Abstract
Theiler's disease is an acute hepatitis in horses that is associated with the administration of equine blood products; its etiologic agent has remained unknown for nearly a century. Here, we used massively parallel sequencing to explore samples from a recent Theiler's disease outbreak. Metatranscriptomic analysis of the short sequence reads identified a 10.5-kb sequence from a previously undescribed virus of the Flaviviridae family, which we designate “Theiler's disease-associated virus” (TDAV). Phylogenetic analysis clusters TDAV with GB viruses of the recently proposed Pegivirus genus, although it shares only 35.3% amino acid identity with its closest relative, GB virus D. An epidemiological survey of additional horses from three separate locations supports an association between TDAV infection and acute serum hepatitis. Experimental inoculation of horses with TDAV-positive plasma provides evidence that several weeks of viremia preceded liver injury and that liver disease may not be directly related to the level of viremia. Like hepatitis C virus, the best characterized Flaviviridae species known to cause hepatitis, we find TDAV is capable of efficient parenteral transmission, engendering acute and chronic infections associated with a diversity of clinical presentations ranging from subclinical infection to clinical hepatitis.
Keywords: epidemiology, veterinary virology, metagenomics, deep sequencing, pathogen discovery
Theiler's disease (also known as acute serum hepatitis, postvaccination hepatitis, or idiopathic acute hepatic disease) is one of the most common causes of acute hepatitis in horses (1). It was first reported in 1919 by Arnold Theiler, who observed symptoms of liver disease in animals vaccinated against African horse sickness with a combination of live virus and equine antiserum (2). In the ensuing decades, numerous outbreaks of Theiler's disease have been reported in North America and Europe, typically following the relatively common practice of administering hyperimmune equine plasma or serum to horses exposed to contagious or life-threatening infectious agents (e.g., Western equine encephalitis virus, Bacillus anthracis, Streptococcus equi, Clostridium perfringens, and equine influenza virus) or toxins (e.g., tetanus). Theiler's disease has also been observed following i.v.-administered plasma as a colloid in horses with colitis or following surgical correction of intestinal strangulation (3–6).
Theiler's disease outbreaks share similarities with historical outbreaks of human posttransfusion hepatitis, which were often associated with contaminated blood products before the identification of hepatitis B and C viruses (HBV and HCV) (3). Horses typically exhibit a rapid onset of acute symptoms, including lethargy, anorexia, and jaundice, as well as an elevation of serum levels of liver enzymes and bilirubin 2–3 mo following administration of blood products. Fever and additional central nervous system signs (cortical blindness, ataxia, aggressive behavior, or coma) may also be present. Subclinical cases exhibiting elevated liver enzymes but no overt clinical signs of hepatitis have also been observed (6). Postmortem studies of Theiler's disease cases have identified significant necrosis and degeneration in the liver, identifying this as the major site of pathology. Morbidity rates associated with Theiler's disease outbreaks vary from slightly more than 1% to as high as 18%. Among symptomatic horses, Theiler's disease results in mortality rates between 50% and 90% (1, 7, 8).
The association between prior treatment with equine serum/plasma and the appearance of Theiler's disease has long suggested an etiologic role for a contaminating toxin or infectious agent. However, numerous experimental studies in both the natural equine host and laboratory animals have failed to convincingly identify an underlying chemical (2, 9) or pathogen (3). The cause of Theiler's disease has therefore remained a mystery for almost a century.
Here, we examined a recent Theiler's disease outbreak, using massively parallel sequencing technology to investigate the possibility of the existence of an associated virus. A previously unknown and highly divergent member of the Flaviviridae family was identified in serum from two overtly clinical index cases of hepatitis and from an equine plasma product administered to the horses before the outbreak. A quantitative reverse transcription–PCR (qRT-PCR) assay was developed and applied to explore the epidemiology of infection. The kinetics of infection and disease symptoms in horses experimentally inoculated with the equine plasma product were also examined. These studies provided evidence that this previously undescribed virus, here designated “Theiler's disease-associated virus” (TDAV), could be a causative agent of Theiler's disease.
Results
Serum Hepatitis Outbreak Following Prophylactic Treatment with Equine Antitoxin.
We investigated an outbreak of acute hepatic disease that occurred on a horse farm following prophylactic treatment of the animals with equine antiserum to botulinum toxin. The farm (Farm A) housed a population of 75 horses, of which 4 were diagnosed with botulism after consumption of contaminated feed. Two of the horses presented with a rapid onset of paralysis and were euthanized. The other two horses displayed milder symptoms of botulism, were treated with equine botulinum antitoxin, and made a full recovery. As a prophylactic measure, antitoxin was then administered to 21 other horses suspected of exposure to the contaminated feed. Four other horses were treated with the same lot of antitoxin (“antitoxin 1”) that was used to treat the two symptomatic horses. Seventeen additional horses were treated with a second, independently sourced lot of botulinum antitoxin (“antitoxin 2”). The rest of the horses (n = 52) on the farm were not treated with botulinum antitoxin. One of the horses that was treated with antitoxin 1 upon presentation with mild botulism symptoms was removed from the farm and was unavailable for further monitoring, leaving a total of 22 horses that were treated with antitoxin 1 (n = 5) or antitoxin 2 (n = 17) for continued observation and testing.
Eight weeks after the administration of antitoxin 2, two of the horses treated with antitoxin 2 (horse A1 and horse A2) displayed signs of acute hepatic insufficiency, including lethargy, poor appetite, jaundice, and photodermatitis. Serum levels of liver enzymes, direct and indirect bilirubin, and bile acids were markedly elevated in both horses. A diagnosis of Theiler's disease was made on the basis of clinical findings, biochemical abnormalities characteristic of the disease, no known exposure to plant or chemical toxins, and knowledge that the horses had been treated with an equine blood product 8 wk earlier. All horses that received antitoxin treatment were subsequently monitored for overt symptoms of hepatitis and weekly serum samples were tested for biochemical evidence of liver injury [elevated levels of aspartate amino transferase (AST), gamma glutamyl transferase (GGT), sorbitol dehydrogenase (SDH), glutamate dehydrogenase (GLDH), and bilirubin]. In total, 8 of the 22 horses that received prophylactic antitoxin contracted biochemically documented hepatitis over the following several weeks (Fig. 1); 3 of the horses had normal biochemistry results on the first week of the investigation, but were abnormal the following week (week 9 after administration of the antitoxin). All eight cases occurred in horses that had been treated with antitoxin 2, raising the possibility that this preparation might have harbored a previously unidentified infectious agent. To address this possibility, we extracted RNA and prepared sequencing libraries from serum specimens of the two index cases (horse A1 and horse A2), as well as from antitoxin 2.
Fig. 1.
