Abstract
The initiation of primary cilium assembly entails the docking of ciliary vesicles presumably derived from the Golgi complex to the distal end of the mother centriole. Distal appendages, which anchor the mother centriole to the plasma membrane, are thought to be involved in the docking process. However, little is known about the molecular players and mechanisms that mediate the vesicle–centriole association. Here we report that coiled-coil domain containing 41 (CCDC41) is required for the docking of ciliary vesicles. CCDC41 specifically localizes to the distal end of the mother centriole and interacts with centrosomal protein 164 (Cep164), a distal appendage component. In addition, a pool of CCDC41 colocalizes with intraflagellar transport protein 20 (IFT20) subunit of the intraflagellar transport particle at the Golgi complex. Remarkably, knockdown of CCDC41 inhibits the recruitment of IFT20 to the centrosome. Moreover, depletion of CCDC41 or IFT20 inhibits ciliogenesis at the ciliary vesicle docking step, whereas intraflagellar transport protein 88 (IFT88) depletion interferes with later cilium elongation steps. Our results suggest that CCDC41 collaborates with IFT20 to support the vesicle–centriole association at the onset of ciliogenesis.
Keywords: ciliopathy, axoneme
Primary cilia function as sensory organelles, detecting chemical and mechanical stimuli from the environment. Moreover, recent studies have demonstrated that primary cilia can modulate the activity of several signaling pathways that play key roles in embryonic development and tumorigenesis (1–3). Accordingly, defects in the formation or function of primary cilia are associated with a wide range of pathological phenotypes, including brain malformation, polydactyly, retinal degeneration, and kidney cysts (4, 5).
The inner core of primary cilia contains an elaborate microtubule-based structure called the axoneme. The assembly of the axoneme is nucleated by the basal body, which is derived from the mother centriole of the centrosome (6, 7). The distal end of the centriole has a cap structure composed of centriolar coiled-coil protein 110 (CP110) and centrosomal protein 97 (Cep97) (8, 9). The cap structure plays a role in suppressing axoneme assembly and aberrant centriole elongation. When cells exit the cell cycle, the cap is removed specifically from the mother centriole, and the transition from centriole to basal body occurs. Both down-regulation of kinesin family member 24 (Kif24) expression and recruitment of tau tubulin kinase 2 (TTBK2) to the basal body appear to be required for the cap removal (10, 11). Subsequent cilium elongation requires intraflagellar transport (IFT), bidirectional motility along axonemal microtubules, to transport building materials from the cell body to the growing cilium (12, 13).
The axoneme is surrounded by a specialized ciliary membrane, which exhibits characteristic protein and lipid compositions distinct from those of the plasma membrane. Membrane trafficking regulators such as ras-related GTP-binding protein 8 and 11 (Rab8 and Rab11) collaborate with the Bardet–Biedl syndrome proteins to control ciliary membrane biogenesis as well as membrane protein targeting to the cilium (14–16). Axoneme assembly is blocked in cells with defects in membrane trafficking machinery, indicating that ciliary membrane biogenesis and axoneme assembly are intimately linked. Interestingly, detailed EM studies have suggested that the docking of a Golgi-derived vesicle, termed the primary ciliary vesicle, to the distal end of the mother centriole establishes membrane–centriole association at the onset of ciliogenesis (17, 18). The assembly of the axoneme, which could be triggered by this docking event, invaginates the primary ciliary vesicle. The deformed ciliary vesicle appears to grow by fusion with “secondary ciliary vesicles” and eventually fuses with the plasma membrane. Although trafficking of Rabin8 to the centrosome is known to be involved in the process (16), the molecules and mechanisms that coordinate the formation and docking of ciliary vesicles remain unclear (19, 20).
Coiled-coil domain containing 41 (CCDC41) is an uncharacterized protein composed of multiple coiled-coil domains. In this study, we identify CCDC41 as a component of the mother centriole required for ciliogenesis. Immunofluorescence staining showed that CCDC41 localizes to the mother centriole and partially overlaps with distal appendage components. RNAi-mediated knockdown of CCDC41 inhibited ciliogenesis in human retinal pigment epithelial (RPE1) cells. Moreover, depletion of CCDC41 in zebrafish displayed embryonic ciliogenesis defects. We further found that a pool of CCDC41 is anchored to the Golgi complex by intraflagellar transport protein 20 (IFT20), which functions in the transport of proteins destined for the ciliary membrane (21, 22). Remarkably, CCDC41 depletion inhibited IFT20 recruitment to the basal body area, and interferes with the formation/docking of ciliary vesicles. Our data suggest a model in which CCDC41 collaborates with IFT20 to mediate ciliary vesicle docking at the onset of ciliogenesis.
Results
CCDC41 Localizes to the Distal End of the Mother Centriole.
