Abstract
Development of an antimalarial subunit vaccine inducing protective cytotoxic T lymphocyte (CTL)-mediated immunity could pave the way for malaria eradication. Experimental immunization with sporozoites induces this type of protective response, but the extremely large number of proteins expressed by Plasmodium parasites has so far prohibited the identification of sufficient discrete T-cell antigens to develop subunit vaccines that produce sterile immunity. Here, using mice singly immunized with Plasmodium yoelii sporozoites and high-throughput screening, we identified a unique CTL response against the parasite ribosomal L3 protein. Unlike CTL responses to the circumsporozoite protein (CSP), the population of L3-specific CTLs was not expanded by multiple sporozoite immunizations. CSP is abundant in the sporozoite itself, whereas L3 expression does not increase until the liver stage. The response induced by a single immunization with sporozoites reduces the parasite load in the liver so greatly during subsequent immunizations that L3-specific responses are only generated during the primary exposure. Functional L3-specific CTLs can, however, be expanded by heterologous prime-boost regimens. Thus, although repeat sporozoite immunization expands responses to preformed antigens like CSP that are present in the sporozoite itself, this immunization strategy may not expand CTLs targeting parasite proteins that are synthesized later. Heterologous strategies may be needed to increase CTL responses across the entire spectrum of Plasmodium liver-stage proteins.
Keywords: vaccination, epitope
Malaria parasites cause tremendous morbidity and mortality worldwide. The only vaccine approach known to induce sterile protection against challenge relies on whole organism sporozoite-stage parasites. This vaccination is experimentally achieved in mice and humans by using radiation-attenuated sporozoites (RAS) (1, 2), genetically attenuated parasites (GAPs) (3), or wild-type (WT) sporozoites with chloroquine prophylaxis (CPS) (4, 5). These approaches induce protective antibodies and T cells, with cytotoxic T lymphocytes (CTLs) particularly important for protection against experimental sporozoite challenge (6).
Because sporozoites are difficult to manufacture, a multicomponent subunit vaccine that induces protective CTLs is desirable; however, protective antigens must be identified first. Because Plasmodia have >5,000 genes, there are many candidates yet few bona fide antigens have been described since the identification of the circumsporozoite protein (CSP) as a critical target more than 24 y ago (7, 8). CSP coats the sporozoite surface, is shed from the motile sporozoite (9), and is transported to the hepatocyte cytoplasm (10). CSP is a target of protective class I-dependent CTL responses (10–12). Depending on the mouse model, CSP can also elicit important CD4+ T-cell responses (13, 14) and MHC class II-dependent IgG responses (15). In some systems, large numbers of adoptively transferred CSP-specific T-cell clones or endogenously generated CTLs can protect mice against sporozoite challenge (8, 12, 14, 16, 17). However, it remains unclear whether CSP-specific CTLs in naïve subjects can be primed to achieve protection with existing vaccination approaches. In addition, the CSP-based RTS,S vaccine in phase 3 human trials does not trigger strong CTL responses (18, 19). The partial protection (20) from RTS,S is instead associated with antibodies and CD4+ T-cell responses (21). Thus, CSP is an important but currently insufficient target for use as a single-antigen vaccine. Because responses to non-CSP antigens can protect in the absence of CSP-specific immunity in mice (11, 22–25), we and others are working to discover novel protective antigens to broaden the immune repertoire.
Single immunization using CPS or late-arresting GAP approaches usually provides greater protection than an equivalent number of RAS parasites (11, 26). RAS parasites arrest shortly after hepatocyte infection, whereas GAPs progress further depending on the specific genetic lesion. CPS completes liver-stage development and progresses to a brief RBC infection halted by drug treatment. GAP and CPS approaches express a wider array of proteins than RAS. Accordingly, late-arresting GAPs were shown to induce more diverse CTL responses than RAS vaccination in mice (27). Thus, proteins expressed de novo in the liver [e.g., by CPS and some GAPs like 3-oxoacyl-ACP synthase I/II; PY04452 (FabB/F)-deficient parasites] and in the red blood cell (RBC) stages (e.g., by CPS) have received attention as candidate antigens. However, despite efforts by several groups, few non-CSP antigens remain confirmed as CTL targets and none are reported to be individually protective (28).
It remains unclear why there is a paucity of protective antigens after sporozoite immunization. The lack of targets may reflect technical limitations in antigen discovery, antigenic bias due to homologous sporozoite vaccination, lack of late-stage antigen expression, and/or CSP immunodominance. Traditionally, pooled synthetic peptides are used in IFN-γ (IFNγ) enzyme-linked immunosorbent spot (ELISPOT) assays to identify T-cell targets (29), but even with pooling strategies (30), these approaches require complex, expensive peptide libraries. In lieu of peptides, plasmid-encoded parasite genes can be transfected into antigen-presenting cells (APCs) (31), but this approach is low throughput and difficult because of the A/T-richness of Plasmodial genes. Instead here, we used a minigene-based high-throughput screen (HTS) for CTLs (32) to study a library of Plasmodium yoelii-derived antigens in sporozoite-vaccinated mice. Our approach identified a unique CTL target antigen that provided surprising insights about CTL responses in multiply-immunized mice.
Results
HTS Reveals Greater CTL Diversity Following Different Sporozoite Immunizations.
To identify novel malaria vaccine candidates, we developed a HTS approach based on massively parallel synthesis of thousands of oligonucleotides that encode candidate peptides (32) (Fig. S1). Liver-stage expressed genes (33) were subjected to H2d class I MHC prediction to produce a sequence library (Dataset S1) encoding 1,988 distinct 17-aa peptides (centered on a predicted 9-mer peptide) in 199 pools of 10 minigenes each. Pools were transfected into H2d P815 cells and used for ELISPOT assays of mouse splenocytes 6 d after immunization.
