Abstract
In multimeric cell-surface receptors, the conformational changes of the extracellular ligand-binding domains (ECDs) associated with receptor activation remain largely unknown. This is the case for the dimeric metabotropic glutamate receptors even though a number of ECD structures have been solved. Here, using an innovative approach based on cell-surface labeling and FRET, we demonstrate that a reorientation of the ECDs is associated with receptor and G-protein activation. Our approach helps identify partial agonists and highlights allosteric interactions between the effector and binding domains. Any approach expected to stabilize the active conformation of the effector domain increased the agonist potency in stabilizing the active ECDs conformation. These data provide key information on the structural dynamics and drug action at metabotropic glutamate receptors and validate an approach for tackling such analysis on other receptors.
Keywords: allostery, biosensor, snap-tag, time-resolved FRET
Many cell-surface receptors are multimers of proteins composed of several domains (1–3), including extracellular domains (ECDs) involved in endogenous ligand recognition and transmembrane domains (TMDs) responsible for intracellular signal transduction. Analysis of the conformational changes of the ECDs associated with receptor activation is crucial to understand the detailed mechanism involved in receptor activation and for the development of new innovative drugs. However, limited information is available on how the conformational changes in these proteins lead to receptor activation, especially in living cells.
The eight glutamate-activated G-protein–coupled receptors (GPCRs), called “metabotropic glutamate receptors” (mGluRs), are key examples of multidomain and multimeric receptors (Fig. 1A). These receptors are strict dimers (4–6), and each subunit is made of a large ECD associated with a seven-helix TMD responsible for G-protein activation and downstream signaling (7). The mGluRs are key elements involved in the regulation of synaptic activity (8), and therefore they represent promising targets in drug development for the treatment of multiple neurologic and psychiatric diseases (9). More generally, the mGluRs are part of the class C GPCR family that contains structurally related receptors such as the receptors for sweet and umami taste, calcium, basic amino acids, and the inhibitory neurotransmitter GABA (10, 11).
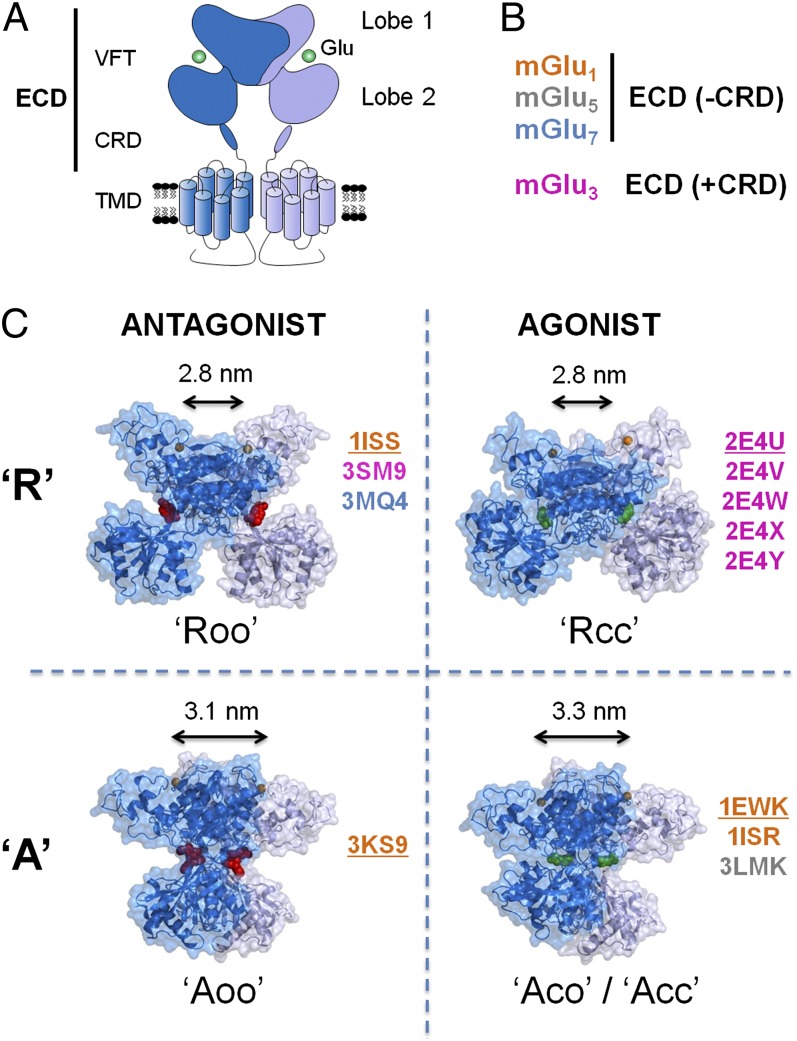
Fig. 1.
Structural organization of the mGluRs and crystal structures of the dimeric ECDs. (A) Cartoon illustrating the full-length dimeric mGluRs in which each subunit is composed of an ECD made of a bilobate VFT and a CRD that is linked directly to the TMD. The glutamate-binding pocket is between the first and second lobes of the VFTs. (B) Crystal structures of the ECD of mGlu1, 3, 5, and 7 (see PDB numbers in C) obtained in presence or absence of the CRD and in complex with agonists and competitive antagonists. (C) In the different structures (7, 14–16), the dimeric ECDs were observed in resting (R) and active (A) orientation whether they were bound to agonist (green) or to antagonist (red), raising the question of the true inactive and active orientations of the ECD. In these structures, the VFTs are in open (o) or closed (c) state, thus defining the major states of the ECDs: Roo, Rcc, Aoo, Aco, and Acc. In the dimer, the VFT in front is represented in dark blue, and the second one in the back is shown in purple. The main difference between the inactive and active states is that the second lobes are distant in the inactive state and closed in the active state. Only one structure representative of each conformation and corresponding to the underlined PDB number is shown with two molecules of agonists or antagonists. Double-headed arrows indicate the distances between the two N termini. The Cα of the first N terminus residue in the crystal structures is highlighted in orange.
Crystallographic studies of the isolated dimeric ECDs and mutagenesis analyses have provided a clear view of the structure of the dimeric ECD and of the initial steps of mGluR activation. The ECD is composed of a Venus flytrap (VFT) bilobate domain containing the agonist binding site (12–15) and a cysteine-rich domain (CRD) that connects the VFT to the TMD (16). The VFTs exist in two major states: an open state (o) in absence of ligand and stabilized by antagonists, and a closed state (c) stabilized by agonists and required for receptor activation (12–15). The VFT dimer is in equilibrium between various conformations, depending on whether one or two VFTs are open or closed. In the absence of ligand or in the presence of antagonists, both VFTs are opened (oo). The binding of one or two agonists triggers the closure of one (co) or two VFTs (cc) in the dimer, resulting in receptor activation (13).