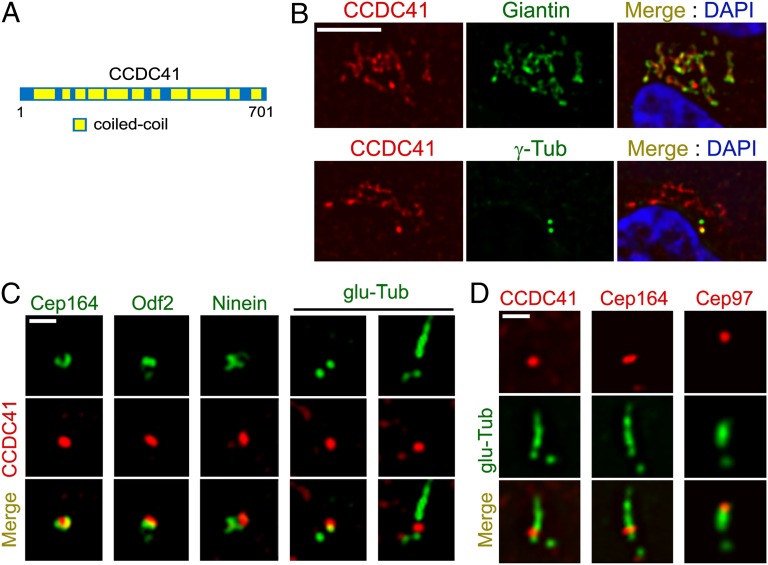
Previously, we reported the result of an RNAi library screen to identify molecules involved in the formation of primary cilia (23). To supplement the data, we recently performed a similar siRNA library screen and identified several ciliary proteins, including CCDC41. CCDC41 is a protein of 701 aa with a predicted molecular weight of 83 kDa. NCOIL secondary structure analysis program (http://embnet.vital-it.ch/software/COILS_form.html) predicts that coiled-coil domains occupy most of the CCDC41 sequence (Fig. 1A). No other obvious functional domains could be identified. Orthologues of CCDC41 have been identified in other vertebrates, including mouse, chicken, Xenopus, and zebrafish.
Fig. 1.
Subcellular localization of CCDC41. (A) Schematic representation of CCDC41 and its domain structure. (B) Double-immunofluorescent localization of CCDC41 in RPE1 cells. (C) RPE1 cells were double-labeled with the indicated antibody pairs. Ciliogenesis was induced by 24 h serum starvation (Right). (D) U2OS cells were transfected with CP110 siRNAs for 3 d to induce abnormal centriole elongation and double-labeled with the indicated antibody pairs. (Scale bars: B, 5 μm; C and D, 1 μm.)
To characterize the localization of CCDC41, we used polyclonal antibodies raised against 100 aa close to the C terminus of CCDC41. As shown in Fig. 1B, CCDC41 immunofluorescence in RPE1 cells was detected at sites partially overlapping Giantin, a Golgi complex marker. In addition, higher levels of CCDC41 immunoreactivity were detected at one of the two centrioles, suggesting that CCDC41 is anchored asymmetrically to the mother or daughter centriole (Fig. 1B). RNAi-mediated knockdown of CCDC41 decreased the levels of CCDC41 immunofluorescence, supporting the specificity of CCDC41 immunostaining results (Fig. S1). Asymmetric localization of CCDC41 at the centrosome was also observed in U2OS (human osteosarcoma), NIH3T3 (mouse embryonic fibroblast), and IMCD3 (mouse inner medullary collecting duct) cells (Fig. S2A). Moreover, exogenously expressed enhanced green fluorescent protein (EGFP)-tagged CCDC41 (GFP-CCDC41) was targeted to one of the two centrioles (Fig. S2B).
Importantly, CCDC41 signal partially overlapped with centrosomal protein 164 (Cep164) (24), a components of centriole distal appendages (Fig. 1C). We also observed a partial overlap between CCDC41 and outer dense fiber of sperm tails 2 (Odf2), which localizes to both distal and subdistal appendages (25). However, CCDC41 did not overlap with a subdistal appendage marker ninein (26), implying that CCDC41 is associated with distal appendages (Fig. 1C). In ciliated RPE1 cells, CCDC41 localizes to the distal portion of the basal body near the transition zone area, but CCDC41 signal was not detected along the ciliary axoneme (Fig. 1C). Because CCDC41 staining persisted in ciliated cells, it is unlikely that CCDC41 is anchored to the distal cap structure that disappears during ciliogenesis. Previously, it was shown that CP110 knockdown causes an abnormal elongation of centriolar microtubules in U2OS cells (9). As shown in Fig. 1D, CCDC41 and Cep164 remained at the position of distal appendages on abnormally elongated centrioles in U2OS cells after CP110 knockdown, whereas a cap component Cep97 moved to the distal end of elongated centrioles.