In general, low frequency T-cell responses were observed for all primary immunization regimens. The background in CPS-immunized mice was significantly higher [22.9 ± 20.2 to 25.5 spot-forming units (SFUs), 95% confidence interval (CI)] than for GAP- (4.7 ± 4.7 to 9.6 SFU, 95% CI) or RAS-immunized mice (5.6 ± 4.5 to 6.7 SFU, 95% CI) (P < 0.0001, unpaired Student's t test, 16 wells for CPS and RAS, 15 wells for GAP), possibly due to natural killer cell activation by infected RBCs (iRBCs) (34). CPS spleens were larger and contained more pigment than normal-appearing spleens of GAP- and RAS-immunized mice, likely due to uptake of drug-killed iRBCs. After normalization, CPS and GAP triggered the most positive results (each with 27 incompletely overlapping pools) followed by RAS immunization (17 pools) at P < 0.05 (Fig. 1A and Fig. S2). Most positive responses produced fewer IFNγ spots than the CSP control well. Only two pools were positive in all screens in CPS, GAP, and RAS immunized mice (pools 79 and 171). All immunizations induced strong ELISPOT responses to the column-synthesized CSP-encoding control minigene (Fig. 1A and Fig. S2). Pool 102 also contained the microarray-synthesized minigene encoding the SYVPSAEQI peptide of CSP and was positive in GAP and RAS immunizations, but statistically negative in the CPS screen because of higher background.
Fig. 1.
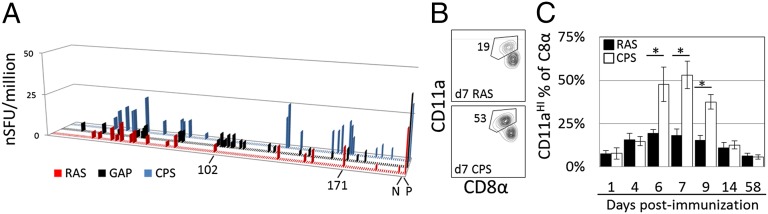
Different sporozoite immunizations result in CD8+ T-cell responses that vary in magnitude and diversity. (A) Normalized HTS results from primary immunization with RAS (red), CPS (blue), and GAP regimens (black). Data are the mean normalized IFNγ spot count per well for 199 screening pools based on duplicate screening wells at 95% confidence. The x axis displays pools 1–199 in numerical order followed by control wells, as indicated. Pools 102 (containing a CSP epitope) and 171 and negative (N) and positive (P) controls are indicated. (B) Flow cytometry of peripheral blood leukocytes 6 d after immunization with 2 × 104 RAS or CPS parasites, assessing CD11a and CD8α to identify P. yoelii sporozoite-induced CD8+ T cells. Values adjacent to gated regions indicate percent of CD11aHICD8aLO cells of all CD8+ T cells. (C) Average percentage of CD11aHICD8αLO cells of all CD8+ T cells at the times after immunization. *P < 0.0001 (two-way ANOVA); error bars, 95% CI, n = 9 mice per group; representative of two experiments. Filled bars, RAS; open bars, CPS.
Microarray synthesized oligonucleotides are known to contain more errors than column-synthesized oligonucleotides (35, 36). Because the CSP peptide and single minigene were better able at detecting T-cell responses than the library-based minigene, efforts are underway to increase the signal-to-noise in the HTS system. Our findings showed that CPS immunization induced the largest increase in peripheral CD8αLO/CD11aHI T cells—indicative of recent antigenic stimulation (37), followed by FabB/f and then RAS (Fig. 1), consistent with earlier data comparing GAP and RAS approaches (27). Thus, it appears that more diverse responses are achieved by GAP and CPS approaches compared with RAS immunization, and we identified two reproducibly positive pools herein.
Identification of a T-Cell Epitope in the P. yoelii Ribosomal Protein L3.
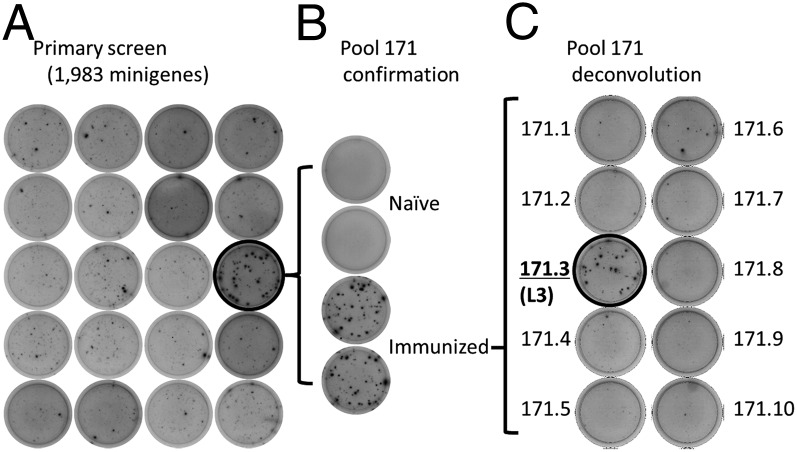
We evaluated pools 79 and 171 for T-cell target antigens by ELISPOT and MHC binding studies. Pool 79 contained 10 coding sequences for proteins PY02320 (six minigenes), PY02325, PY02338, and PY02348 (two minigenes) and pool 171 contained 10 coding sequences for proteins PY05859 (two minigenes), PY05881, PY05897 (five minigenes), and PY05908 (two minigenes). Upon 9-mer peptide deconvolution, no single response accounted for the reactivity of Pool 79, possibly indicating that targets outside of the 9-mer core were targeted or that multiple responses contributed to the pool positivity. In contrast, Pool 171 was reproducibly reactive in immunized but not naïve mice (Fig. 2 A and B) and a single peptide from the PY05881 ribosomal protein L3 (GYKSGMSHI) predicted to bind H2-Kd generated ELISPOT responses (Fig. 2C).
Fig. 2.
HTS identifies the P. yoelii ribosomal protein L3 as a T-cell antigen. (A) Subset of wells from primary HTS showing positive result in pool 171. (B) Pool 171-specific responses in naïve vs. sporozoite-immunized mice. (C) Deconvolution of pool 171 using individual minigenes shows that minigene 171.3 (from L3 ribosomal protein, PY05881) was the antigenic component of the pool.