However, how the closure of at least one VFT affects the conformational state of the TMD during receptor activation remains controversial. Based on the initial X-ray structures (7, 14–16), the predominant model proposes that the closure of the VFTs in the dimer is associated with a large change in the relative orientation of the two VFTs, from a resting (R) to an active (A) orientation defining three major states: Roo, Aco, and Acc (Fig. 1C). The major consequence of this reorientation is that the second lobes of the VFTs, which are distant in the R state, become closer in the A conformation, stabilizing the active state of the TMDs. However, as shown in Fig. 1C, if we consider all structures deposited in the Protein Data Bank (PDB) (SI Appendix, Table 1), no correlation emerges between the specific conformations of the dimeric VFTs and the nature (i.e., agonist or antagonist) of the cocrystallized ligand. Indeed, agonist-bound VFTs can be found in the R orientation, and the A orientation also has been observed with bound antagonists (Fig. 1C).
To understand mGluR dynamics better and to resolve this apparent paradox, we examine here the conformational changes of the ECDs associated with the activation of mGluRs at the surface of living cells. To do so, we used a combination of innovative technologies enabling the exclusive labeling of cell-surface receptors with fluorophores compatible with time-resolved Förster (fluorescence-based) resonance energy transfer (trFRET) measurements. With this approach, we clearly demonstrate that a large movement between the ECDs is associated with receptor and G-protein activation. Our approach also allows the detection of partial agonists and reveals precise allosteric interactions between the ECD and TMD of the mGluRs. Finally, it supplies important information on the mode of action of allosteric regulators, either small molecules or associated proteins such as G proteins.
Results
Engineering of mGluRs to Analyze Conformational Changes of the Dimeric ECDs.
To study the conformational changes of the ECDs in the context of the full-length mGluRs, we developed a trFRET-based strategy. We fused the 20-kDa SNAP-tag enzyme which can be covalently labeled with fluorophores (17) to the N-terminal end of the receptor. This strategy has a number of advantages. First, because mGluRs are strict homodimers (4–6), the FRET signal results exclusively from the energy transfer between the two protomers (5). Second, according to the available crystal structures of mGlu ECDs (14, 15), the distance between the N termini varies from 2.8 nm in the R conformation to 3.3 nm in the A conformation, a variation compatible with changes in FRET efficiency (Fig. 1C). Thus, we hypothesized that if the N termini are labeled with FRET-compatible probes, the R-to-A conformation switch may result in a decrease in the FRET efficiency. Third, because of its accessibility, the labeling of the N termini of the mGluR does not interfere with the receptor function as measured by G-protein coupling (5). Fourth, and most importantly, the SNAP-tag enzyme can be labeled selectively and irreversibly using cell-impermeant benzylguanine-derived fluorescent substrates allowing a specific labeling of cell-surface receptors only (5, 6, 17). This latter approach prevents any FRET signal originating from intracellular receptors that cannot be activated by agonists.
We expressed SNAP-mGlu2 in HEK293 cells and labeled them with a trFRET donor, SNAP-Lumi4-Tb, and a green fluorescent acceptor, SNAP-Green (Fig. 2A). Concentrations were chosen to label surface subunits with both fluorophores equivalently. Consequently, half of all mGlu2 homodimers at the surface carry one donor and one acceptor molecule (5). We then recorded the trFRET signal as the Green-labeled acceptor delayed emission at 520 nm following Lumi4-Tb excitation at 337 nm.
Fig. 2.
Glutamate induces a relative movement of the ECDs in the full-length mGluRs. (A) Cartoon illustrating the full-length SNAP-mGlu2 (ST-mGlu2) labeled with the Lumi4-Tb donor and the Green fluorescent acceptor, with a high FRET signal in the absence of ligand or in presence of the antagonist LY341495 (LY34) and a low FRET signal in the presence of glutamate or the agonist LY379268 (LY37). (B) trFRET signal between the two ECDs after cell-surface labeling of SNAP-mGlu2–expressing cells with SNAP-Lumi4-Tb and SNAP-Green in the presence of a saturating concentration of glutamate (injection at t = 30 s) and then in the presence of the competitive antagonist LY341495 (injection at t = 60 s). The trFRET signal is represented as the ratio of the acceptor-sensitized emissions measured in two time windows (Materials and Methods) and is normalized to the maximum signal obtained in the presence of a saturating concentration of antagonist. (C) trFRET signal of wild-type SNAP-mGlu2, the ligand-binding–deficient SNAP-mGlu2-YADA mutant in which the two residues Y216 and D295 important for agonist binding in the VFT are mutated to alanine, and wild-type SNAP-mGlu1 and SNAP-mGlu3. All receptors were labeled with Lumi4-Tb and Green in the presence of a saturating concentration of competitive antagonist (LY34) or agonist (LY37). (D and E) Intramolecular trFRET signal measured on donor- and acceptor-labeled SNAP-mGlu1 and SNAP-mGlu3 in the presence of the indicated antagonists or agonist found in the crystal structures of mGlu1 and mGlu3.
Glutamate Induces a Relative Movement of the ECDs.
We observed that glutamate application resulted in a large decrease in the FRET signal (Fig. 2B), a variation that was independent of the number of cell-surface receptors (SI Appendix, Fig. S1). Importantly, a competitive antagonist, LY341495, completely restored the emission signal, ruling out any nonspecific effect of glutamate on the FRET efficiency. Of note, the FRET signal recorded after antagonist application is higher than that recorded in the control condition, suggesting either a slight constitutive activity of the receptor or ambient glutamate in the assay medium. Eventually, no such agonist-mediated FRET change was observed with a mutant mGlu2 insensitive to glutamate, mGlu2-YADA (Fig. 2C) (18). Finally, agonist application is associated with an increase in lifetime of the donor fluorophore, consistent with a decrease in FRET efficiency (SI Appendix, Fig. S2).
Three main points are consistent with the decrease in FRET efficiency originating from a relative movement of the ECDs (Fig. 2B). First, the kinetics of the change is very rapid (t1/2 less than 1 s), a characteristic that suggests a conformational change rather than the recruitment or the dissociation of a partner subunit or even the internalization of the receptor (19). Second, with the rare earth cryptate Lumi4-Tb, FRET efficiency depends almost exclusively on the distance between the two fluorophores and not on the orientation (20). Third, as expected from an energy transfer, the lifetime of the donor is decreased significantly in the presence of the acceptor under the control condition but to a lesser extent in the presence of agonist, consistent with a lower FRET efficiency when the receptor is activated (SI Appendix, Fig. S2).