CCDC41 localizes to centrioles at all stages of the cell cycle, including mitosis (Fig. S2C). Increasing levels of CCDC41 immunofluorescence staining was detected in a second parental centriole at the onset of centrosome separation (Fig. S2C), indicating that CCDC41 is recruited to centrioles at early stages of the centriole maturation. Centriolar CCDC41 localization was unaffected by nocodazole treatment, which disrupts cytoskeletal microtubules, whereas Golgi-associated CCDC41 staining was largely undetectable (Fig. S2D). Brefeldin A, which disrupts the Golgi complex, also selectively affected Golgi-associated CCDC41 immunoreactivity (Fig. S2D). Collectively, these observations suggest that CCDC41 is a stable, intrinsic component of the mother centriole.
CCDC41 Is Required for Ciliogenesis.
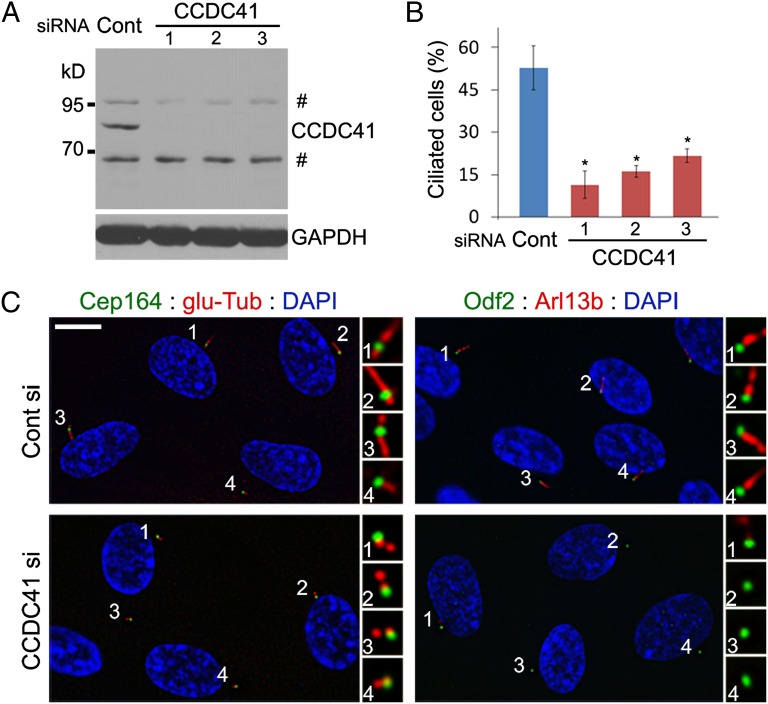
Next, we investigated the functional consequences of CCDC41 knockdown. CCDC41 antibody recognized an expected band of ∼83 kDa as well as two extra bands on Western blots performed with lysates from RPE1 cells (Fig. 2A). We transfected three distinct siRNA oligonucleotides targeting CCDC41 and found that CCDC41 levels were substantially reduced 48 h after transfection (Fig. 2A). Cilia were visualized by antibodies detecting polyglutamylated tubulin or ADP-ribosylation factor-like 13b (Arl13b) (27), and the two antibodies produced similar results. As shown in Fig. 2B, depletion of CCDC41 led to a significant decrease in the percentage of cells displaying the primary cilium in serum-starved RPE1 cells. In contrast, CCDC41 knockdown did not cause a noticeable change in cell cycle profile, as determined by flow cytometry (Fig. S3A). In addition, cytoskeletal microtubule organization and spindle bipolarity appear normal in CCDC41-depleted cells (Fig. S3 B and C). To determine whether mother centrioles without CCDC41 are competent to elongate in response to CP110 depletion, we cotransfected U2OS cells with CCDC41 and CP110 siRNAs. Efficient CCDC41 depletion in U2OS cells was confirmed by Western blot (Fig. S4A). As shown in Fig. S4B, CP110-depleted cells showed elongated centrioles regardless of the presence or absence of CCDC41, suggesting that centrioles are competent for elongation without CCDC41 function. Furthermore, CCDC41 depletion had no effect on extracentriole duplication in U2OS cells treated with hydroxyurea (28) (Fig. S4C). These results suggest that CCDC41 is required for ciliogenesis.
Fig. 2.
CCDC41 depletion prevents primary cilia formation. (A) Western blot illustrating efficient depletions of CCDC41 in RPE1 cells by each of three distinct siRNAs. Number sign indicates nonspecific bands. (B) RPE1 cells were shifted from 10% serum to serum-free media 48 h after siRNA transfection and cultured for an additional 24 h. Ciliated cells were identified by double labeling with anti–glu-Tub and anti–γ-Tub antibodies. Error bars represent SD (n = 3 independent experiments; *P < 0.01, t test). (C) CCDC41 knockdown efficiently inhibits ciliogenesis in most transfected cells without causing apparent changes in the appendage components Cep164 and Odf2. (Scale bar: 10 μm.)