Overview of a Theiler's disease outbreak. Twenty-two horses on Farm A suspected of exposure to botulinum toxin were prophylactically treated with i.v. equine antibotulinum toxin hyperimmune plasma. Five horses received antitoxin from one source (gray horses, antitoxin 1), whereas 17 horses received an independently sourced batch (black horses, antitoxin 2). Fifteen horses followed in this study went untreated (white horses, untreated). Within 8 wk of antitoxin administration, 8 horses treated with antitoxin 2 showed signs of acute hepatitis (yellow boxes). All other horses were clinically asymptomatic (no boxes).
Viral Sequence Detection Leads to Assembly of a Highly Divergent Flaviviridae Genome.
Massively parallel sequencing of libraries from horse A1, horse A2, and antitoxin 2 resulted in an average of 22 million 100-nt sequence reads for each sample. For the two serum specimens, complexity and host filtering of the raw sequence data removed ∼95% of the reads from downstream analyses. For the antitoxin 2 plasma sample, complexity and host filtering removed ∼50% of the reads. The remaining reads were mapped to all viral protein sequences in RefSeq. For horses A1 and A2 ∼0.01% of sequence reads (2,954 and 3,522 reads, respectively) mapped to members of the Flaviviridae; 0.06% (9,116) of antitoxin 2 reads mapped to the same virus family. In addition to the abundance of Flaviviridae reads in each sample, horse A1 had a single read pair mapping to the Baculoviridae, a family of insect viruses (10), and another read pair derived from the Adenoviridae family, mapping to the region found in laboratory recombination vectors (11–13); horse A2 contained 80 read pairs deriving from a 298-nt contiguous region in pepper mild mottle virus, a plant virus from the Virgaviridae family. These reads likely represent artifactual contamination introduced during sample collection and library preparation. Horse A2 additionally contained four read pairs derived from equid herpesvirus 2, a common equine virus sometimes associated with mild respiratory disease in horses (14, 15). No additional viral reads were detected in the antitoxin 2 specimen.
Sequence reads mapping to members of the Flaviviridae were used as the starting point for de novo genome assemblies, using the paired-read iterative contig extension (PRICE) assembly algorithm (Materials and Methods) (16). A 10.5-kb contig corresponding to a previously unknown and divergent genome of the Flaviviridae family was assembled from the horse A1 sequences, and long contigs from an essentially identical (>99% nucleotide sequence identity) genome were assembled from the horse A2 and antitoxin 2 samples (Fig. 2A). The depicted protein cleavage sites are putative and were determined on the basis of a multiple-sequence alignment with members of the Pegivirus and Hepacivirus genera (Fig. S1). Mapping the reads from each sample back to the assembled genome revealed that the true viral sequence coverage was two orders of magnitude greater than initially perceived: 0.8% of reads from horse A1, 1.4% of reads from horse A2, and 2.4% of reads from antitoxin 2 mapped to the recovered genome (Fig. 2B). We provisionally designate the genome Theiler's disease-associated virus (TDAV).
Fig. 2.
Genome of Theiler's disease-associated virus (TDAV). (A) Schematic of the TDAV genome. Protein cleavage sites are putative and were annotated on the basis of homologous inference from HCV (Fig. S1). The “*” gene corresponds to HCV p7, GBV-B p13, GBV-D X, and GBV-A 21 kDa protein (19). (B) Coverage map of sequencing reads from horse A1, horse A2, and antitoxin 2. (C) Median calculated amplicon size based on the distance between the 5′ ends of paired-end sequencing reads mapping to each nucleotide. (D) Overlapping clones recovered and sequenced to confirm the genome assembly (black) and the location of amplicons used in the TDAV detection assay (green; primer pairs labeled below). (E) Pairwise amino acid percentage of identity plot (100-aa windows) of TDAV compared with HCV-gt1, NPHV, and GBV-D. Genome position scale (Lower) refers to A–E.
Validation of TDAV Genome Sequence Assembly.
To validate the computer-generated genome assembly, we first investigated the potential presence of artificial structural rearrangements. In an assembly free of artifacts, the distance between paired-end reads should match the amplicon size selected during library preparation. This was evaluated by calculating the distance between read pairs mapping back to the TDAV genome assembly and by plotting the median value of all calculated amplicon sizes at each position. The calculated amplicon sizes based on the horse A1 data closely matched the experimentally selected insert size of 300 ± 30 nt (Fig. 2C).
To obtain empirical support for the presence of the predicted TDAV sequence in clinical samples, RT-PCR was performed. RNA prepared from horse A1 serum was reverse transcribed and amplified with primer pairs predicted to yield 10 overlapping amplicons encompassing nearly the complete TDAV genome assembly (Fig. 2D). All 10 amplicons were successfully recovered. Cloning and Sanger sequencing of three independent clones for each amplicon revealed >99% nucleotide identity with the in silico-generated TDAV assembly.
The extreme 5′ and 3′ ends of the TDAV genome assembly were underrepresented in the initial library (Fig. 2B). The 5′ end was investigated by 5′ rapid amplification of cDNA ends (RACE), using RNA samples from horse A1, horse A2, and antitoxin 2. This method extended the initial sequence assembly by 24 nt. The reported 5′-terminal nucleotide (“T”) was found in 21 of 35 clones, whereas 9 clones contained a different terminal nucleotide in that position, and 5 clones contained a single additional upstream nucleotide (“T”) (Fig. S2). We were unable to extend the sequence of the 3′ end beyond the sequencing read assembly.
Genome Features of TDAV.
The TDAV genome assembly is 10,479 nt in length (GenBank accession no. KC145265), encompassing a 5′-untranslated region (UTR) of 617 nt, a single ORF initiating from an AUG at position 618 and encoding a putative polyprotein 3,189 aa residues in length, and 292 nt of the 3′-UTR. By comparison with related Flaviviridae, the TDAV genome encodes three putative structural (a truncated protein corresponding to core, E1, and E2) and seven putative nonstructural (a gene product between E2 and NS2, NS2, NS3, NS4A, NS4B, NS5A, and NS5B) proteins (Fig. 2 and Fig. S1).
Although TDAV appears most closely related to the newly proposed Pegivirus species (GB viruses A, C, and D) and the Hepacivirus species (hepatitis C virus) within the Flaviviridae phylogeny (Fig. 3 and Fig. S3), it is highly divergent from both groups. TDAV shares only 35.3% amino acid sequence identity over the length of the entire polyprotein with its nearest relative, GB virus D (GBV-D) (17), 20.5% identity with hepatitis C virus (genotype 1) (HCV-gt1) (18), and 20.4% identity with the most closely related equine member of the Flaviviridae, the nonprimate hepacivirus (NPHV) species (19). Pairwise sequence identity between TDAV and these species varies dramatically according to genome locus (Fig. 2E and Fig. S1). The putative serine protease, NS3, and the RNA-dependent RNA polymerase, NS5B, are the most conserved regions of the polyprotein, with some regions exceeding 75% amino acid identity to GBV-D (Fig. 2E).
Fig. 3.