Taken together, our data show that the decrease in FRET efficiency induced by glutamate and reversed by a competitive antagonist likely reflects a reorientation of the dimeric ECDs.
Intramolecular FRET Is Linked to Receptor Activation.
To prove that the change in FRET is related to the activation process of mGluRs, we tested four different selective and nonselective mGlu2 agonists on our FRET sensor (Fig. 3A). They all consistently induced a decrease in the FRET efficiency in a dose-dependent manner with potencies (fitted EC50) in the range of those measured using cellular functional assays based on the coupling of mGlu2 to the phospholipase C signaling pathway through the use of the chimeric G protein Gqi9 (Fig. 3B). The EC50 measured in the inositol phosphate (IP) accumulation assay and in the calcium mobilization assay correlated strongly with those determined in the FRET assay (Fig. 3B). Of note, the EC50 values for IP accumulation and calcium assays are lower (reflecting higher potency) than those obtained using the FRET sensor, showing that higher concentrations of agonist are needed to elicit a full FRET response. However, the Ki values for the FRET assay perfectly matched those obtained in radioactive ligand-binding experiments (Fig. 3C). Eventually, the agonist-mediated change in FRET is fully reversed by a series of antagonists with Ki values consistent with those reported in the literature (SI Appendix, Fig. S3) (21).
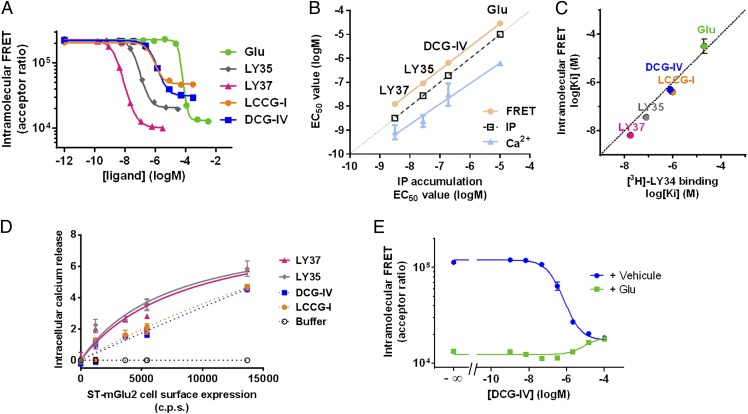
Fig. 3.
The relative movement of the ECDs is correlated with receptor function. (A) Intramolecular trFRET signal measured on donor- and acceptor-labeled SNAP-mGlu2 in the presence of increasing concentrations of the indicated selective (LY35 and LY37) and nonselective group II mGluRs agonists (glutamate, LCCG-I, and DCG-IV). (B) Correlation between the potency of the agonists determined by IP accumulation and their potency in intramolecular trFRET and in calcium mobilization assays. (C) Correlation between the Ki of the agonists determined in intramolecular trFRET in the presence of 3 nM LY34 and the Ki determined in a competitive radioligand-binding study using 3 nM of the tritiated LY34. (D) Response in the calcium mobilization assay with the indicated agonists (10 μM LY37 and 100 μM LY35, DCG-IV, and LCCG-I) for the quantities of SNAP-mGlu2 at the cell surface measured by the fluorescence emission of the Lumi4-Tb bound to the receptor. (E) Intramolecular trFRET signal for labeled SNAP-mGlu2 with increasing concentrations of the partial agonist DCG-IV in the presence or absence of 100 µM glutamate.
Interestingly, (2S,2′R,3′R)-2-(2′,3′-Dicarboxycyclopropyl)glycine (DCG-IV) and (2S,1′S,2′S)-2-(Carboxycyclopropyl)glycine (LCCG-I) lead to a partial reduction in FRET efficiency (Fig. 3A), suggesting partial agonist activities. This reduction was confirmed in a functional calcium assay by testing the effects of maximal doses of DCG-IV and LCCG-I on cells transfected with low amounts of receptor plasmid DNA to avoid saturation of the response and receptor reserve (Fig. 3D). In addition, as expected for a partial agonist, DCG-IV partly inhibited the maximal effect of glutamate (Fig. 3E).
FRET Change Is Associated with ECDs Reorientation and Receptor Activation.
Two sets of data demonstrate that the FRET change is correlated with a reorientation of the ECDs and the activation of the receptor. First, we locked the R conformation by introducing a glycan wedge expected to prevent the association of the second lobes (Fig. 4A), a strategy that has been used previously to block the GABAB receptor in its inactive conformation by steric hindrance generated by the engineered glycosylation (22). We introduced an NXS motif, which is recognized by the endoplasmic reticulum machinery as being modified with N-linked glycosylation, at residue 515, at the level of the CRD dimer interface (23). In the absence of ligand, we found that the FRET efficiency measured with this mutant is similar to that measured on the wild-type receptor but that the effects of glutamate or the most potent agonist LY379268 on the FRET efficiency are strongly impaired (Fig. 4A). Accordingly, the activation of this mutant measured in the IP assay is strongly impaired (Fig. 4A). Second, we used a mutant (L521C) that is locked in an active conformation by an engineered disulfide bond that cross-links the two CRDs (23). We found that the FRET signal intensity of the mutant L521C is similar to that measured on the wild-type receptor in the presence of saturating concentrations of agonist (Fig. 4B). Interestingly, the competitive antagonist LY341495 significantly increased the FRET efficiency of the constitutively active mutant L521C (Fig. 4B) without reaching the level of the inactive receptor and without specifically changing the basal activity of this mutant receptor.
Fig. 4.
The relative movement of the ECDs is necessary and sufficient for mGlu2 activation. Intramolecular trFRET signal (Upper) and IP accumulation (Lower) measured for the mGlu2 receptor mutant 515NGlyc blocked in the resting conformation (A) and the mutant L521C locked in the constitutively active state (B) in transfected cells incubated with the agonist LY37 or the competitive antagonist LY34 and in the presence of the chimeric Gqi9 protein.
In conclusion, these data indicate that a relative movement of the ECDs, especially at the level of the second lobes, is both necessary and sufficient to control the activation of the TMDs by orthosteric ligands. They also are consistent with the initial structural observation of an R-to-A conformational change of the dimeric VFT being associated with receptor activation.
ECDs Movement in Other mGlu Receptors.