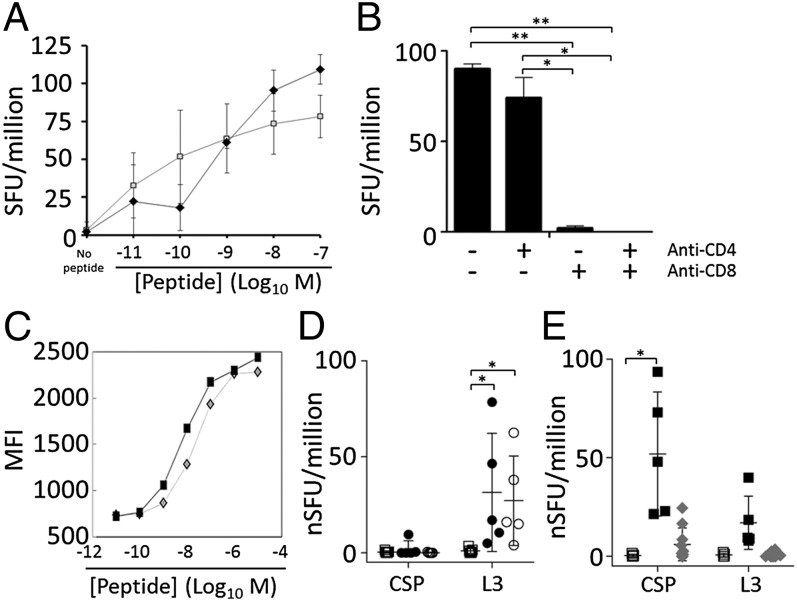
The P. yoelii L3 ribosomal protein is expressed in liver and RBC stages (33) and is conserved in Plasmodium berghei ANKA (PBANKA_051190), Plasmodium falciparum (PF10_0272), and Plasmodium vivax (PVX_111330). By ELISPOT, L3-specific cells responded to low nanomolar peptide concentrations, similar to the behavior of CSP-specific cells (Fig. 3A). The L3 response was mediated by CD8+ T cells because anti-CD8 but not anti-CD4 antibodies blocked the response (Fig. 3B). By H2-Kd binding studies using RMA/S lymphoma cells, both L3 and CSP peptides demonstrated low nanomolar-strength MHC-specific binding to H2-Kd (L3 Kd = 0.7 nM compared with CSP Kd 1.6 nM) (Fig. 3C). The L3-specific ELISPOT response could also be blocked by using anti–H2-Kd antibodies (Fig. S3).
Fig. 3.
The L3-specific response is mediated by CD8+ T cells and can be induced by cross-priming. (A) IFNγ ELISPOT of CPS-immunized BALB/c mice with varied concentrations of CSP (SYVPSAEQI, open squares) or L3 (GYKSGMSHI, filled diamonds) peptides. SFU, IFNγ spot forming units (SFU) per million splenocytes. Compared with no peptide controls, all results were significant at P < 0.05 for all peptide concentrations >1 × 10−9 M (unpaired t test; three samples per data point). (B) Antibodies were used to block CD4 and/or CD8 during the ELISPOT. Error bars show 95% CI; *P < 0.05, **P < 0.001; two samples per group. (C) RMA/S cells expressing H2-Kd were incubated with the designated concentration of CSP (diamonds) or L3 (squares) peptide and monitored to determine the stabilization of H2-Kd. A–C are representative of two experiments each. (D) Mice were immunized with heat-treated uninfected (squares) or infected-erythrocytes (P. yoelii, filled circles; P. berghei, open circles), and 7 d later ELISPOT was performed as in A by using 1 × 10−7 M peptides. Data compiled from two experiments and presented as normalized SFU per million splenocytes (nSFU = SFU minus SFU of mock-treated controls). *P < 0.05 (unpaired t test). (E) Mice immunized with live- (black squares) or heat-treated (gray diamonds) GAP sporozoites were compared with naïve mice (white squares) by ELISPOT as in D. *P < 0.0001 (unpaired t test). In D and E, error bars, SEM; n = 2–3 per experiment for naïve mice and live GAP; n = 5 per experiment for heat-treated iRBCs and heat-treated GAP.
CSP- and L3-specific responses could be detected in liver lymphocytes from CPS- and RAS-immunized mice by ELISPOT 6 d after immunization (Fig. S4). Because L3 lacks a canonical signal sequence and may not traffic to the parasitophorous vacuole or hepatocyte cytosol, we tested responses to cross-presented L3 as well. L3-specific T cells but not CSP-specific T cells were induced by using heat-treated iRBCs from P. yoelii- and P. berghei-infected mice (Fig. 3D). In contrast, CSP-specific but not L3-specific T cells were induced by heat-treated GAP sporozoites (Fig. 3E), consistent with the observation that sporozoites unable to invade hepatocytes can still cross-present CSP (38). Our findings suggest that L3 is not potently cross-presented by dead or dying sporozoites and instead is probably targeted after synthesis by infected hepatocytes. This finding agrees with most expression studies in Plasmodium spp., which consistently find abundant L3 transcripts and protein in liver (39, 40) and iRBC stages (41). Although low abundance L3 transcripts were detected in sporozoites (42, 43), L3 protein was undetectable (44) or nearly undetectable (42, 43) by mass spectrometry compared with later timepoints. L3 is a reported discrete malaria CD8+ T-cell epitope carried on BALB/c malaria-infected erythrocytes; a few additional epitopes were reported in C57BL/6 mice (45). These findings collectively show that the P. yoelii/berghei L3 peptide GYKSGMSHI is a CD8+ T-cell target in BALB/c mice.
L3-Specific Response Fails to Boost in Sporozoite Hyperimmunized Mice.
