2010;6:155–179. doi: 10.1146/annurev.clinpsy.3.022806.091532. [DOI] [PubMed] [Google Scholar]
- 13.Buss KA, Davidson RJ, Kalin NH, Goldsmith HH. Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Dev Psychol. 2004;40(4):583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- 14.Barrett LF, Ochsner KN, Gross JJ. On the automaticity of emotion. In: Bargh J, editor. Social Psychology and the Unconscious: The Automaticity of Higher Mental Processes. NY: Psychology Press; 2007. pp. 173–217. [Google Scholar]
- 15.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: Environmental cues and neurochemical regulation. Science. 1989;243(4899):1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 16.Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. NY: Guilford; 2009. pp. 3–42. [Google Scholar]
- 17.Gibbs RA, et al. Rhesus Macaque Genome Sequencing and Analysis Consortium Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316(5822):222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 18.Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Sytems. Vol 4. NY: Elsevier; 2007. pp. 3–34. [Google Scholar]
- 19.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240(4849):167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 20.Feinstein JS, Adolphs R, Damasio A, Tranel D. The human amygdala and the induction and experience of fear. Curr Biol. 2011;21:1–5. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 24.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8(7):2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36(2):529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62(6):757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22(4):717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12(2):118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20(12):4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coudé G, et al. Neurons controlling voluntary vocalization in the macaque ventral premotor cortex. PLoS ONE. 2011;6(11):e26822. doi: 10.1371/journal.pone.0026822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viviani D, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333(6038):104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 32.Davidson RJ. Specificity and patterning in biobehavioral systems. Implications for behavior change. Am Psychol. 1978;33(5):430–436. doi: 10.1037//0003-066x.33.5.430. [DOI] [PubMed] [Google Scholar]
- 33.Bystritsky A. Treatment-resistant anxiety disorders. Mol Psychiatry. 2006;11(9):805–814. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- 34.Oler JA, et al. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. J Neurosci. 2009;29(32):9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- 36.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox AS, et al. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30(20):7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: Treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 40.Krystal JH, et al. Neuroplasticity as a target for the pharmacotherapy of anxiety disorders, mood disorders, and schizophrenia. Drug Discov Today. 2009;14(13–14):690–697. doi: 10.1016/j.drudis.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darwin C. The Expression of the Emotions in Man and Animals. 4th Ed. NY: Oxford Univ Press; 2009. [Google Scholar]
- 42.Ekman P. An argument for basic emotions. Cogn Emotion. 1992;6(3–4):169–200. [Google Scholar]
- 43.Barrett LF. Are emotions natural kinds? Perspect Psychol Sci. 2006;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- 44.Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.