As shown in Fig. 1, the structures that do not fit with the R-to-A model for receptor activation are those of the mGlu1 VFTs bound to the antagonist LY341495 (PDB 3KS9), which are found in a relative A orientation, and those of the mGlu3 VFTs occupied by five different agonists, including glutamate and DCG-IV, which are all in the R orientation. Thus it is possible that our observations with mGlu2, may not apply to either mGlu1 or mGlu3 receptors. However, all three SNAP-tagged mGlu1, mGlu2, and mGlu3 receptors show a high FRET efficiency in their inactive conformation when bound to the antagonist LY341495 and a low FRET efficiency in the presence of agonist (Fig. 2C and SI Appendix, Fig. S4). For mGlu1, the low FRET efficiency measured in the presence of glutamate is dose-dependently reversed in high values by increasing LY341495 concentrations (Fig. 2D). For mGlu3, all four agonists observed in the crystal structure in the resting state —glutamate; 1S,3R-ACPD; 2R,4R-APDC; and DCG-IV—decrease the FRET efficiency, as does glutamate on mGlu2 and mGlu1 (Fig. 2E), with potencies similar to those measured with other assays on this receptor (21). Of interest, and as observed with mGlu2, DCG-IV displays a partial effect. Taken together, our data are consistent with a similar reorientation of the ECDs of all three mGluRs tested (mGlu1, mGlu2, and mGlu3) during receptor activation, from the R orientation in the inactive state to the A orientation in the active state.
Allosteric Control of the Relative Movement of the ECDs by the TMD.
If the movement of the ECDs is critical for the allosteric control of the conformational state of the TMD, then such coupling also should occur in the other direction, from the TMD to the ECD. In other words, stabilizing the active or inactive state of the TMD should affect the dynamics of the ECD movement. In mGluRs, synthetic negative and positive allosteric modulators (NAMs and PAMs, respectively) interacting in the TMD have been identified (Fig. 5A). NAMs act as noncompetitive inhibitors and often display inverse agonist activity (24, 25), whereas PAMs enhance the potency or/and efficacy of agonists (26) but have no or weak direct agonist activity when applied alone in the absence of agonist.
Fig. 5.
The relative movement of the ECDs is controlled allosterically by the TMD. (A) Cartoon illustrating that the PAM stabilizes the active conformation of mGlu2 in presence of the agonist (Left), and the NAM stabilizes the resting conformation (Right). (B) Intramolecular trFRET signal measured for the indicated agonists in the presence of either 1 μM of the PAM LY487379 or 1 μM of the NAM Ro64-5229. (C) Potency (Left) and efficacy (Right) of the indicated agonists in absence or presence of the NAM or PAM in the conditions described in A. Data in A–C are mean ± SEM of three individual experiments, each performed in triplicate. *P < 0.05, one-way ANOVA followed by a Bonferroni’s multiple comparison test. (D) Intramolecular trFRET signal measured for the isolated mGlu2 ECD (SNAP-mGlu2-499GPI) at the surface of living cells.
We examined the effect of both the mGlu2 NAM Ro64-5229 and the mGlu2 PAM LY487379 on the agonist-induced FRET change (Fig. 5A). As illustrated in Fig. 5 B and C, the NAM slightly decreased the agonist efficacies and slightly reduced agonist potencies; meanwhile the PAM enhanced both agonist efficacies and potencies. Of note, the partial agonists DCG-IV and LCCG-I become full agonists in the presence of PAM. Consistent with the absence of agonist activity of the PAM in the concentration range tested, no significant effect of the PAM applied alone was observed in the FRET assay (SI Appendix, Fig. S5). This finding further supports the proposal that, in the absence of agonist, the inactive ECD dimer prevents this mGlu2 PAM from activating the receptor.
We also examined whether the TMD per se had some influence on the conformational dynamics of the ECD dimer by expressing the mGlu2 ECD or the mGlu2 VFT attached to the plasma membrane through a glycosyl phosphatidylinositol (GPI) anchor (Fig. 5D) (27). Interestingly, such constructs behave like the full-length mGlu2 with almost the same basal and agonist-induced FRET efficiency and with the same agonist potencies. However, as expected, neither the PAM nor the NAM had any influence on the effect of agonists on these truncated receptors lacking the TMD.
These data reveal that the active conformation of the ECD dimer is stabilized further by compounds stabilizing the active form of the TMD and that the TMD per se is not required for the agonist to induce or stabilize the A conformation of the ECD dimer.
Mutations in the TMD Control the Conformation of the Dimeric ECD.
A number of mutations in the TMD of class C GPCRs which result in either a gain or a loss of function have been reported, either through site-directed mutagenesis (28) or through the analysis of human gene sequences (29–32). For example, both gain- and loss-of-function mutations identified in the calcium-sensing receptor gene are responsible for autosomal dominant hypocalcemia and familial hypocalciuric hypercalcemia, respectively (33). We examined whether similar mutations introduced in the mGlu2 TMD can affect the dynamics of the ECD dimer.
We first examined the effect of two mutations in the mGlu2 TMD (Q679V and C770A), equivalent to activating mutations identified in the mGlu8 receptor (Fig. 6A) (34, 35). Both mutants display a small but significant basal activity and higher agonist potency in the IP assay (Fig. 6B, Upper). In the ECD FRET assay (Fig. 6B, Middle) neither mutant displays strong basal activity, but both display higher agonist potency. Consistent with this effect (possibly resulting from a better stabilization of the active conformation of the TMD by the mutations), the addition of a NAM restores a wild-type potency for agonists (Fig. 6B, Middle). In addition, and as observed with the PAM on the wild-type receptor, both mutations converted the partial agonist LCCG-I into a full agonist (Fig. 6B, Lower), again as is consistent with the proposal that these mutations further stabilize an active conformation of the TMD upon agonist activation.
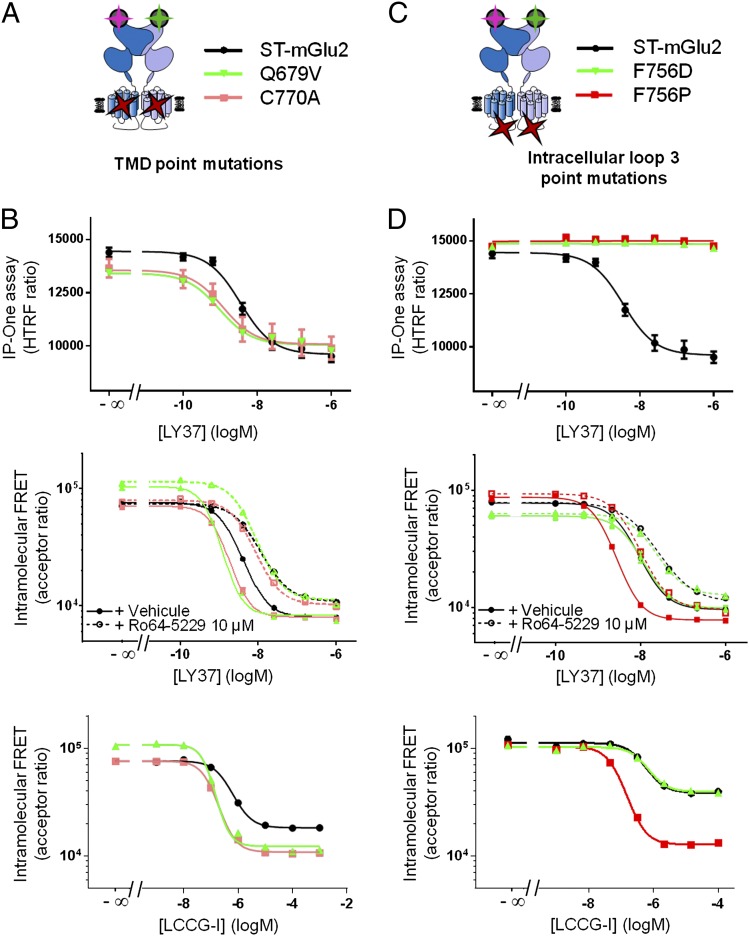
Fig. 6.