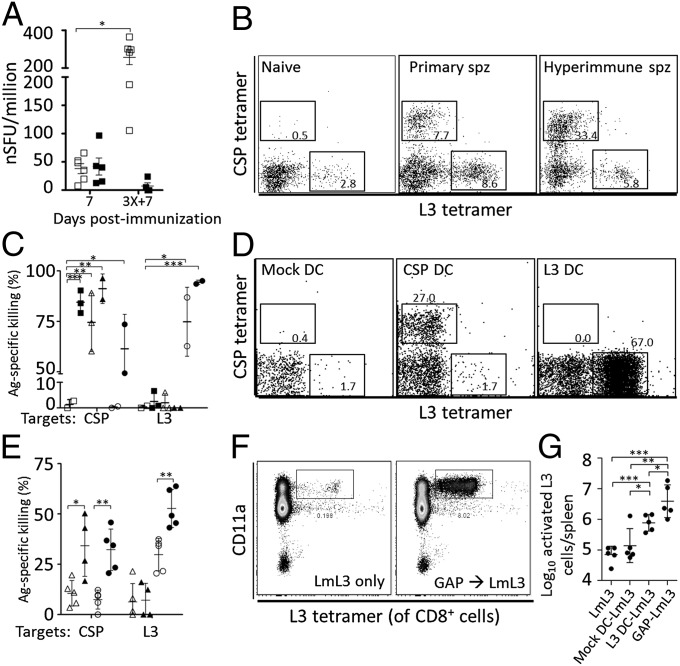
Because L3-specific responses were detected after primary immunization, L3- and CSP-specific responses were monitored over the course of multiple immunizations. In all regimens, the CSP-specific ELISPOT response increased in frequency with each round of immunization, whereas the L3-specific response decreased after repeated immunizations (Fig. 4A; CPS shown). Phycoerythrin (PE)-labeled CSP280–288/Kd and allophycocyanin (APC)-labeled L348–56/Kd tetramers were used to isolate antigen-specific cells from naïve, acutely immunized and multiply immunized mice. In naïve mice, the precursor frequency of L3-specific cells routinely exceeded that of CSP-specific cells (Fig. 4B). In all sporozoite immunizations, the frequency of CSP tetramer-stained cells continued to rise with each immunization, whereas L3-specific cells always decreased after reaching a peak after a single immunization. This finding was irrespective of whether RAS, GAP, or CPS regimens were used. Following primary sporozoite immunization or immunization with peptide-pulsed dendritic cells (DCs), CSP- and L3-specific tetramer-purified cells were CD11aHI, CD44HI, CD62LLO with increasing killer cell lectin-like receptor subfamily G member 1 (KLRG1) (Fig. S5). In contrast, the expanded CSP-specific CTLs in hyperimmunized mice displayed a CD11aHI/CD62LLO phenotype with some persistent KLRG1HI cells, whereas similar L3-specific cells became increasingly rare (Fig. S5). Thus, despite similar phenotypes during primary immunization, CSP-specific T cells were increased and activated in multiply sporozoite-immunized mice, whereas L3-specific T cells failed to secondarily expand after additional sporozoite exposures.
Fig. 4.
L3-specific CTLs are not boosted by multiple sporozoite injections but can be boosted by heterologous immunization. (A) ELISPOT from BALB/c mice homologously immunized one or three times at 30-d intervals with 1 × 104 CPS. ELISPOT was performed on the day listed by using 1 × 10−7 M CSP (open squares) or L3 (filled squares) peptides. *P = 0.002 (unpaired t test, 4–6 mice per group). (B) Flow cytometry for MACS tetramer-purified splenocytes from naïve mice or mice immunized once or three times with 2 × 104 GAP. Gates and values denote the percentage of tetramer-positive CD8+CD4−B220− single cells. (C) In vivo killing of CSP- or L3-coated target cells in naïve mice (open squares) or mice immunized with 3× RAS (filled squares), CSP peptide-pulsed DCs (open triangles), 3× RAS then CSP peptide-pulsed DCs (filled triangles), L3 peptide-pulsed DCs (open circles) or 3× RAS then L3 peptide-pulsed DCs (filled circles). *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired t test; 2–3 mice per group). (D) Flow cytometry for MACS tetramer-purified splenocytes as in B from mice immunized 6 d earlier with mock-, CSP- or L3-peptide treated DCs. Gates and values as in B. (E) In vivo killing of CSP- or L3-coated target cells as in C in mice receiving CSP-pulsed DC priming and no booster immunization (open triangles), CSP DC priming and single RAS booster (filled triangles), L3 peptide-pulsed DC priming and no booster (open circles), or L3 peptide-pulsed DC priming and single RAS booster (filled circles). *P < 0.05, **P < 0.01 (unpaired t test); 4–5 mice per group. (F and G) Flow cytometry of total splenocytes showing CD11aHIL3 tetramer-stained CD8+ T cells in mice immunized with mock- or L3 peptide-pulsed DCs or 2 × 104 GAP parasites followed by L3-expressing Listeria infection 30 d later. The number of activated L3-specific cells per spleen was calculated and plotted in G. *P < 0.05, **P < 0.01, ***P < 0.001 (unpaired t test; 5 mice per group).
Sporozoite-Induced L3 Cells form Functional Memory CTLs.
Using in vivo killing assays, we evaluated the function of CSP- and L3-specific cells induced by sporozoite immunizations to look for differences between these CTLs. With repeated sporozoite exposures, CSP-specific killing significantly increased, whereas L3-specific killing did not (Fig. 4C). To determine whether sporozoite-primed, L3-specific CTLs were functionally defective, we immunized naïve or RAS-hyperimmunized mice with a booster of peptide-pulsed DCs and tested for killing. When RAS-hyperimmunized mice were boosted with L3 peptide-pulsed DCs, antigen-specific killing was increased compared with naïve mice primed with alike DCs alone, suggesting that sporozoite immunization formed memory CTLs. Flow cytometry of splenocytes from mice immunized with CSP or L3 peptide-pulsed DCs showed consistently expanded populations of tetramer-stained CTLs (Fig. 4D). Despite their functionality, mice were not protected from sporozoite challenge by a single immunization with L3-pulsed DCs (Fig. S6). To ascertain whether sporozoites could recall DC-primed CTLs, we reversed the order of immunizations and repeated in vivo killing assays. L3-specific killing in mice primed with L3 peptide-pulsed DCs was boosted by a secondary sporozoite immunization (Fig. 4E). In all of these studies, CSP-specific responses could be potently triggered by any combination of DC and/or sporozoite immunizations.