Most mother centrioles in CCDC41-depleted cells exhibit normal levels of Cep164 and Odf2 (Fig. 2C), excluding the possibility that loss of distal appendages might underlie the ciliogenesis defect. However, we observed a small increase in the number of cells that did not show detectable levels of centriolar Cep164 in response to CCDC41 knockdown (Fig. S5 A and B). In contrast, Cep164 depletion exerted no detectable effect on CCDC41 localization (Fig. S5C). To test potential interactions between CCDC41 and Cep164, we cotransfected RPE1 cells with GFP-CCDC41 and Myc epitope-tagged Cep164 (Cep164-myc) constructs. Noticeably, GFP-CCDC41 recruited Cep164-myc to ectopic cytosolic sites (Fig. S5D). In contrast, Cep164-myc proteins did not form extracentrosomal aggregates in the absence of exogenous GFP-CCDC41 (Fig. S5D). Moreover, we found that cytosolic aggregates of GFP-CCDC41 efficiently recruit endogenous Arl13b, which is thought to form a functional network with Cep164 (29) (Fig. S5E). However, GFP-CCDC41 did not affect γ-tubulin localization (Fig S5F). These results suggest that CCDC41 plays a certain role in Cep164 recruitment to the distal appendages.
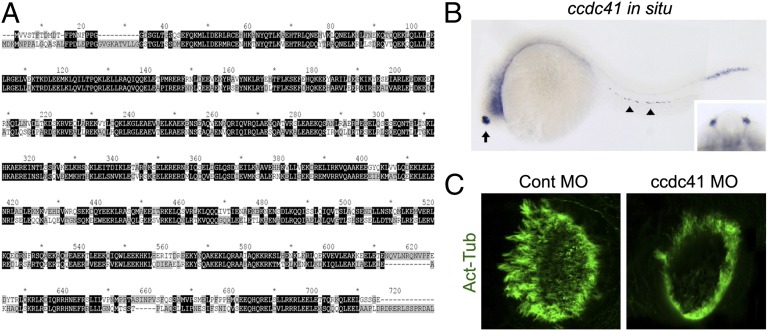
As a further test of the involvement of CCDC41 in ciliogenesis, we examined roles of CCDC41 in zebrafish, which have become a standard model system to test basic ciliary function in vivo (30, 31). Human and zebrafish CCDC41 amino acid sequences share 76% similarity (Fig. 3A). Expression of ccdc41 mRNA in developing zebrafish embryos was analyzed by whole-mount in situ hybridization. The olfactory placodes exhibited the strongest ccdc41 signals, and the expression was also detected in the pronephric duct and tail myotomes (Fig. 3B). In accord with the expression patterns, morpholino-mediated knockdown of ccdc41 resulted in a reduction of olfactory cilia formation, demonstrating that ccdc41 has a role in ciliogenesis in zebrafish (Fig. 3C). No defect in left/right body asymmetry was observed (Fig. S6A). Knockdown efficiency was verified by coinjection of GFP-ccdc41 vector and ccdc41 morpholino (Fig. S6B).
Fig. 3.
ccdc41 is expressed in ciliated tissues and its depletion induces olfactory ciliogenesis defects in zebrafish. (A) Alignment of human and zebrafish CCDC41 protein sequences by using the ClustalX program. (B) ccdc41 whole-mount in situ hybridization shows that ccdc41 mRNA is expressed in olfactory placodes (arrow), pronephric duct (arrowhead), and tail myotomes at 24 h postfertilization. (Inset) Frontal view of the head shows ccdc41 expression in bilateral olfactory placodes. (C) Anti–acetylated-tubulin (Act-Tub) antibody staining of the zebrafish nasal pit at 5 d postfertilization. MO, morpholino.
CCDC41 Interacts with IFT20.
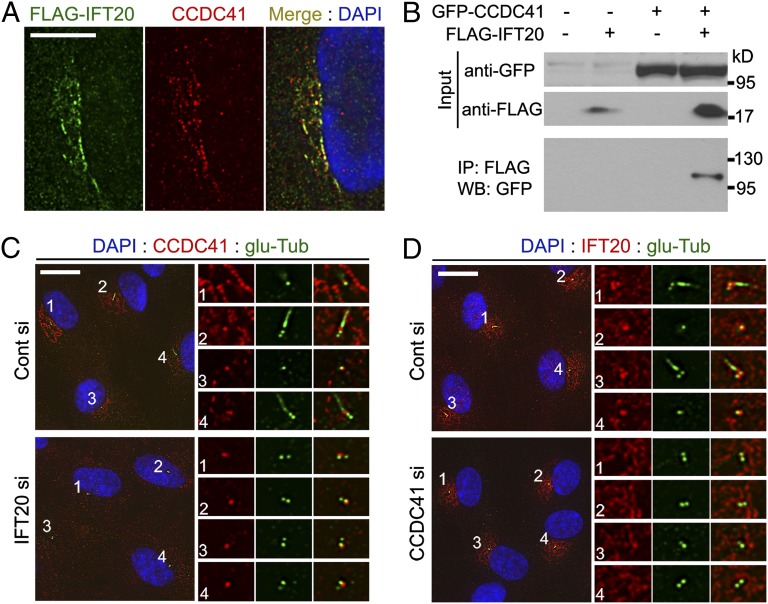
Unlike other IFT proteins, IFT20 localizes to the Golgi complex in addition to the centrosome and cilia (21, 22). Detection of CCDC41 at the Golgi area (Fig. 1B) prompted us to examine a physical association between IFT20 and CCDC41. As shown in Fig. 4A, immunofluorescence staining revealed a partial overlap between exogenously expressed FLAG-tagged IFT20 (FLAG-IFT20) and endogenous CCDC41. Golgi localization of FLAG-IFT20 was confirmed by double labeling with anti-Giantin antibody (Fig. S7A). To further verify the interaction, we performed an immunoprecipitation experiment. HEK293 cells were cotransfected with FLAG-IFT20 and GFP-CCDC41, and cell extracts were precipitated with agarose beads conjugated with Flag-antibodies. As shown in Fig. 4B, GFP-CCDC41 was detected from the immunoprecipitates, indicating that CCDC41 may physically interact with IFT20.