Mutations in TMD can influence the relative movement of the ECDs. (A) Cartoon illustrating the TMD punctual mutations equivalent to activating mutations in mGlu8. (B) IP accumulation (Upper) and intramolecular trFRET for the wild type and the indicated SNAP-mGlu2 constructs for the full agonist in presence or absence of 10 μM of the NAM Ro64-5229 (Middle) or for the partial agonist (Lower). (C) Cartoon to illustrate the mutations in the third intracellular loop at the residue F756 of mGlu2. (D) IP accumulation (Upper) and intramolecular trFRET for the wild type and the indicated SNAP-mGlu2 constructs for the full agonist in presence or absence of 10 μM Ro64-5229 (Middle) or for the partial agonist (Lower).
We also examined the effect of two loss-of-function mutations of the highly conserved residue Phe756 located in the third intracellular loop of class C GPCRs (Fig. 6B) (36, 37). The F756D mutant behaves like the wild type in the ECD FRET assay for agonist potency and efficacy measured in the absence and in the presence of either a PAM or a NAM, even though a complete loss of coupling to the G protein was observed (Fig. 6D, Upper). Surprisingly, the F756P mutant, which also prevents G-protein coupling, displays increased agonist potency in affecting the ECD FRET sensor (Fig. 6D, Middle), an effect not significantly potentiated by a PAM (SI Appendix, Fig. S6) but largely inhibited by a NAM (Fig. 6D, Middle). Moreover, as observed with the wild-type receptor in the presence of a PAM, the efficacy of the partial agonist LCCG-I is increased greatly to reach that of a full agonist (Fig. 6D, Lower). These data are consistent with the constraints brought by the Pro residue stabilizing the overall TMD in an active-like conformation but at the same time preventing G-protein interaction and activation.
G Protein Controls the Conformation of the Dimeric ECD.
Many proteins are known to interact directly with mGluRs for cell signaling or for modulating receptor activity (38). We analyzed the influence of the Go protein coupling on the conformation of the ECDs of mGlu2 (Fig. 7A). To improve the coupling between Gαo and mGlu2, we used the Gαo mutant G203T known to have a low affinity for both GDP and GTP and resulting in a stabilization of the Gα empty state that displays dominant negative properties (39). The coexpression of this G-protein mutant alone (SI Appendix, Fig. S7) or in combination with the subunit Gβ2γ2 (Fig. 7B) enhanced the potency of agonists for SNAP-mGlu2, showing that the G-protein coupling increases agonist potency.
Fig. 7.
G protein controls the conformation of the ECDs. (A) Cartoon illustrating the key intermediate of the GDP-to-GTP exchange, the agonist-receptor-Gempty complex, in which the agonist displays a reduced dissociation rate and therefore a higher apparent affinity. This intermediate state is mimicked by a Gα mutant, Gαo (G203T) that has a low affinity for GDP and GTP. (B) Intramolecular trFRET signal on donor- and acceptor-labeled SNAP-mGlu2 in the presence of the Gαo mutant cotransfected with Gβ2γ2. (C) Intramolecular trFRET signal for the constructs SNAP-mGlu2 mutated in the third intracellular loop in the presence of the wild type or the mutated Gαo(G203T) protein cotransfected with Gβ2γ2.
Consistent with the mutation F756P preventing G-protein coupling when already in an active-like conformation, the agonist potency on the ECD FRET-based assay is similar to that observed with the wild-type receptor coexpressed with the active form of the G protein (Gαo G203T), whereas the coexpression of this mutant G protein had no effect on agonist potency on the F756P mutant (Fig. 7C). In contrast, the agonist potency of the other loss-of-function mutant, F756D, in the ECD FRET-based assay is similar to that of the wild type. As is consistent with its inability to couple to G protein, the addition of Gαo G203T had no effect (Fig. 7C).
Discussion
The present study reports important information on the activation process of the dimeric mGluRs, and solves an issue not yet clarified through X-ray crystallography studies. Our approach that allowed the efficient analysis of cell-surface conformational changes by FRET showed that a large change in conformation at the level of the ECD dimer is intimately linked with mGluRs and G-protein activation, led to the identification of partial agonist, and revealed the allosteric control of the ECD dynamics by the TMD. This finding clarifies our view on the activation mechanism of mGluRs and its regulation by G proteins and helps in understanding the allosteric communication among the different domains of these dimeric receptors.
The agonist-induced decrease in intramolecular FRET efficiency is caused by a large change in the distance that separates both fluorophores because trFRET is barely sensitive to the relative fluorophore dipole orientation (20). Such movement is well related to receptor activation because (i) agonist potencies correlate with those measured using cell-based functional assays; (ii) mutations expected to prevent ECD movement prevented both agonist-induced FRET change and receptor activation; and (iii) locking the receptor in its active state resulted in agonist-like trFRET efficiency. These data are consistent with the conformational change from resting R to active A orientation resulting from glutamate binding, as initially proposed based on the first mGlu1 VFT dimer structures (40). Our finding that similar changes in trFRET are observed with the full-length mGlu1, mGlu2, and mGlu3 receptors upon agonist binding indicate that a similar conformational change occurs in all mGluRs upon activation. Of note, the same change is observed with VFTs inserted in the plasma membrane with a GPI anchor. Two possibilities could explain the two nonpreferential conformations observed in crystals, i.e., the Rcc stabilized by an agonist and the Aoo in presence of antagonist for mGlu3 and mGlu1, respectively (Fig. 1C). These conformations could result from constraints in the packing mode of the proteins in the crystal that stabilize a less stable conformation of the mGluRs. Alternately, they could result from a lack of constraints in the absence of the plasma membrane attachment of the VFTs.