To test whether heterologous prime-boost immunization could overcome the poor L3 response in sporozoite immunized mice, we primed mice with L3 peptide-pulsed DCs or GAP sporozoites and boosted with recombinant Listeria monocytogenes (Lm) expressing the L3 epitope (Lm-L3). Similar to the behavior of CSP-specific cells following multiple sporozoite immunizations, these heterologous prime-boost approaches expanded the population of activated (CD11aHICD62LLOCD44HI) L3-specific T cells by 0.74 or 1.67 log10 cells per spleen for L3 peptide-pulsed DC or GAP priming, respectively, compared with the appropriately matched Lm-L3 priming immunization alone (Fig. 4 F and G). Thus, in contrast to multiple sporozoite immunizations, a heterologous prime-boost approach expanded the L3-specific population and maintained its activated phenotype.
Prior Sporozoite Immunization Eliminates L3 Expression and Liver Stage Parasite Development and Prevents the Boosting of Responses Against Late Liver Stage Antigens.
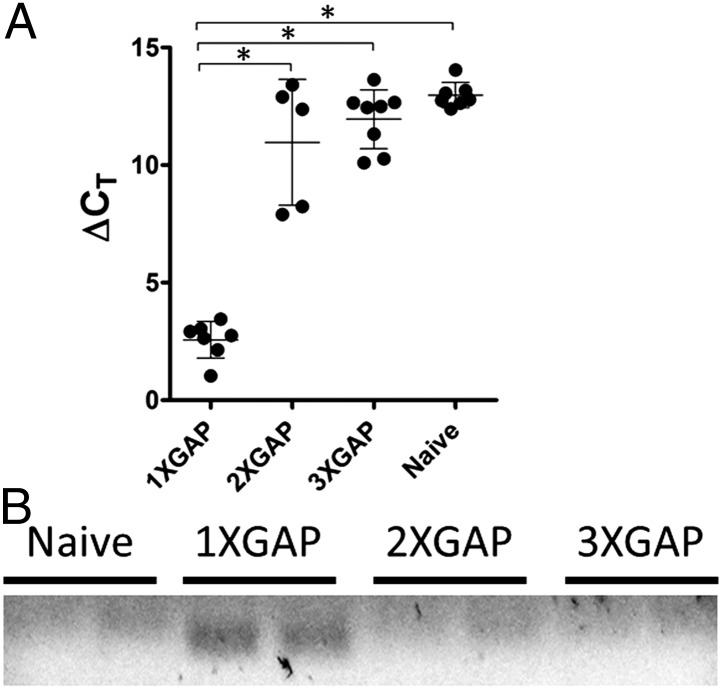
Heterologous prime-boost studies presented above suggested that functional memory L3 CTLs were formed by priming with sporozoites. To explain the lack of L3 CTL boosting in response to subsequent sporozoite immunizations, we hypothesized that L3 antigen was insufficiently presented during secondary or later sporozoite immunizations because the developing adaptive immune response constrained the parasite such that L3 expression was suppressed during later immunizations. To test this hypothesis, we immunized mice one to three times with 2 × 104 P. yoelii GAP at 1- or 3-wk intervals. Unimmunized mice and mice 44 h after the last immunization were killed and total RNA was extracted from livers to evaluate parasite abundance and transcript expression. Regardless of the interval between immunizations (1 or 3 wk), prior sporozoite immunization reduced the liver parasite burden >1,000-fold compared with the first exposure (Fig. 5A and Table 1). In many multiply-immunized mice, parasite 18S rRNA was undetectable.
Fig. 5.
Prior sporozoite immunization significantly reduces the load of liver-stage parasites and eliminates detectable L3 transcript expression. (A) The ΔCT of murine GAPDH and P. yoelii 18S rRNA was determined by real-time RT-PCR 44 h after immunization. *P < 0.0001 (unpaired t test; 5–8 mice per group). (B) Electrophoresis of L3-specific RT-PCR product obtained as in A. A specific-band was only detected in mice immunized once with sporozoites.
Table 1.
P. yoelii 18S rRNA liver-stage burden in GAP-immunized BALB/c mice
| Interval, d | Sporozoite doses | ΔCT 18S (95% CI) | n mice (n exp) |
| 0 (naïve) | 12.98 (12.58–13.37) | 7 (3) | |
| 7 | 1 | 2.56 (1.98–3.14)* | 7 (3) |
| 2 | 10.97 (8.62–13.31) | 5 (2) | |
| 3 | 11.95 (11.08–12.81) | 8 (3) | |
| 0 (naïve) | 13.06 (11.58–14.54) | 6 (2) | |
| 21 | 1 | 2.60 (0.40–4.80)* | 8 (2) |
| 2 | 7.40 (6.37–8.43)* | 8 (2) | |
| 3 | 12.10 (11.40–12.80) | 8 (2) |
BALB/c mice were immunized one, two, or three times at 7- or 21-d intervals and then killed 44 h after the final sporozoite injection. Naïve mice were also killed as controls. Total liver RNA was purified and real-time RT-PCR was performed to measure mouse GAPDH mRNA and P. yoelii 18S rRNA. The parasite burden is shown as the ΔCT (CT Py 18S rRNA – CT mouse GAPDH mRNA), which is inversely proportional to the parasite load. CI, confidence interval.
P < 0.0001 (unpaired t test comparison with unimmunized).
To study L3 expression and liver-stage development, additional early and late liver-stage parasite transcripts were analyzed by real-time RT-PCR 44 h after the last immunization. Most of the transcripts listed in Table S1 were detected at 44 h after a single GAP exposure, but were undetectable after subsequent immunizations (L3/PY05881, PY00204, PY02405, PY03995, PY02619, PY05756, PY05060, PY07509). One transcript (PY05756) was detected after primary and secondary but not tertiary exposure (Table S1). L3 expression after one immunization was also confirmed by gel electrophoresis showing an amplified band of the appropriate size (Fig. 5B). These studies confirm the absence of developing parasites in multiply-immunized mice. The lack of L3 expression in later immunizations explains why L3 CTLs are primed by sporozoite immunization but not boosted by homologous sporozoite immunization and further supports heterologous strategies for inducing T-cell responses against malaria parasite antigens.