Fig. 4.
CCDC41 and IFT20 interact with each other for proper localization. (A) RPE cells were transfected with FLAG-IFT20 plasmid and doubled-labeled with anti-FLAG and anti-CCDC41 antibodies. (B) HEK293T cells were transfected with the indicated plasmid vectors for 16 h. Cell lysates were immunoprecipitated with anti-FLAG antibody conjugated with agarose beads, and the resulting precipitates were immunoblotted with anti-GFP antibody. (C) RPE1 cells were transfected with siRNAs for 3 d (cells were serum-starved for the final 24 h), and double-stained with anti-CCDC41 and anti–glu-Tub antibodies. (D) RPE1 cells were transfected with siRNAs for 3 d (cells were serum-starved for the final 24 h), and double-stained with anti-IFT20 and anti–glu-Tub antibodies. (Scale bars: A, 10 μm; C and D, 20 μm.)
Next, we tested whether these two proteins depend on each other for cellular localization. As previously reported (21), siRNA-mediated depletion of IFT20 did not affect the integrity of the Golgi complex (Fig. S7 B and C). However, cells depleted of IFT20 exhibited a decrease in the levels of CCDC41 immunoreactivity at the Golgi complex (Fig. 4C). This indicates that IFT20 may be required for Golgi localization of CCDC41. In contrast, IFT20 depletion did not perturb centriolar localization of CCDC41, suggesting that CCDC41 is recruited to centrioles independently of IFT20. Interestingly, the levels of centrosomal IFT20 staining were decreased in cells transfected with CCDC41 siRNAs, whereas the Golgi-associated pool of IFT20 appeared to be unaffected (Fig. 4D). These results suggest that IFT20 plays a role in CCDC41 recruitment to the Golgi complex and CCDC41 is involved in IFT20 recruitment to the centrosome. As shown in Fig S7D, CCDC41 appears to be dispensable for centrosomal recruitment of intraflagellar transport protein 88 (IFT88).
CCDC41 May Mediate Ciliary Vesicle Docking to the Mother Centriole.
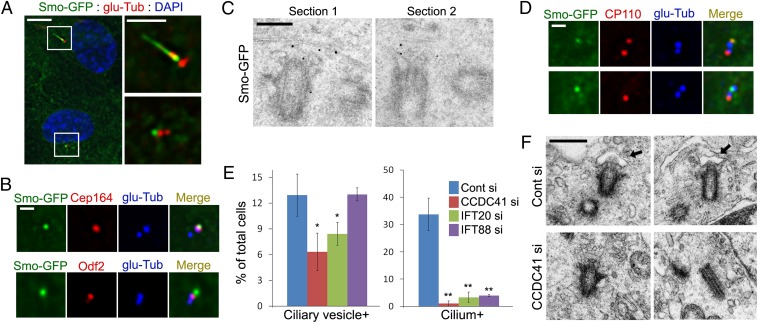
Certain components of distal appendages that have potential to interact with membranes may mediate the docking of the primary ciliary vesicle to the mother centriole at the onset of ciliogenesis. To test whether CCDC41 is involved in the docking step, we searched for a method to visualize the primary ciliary vesicle. We examined whether EGFP-tagged smoothened (Smo-GFP), which is specifically targeted to ciliary membranes (23), can also label ciliary vesicles. As expected, Smo-GFP was detected in the ciliary membrane (Fig. 5A). In addition, a subset of cells exhibited a solitary Smo-GFP dot adjacent to a centriole (Fig. 5A). Costaining with appendage markers revealed that Smo-GFP dots were indeed anchored to distal appendages of the mother centriole (Fig. 5B and Fig. S8A). Immuno-EM analysis further confirmed that Smo-GFP is targeted to the distal end of the mother centriole before the onset of axoneme assembly (Fig. 5C). Consistent with the fact that U2OS cells do not develop cilia (9), Smo-GFP dots specifically anchored to the mother centriole were not observed in U2OS cells stably expressing Smo-GFP (Fig. S8B). Elongated centrioles in CP110-depleted U2OS-SmoGFP cells also did not exhibit specific Smo-GFP dots. Together, these results suggest that centriole-associated Smo-GFP dots in RPE1 cells represent the primary ciliary vesicle. Optical sectioning of live cells revealed that Smo-GFP dots locate below the plasma membrane (Fig. S8C), confirming the internal development of rudimentary cilia.