Our FRET data also provide important information on how the ECD’s reorientation is related to the efficacy of the different agonists. We found that the well-known group II mGluR agonists DCG-IV and LCCG-I display a lower efficacy than the other agonists tested in the intramolecular FRET assay. Our additional functional studies confirmed that these drugs indeed are partial agonists. Partial activity usually is difficult to detect for drugs that still have a high efficacy close to that of a full agonist, as observed with DCG-IV and LCCG-I, especially with receptors overexpressed in cell lines and when measuring amplified GPCR-mediated responses. This difficulty highlights the power of our trFRET-based sensor for ligand characterization. Such a partial agonist action may be explained by a partial VFT closure and then a partial reorientation of the ECDs. Alternatively, partial agonism may be caused by a lower stability of the closed state of the VFTs so that only a fraction of the agonist-occupied receptors reach the A orientation. Because the mGlu3 VFT structure bound to the partial agonist DCG-IV (Fig. 2E) was found in a fully closed state, as observed with the full agonists, it is likely that the highly homologous mGlu2 VFT also can reach a fully closed state upon DCG-IV binding. Accordingly, the partial DCG-IV and LCCG-I activities likely result from a lower stability of the fully closed state. Further experiments analyzing single molecules may be needed to confirm this proposal.
Our data also bring information on the structural dynamics of the ECD when the receptor is blocked in the active conformation. Interestingly, the antagonist LY341495 does increase the intramolecular FRET efficiency in the receptor locked in an active state through an engineered intersubunit disulfide bridge. Such a change in FRET is not associated with a decrease in receptor activity, indicating that even though the receptor is locked in the active state at the level of the CRD and TMD, some flexibility still remains at the level of the VFTs. Because LY341495 is not accepted in a closed VFT, it is likely that even though the VFT dimer is maintained in the A orientation, binding of LY341495 forces both VFTs to reach an open conformation, as observed in the mGlu1 ECD structure with bound LY341495 (PDB 3KS9; Fig. 1C). Accordingly, the increase in FRET efficiency measured with LY341495 may result from a change from the Acc state to an Aoo state and suggests that both VFTs are in the closed form (Acc) in this constitutively active mutant. This proposal is supported by the low potency of LY341495 in inducing a FRET change, 200 times lower than its potency as an antagonist on the wild-type receptor.
Our results also reveal a clear allosteric communication between the TMD and the ECD, a molecular mechanism crucial for the functioning of the mGluRs. Indeed, stabilization of the TMD in an active conformation, using either a PAM or introducing mutations, influences the ECD dimer dynamics, as illustrated by the increased potency of agonists in the intramolecular FRET assay. However, in the absence of agonist neither these mutations nor PAM application leads to activation of the receptor as measured through the intracellular functional assay and does not induce a change in the VFT dimer conformation as revealed by the absence of FRET change. This result confirms that the receptor activation is dependent on the conformational change of the ECD dimer and highlights the important energy barrier brought by the ECD that limits the ability of the dimeric TMD to be fully activated (41, 42).
NAMs that are known to stabilize the TMD in the inactive state only slightly decrease agonist potency in changing the ECD dimer orientation. Thus the A orientation of the VFT dimer can be obtained even when both TMDs are in the inactive state. This finding is consistent with our recent observation on the mechanism of the TMDs’ activation in which a relative movement of the TMDs occurs first, followed by a change in conformation in the TMD (43).
Our study also revealed that loss-of-function mutations can have different “phenotypes,” as illustrated by the F756P and F756D mutations in the third intracellular loop. Although both mutants are unable to activate G proteins, the F756P mutant appears to lower the energy barrier to reach an active-like state, increasing agonist potency in the ECD FRET-based assay, increasing partial agonist efficacy and being insensitive to the PAM while returning to an inactive-like behavior in the presence of a NAM.
Our experimental system also is able to detect the conformational change of the ECDs induced by interacting proteins such as G proteins. Indeed, the G protein in its empty form is well known to stabilize the high agonist affinity state of GPCRs and then the active state of the receptor (44). Consistent with this model, we found that overexpressing the Gαo dominant negative mutant that displays a low affinity for both GDP and GTP has a direct effect on the ECD dimer dynamics and, indeed, increases agonist potency. The effect observed is consistent with the small decrease in agonist-binding affinity upon GTPγS treatment reported for many mGlu receptors (45).
When comparing agonist potencies in the intramolecular FRET assay and in the IP accumulation and calcium release functional assays, we found that potencies are 3–10 times higher (lower EC50s) in the functional assays based on signaling. One should note that, because the FRET-based assay is intimately linked to the conformational change of the receptor, potencies measured in such an assay are very close to the binding affinity of the agonist. In contrast, signal amplification resulting from the measurement of downstream signaling events likely increases agonist potencies. One also may consider another, not exclusive possibility. Because of the increase in agonist affinity resulting from the formation of the receptor–G protein (RG) complex that likely corresponds to the real active form of the complex, it is likely that the agonist potency measured in any functional assay is related to the agonist affinity on this RG complex. Instead, the intramolecular FRET assay measured agonist affinity on all cell-surface receptors, only a fraction of which are in the active RG state, and found a lower apparent affinity than on any other functional assays.
Finally, our experimental approach demonstrates an efficient and robust tool to analyze the effect of ligands on the mGluRs at the surface of living cells. Both full-length receptors and isolated VFTs can be analyzed to define whether the ligand binds to the ECD or the TMD of the mGluRs. Our approach, which is easy to use and allows intramolecular FRET measurements in microtiter plates, likely will be useful in studying the activation process of other cell-surface multimeric complexes. It may be developed as a reliable assay for drugs screening.
Taken together, our data bring clear evidence that a change in the relative orientation of the mGlu ECDs is a key step in receptor activation. Consistent with such a movement controlling the active state of the receptor, we found that stabilizing the TMD in an active state further stabilizes the ECD dimer in its active orientation, as illustrated by the large increase in agonist potency. These data provide key information on the structural dynamics and drug action at mGluRs and validate using such an approach to perform such analysis on other receptors.
Materials and Methods
Materials.
Glutamate was purchased from Sigma-Aldrich. LY379268, LY354740, and LY341495 were from Abcam Biochemicals; DCG-IV, LY487379, Ro64-5229, and (2S)-α-Ethylglutamic acid (EGLU) were from Tocris Bioscience; and [3H]-LY341495 was from the American Radiolabeled Chemicals. Lipofectamine 2000 and Fluo4-AM were from Life Technologies. SNAP-Lumi4-Tb, SNAP-Green, and Tag-Lite buffer were from Cisbio Bioassays.