Discussion
Whole sporozoite immunization can protect animal and human subjects, with major contributions from antibody and T-cell–mediated responses to CSP (46). However, as described earlier, CSP-specific immunity is not absolutely necessary or sufficient to attain immunological protection against challenge (11, 21–24). Thus, there is a need to identify individual immunogenic antigens so that they can be evaluated for inclusion in multicomponent subunit vaccines. Using a unique HTS system for CD8+ T-cell antigen identification (32), we, like others (28), detected many low frequency T-cell responses to sporozoite vaccinations, with a trend toward more diversity in less-attenuated parasites (e.g., CPS and GAP > RAS), as has been reported (27). We identified a peptide from the L3 ribosomal protein as a CD8+ T-cell target in all sporozoite vaccination regimens and following immunization with heat-treated infected erythrocytes. Unlike the well-studied CSP antigen that coats the infectious sporozoite, the L3 protein is not highly expressed until later in the liver stage. Thus, this work identifies a specific CD8+ T-cell epitope carried on liver-stage and infected erythrocytes in BALB/c mice.
In this study, homologous sporozoite immunizations resulted in boosting of CSP-specific, but not L3-specific, CD8+ T-cell responses. Previous reports show that when multiple homologous sporozoite immunizations were given at 48-h intervals, there was no significant increase in CSP-specific CD8 T-cell expansion compared with a single dose of sporozoites (47). This so-called “self-regulation” was due to antigen-specific CD8+ T cells that could eliminate antigen-presenting DCs (48) and prevent the further expansion of naive CD8+ T cells specific for the same antigen. This phenomenon is thought to mimic the situation in hyperendemic field settings, where humans sometimes receive multiple malaria-infected mosquito bites daily. When the interval between immunizations is extended to a week or more as in our studies, we and others (27) find that endogenous CSP-specific CD8+ T cells in fact increase in frequency after repeated sporozoite immunizations. Thus, CSP-specific T cells do not appear to be affected by self-regulation using our vaccination approach. Looking beyond CSP-specific cells, previous phenotyping of bulk CD8+ T cells showed that some portion of memory T-cell responses could be boosted by repeated sporozoite exposures. Lymphocytes from mice treated with homologous sporozoite immunizations showed an increase in the frequency of memory CD8+ T cells at a memory timepoint (37). We predict that such increases following repeat sporozoite immunizations are responses against preformed or early liver stage antigens. Thus, with the right immunization interval, some CD8+ T-cell responses appear to boost in response to homologous sporozoite exposures.
In contrast to CSP-specific T cells, L3-specific CD8+ T cells failed to boost after repeated sporozoite immunizations. We first looked to see whether there were functional defects in L3-specific cells, but these cells were capable of killing peptide-coated target cells in an antigen-specific manner and could be restimulated with other forms of antigen. Instead, we found that the lack of L3-specific CTL expansion following secondary sporozoite immunization was likely due to the strong antisporozoite immune response that essentially prevents L3 expression. We do not think that failed secondary L3 responses are due to self-regulation (i.e., killing or blocking) of L3 antigen-presenting cells—rather we think that the antigen is altogether absent because the parasite is killed before L3 expression is evident. L3 is therefore unlike most preerythrocytic malaria antigens studied to date—it is not abundant on the mosquito-derived sporozoite and likely requires productive infection of hepatocytes to be highly expressed. For such antigens, the first and second immunizations with homologous sporozoites occur in very different immunological settings.
Our data suggest that the loss of parasite antigen expression in multiply sporozoite immunized mice may complicate efforts to identify novel late-stage antigenic targets. The relative protection afforded by previous sporozoite immunization eliminates mRNA expression for numerous proteins known to be expressed during liver-stage development. Thus, late-stage antigens may not be sufficiently exposed during secondary or later immunizations such that responses against these antigens may fail to boost, whereas dominant immune responses progressively focus on preformed antigens like CSP and others expressed by the incoming sporozoite. Certainly CSP-specific responses are known to dominate the BALB/c immune response in sporozoite-immunized mice (22). However, recent studies suggest an important role for non-CSP responses as well. Mouse studies suggest that fewer CPS parasites and fewer CPS immunizations are required for protection compared with the RAS regimen (26). Similarly, a single CPS immunization via the bites of 12–15 P. falciparum NF54-infected mosquitoes protected 4 of 10 human subjects from developing detectable blood-stage parasitemia following a second CPS immunization and reduced the peak parasitemia >20-fold in the remaining infected individuals (5). Although this human study was designed to test the efficacy of three CPS immunizations, relative protection from the second immunization was apparent even after the first immunization, similar to our findings here using GAP parasites. The increased potency of late-arresting or WT sporozoites compared with heat-killed or early arresting sporozoites is thought to be due to expression of antigens found later in the liver stage or even in the erythrocyte stage. It would be desirable to identify such proteins because they could be combined with CSP to produce multicomponent subunit vaccines capable of acting at multiple checkpoints throughout parasite liver-stage development. However, in retrospect it appears that multiple homologous sporozoite immunizations may have hamstrung screening efforts by biasing immune responses in favor of preformed antigens like CSP. Because of this effect, efforts that screen multiply-immunized mice with late liver-stage antigen libraries could be destined to fail.
Although the L3 antigen identified herein does not appear to be protective alone, L3-specific T-cell responses were boosted by using heterologous prime-boost immunization regimens. The lack of L3 boosting by repeated sporozoite exposures may be emblematic of a phenomenon affecting other liver-stage parasite antigens as well. Heterologous prime-boost approaches are being used for malaria vaccination-challenge studies in mice and humans (49). Our findings make the case for heterologous approaches at the antigen discovery phase as well. By inducing a broad immune repertoire, we may be able to generate responses capable of eradicating all malaria-infected hepatocytes, including those missed by CSP- or other sporozoite-limited responses.