Fig. 5.
CCDC41 may be involved in the docking of the primary ciliary vesicle. (A) RPE1 cells stably expressing Smo-GFP (RPE1-SmoGFP) were serum-starved for 16 h and stained with anti–glu-Tub antibody. (Right) Higher-magnification view of the boxed areas at Left. (B) RPE1-SmoGFP cells were double-stained with the indicated antibodies. (C) PRE1-SmoGFP cells were serum-starved for 16 h, and processed for immuno-EM with anti-EGFP antibody. (D) RPE1-SmoGFP cells were serum starved (Upper, 8 h; Lower, 16 h), and double stained with anti-CP110 and anti–glu-Tub antibodies. (E) RPE1-SmoGFP cells were transfected with the indicated siRNAs for 3 d (cells were serum-starved for the final 16 h) and stained with anti–glu-Tub antibody. Error bars represent SD (n = 3 independent experiments; *P < 0.05 and **P < 0.01, t test). (F) RPE1 cells were transfected with siRNAs for 3 d (cells were serum-starved for the final 16 h) and processed for transmission EM analysis. Arrows indicate vesicles anchored to the distal end of the centriole. (Scale bars: A, Left, 10 μm; A, Right, 5 μm; B and D, 1 μm; C, 200 nm; F, 500 nm.)
To further characterize the presumptive primary ciliary vesicle, we examined Arl13b, which is thought to regulate the distribution of Smo within cilia (32). As shown in Fig. S8D, Arl13b is detected on the presumptive primary ciliary vesicles. This observation further suggests that Smo-GFP dots are not random Smo-GFP aggregates but vesicles specifically targeted to the basal body. Removal of the CP110 cap structure from the mother centriole is thought to be a prerequisite for the assembly of the axoneme (8, 10, 11). We found that only approximately 6% of centriole-anchored Smo-GFP dots (five of 78) exhibited CP110 immunoreactivity (Fig. 5D). Thus, it is likely that the docking of the primary ciliary vesicle is coincident with the cap removal. After 8 h of serum starvation, approximately 10% of cells showed the presumptive primary ciliary vesicle (Fig. S8E). The number of primary ciliary vesicle-positive cells increased slightly after 16 h of serum starvation and then decreased. In contrast, the number of ciliated cells steadily increased during serum starvation.
Next, we tested whether CCDC41 is required for the formation or anchoring of the primary ciliary vesicle. As shown in Fig. 5E, depletion of CCDC41 significantly decreased the number of cells exhibiting the presumptive primary ciliary vesicle. To confirm this result, we performed a transmission EM analysis. As expected, we observed cells containing the mother centriole, which is associated with vesicular structures from control siRNA transfected cultures (Fig. 5F). Although not quantitative, centriole-associated vesicles were not observed in cells transfected with CCDC41 siRNA (Fig. 5F). Moreover, we found that CCDC41 depletion inhibits the accumulation of a transition zone marker tectonic family member 1 (TCTN1) (19) at the distal end of the mother centriole upon serum starvation (Fig. S9). This result further demonstrates that, in the absence of CCDC41, the mother centriole fails to localize with the membrane. Consistent with its interaction with CCDC41, depletion of IFT20 also resulted in a similar decrease in number of cells containing ciliary vesicles (Fig. 5E). In contrast, knockdown of IFT88 did not change the number of ciliary vesicle-positive cells even though most cells failed to develop cilia (Fig. 5E). These results suggest that CCDC41 and IFT20 play a specific role in the establishment of centriole–membrane association. The fact that the decrease in ciliated cell numbers after IFT88 knockdown was not accompanied by a reciprocal increase of ciliary vesicle-positive cells implies that centriole–ciliary vesicle connection is unstable unless IFT-mediated cilium assembly is activated.
Discussion
Ciliogenesis involves the establishment of microtubule-membrane association, which apparently precedes the onset of axoneme assembly (19, 20). In RPE1 cells, fibroblasts and smooth muscle cells, initial microtubule–membrane association is achieved by the docking of the primary ciliary vesicle to the distal end of the mother centriole (17, 18). CCDC41 is in a unique position to coordinate the docking process. First, CCDC41 is stably incorporated into the distal end of the mother centriole and interacts with a distal appendage component Cep164. In addition, a pool of CCDC41 localizes to the Golgi complex and physically interacts with IFT20, which is thought to function in the sorting or transport of proteins destined for the ciliary membrane (21, 22). Moreover, CCDC41 can interact with Arl13b (Fig. S5E), which functions at the primary ciliary vesicle (Fig. S8D) as well as at ciliary membranes (33). We further demonstrate that CCDC41 knockdown interferes with the formation or docking of the primary ciliary vesicle, effectively blocking ciliogenesis. Therefore, we suggest a model in which CCDC41 collaborates with IFT20 for the formation and transport of the primary ciliary vesicle from the Golgi complex to the centrosome. A pool of CCDC41 is then stably incorporated into distal appendages, mediating the docking of the ciliary vesicle.