Plasmids and Transfection.
pRK5 plasmids encoding the HA-SNAP–tagged wild-type mGlu1, 2, and 3 from rat and L521C mutant mGlu2 were described previously (5, 23). The single mutations in the pRK5 plasmid encoding rat Gαo protein and HA-SNAP–tagged mGlu2 and the 499GPI construct were generated using QuikChange mutagenesis protocol (Agilent Technologies) or were obtained from synthetic cDNA from Genecust. HEK293 cells were cultured in DMEM (Life Technologies) supplemented with 10% (vol/vol) fetal calf serum (Lonza). Cells were transfected by electroporation or by the reverse Lipofectamine 2000 protocol as previously described (5). To maintain a low concentration of ambient glutamate, cells were cotransfected with the cDNA of the glutamate transporter EAAC1 and incubated in DMEM Glutamax medium (Life Technologies) at least 2 h before the different assays were performed.
IP and Intracellular Calcium Measurements.
Measurement of IP accumulation in HEK293 cells was determined using the IP-One HTRF kit (CisBio Bioassays) according to the manufacturer’s recommendations, and the calcium mobilization signal was determined in HEK293 cells with a calcium-sensitive fluorescent dye (Fluo-4, Life Technologies), as previously described (46).
Cell-Surface Quantification and Ligand-Binding Assay.
Amounts of SNAP-tagged constructs at the cell surface were quantified as previously described (5). HEK293 cells were incubated at 37 °C for 1 h with a solution of 300 nM SNAP-Lumi4-Tb and were washed three times with tag-Lite buffer. After excitation with a laser at 337 nm, the fluorescence of the Lumi4-Tb was collected at 620 nm for 450 μs after a 50-μs delay on a PHERAstar FS (BMG Labtech).
For the ligand-binding assay, intact HEK293 cells in 10-cm dishes were collected 24 h after transfection and incubated with 3 nM [3H]-LY341495 (corresponding to the Kd of this radioligand) and various concentrations of cold ligands for 1 h at room temperature. Cells then were collected on filters and washed three times with a solution of 0.9% NaCl. Filters were placed in tubes containing a liquid scintillation mixture solution, and radioactivity was measured with a Packard beta counter (Perkin Elmer). Unspecific binding was determined in presence of 10 µM LY379268. Ki values were calculated according to the following equation: Ki = IC50/(1 + [RL]/Kd), where [RL] and Kd are the concentration and dissociation constant of the radioligand, respectively.
FRET Measurements.
For labeling, 24 h after transfection, HEK293 cells were incubated at 37 °C for 1 h with a solution of 100 nM of SNAP-Lumi4-Tb and 60 nM of SNAP-Green in Tag-Lite buffer (5). After being labeled, cells were washed three times with Tag-Lite, and drugs were added. The trFRET measurements were performed in Greiner black 96-well plates on a PHERAstar FS microplate reader with the following setup: after excitation with a laser at 337 nm (40 flashes per well), the fluorescence was collected at 520 nm for a 50-µs reading after a 50-µs delay after excitation (window 1) or for a 400-µs reading after a 1,200-µs delay (window 2) (SI Appendix, Fig. S2). The acceptor ratio was determined by dividing the signal measured in window 1 by the signal measured in window 2 and then was plotted on logarithmic scale. The intensity level of window 2 was above noise level by at least a factor of 5 to avoid erroneous divisions. We chose this representation because, in the range of acceptor ratio measured, the log of the ratio is correlated linearly with the distance between Lumi4Tb and the Green acceptor used (Förster radius of pair is 4.6 nm). Kinetic trFRET measurements were performed with the same setup, with signal captured every 1 s during 90 s.
Statistical Analysis.
When indicated, statistical analyses of the data were performed on results from three individual experiments, each performed in triplicate. Mean ± SEM were plotted, and a one-way ANOVA test was performed followed by a Bonferroni’s multiple comparison test using the analysis software GraphPad Prism. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. Guillaume Lebon, Pierre Paoletti, and Greg Stewart for constructive discussions. The intracellular calcium release, inositol phosphate assays, and homogeneous time-resolved fluorescence experiments were performed on the ARPEGE (Pharmacology Screening-Interactome) platform facility at the Institut de Génomique Fonctionnelle. E.D. was supported by a fellowship from the Fondation pour la Recherche Médicale and from the Association Schizo Oui. P.R. and J.-P.P. were supported by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, by grants from the Agence Nationale de la Recherche [G-protein–coupled receptor dimers ANR-BLAN06-3_135092, mGluPatho (ANR-08-NEUR-006-02 in the frame of ERA-Net NEURON), and Glusense (ANR-09-BIOT-018)] and the Fondation Bettencourt Schueller, and by an unrestricted grant from Senomyx.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 5742.
See Author Summary on page 5754 (volume 110, number 15).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215615110/-/DCSupplemental.