Phylogenetic analysis of Flaviviridae based on polyprotein sequences. Multiple-sequence alignments (available upon request) were generated using MUSCLE (39) and a neighbor-joining phylogenetic tree with 100 bootstrap replicates was generated in MEGA5 (40). TDAV is in boldface type for emphasis. All branches are numbered starting at “*” and incremented clockwise (see Table S1 for key). Closer branch groups are labeled with a range of sequence numbers; a representative member of a group is listed in parentheses with a thicker line illustrating the branch for the representative member. WNV, West Nile virus; DENV, Dengue virus 1; DV, Donggang virus; YFV, Yellow fever virus; MV, Modoc virus; TBEV, Tick-borne encephalitis virus; KRV, Kamiti River virus; TBV, Tamana bat virus; BVDV, Bovine viral diarrhea virus 1; TDAV, Theiler's disease-associated virus; GBV-(A-D), GB virus (A-D); HCV, Hepatitis C virus genotype 1; NPHV, NPHV #1 (AFJ20709.1). “†” indicates nearly identical branches 8 and 9, corresponding to Tembusu virus and Duck flavivirus TA, respectively. Species text color denotes genus classification: Pegivirus (blue), Hepacivirus (orange), Pestivirus (green), and Flavivirus (black). The NPHVs and TDAV [gray and black (in boldface type), respectively] have yet to be classified into a genus.
At 617 nt and 292 nt, the TDAV 5′-UTR and (possibly partial) 3′-UTR are longer than those found in the Hepacivirus species (∼340 nt and 200–235 nt, respectively). Although there appears to be significant potential for secondary structure predicted in each UTR, canonical IRES or other RNA structural motifs typical of other Flaviviridae are not readily apparent (Fig. S4). The core protein, which serves as the nucleocapsid for HCV, is truncated or absent in GBV-A, GBV-C, and GBV-D (20). In TDAV, its predicted size is also small (26 aa), with a putative signal peptidase cleavage site between amino acids 15 and 16. Likewise, the fourth predicted TDAV protein, which lies between E2 and NS2, appears more similar to Pegivirus than to Hepacivirus species. In HCV, this region encodes p7, a 7-kDa (63-aa) protein that is important for virus assembly and release. In the Pegivirus species, the protein varies from 6 kDa (in GBV-C) to 21 kDa (in GBV-A), and its role in the virus life cycle is not clear. The corresponding TDAV protein has a predicted molecular weight of ∼10.8 kDa. Finally, unlike the Pegivirus species and Hepacivirus species, the TDAV NS5A protein contains three putative insertions spanning ∼160 aa not found in any other members of the Flaviviridae, including a large insertion of nearly 100 aa in domain 1b of NS5A and two shorter insertions in domain 2 (Fig. S1). The functional role of these insertions is unknown.
Epidemiological Survey for the Presence of TDAV.
An epidemiological survey was conducted to understand the prevalence of TDAV in horses, to investigate the correlation between clinical symptoms and viremia, and to determine whether there is natural animal-to-animal transmission. A qRT-PCR–based TDAV assay using two distinct sets of primer pairs (EVT-146/147 and EVT-162/163, Fig. 2D) was developed (Materials and Methods and SI Materials and Methods) and used to screen 60 horses from three geographically distant farms. Farm A, on which the Theiler's disease outbreak took place, provided serum samples from 37 horses, which sometimes grazed in pastures together or were housed near each other in common stables. These horses stratified into three subgroups: 15 had not been treated with botulinum antitoxin, 5 had received antitoxin 1, and 17 (including horse A1 and horse A2) had received the TDAV-positive antitoxin 2. Sera from 20 Farm B horses with no history of exposure to antitoxin from either source or any contact with Farm A horses were also tested. Serum samples from the three individual horses used to produce antitoxin 2 were also provided for testing (Farm D).
The results of the TDAV qRT-PCR detection assay are displayed as Ct values plotted on an inverted y axis (Fig. 4A). Of the 17 horses exposed to antitoxin 2 on Farm A, 15 displayed low Ct values, indicating TDAV positivity. The viral load in these 15 horses ranged from 107 to 108 genomes per milliliter. Two antitoxin 2-treated horses (A7 and A11) were seemingly negative by the qRT-PCR assay, using primers EVT-146/147; however, in parallel analysis of these samples with the EVT-162/163 assay, a subset of the qPCR replicates displayed a positive signal (Dataset S1). Given that not a single replicate qPCR from an animal that was not exposed to antitoxin 2 displayed a positive signal in the EVT-162/163 TDAV assay, this suggests that A7 and A11 were infected with TDAV, but have viral titers hovering around the detection limit of the second assay. All (40/40) untreated horses or horses treated with antitoxin 1 from Farms A and B were negative for TDAV as judged by high Ct values in the EVT-146/147 assay and undetectable signal in the EVT-162/163 assay. Of the 3 donor horses used to produce antitoxin 2, 2 animals were TDAV negative, whereas 1 was positive; this animal is presumably the source of TDAV in antitoxin 2. This TDAV-positive donor horse was retested 7 mo later and was virus negative. These results establish a strong association of TDAV infection with antitoxin 2 exposure and additionally suggest the virus is not highly prevalent in untreated horses. Furthermore, the fact that TDAV was not detected in horses on Farm A that did not receive antitoxin 2, despite contact with TDAV-infected horses, suggests that TDAV was not readily spread between horses. Clinical status, antitoxin treatment, viral loads, and raw data from both qRT-PCR assays are provided in Dataset S1.
Fig. 4.

Quantitative PCR-based assay to detect TDAV in different cohorts. Cycles to threshold (Ct) from the qRT-PCR TDAV assay (primers EVT-146/147) are plotted on an inverted y axis; a lower Ct value represents a greater viral load. (A) Serum/plasma from horses on Farms A, B, and D with antitoxin treatment status indicated. All samples were analyzed in parallel with a second qRT-PCR TDAV assay (primers EVT-162/163). Those samples in which a qPCR signal was absent by the second assay are denoted as “confirmed negative” by gray circles. (B) Antitoxin 2-treated horses from Farm A were categorized as “clinical” when they had characteristic signs of acute hepatitis including icterus, lethargy, and anorexia and when there were elevations in the serum activity of at least three of the tested liver enzymes (AST, GGT, SDH, and GLDH). Horses classified as “subclinical” had no overt clinical manifestations of acute hepatitis but had significant elevations in the serum activity of at least three of the tested liver enzymes. Asymptomatic cases had neither clinical signs of acute hepatitis nor abnormalities in the serum activities of the tested liver enzymes.