Mother centrioles are characterized by associated appendage structures. Although the formation of appendages occur independently of ciliogenesis, appendages are absolutely required for the generation of primary cilia in animal cells. Distal appendages appear to be converted into transition fibers, which anchor the basal body to the membrane (19, 20). In addition, distal appendages/transition fibers are thought to be required for the recruitment of IFT proteins to basal bodies (34). The exact roles of CCDC41 in the formation of distal appendages remain to be elucidated, but at least CCDC41 can interact with a distal appendage component Cep164 (Fig. S5 A, B, and D). However, CCDC41 knockdown causes a ciliogenesis defect even in the majority of cells exhibiting normal levels of distal appendage component Cep164 and Odf2 (Fig. 2C). Therefore, it is likely that CCDC41 may play a specific role in ciliogenesis other than supporting distal appendage formation.
Considering its Golgi localization and roles in protein targeting to the ciliary membrane, IFT20 is a good candidate for mediating the formation and transport of ciliary vesicles. Consistent with this idea, depletion of IFT20 significantly reduced the number of cells exhibiting the presumptive primary ciliary vesicle anchored to the centriole (Fig. 5E). Remarkably, CCDC41 knockdown resulted in a decrease in the number of cells exhibiting high levels of IFT20 immunoreactivity at the centrosome/basal body (Fig. 4D). Thus, it is possible that the major role of CCDC41 in ciliogenesis is to recruit IFT20 to the basal body. Alternatively, CCDC41 may directly contribute to the connection between the primary ciliary vesicle and distal appendages through homophilic or heterophilic binding activities of its coiled-coil domains. Because CCDC41 and IFT20 depend on each other for correct localization, we were not able to test whether CCDC41 plays a role independent of IFT20 in the formation or docking of ciliary vesicles. Previously it was shown that hook homolog 2 (Hook2) accumulates at the centrosome and plays a role in the formation of pericentriolar vesicles (35). However, because it accumulates broadly at the pericentriolar area, Hook2 is unlikely to be sufficient for directing the final docking step of the vesicle to distal appendages. Future studies are needed to elucidate the molecular mechanisms by which CCDC41/IFT20 function is coordinated with Rab proteins and Hook2.
In conclusion, this study identifies CCDC41 as a mother centriole component that is involved in earliest steps of ciliogenesis. Our results provide a clue to understand the formation and docking of the primary ciliary vesicle. While this paper was under review, Schmidt et al. (36) reported that Cep164 is required for vesicular docking to the mother centriole and Tanos et al. (37) reported that CCDC41 promotes centriole-to-membrane docking, consistent with our data described here.
Materials and Methods
Cell culture and Transfection.
RPE1 cells stably expressing Smo-GFP were established as previously reported (23). Cells were transfected with 5 to 10 nM siRNAs by using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s reverse transfection protocol. The siRNA sequences used in this study are shown in SI Materials and Methods. Nontarget siRNAs were used for control transfection (Dharmacon and Bioneer). Human CCDC41 cDNA was amplified by RT-PCR and cloned into pEGFP-C2 vector (Clontech). Cells were transfected with plasmid DNAs by using Lipofectamine LTX (Invitrogen) according to the manufacturer’s forward transfection protocol.
Immunofluorescence.
For indirect immunofluorescence, cells were fixed in methanol for 4 min at −20 °C. Primary antibodies were applied for 1 h at room temperature. Antibodies used are shown in SI Materials and Methods. Alexa 488-, Alexa 594-, or Alexa 647-conjugated secondary antibodies (Molecular Probes) were applied for 1 h at room temperature. Cell surface labeling with Alexa 594-conjugated wheat germ agglutinin (Invitrogen) was performed according to the manufacturer’s instructions.
Immunoprecipitation.
Cells were extracted with Nonidet P-40 lysis buffer 16 h after plasmid DNA transfection, and cell lysates were incubated with anti-FLAG M2 affinity gel (Sigma) for 4 h at 4 °C. Immunoprecipitates were boiled with SDS sample buffer, and the same volumes of samples were loaded for Western blot analysis.
Zebrafish Experiments.
For whole-mount in situ hybridization, the zebrafish ccdc41 gene was cloned by RT-PCR and used for antisense RNA probe synthesis. For antisense knockdown experiment, translation-blocking morpholino oligonucleotide ccdc41 translation start site morpholino (5′-GGGTTCATCTTATCCATAGCAGAAA-3′) was synthesized (Gene Tools) and microinjected into zebrafish embryos at one- to four-cell stages.