Materials and Methods
Reagents.
Chemicals and antibodies are detailed in SI Materials and Methods. Recombinant Lm-L3 within the ovalbumin protein was a gift of Dietmar Zehn (Swiss Vaccine Research Institute, Lausanne, Switzerland) and is described in SI Materials and Methods.
Mice and Py17XNL Infection.
Balb/cj and Thy1.1+ BALB/c mice from Jackson Laboratories (6–8 wk old) were housed in approved facilities at the University of Washington for studies approved by the Institutional Animal Care and Use Committee. Mice were infected i.v. with 1 × 102 to 2 × 105 WT, FabB/F (GAP), or irradiated (10,000 rad) P. yoelii 17XNL sporozoites obtained from the Center for Mosquito Production and Malaria Infection Research (CeMPMIR, Seattle Biomedical Research Institute, Seattle). The CPS protocol used 0.8 mg of chloroquine diphosphate i.p. daily for 10 d. Some mice received heat-treated parasites or additional sporozoite immunizations as described in the text and SI Materials and Methods.
In Silico Library Protein Selection and Minigene Library Synthesis.
Design, synthesis, pooling, and use of a minigene library containing 1,988 components predicted to bind BALB/c MHC is in SI Materials and Methods. The technique was as described (32).
IFNγ ELISPOT.
Erythrocyte-depleted cell suspensions from spleens or livers were subjected to ELISPOT as described in SI Materials and Methods by using either peptides or minigene-transfected P815 cells. Antibodies against CD4, CD8, or H2-Kd were used in some experiments. Counting is as described in SI Materials and Methods.
MHC Binding.
H2-Kd-expressing RMA/S cells were used to test MHC binding as reported (50).
Magnetic-Activated Cell Sorting Purification and T-Cell Phenotyping.
CD8+ T-cell purification was performed by negative selection magnetic-activated cell sorting (MACS; Miltenyi Biotech). Tetramer pulldown assays were as described (51) by using reagents described in SI Materials and Methods.
In Vivo Cytotoxicity Assay.
Assays were performed after 8 h as reported (52).
Immunization with Peptide-Pulsed DCs or Lm-L3.
Bone marrow-derived DCs were cultured by using BALB/c serum, treated with LPS and peptides, washed, and injected as in SI Materials and Methods. For Lm-L3 immunizations, stock bacteria were grown and injected i.v. as reported (53).
Protection and Liver-Stage RT-PCR Studies.
As described in SI Materials and Methods, RNA was harvested from livers of mice immunized with GAP or challenged with WT sporozoites 40–44 h after injection. Quantitative RT-PCR was performed for mouse GAPDH and P. yoelii 18S rRNA, and the ΔCT was calculated as in SI Materials and Methods. Expression of other transcripts was also tested as in SI Materials and Methods.
Statistical Analysis.
Data were tabulated by using Microsoft Excel; P values were calculated by using Prism software (GraphPad). Unless noted, error bars depict ±1 SD.
Supplementary Material
Acknowledgments
We thank Crystal Rawlings, Jay Shendure, Nick Crispe, Stefan Kappe, and the Center for Mosquito Production and Malaria Infection Research (Seattle Biomedical Research Institute) for assistance. S.C.M. is supported by National Institute of Allergy and Infectious Diseases Grant 1K08AI097238-01, and B.C.S. and S.C.M. are supported by the Bill and Melinda Gates Foundation. M.J.B. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303834110/-/DCSupplemental.
References
- 1.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 2.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266(3):169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 4.Putrianti ED, Silvie O, Kordes M, Borrmann S, Matuschewski K. Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. J Infect Dis. 2009;199(6):899–903. doi: 10.1086/597121. [DOI] [PubMed] [Google Scholar]
- 5.Roestenberg M, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361(5):468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 6.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165(3):1453–1462. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, et al. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- 8.Romero P, et al. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341(6240):323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 9.Stewart MJ, Vanderberg JP. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J Protozool. 1988;35(3):389–393. doi: 10.1111/j.1550-7408.1988.tb04115.x. [DOI] [PubMed] [Google Scholar]
- 10.Bongfen SE, Torgler R, Romero JF, Renia L, Corradin G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J Immunol. 2007;178(11):7054–7063. doi: 10.4049/jimmunol.178.11.7054. [DOI] [PubMed] [Google Scholar]
- 11.Kumar KA, Baxter P, Tarun AS, Kappe SH, Nussenzweig V. Conserved protective mechanisms in radiation and genetically attenuated uis3(-) and uis4(-) Plasmodium sporozoites. PLoS ONE. 2009;4(2):e4480. doi: 10.1371/journal.pone.0004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balam S, Romero JF, Bongfen SE, Guillaume P, Corradin G. CSP—a model for in vivo presentation of Plasmodium berghei sporozoite antigens by hepatocytes. PLoS ONE. 2012;7(12):e51875. doi: 10.1371/journal.pone.0051875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rénia L, et al. In vitro activity of CD4+ and CD8+ T lymphocytes from mice immunized with a synthetic malaria peptide. Proc Natl Acad Sci USA. 1991;88(18):7963–7967. doi: 10.1073/pnas.88.18.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rénia L, et al. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J Immunol. 1993;150(4):1471–1478. [PubMed] [Google Scholar]
- 15.Molano A, et al. Cutting edge: The IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: Exploring the role of GPIs in NK T cell activation and antimalarial responses. J Immunol. 2000;164(10):5005–5009. doi: 10.4049/jimmunol.164.10.5005. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues MM, et al. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3(6):579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt NW, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci USA. 2008;105(37):14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbosa A, et al. Plasmodium falciparum-specific cellular immune responses after immunization with the RTS,S/AS02D candidate malaria vaccine in infants living in an area of high endemicity in Mozambique. Infect Immun. 2009;77(10):4502–4509. doi: 10.1128/IAI.00442-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pichyangkul S, et al. Preclinical evaluation of the safety and immunogenicity of a vaccine consisting of Plasmodium falciparum liver-stage antigen 1 with adjuvant AS01B administered alone or concurrently with the RTS,S/AS01B vaccine in rhesus primates. Infect Immun. 