During the Theiler's disease outbreak on Farm A, animals were broadly classified as being hepatitis positive or hepatitis negative on the basis of laboratory biochemical test results with further stratification of the positive horses as “clinical” or “subclinical” (Fig. 4 legend). The four clinical cases displayed significant elevation of liver enzymes in the serum along with common clinical signs of jaundice, lethargy, poor appetite, and photodermatitis. The subclinical cases displayed varying degrees of elevation of liver enzymes in the serum, but without overt clinical manifestations of liver disease except for one horse that developed photodermatitis. To investigate the correlation between viral load and the severity of clinical manifestations, the qRT-PCR assay data from the antitoxin 2-exposed animals were stratified by hepatitis status and symptom level (Fig. 4B). Because the range of Ct values (representing a viral load of 107–108 genomes per milliliter; SI Materials and Methods) was similar for both hepatitis-positive and hepatitis-negative horses, a clear relationship between viral load and hepatitis was not apparent. Moreover, in each group (hepatitis positive and negative) there was a single horse (A7 and A11, respectively) with viral loads that fell below this range, consistent with the conclusion that the viral load is not predictive of the extent of hepatic injury.
Experimental Inoculation Study.
To better understand the dynamics of TDAV infection and the role of the virus in disease progression, a small experimental inoculation study was performed. Four horses that were healthy and free of evidence of liver disease according to initial laboratory evaluation (Materials and Methods) received standard 500-mL doses of antitoxin 2 (the same lot involved in the Farm A outbreak) via the i.v. route. For the next 10–14 wk, the animals were monitored daily for overt signs of illness. Serum specimens were collected weekly and tested for biochemical evidence of liver injury and quantification of TDAV serum levels.
Over the course of the study, none of the four animals displayed clinical signs of illness. However, horse C1 had elevated liver enzyme levels in the serum that peaked between weeks 8 and 10 postinoculation (Fig. 5 B–E). In contrast, serum liver enzymes for horses C2–C4 remained almost unchanged over the course of the study, with the exception of a transient elevation in GLDH and SDH levels in horse C3 at week 7.
Fig. 5.
TDAV viral load and liver enzyme function in animals experimentally inoculated with antitoxin 2. (A) qRT-PCR quantification of TDAV RNA. Cycles to threshold (Ct) values from the qRT-PCR assay [average values and SD (n = 4) with primers EVT-146/147] are plotted on an inverted y axis; a lower Ct value represents a greater viral load. (B–E) Biochemical tests for AST, GGT, SDH, and GLDH. Samples were taken at time 0 and weekly for 10–14 wk after inoculation with 500 mL of the same lot of antitoxin 2 linked to the serum hepatitis outbreak. One horse became symptomatic (horse C1, blue), whereas the other three did not (horse C2, green; horse C3, orange; and horse C4, black).
The dynamics of TDAV infection were diverse among the four inoculated animals (Fig. 5A), with viral loads falling in the same range as those observed in the outbreak (Fig. 4A). In horses C1 and C3, TDAV levels climbed rapidly and remained elevated from week 4 for the duration of the study. Importantly, the near-maximal viral load in horse C1 predated the onset of peak biochemical evidence of liver disease by at least 5 wk. Indeed, there were 3 wk (weeks 4–7) during which viral load was maximal but no signs of hepatic injury were evident, as judged by AST, GGT, SDH, and GLDH levels. In horse C4, viral load gradually increased and peaked later (week 8) and at lower levels than those in horses C1 and C3. In horse C2, TDAV was detected from week 2 onward; however, the viral load was very low relative to that in the other animals and did not rise substantially over of the course of the study.
Acute TDAV Infection Can Become Chronic.
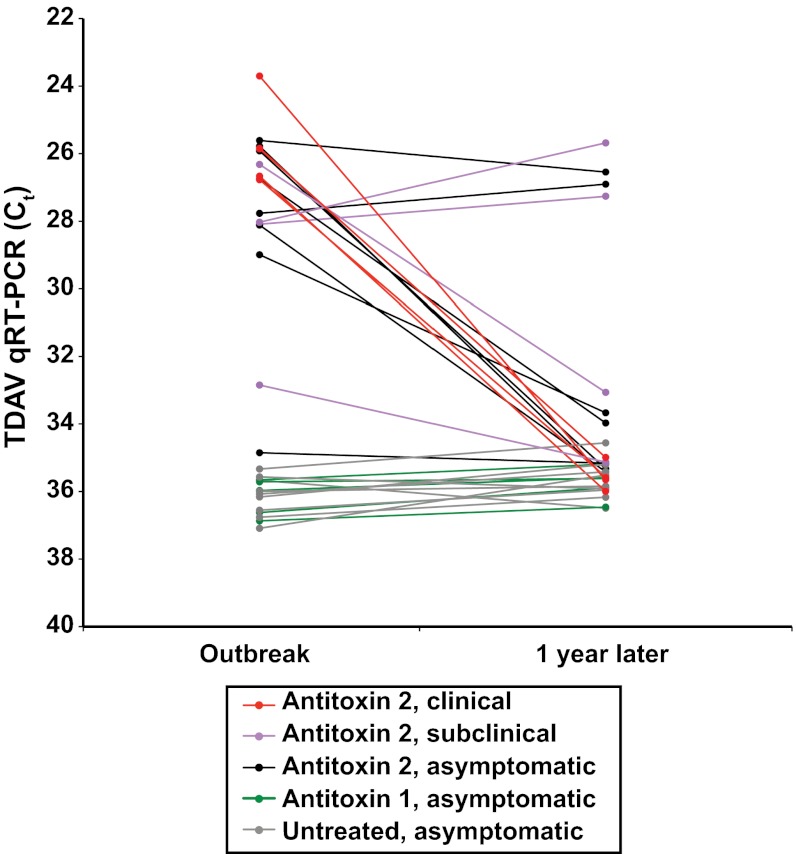
Both the recently proposed Pegivirus species and the Hepacivirus species are known to cause chronic infection in some individuals (20). To examine the potential of TDAV to progress to chronicity, serum samples from 30 animals on Farm A were collected approximately 1 y after the initial Theiler's disease outbreak (Fig. 6). Characteristically, Theiler's disease outbreaks are associated with some mortality but in this outbreak there were no deaths, allowing all but 1 of the 17 infected horses (one nondiseased horse had left the farm) to be retested 1 y later. In both index cases (horses A1 and A2) that were TDAV positive and displayed clinical signs of illness during the outbreak, the virus was not detectable in serum samples collected 1 y later. Similarly, 10 other animals that had been TDAV positive during the outbreak (including horses A7 and A11, which had a very low viral load during the outbreak) were negative for the virus after 1 y. In contrast, 4 horses without overt clinical symptoms during the outbreak were identified as asymptomatic carriers of TDAV 1 y later, demonstrating that TDAV can establish chronic infection in some animals. Finally, all Farm A horses that were TDAV negative during the outbreak continued to be negative at 1 y, again suggesting that horizontal transmission from infected animals on the same farm is at most very inefficient.
Fig. 6.
TDAV can cause chronic infection. TDAV was monitored in paired samples harvested during the outbreak and approximately 1 y later. Cycles to threshold (Ct) from the qRT-PCR TDAV assay (primers EVT-146/147) are plotted on an inverted y axis; a lower Ct value represents a greater viral load. The qRT-PCR assays were performed in parallel on paired samples, permitting direct comparison of Ct values.