Transmission EM.
RPE1 cells were grown on gridded coverslips (MatTek) and serum-starved for 16 h. Cells were fixed with 2.5% (wt/vol) glutaraldehyde for 20 min, postfixed in 1% (wt/vol) OsO4 for 1 h, dehydrated in a graded series of ethanol, and then embedded in the resin. Serial sections were examined on a transmission electron microscope (Tecnai G2 Spirit Twin; FEI). For the preembedding immuno-EM, RPE1-SmoGFP cells were serum starved for 16 h, fixed with 4% (wt/vol) paraformaldehyde/0.05% glutaraldehyde for 15 min, rinsed with 50 mM glycine, and permeabilized with 0.1% Triton X-100 for 1 min. Cells were incubated with rabbit anti-EGFP antibody (1:2,000; Abcam) for 1 h, and bound primary antibody was visualized by goat anti-rabbit IgG Nanogold (1:50; Nanoprobes).
Supplementary Material
Acknowledgments
We thank Dr. Erich Nigg (University of Basel) for Cep164-myc plasmid and Cep164 antibody and Dr. Gregory Pazour (University of Massachusetts Medical School) for FLAG-IFT20 plasmid. This study was supported by Outstanding Young Investigator Grant 20120004114 from the National Research Foundation of Korea; Korean Health Technology R&D Project A120017 from the Ministry of Health and Welfare of Korea; and National Cancer Center of Korea Research Grant NCC1210360-1 (to C.-H.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220927110/-/DCSupplemental.
References
- 1.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137(1):32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetz SC, Anderson KV. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20(1):58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster MA, Gleeson JG. The primary cilium as a cellular signaling center: lessons from disease. Curr Opin Genet Dev. 2009;19(3):220–229. doi: 10.1016/j.gde.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettencourt-Dias M, Hildebrandt F, Pellman D, Woods G, Godinho SA. Centrosomes and cilia in human disease. Trends Genet. 2011;27(8):307–315. doi: 10.1016/j.tig.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Dynlacht BD. Regulating the transition from centriole to basal body. J Cell Biol. 2011;193(3):435–444. doi: 10.1083/jcb.201101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigg EA, Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol. 2011;13(10):1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and CP110 suppress a cilia assembly program. Cell. 2007;130(4):678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt TI, et al. Control of centriole length by CPAP and CP110. Curr Biol. 2009;19:1005–1011. doi: 10.1016/j.cub.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht BD. Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell. 2011;145(6):914–925. doi: 10.1016/j.cell.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Goetz SC, Liem KF, Jr, Anderson KV. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell. 2012;151(4):847–858. doi: 10.1016/j.cell.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa H, Marshall WF. Ciliogenesis: Building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12(4):222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3(11):813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 14.Knödler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA. 2010;107(14):6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: How to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westlake CJ, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA. 2011;108(7):2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molla-Herman A, et al. The ciliary pocket: An endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123(pt 10):1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- 19.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13(7):608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: Mechanisms of ciliogenesis and ciliary access. J Cell Biol. 2012;197(6):697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17(9):3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keady BT, Le YZ, Pazour GJ. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol Biol Cell. 2011;22(7):921–930. doi: 10.1091/mbc.E10-09-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, et al. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464(7291):1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graser S, et al. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179(2):321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa H, Kubo A, Tsukita S, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7(5):517–524. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- 26.Brito DA, Gouveia SM, Bettencourt-Dias M. Deconstructing the centriole: Structure and number control. Curr Opin Cell Biol. 2012;24(1):4–13. doi: 10.1016/j.ceb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12(5):767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Lange BM. Integration of the centrosome in cell cycle control, stress response and signal transduction pathways. Curr Opin Cell Biol. 2002;14(1):35–43. doi: 10.1016/s0955-0674(01)00291-5. [DOI] [PubMed] [Google Scholar]
- 29.Humbert MC, et al. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci USA. 2012;109(48):19691–19696. doi: 10.1073/pnas.1210916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wessely O, Obara T. Fish and frogs: Models for vertebrate cilia signaling. Front Biosci. 2008;13:1866–1880. doi: 10.2741/2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer-Zucker AG, et al. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development. 2005;132(8):1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 32.Larkins CE, Aviles GD, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 2011;22(23):4694–4703. doi: 10.1091/mbc.E10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cevik S, et al. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J Cell Biol. 2010;188(6):953–969. doi: 10.1083/jcb.200908133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11(20):1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 35.Baron Gaillard CL, et al. Hook2 is involved in the morphogenesis of the primary cilium. Mol Biol Cell. 2011;22(23):4549–4562. doi: 10.1091/mbc.E11-05-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt KN, et al. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol. 2012;199(7):1083–1101. doi: 10.1083/jcb.201202126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanos BE, et al. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013;27(2):163–168. doi: 10.1101/gad.207043.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.