References
- 1.Janovjak H, Sandoz G, Isacoff EY. A modern ionotropic glutamate receptor with a K(+) selectivity signature sequence. Nat Commun. 2011;2:232. doi: 10.1038/ncomms1231. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pin JP, Galvez T, Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol Ther. 2003;98(3):325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 4.Brock C, et al. Activation of a dimeric metabotropic glutamate receptor by intersubunit rearrangement. J Biol Chem. 2007;282(45):33000–33008. doi: 10.1074/jbc.M702542200. [DOI] [PubMed] [Google Scholar]
- 5.Doumazane E, et al. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25(1):66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- 6.Maurel D, et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: Application to GPCR oligomerization. Nat Methods. 2008;5(6):561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rondard P, Goudet C, Kniazeff J, Pin JP, Prézeau L. The complexity of their activation mechanism opens new possibilities for the modulation of mGlu and GABAB class C G protein-coupled receptors. Neuropharmacology. 2011;60(1):82–92. doi: 10.1016/j.neuropharm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Niswender CM, Conn PJ. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bräuner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets. 2007;8(1):169–184. doi: 10.2174/138945007779315614. [DOI] [PubMed] [Google Scholar]
- 11.Kniazeff J, Prézeau L, Rondard P, Pin JP, Goudet C. Dimers and beyond: The functional puzzles of class C GPCRs. Pharmacol Ther. 2011;130(1):9–25. doi: 10.1016/j.pharmthera.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Bessis AS, et al. Closure of the Venus flytrap module of mGlu8 receptor and the activation process: Insights from mutations converting antagonists into agonists. Proc Natl Acad Sci USA. 2002;99(17):11097–11102. doi: 10.1073/pnas.162138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kniazeff J, et al. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol. 2004;11(8):706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- 14.Kunishima N, et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407(6807):971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ Proc Natl Acad Sci USA. 2002;99(5):2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muto T, Tsuchiya D, Morikawa K, Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci USA. 2007;104(10):3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keppler A, et al. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21(1):86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- 18.Malherbe P, et al. Identification of essential residues involved in the glutamate binding pocket of the group II metabotropic glutamate receptor. Mol Pharmacol. 2001;60(5):944–954. doi: 10.1124/mol.60.5.944. [DOI] [PubMed] [Google Scholar]
- 19.Lohse MJ, et al. Optical techniques to analyze real-time activation and signaling of G-protein-coupled receptors. Trends Pharmacol Sci. 2008;29(3):159–165. doi: 10.1016/j.tips.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Selvin PR. Principles and biophysical applications of lanthanide-based probes. Annu Rev Biophys Biomol Struct. 2002;31:275–302. doi: 10.1146/annurev.biophys.31.101101.140927. [DOI] [PubMed] [Google Scholar]
- 21.Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38(10):1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 22.Rondard P, et al. Functioning of the dimeric GABA(B) receptor extracellular domain revealed by glycan wedge scanning. EMBO J. 2008;27(9):1321–1332. doi: 10.1038/emboj.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, et al. Interdomain movements in metabotropic glutamate receptor activation. Proc Natl Acad Sci USA. 2011;108(37):15480–15485. doi: 10.1073/pnas.1107775108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll FY, et al. BAY36-7620: A potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol Pharmacol. 2001;59(5):965–973. [PMC free article] [PubMed] [Google Scholar]
- 25.Pagano A, et al. The non-competitive antagonists 2-methyl-6-(phenylethynyl)pyridine and 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester interact with overlapping binding pockets in the transmembrane region of group I metabotropic glutamate receptors. J Biol Chem. 2000;275(43):33750–33758. doi: 10.1074/jbc.M006230200. [DOI] [PubMed] [Google Scholar]
- 26.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: A novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, et al. Molecular determinants involved in the allosteric control of agonist affinity in the GABAB receptor by the GABAB2 subunit. J Biol Chem. 2004;279(16):15824–15830. doi: 10.1074/jbc.M313639200. [DOI] [PubMed] [Google Scholar]
- 28.Wellendorph P, Bräuner-Osborne H. Molecular basis for amino acid sensing by family C G-protein-coupled receptors. Br J Pharmacol. 2009;156(6):869–884. doi: 10.1111/j.1476-5381.2008.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank RA, et al. Clustered coding variants in the glutamate receptor complexes of individuals with schizophrenia and bipolar disorder. PLoS ONE. 2011;6(4):e19011. doi: 10.1371/journal.pone.0019011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pidasheva S, D’Souza-Li L, Canaff L, Cole DE, Hendy GN. CASRdb: Calcium-sensing receptor locus-specific database for mutations causing familial (benign) hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat. 2004;24(2):107–111. doi: 10.1002/humu.20067. [DOI] [PubMed] [Google Scholar]
- 31.Prickett TD, et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet. 2011;43(11):1119–1126. doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeitz C, et al. Night blindness-associated mutations in the ligand-binding, cysteine-rich, and intracellular domains of the metabotropic glutamate receptor 6 abolish protein trafficking. Hum Mutat. 2007;28(8):771–780. doi: 10.1002/humu.20499. [DOI] [PubMed] [Google Scholar]
- 33.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4(7):530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita T, Kai T, Terakita A, Shichida Y. A novel constitutively active mutation in the second cytoplasmic loop of metabotropic glutamate receptor. J Neurochem. 2004;91(2):484–492. doi: 10.1111/j.1471-4159.2004.02739.x. [DOI] [PubMed] [Google Scholar]
- 35.Yanagawa M, Yamashita T, Shichida Y. Activation switch in the transmembrane domain of metabotropic glutamate receptor. Mol Pharmacol. 2009;76(1):201–207. doi: 10.1124/mol.109.056549. [DOI] [PubMed] [Google Scholar]
- 36.Chang W, Chen TH, Pratt S, Shoback D. Amino acids in the second and third intracellular loops of the parathyroid Ca2+-sensing receptor mediate efficient coupling to phospholipase C. J Biol Chem. 2000;275(26):19955–19963. doi: 10.1074/jbc.M909613199. [DOI] [PubMed] [Google Scholar]
- 37.Francesconi A, Duvoisin RM. Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem. 1998;273(10):5615–5624. doi: 10.1074/jbc.273.10.5615. [DOI] [PubMed] [Google Scholar]
- 38.Bockaert J, Perroy J, Bécamel C, Marin P, Fagni L. GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annu Rev Pharmacol Toxicol. 2010;50:89–109. doi: 10.1146/annurev.pharmtox.010909.105705. [DOI] [PubMed] [Google Scholar]
- 39.Barren B, Artemyev NO. Mechanisms of dominant negative G-protein alpha subunits. J Neurosci Res. 2007;85(16):3505–3514. doi: 10.1002/jnr.21414. [DOI] [PubMed] [Google Scholar]
- 40.Jingami H, Nakanishi S, Morikawa K. Structure of the metabotropic glutamate receptor. Curr Opin Neurobiol. 2003;13(3):271–278. doi: 10.1016/s0959-4388(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 41.Goudet C, et al. Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors. Proc Natl Acad Sci USA. 2004;101(1):378–383. doi: 10.1073/pnas.0304699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rondard P, et al. Coupling of agonist binding to effector domain activation in metabotropic glutamate-like receptors. J Biol Chem. 2006;281(34):24653–24661. doi: 10.1074/jbc.M602277200. [DOI] [PubMed] [Google Scholar]
- 43.Hlavackova V, et al. Sequential inter- and intrasubunit rearrangements during activation of dimeric metabotropic glutamate receptor 1. Sci Signal. 2012;5(237):ra59. doi: 10.1126/scisignal.2002720. [DOI] [PubMed] [Google Scholar]
- 44.Yao XJ, et al. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc Natl Acad Sci USA. 2009;106(23):9501–9506. doi: 10.1073/pnas.0811437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schweitzer C, et al. Characterization of [(3)H]-LY354740 binding to rat mGlu2 and mGlu3 receptors expressed in CHO cells using semliki forest virus vectors. Neuropharmacology. 2000;39(10):1700–1706. doi: 10.1016/s0028-3908(99)00265-8. [DOI] [PubMed] [Google Scholar]
- 46.Monnier C, et al. Trans-activation between 7TM domains: Implication in heterodimeric GABAB receptor activation. EMBO J. 2011;30(1):32–42. doi: 10.1038/emboj.2010.270. [DOI] [PMC free article] [PubMed] [Google Scholar]