Discussion
We have applied massively parallel sequencing and subsequent nucleic acid-based screening to identify a previously unknown, highly divergent member of the Flaviviridae. This virus, which we have termed TDAV, was identified in an outbreak of Theiler's disease, and represents a strong etiologic candidate for equine serum hepatitis.
Several lines of evidence implicate TDAV as the causative agent in this outbreak of Theiler's disease in horses. First, metatranscriptomic analysis demonstrated that TDAV was the only detectable virus common to both the antitoxin 2 material and the index cases, and there were few non-TDAV viral sequences detected in any of the ∼60 million sequence reads we analyzed in this study. Second, although not all TDAV-positive horses had hepatitis, TDAV was detectable in every animal in the outbreak with clinical or biochemical evidence of hepatitis. Third, TDAV positivity was exclusively associated with antecedent exposure to antitoxin 2, supporting the hypothesis that infection had originated from exposure to this previously unknown virus in the equine plasma product and not from a virus routinely found in farm horses. Fourth, the monitoring of serum biochemistry of Farm A horses both immediately following the onset of disease in the first two clinical cases and in the subsequent inoculation study indicated that TDAV infection precedes liver injury, excluding the possibility that the association of virus with hepatitis on Farm A reflects preferential infection of animals with antecedent liver injury.
We emphasize that our inoculation study does not formally fulfill Koch’s postulates, as the TDAV in the inoculum was not first purified by cultivation in vitro (because we have yet to develop suitable culture systems). The variability we observed in the clinical presentation among the TDAV-infected horses merits further investigation as it raises the possibility of a potential role for host factors or additional as-yet unidentified infectious agents to influence the clinical course of Theiler's disease. Indeed, although our metatranscriptomic approach was sufficiently sensitive to allow the detection of an equid herpesvirus infection within the serum of one of the sequenced index cases with as few as four read pairs (0.0004% of the total reads sampled), we cannot rule out an additional infectious agent present at low levels that is relevant to Theiler's disease. Likewise, we cannot rule out the presence of an agent so highly diverged from any previously described virus that it would go unrecognized. Thus, the association of TDAV with Theiler's disease, although very strong, remains inferential.
It is also unclear whether TDAV is the sole virus or merely one of several infectious agents that may ultimately be associated with Theiler's disease—as is the case in human hepatitis, which is linked to at least five unrelated viruses (hepatitis viruses A–E). In this regard we note that, consistent with a recent report (21), we have been unable to link NPHV, the only known hepaci-like virus detected in horses, to hepatitis in this outbreak (19). Parallel qRT-PCR assays for NPHV RNA performed in this study (as described in ref. 19) identified the virus in only three horses: two asymptomatic TDAV-positive horses (Farm A) and one TDAV-negative horse with no history of antitoxin treatment (Farm B) (Table S2). Of course, this does not exclude a role for NPHV in hepatic (or extrahepatic) disease in other epidemiological settings.
The closest relatives of TDAV appear to be the GB viruses that make up the newly proposed Pegivirus genus. Until recently, GB viruses were considered a group of highly prevalent unclassified Flaviviridae species that produce persistent, but typically subclinical or asymptomatic infections in their cognate host species. GB viruses A, B, and C were first identified in studies of non-A non-B serum hepatitis in humans and nonhuman primates in the early 1990s, although none have been unequivocally linked to human liver injury in subsequent epidemiological investigations. GBV-D, also thus far unlinked to disease, was recently identified in a serum survey of frugivorous bats native to south-central Asia (17). On the basis of observed similarities in genome organization and sequence, tissue tropism, lack of detectable pathogenicity in humans, and the persistent nature of infection, it has been proposed to classify GBV-A, GBV-C, and GBV-D together in a new Flaviviridae genus termed Pegivirus (persistent GB or G virus), whereas GB virus B has been proposed as a second type member in the Hepacivirus genus, on the basis of its parallel genome organization and sequence similarity, apparent hepatotropism, and the potential for pathogenicity that it shares with the current Hepacivirus genus-type member, HCV (20). At present, on the basis of sequence features, TDAV would likely be considered a member of the proposed Pegivirus genus; however, the potential pathogenicity we observe here would make TDAV the sole member of this proposed genus for which a disease association has been identified.
Membership of a virus in a given genus does not necessarily connote its natural host. We note that although we have discovered TDAV in horses, the natural reservoirs and hosts of this virus are unknown; it could be an exclusively equine virus or an agent introduced into the equine population from another source and amplified there by the practice of transfusion. Further epidemiological work will be required to answer this question.
Divergence from Flaviviridae family members is evident in two main interesting features of the TDAV genome. First, the virus harbors three amino acid insertions in the NS5A protein corresponding to ∼160 aa not found in any of the related hepacivirus or GB virus species. HCV NS5A is a phosphoprotein with no known enzymatic activity that plays an essential, although poorly understood, role in the viral life cycle. NS5A is required for RNA replication, for infectious HCV assembly, and for interactions with a variety of cellular proteins (22–24); the NS5A protein is also the target of the most potent anti-HCV inhibitors discovered to date (25). The largest TDAV NS5A insertion resides in the equivalent of HCV NS5A domain I, a zinc-binding region with RNA-binding activity (26, 27); the relevance of the insertions in the TDAV life cycle is not known. Second, TDAV lacks a microRNA (miR)-122 binding site in the 5′-UTR that has been detected in the hepaciviruses (19, 28, 29). In humans, miR-122 is an abundant liver-specific miRNA, which is essential for HCV replication (28). The role, if any, that miRNAs play in TDAV replication remains to be determined.
Identification of a divergent Flaviviridae species associated with hepatitis invites comparison with HCV, the only member of this viral family thus far confirmed to cause hepatitis in humans. TDAV resembles HCV in several elements of its biology, including the ability to engender both acute and chronic infections that can present with symptomatic or asymptomatic infection (30, 31). TDAV is a blood-borne virus that showed no evidence of horizontal transmission via normal contact between uninfected and infected horses on Farm A over 1 y. This also resembles HCV, which is rarely transmitted by casual, nonparenteral human contact and is inefficiently transmitted by sexual contact; indeed, for HCV as for TDAV, parenteral exposure appears to be the most efficient route of transmission (32, 33). Of course, the lack of detectable horizontal TDAV transmission in this study does not entirely preclude its occurrence or the possibility of vertical transmission. As observed for HCV, TDAV-positive individuals may be asymptomatic for prolonged periods. Furthermore, as in HCV (34, 35), TDAV serum RNA levels and corresponding severity of clinical disease are not strongly correlated in all infected animals. For TDAV, we observed a slightly higher proportion of asymptomatic, TDAV-positive horses than is typically observed for HCV (30%). This may indicate that TDAV viral replication itself is not cytocidal and that damage to hepatocytes, where present, may result from secondary immune or inflammatory mechanisms. Alternatively, additional factors or exposures that vary across hosts may influence the course of disease associated with TDAV infections. Finally, acute HCV infections can clear without any intervention in some individuals, although ∼70% can progress to chronicity (36); in this study, we observed a TDAV chronicity rate of 4 of 16 (25%) animals after 1 y.