2008;76(1):229–238. doi: 10.1128/IAI.00977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kester KE, et al. RTS,S Vaccine Evaluation Group Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: Safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 21.Ndungu FM, et al. A statistical interaction between circumsporozoite protein-specific T cell and antibody responses and risk of clinical malaria episodes following vaccination with RTS,S/AS01E. PLoS ONE. 2012;7(12):e52870. doi: 10.1371/journal.pone.0052870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar KA, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444(7121):937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 23.Grüner AC, et al. Sterile protection against malaria is independent of immune responses to the circumsporozoite protein. PLoS ONE. 2007;2(12):e1371. doi: 10.1371/journal.pone.0001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauduit M, et al. Minimal role for the circumsporozoite protein in the induction of sterile immunity by vaccination with live rodent malaria sporozoites. Infect Immun. 2010;78(5):2182–2188. doi: 10.1128/IAI.01415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauduit M, et al. A role for immune responses against non-CS components in the cross-species protection induced by immunization with irradiated malaria sporozoites. PLoS ONE. 2009;4(11):e7717. doi: 10.1371/journal.pone.0007717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belnoue E, et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172(4):2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 27.Butler NS, et al. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe. 2011;9(6):451–462. doi: 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra S, et al. Identification of non-CSP antigens bearing CD8 epitopes in mice immunized with irradiated sporozoites. Vaccine. 2011;29(43):7335–7342. doi: 10.1016/j.vaccine.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyes-Sandoval A, Pearson FE, Todryk S, Ewer K. Potency assays for novel T-cell-inducing vaccines against malaria. Curr Opin Mol Ther. 2009;11(1):72–80. [PubMed] [Google Scholar]
- 30.Doolan DL, et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Natl Acad Sci USA. 2003;100(17):9952–9957. doi: 10.1073/pnas.1633254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, et al. Immune responses to Plasmodium vivax pre-erythrocytic stage antigens in naturally exposed Duffy-negative humans: A potential model for identification of liver-stage antigens. Eur J Immunol. 2005;35(6):1859–1868. doi: 10.1002/eji.200425807. [DOI] [PubMed] [Google Scholar]
- 32.Hondowicz BD, et al. Discovery of T cell antigens by high-throughput screening of synthetic minigene libraries. PLoS ONE. 2012;7(1):e29949. doi: 10.1371/journal.pone.0029949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacci JB, Jr, Aguiar JC, Lau AO, Hoffman SL. Laser capture microdissection and molecular analysis of Plasmodium yoelii liver-stage parasites. Mol Biochem Parasitol. 2002;119(2):285–289. doi: 10.1016/s0166-6851(01)00411-x. [DOI] [PubMed] [Google Scholar]
- 34.Artavanis-Tsakonas K, Riley EM. Innate immune response to malaria: Rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol. 2002;169(6):2956–2963. doi: 10.4049/jimmunol.169.6.2956. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz JJ, Lee C, Shendure J. Accurate gene synthesis with tag-directed retrieval of sequence-verified DNA molecules. Nat Methods. 2012;9(9):913–915. doi: 10.1038/nmeth.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma S, Saaem I, Tian J. Error correction in gene synthesis technology. Trends Biotechnol. 2012;30(3):147–154. doi: 10.1016/j.tibtech.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt NW, Butler NS, Badovinac VP, Harty JT. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 2010;6(7):e1000998. doi: 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hafalla JC, et al. Priming of CD8+ T cell responses following immunization with heat-killed Plasmodium sporozoites. Eur J Immunol. 2006;36(5):1179–1186. doi: 10.1002/eji.200535712. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, et al. Evidence-based annotation of the malaria parasite's genome using comparative expression profiling. PLoS One. 2008;3(2):e1570. doi: 10.1371/journal.pone.0001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarun AS, et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci USA. 2008;105(1):305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Roch KG, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301(5639):1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 42.Florens L, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419(6906):520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 43.Le Roch KG, et al. Global analysis of transcript and protein levels across the Plasmodium falciparum life cycle. Genome Res. 2004;14(11):2308–2318. doi: 10.1101/gr.2523904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall N, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307(5706):82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 45.Lau LS, et al. Blood-stage Plasmodium berghei infection generates a potent, specific CD8+ T-cell response despite residence largely in cells lacking MHC I processing machinery. J Infect Dis. 2011;204(12):1989–1996. doi: 10.1093/infdis/jir656. [DOI] [PubMed] [Google Scholar]
- 46.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012;33(5):247–254. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Hafalla JC, et al. Early self-regulatory mechanisms control the magnitude of CD8+ T cell responses against liver stages of murine malaria. J Immunol. 2003;171(2):964–970. doi: 10.4049/jimmunol.171.2.964. [DOI] [PubMed] [Google Scholar]
- 48.Cockburn IA, Chakravarty S, Overstreet MG, García-Sastre A, Zavala F. Memory CD8+ T cell responses expand when antigen presentation overcomes T cell self-regulation. J Immunol. 2008;180(1):64–71. doi: 10.4049/jimmunol.180.1.64. [DOI] [PubMed] [Google Scholar]
- 49.Porter DW, et al. A human Phase I/IIa malaria challenge trial of a polyprotein malaria vaccine. Vaccine. 2011;29(43):7514–7522. doi: 10.1016/j.vaccine.2011.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müllbacher A, Lobigs M, Kos FJ, Langman R. Alloreactive cytotoxic T-cell function, peptide nonspecific. Scand J Immunol. 1999;49(6):563–569. doi: 10.1046/j.1365-3083.1999.00568.x. [DOI] [PubMed] [Google Scholar]
- 51.Moon JJ, et al. Tracking epitope-specific T cells. Nat Protoc. 2009;4(4):565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sano G, et al. Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J Exp Med. 2001;194(2):173–180. doi: 10.1084/jem.194.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458(7235):211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.