We note, however, that aside from the observed association with hepatitis, many of the biological and epidemiological parallels drawn between TDAV and HCV can also be drawn between TDAV and members of the newly proposed Pegivirus genus. Further studies will be required to clarify the biology and epidemiology of TDAV. If TDAV is confirmed as an infectious cause of Theiler's disease, the availability of a qRT-PCR–based TDAV diagnostic assay and the future development of serology-based assays will permit screening of equine biologic products, as well as further investigations of TDAV prevalence, exposure, and biology. Our studies provide further insight into the diverse world of Flaviviridae, open opportunities to investigate a potentially important pathogen of horses, and provide critical information for the control and potential eradication of equine serum hepatitis.
Materials and Methods
Sample Collection.
Serum and plasma samples were collected from horses at three locations. Farm A was the facility on which serum hepatitis cases were observed and the serum or plasma samples were obtained for the clinical biochemical evaluation, monitoring and screening of horses during the course of the outbreak. Farm B was a horse facility geographically distant with no relationship to Farm A and collection of blood from normal horses was authorized by Farm B's Institutional Animal Care and Use Committee (IACUC). Farm D is a United States Department of Agriculture licensed research facility and the experimental study was performed after approval by the Farm D IACUC. All horses followed in this study had blood collected from the jugular vein into sterile vacutainer tubes with no anticoagulant for both molecular testing and biochemical analyses. Samples were shipped overnight to Cornell University for serum separation and biochemical testing (GGT, AST, SDH, GLDH, bilirubin, and bile acids) with a Hitachi Mod P-800 (Roche Diagnostics). A liver enzyme was considered elevated if it exceeded the upper limit of the normal range [GGT, 29 international units (IU)/L; AST, 374 IU/L; SDH, 11 IU/L; and GLDH, 8 IU/L] for the testing laboratory. Serum for molecular viral testing was frozen at −80 °C before testing. An aliquot of the antitoxin 2 plasma that had been administered to 17 horses was also obtained.
RNA Extraction.
Horse sera were stored at −80 °C before RNA extraction, using the TRIzol LS reagent (Life Technologies) according to the manufacturer’s protocol. Briefly, 900 μL of TRIzol LS was added to 300 μL serum and the homogenized sample was incubated for 5 min at room temperature. Chloroform (0.24 mL) was then added and the sample was shaken vigorously before centrifugation at 12,000 × g for 15 min at 4 °C. Fifteen micrograms of GlycoBlue (Life Technologies) and 600 μL of 2-propanol were added to the isolated aqueous phase and the sample was incubated at room temperature for 10 min. Following centrifugation at 12,000 × g for 10 min at 4 °C, the pellet was washed and dissolved in 20 μL H2O. Extracted RNA was subjected to DNase treatment, using Turbo DNA-free (Life Technologies) according to the manufacturer’s protocol with the addition of an RNase inhibitor. Briefly, 2.9 μL Turbo DNase buffer, 5 μL H2O, 0.25 μL RNase Inhibitor (Life Technologies), and 1 μL Turbo DNase were added to 20 μL RNA. Samples were incubated at 37 °C for 20 min; 3 μL DNase inactivation reagent was then added for 5 min at room temperature. Samples were centrifuged at 10,000 × g for 1.5 min at room temperature and the RNA-containing supernatant was saved for further analysis.
Library Preparation and Sequencing.
Library preparation was performed as described previously (37). Briefly, 4 μL of DNase-treated RNA was used for RT with the following conditions: 3Sol_N primer (final concentration, 8 μM) was mixed with RNA and denatured at 65 °C for 5 min before incubation on ice for 5 min. Superscript III buffer (1×), MgCl2 (5 mM), DTT (10 mM), dNTP (500 μM), RNaseOUT (0.4 unit/μL), and SuperScript III (10 units/μL) were added and sample was incubated at 42 °C for 60 min followed by 94 °C for 4 min. For second-strand synthesis, a 5-μL mix of Sequenase buffer (0.33×) and Sequenase 2.0 (0.13 unit/μL) was added. Reaction temperature was ramped from 4 °C to 37 °C over 8 min and then held at 37 °C for 8 min. In an initial PCR step, 5 μL of this cDNA was amplified with Klentaq buffer (1×), dNTP (200 μM), 3_Sol primer (2 μM), and Klentaq LA DNA polymerase (0.1 unit/μL), and PCR was performed under the following cycling conditions: 94 °C for 3 min; 25 cycles of 94 °C for 30 s, 40 °C for 1 min, and 68 °C for 1 min; and then 68 °C for 7 min. A final 4-primer PCR used 10 ng of the initial PCR as a template with Klentaq buffer (1×), dNTP (200 μM), SolM1 primer (10 nM), SolM2_barcode (10 nM), 5SolM1_18* (200 nM), 5SolM2_19* (200 nM), and Klentaq LA DNA polymerase (0.1 unit/μL). PCR was performed under the following cycling conditions: 94 °C for 1 min; two cycles of 94 °C for 30 s, 40 °C for 30 s, and 68 °C for 1 min; 94 °C for 10 min; seven cycles of 94 °C for 30 s, 58 °C for 30 s, and 68 °C for 1 min; and then 72 °C for 5 min. Resulting sequencing libraries were purified using DNA Clean & Concentrator-5 (Zymo Research) according to the manufacturer’s protocol and eluted with 20 μL H2O. Ten microliters of purified libraries were size selected (423 bp ± 7%) using LabChip XT (DNA 750 assay kit; Caliper/Perkin-Elmer) and further purified by a Zymo column.
Individual library concentrations were determined by qPCR, using a PhiX control library (Illumina) with primers 1.1 and 2.1 and Fast SYBR Green Master Mix (Life Technologies), according to manufacturer’s protocol. Libraries were then pooled in equimolar concentrations and requantified before sequencing. Paired-end sequencing (100 cycles for each paired end) on the HiSeq2000 (Illumina) with v3 cluster generation reagents and SBS reagents was performed according to manufacturer’s instructions.
Sequence Analysis.
The initial FASTQ sequence data were binned by sequencing index for each of the three samples. Sequence read pairs for which one of the reads had 10 or more uncalled bases (Ns) were removed. Low-complexity reads were identified by analyzing the size of the compressed Lempel–Ziv–Welch (38) sequence string after removing Ns from the string to avoid artificial increases in complexity. Sequence read pairs for which one of the reads had a compressed size below 37 were removed. The remaining sequencing reads were aligned to the horse genome (EquCab2.0, GCF_000002305.2) (39) by nucleotide BLAST (40) (blastn) with default word size and E value (11 and 10, respectively). Sequence read pairs for which one of the reads had at least 80/100 nt (80% total read identity) mapping identically to the horse genome were considered host derived and removed from downstream analysis. The remaining sequencing reads were aligned by both blastn and translated BLAST (blastx) to all RefSeq (March 2012) viral genomes and protein sequences, respectively, with default word size (11 for blastn, 3 for blastx) and E value (10 for both blastn and blastx). Sequences matching the viral database were isolated and considered candidates. To confirm viral origin in an unbiased context, candidate viral sequences were aligned to the nonredundant nucleotide database (NT; April 2012) by blastn, using the default parameters. Candidate viral reads that did not have an alignment to NT were aligned to the nonredundant protein database (NR; April 2012) by blastx, using the default parameters. Candidate viral reads whose highest-scoring alignments mapped only to viral genomes were deemed viral in origin.
Genome Assembly.
All reads mapping to the Flaviviridae were consolidated into FASTA files. These reads were used as seeds for a targeted metatranscriptomic assembly, using an α-version (v0.16.2) of the open-source PRICE assembler software (16). PRICE uses paired-end information to generate local assemblies that extend existing contigs; in this case, the initial contigs were the reads with blastx matches to the Flaviviridae, and the contigs were extended through as many cycles as possible. In the event that contigs stopped extending before being joined, the full unfiltered set of sequence reads for the sample was mapped to the partial assembly, and reads with a high percentage of identity to the draft assembly were culled and used as seeds for another attempted assembly.
Phylogenetic Analysis.
All RefSeq Flaviviridae polyprotein sequences, in addition to the polyprotein sequences from the recently discovered nonprimate hepaciviruses (NPHVs) (19) (Table S1), were aligned with the TDAV polyprotein sequence using MUSCLE (41). The multiple sequence alignment (available upon request) was imported into MEGA5 (42), and a neighbor-joining phylogenetic tree was constructed with 100 bootstrap replications and viewed as a radiation tree (Fig. 3).
Physical Recovery and Validation of TDAV Sequence.
The TDAV genome assembly was validated by physical recovery and Sanger sequencing of 10 overlapping RT-PCR amplicons that span nucleotide positions 57–10,127 directly from the clinical sample derived from horse A1. Successful recovery of each amplicon required optimization of the RT and PCR conditions (Tables S3 and S4). Amplicons for each primer pair were cloned into the TOPO T/A pCR4.0 vector (Life Technologies) and the forward and reverse strands of at least three independent clones were sequenced (Elim Biopharma).
RACE.
The extreme 5′ sequence of TDAV was recovered by performing 5′ rapid amplification of cDNA ends (Life Technologies) according to the manufacturer’s protocol. Briefly, 2 μL of RNA from horses A1 and A2 and antitoxin 2 was used as input for reverse transcription primed by EVT-145 (Table S3). Purified cDNAs were subjected to a tailing reaction with dCTP before being used as a template for PCR with EVT-147 (Table S3) and the manufacturer-supplied Abridged Anchor Primer. PCR was performed according to the Taq polymerase manufacturer’s protocol (New England BioLabs) with the following cycling conditions: 94 °C for 2 min; 32 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min; and then 72 °C for 7 min. PCR products were purified and concentrated by DNA Clean & Concentrator-5 (Zymo Research) and size selected (292–438 bp) using the LabChip XT (DNA 750 assay kit; Caliper/Perkin-Elmer).
qRT-PCR–Based TDAV Assay.
The TDAV qRT-PCR assay used RNA isolated from 300 μL equine serum or plasma, as described above. Two microliters of RNA was used for random-primed reverse transcription performed according to the manufacturer’s protocol (SuperScript III First-Strand Synthesis System; Life Technologies). Diluted cDNA (1:10) was added to qPCR reactions with Fast SYBR Green Master Mix (Life Technologies) and one of the following primer pairs: EVT-162/-163 (TDAV), EVT-146/147 (TDAV), and EVT-192/-193 (equine 18S rRNA). All samples were blinded and reactions were performed at least in triplicate. TDAV primers were designed to separate known positive and known negative samples by at least 9 Cts (SI Materials and Methods and Table S3). Predefined Ct threshold values for TDAV positivity could not be set because of small day-to-day variability in the assay; thus positivity was defined as (i) detecting equine 18S rRNA with Cts substantially lower than the Cts of the blinded samples predicted to be H2O and (ii) detecting viral RNA using the EVT-146/147 primer set with Cts substantially lower than in blinded samples predicted to be negative controls. In the EVT-162/163 TDAV assay, negative samples did not give a qPCR signal, which allowed simple identification of TDAV-positive samples in any sample that did give a qPCR signal. Serum viral loads were determined using a standard curve of an in vitro transcribed product, which spanned the region of the TDAV genome that is detected by EVT-146/147. Sample Ct values that fell in the same range as those of water samples were considered too low to quantify. Additionally, viral loads were not computed for samples that were confirmed TDAV negative.
Experimental Inoculation Study.
Four healthy adult horses, 2–3 y of age, were inoculated i.v. with 500 mL of antitoxin 2. Immediately before the inoculation and weekly for 10–14 wk, blood was collected and assayed as described above. The four horses were kept in individual stalls and observed closely for any abnormal clinical signs during the study period.
Supplementary Material
Acknowledgments
Karen Warner is acknowledged for creation of the Theiler's disease serum archive. We thank Catherine Jones and Christopher Jones for valuable input during manuscript preparation. Funding for sample collection and biochemistry testing was provided by the Jack Lowe Equine Research Fund at Cornell University. The study also was supported, in part, by the Cornell University Agricultural Experiment Station Federal Formula Funds, Project NYC-480899 received from the National Institutes for Food and Agriculture, US Department of Agriculture.
Footnotes
Conflict of interest statement: A.L.K., S.C., P.S.-C., T.J.D., and B.C.T. are coinventors on a patent application relating to the results herein. A.L.K., S.C., P.S.-C., D.E.G., and W.Z. are employees of the Novartis Institutes for BioMedical Research.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession no. KC145265).
See Author Summary on page 5752 (volume 110, number 15).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219217110/-/DCSupplemental.
References
- 1.Kahn CM, Line S, editors. 2011. Merck Veterinary Manual (Merck, Whitehouse Station, NJ), 9th Ed. Available at www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/22802.htm.
- 2.Theiler A. 1919. Acute liver-atrophy and parenchymatous hepatitis in horses. The Fifth and Sixth Reports of the Director of Veterinary Research, April, 1918. Department of Agriculture, Union of South Africa (The Government Printing and Stationery Office, Pretoria, Union of South Africa), pp. 7–164.
- 3.Panciera RJ. Serum hepatitis in the horse. J Am Vet Med Assoc. 1969;155(2):408–410. [PubMed] [Google Scholar]
- 4.Hjerpe CA. Serum hepatitis in the horse. J Am Vet Med Assoc. 1964;144:734–740. [PubMed] [Google Scholar]
- 5.Aleman M, Nieto JE, Carr EA, Carlson GP. Serum hepatitis associated with commercial plasma transfusion in horses. J Vet Intern Med. 2005;19(1):120–122. doi: 10.1892/0891-6640(2005)19<120:shawcp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Guglick MA, MacAllister CG, Ely RW, Edwards WC. Hepatic disease associated with administration of tetanus antitoxin in eight horses. J Am Vet Med Assoc. 1995;206(11):1737–1740. [PubMed] [Google Scholar]
- 7.Divers TJ. Acute hepatic failure (Theiler’s disease) In: Robinson NE, editor. Current Therapy in Equine Medicine. 2nd Ed. Toronto: WB Saunders; 1987. pp. 110–112. [Google Scholar]
- 8.Thomsett LR. Acute hepatic failure in the horse. Equine Vet J. 1971;3(1):15–19. doi: 10.1111/j.2042-3306.1971.tb04433.x. [DOI] [PubMed] [Google Scholar]
- 9.Cox HR, Philip CB, Marsh H, Kilpatrick JW. Observations incident to an outbreak of equine encephalomyelitis in the Bitterroot valley of western Montana. J Am Vet Med Assoc. 1938;93:225–232. [Google Scholar]
- 10.Herniou EA, Olszewski JA, O’Reilly DR, Cory JS. Ancient coevolution of baculoviruses and their insect hosts. J Virol. 2004;78(7):3244–3251. doi: 10.1128/JVI.78.7.3244-3251.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 12.Solnick D. Construction of an adenovirus-SV40 recombinant producing SV40 T antigen from an adenovirus late promoter. Cell. 1981;24(1):135–143. doi: 10.1016/0092-8674(81)90509-2. [DOI] [PubMed] [Google Scholar]
- 13.Thummel C, Tjian R, Grodzicker T. Expression of SV40 T antigen under control of adenovirus promoters. Cell. 1981;23(3):825–836. doi: 10.1016/0092-8674(81)90447-5. [DOI] [PubMed] [Google Scholar]
- 14.Borchers K, et al. Virological and molecular biological investigations into equine herpes virus type 2 (EHV-2) experimental infections. Virus Res. 1998;55(1):101–106. doi: 10.1016/s0168-1702(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 15.Kemeny L, Pearson JE. Isolation of herpesvirus from equine leukocytes: Comparison with equine rhinopneumonitis virus. Can J Comp Med. 1970;34(1):59–65. [PMC free article] [PubMed] [Google Scholar]
- 16.Earl D, et al. Assemblathon 1: A competitive assessment of de novo short read assembly methods. Genome Res. 2011;21(12):2224–2241. doi: 10.1101/gr.126599.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein JH, et al. Identification of GBV-D, a novel GB-like flavivirus from old world frugivorous bats (Pteropus giganteus) in Bangladesh. PLoS Pathog. 2010;6:e1000972. doi: 10.1371/journal.ppat.1000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cha TA, et al. At least five related, but distinct, hepatitis C viral genotypes exist. Proc Natl Acad Sci USA. 1992;89(15):7144–7148. doi: 10.1073/pnas.89.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burbelo PD, et al. Serology-enabled discovery of genetically diverse hepaciviruses in a new host. J Virol. 2012;86(11):6171–6178. doi: 10.1128/JVI.00250-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stapleton JT, Foung S, Muerhoff AS, Bukh J, Simmonds P. The GB viruses: A review and proposed classification of GBV-A, GBV-C (HGV), and GBV-D in genus Pegivirus within the family Flaviviridae. J Gen Virol. 2011;92(Pt 2):233–246. doi: 10.1099/vir.0.027490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons S, et al. Nonprimate hepaciviruses in domestic horses, United Kingdom. Emerg Infect Dis. 2012;18(12):1976–1982. doi: 10.3201/eid1812.120498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, et al. Hepatitis C virus nonstructural protein 5A (NS5A) is an RNA-binding protein. J Biol Chem. 2005;280(43):36417–36428. doi: 10.1074/jbc.M508175200. [DOI] [PubMed] [Google Scholar]
- 23.Lohmann V, Körner F, Dobierzewska A, Bartenschlager R. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J Virol. 2001;75(3):1437–1449. doi: 10.1128/JVI.75.3.1437-1449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macdonald A, Harris M. Hepatitis C virus NS5A: Tales of a promiscuous protein. J Gen Virol. 2004;85(Pt 9):2485–2502. doi: 10.1099/vir.0.80204-0. [DOI] [PubMed] [Google Scholar]
- 25.Gao M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465(7294):96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tellinghuisen TL, Marcotrigiano J, Rice CM. Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature. 2005;435(7040):374–379. doi: 10.1038/nature03580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tellinghuisen TL, Marcotrigiano J, Gorbalenya AE, Rice CM. The NS5A protein of hepatitis C virus is a zinc metalloprotein. J Biol Chem. 2004;279(47):48576–48587. doi: 10.1074/jbc.M407787200. [DOI] [PubMed] [Google Scholar]
- 28.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309(5740):1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 29.Kapoor A, et al. Characterization of a canine homolog of hepatitis C virus. Proc Natl Acad Sci USA. 2011;108(28):11608–11613. doi: 10.1073/pnas.1101794108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esteban JI, et al. Evaluation of antibodies to hepatitis C virus in a study of transfusion-associated hepatitis. N Engl J Med. 1990;323(16):1107–1112. doi: 10.1056/NEJM199010183231605. [DOI] [PubMed] [Google Scholar]
- 31.Uto H, Mawatari S, Kumagai K, Ido A, Tsubouchi H. Clinical features of hepatitis C virus carriers with persistently normal alanine aminotransferase levels. Hepat Mon. 2012;12(2):77–84. doi: 10.5812/hepatmon.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong GL, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 33.Alter MJ. HCV routes of transmission: What goes around comes around. Semin Liver Dis. 2011;31(4):340–346. doi: 10.1055/s-0031-1297923. [DOI] [PubMed] [Google Scholar]
- 34.Anand B-S, Velez M. Assessment of correlation between serum titers of hepatitis C virus and severity of liver disease. World J Gastroenterol. 2004;10(16):2409–2411. doi: 10.3748/wjg.v10.i16.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puoti C, et al. Clinical, histological, and virological features of hepatitis C virus carriers with persistently normal or abnormal alanine transaminase levels. Hepatology. 1997;26(6):1393–1398. doi: 10.1053/jhep.1997.v26.pm0009397976. [DOI] [PubMed] [Google Scholar]
- 36.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 37.Runckel C, et al. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS ONE. 2011;6(6):e20656. doi: 10.1371/journal.pone.0020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch TA. A technique for high-performance data compression. Computer. 1984;17:8–19. [Google Scholar]
- 39.Wade CM, et al. Broad Institute Genome Sequencing Platform Broad Institute Whole Genome Assembly Team Genome sequence, comparative analysis, and population genetics of the domestic horse. Science. 2009;326(5954):865–867. doi: 10.1126/science.1178158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 41.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamura K, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]