Abstract
We previously demonstrated that cardiac myosin can use 2-deoxy-ATP (dATP) as an energy substrate, that it enhances contraction and relaxation with minimal effect on calcium-handling properties in vitro, and that contractile enhancement occurs with only minor elevation of cellular [dATP]. Here, we report the effect of chronically enhanced dATP concentration on cardiac function using a transgenic mouse that overexpresses the enzyme ribonucleotide reductase (TgRR), which catalyzes the rate-limiting step in de novo deoxyribonucleotide biosynthesis. Hearts from TgRR mice had elevated left ventricular systolic function compared with wild-type (WT) mice, both in vivo and in vitro, without signs of hypertrophy or altered diastolic function. Isolated cardiomyocytes from TgRR mice had enhanced contraction and relaxation, with no change in Ca2+ transients, suggesting targeted improvement of myofilament function. TgRR hearts had normal ATP and only slightly decreased phosphocreatine levels by 31P NMR spectroscopy, and they maintained rate responsiveness to dobutamine challenge. These data demonstrate long-term (at least 5-mo) elevation of cardiac [dATP] results in sustained elevation of basal left ventricular performance, with maintained β-adrenergic responsiveness and energetic reserves. Combined with results from previous studies, we conclude that this occurs primarily via enhanced myofilament activation and contraction, with similar or faster ability to relax. The data are sufficiently compelling to consider elevated cardiac [dATP] as a therapeutic option to treat systolic dysfunction.
Keywords: metabolism, inotropy, cross-bridge cycling
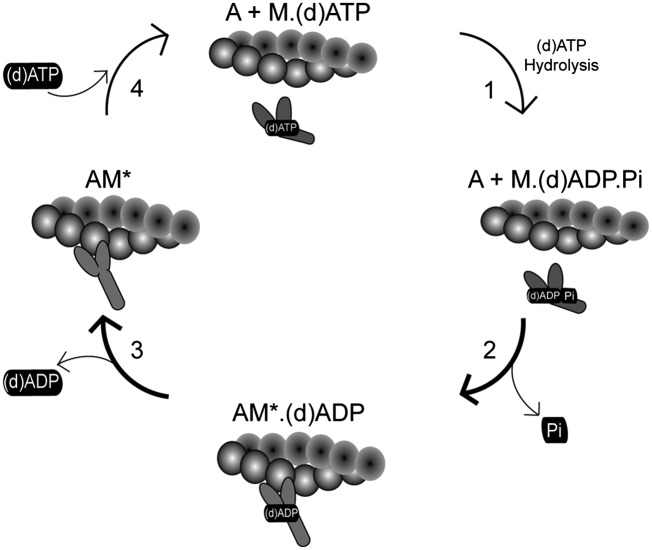
A wide variety of cardiac pathologies such as myocardial infarct, dilated cardiomyopathy, and congestive heart failure involve reduced systolic function of ventricles that often results from altered ATP-mediated actin–myosin (cross-bridge) cycling (1–4). The schematic shown in Fig. 1 outlines the basic components of this chemomechanical cycle that are critical to understand how cardiomyocytes use energy to develop tension and shortening: (i) ATP bound to detached myosin is hydrolyzed to ADP and inorganic phosphate (Pi); (ii) myosin binds to actin and undergoes the power-stroke associated with Pi release, tension development, and shortening; (iii) ADP is released from myosin; and (iv) ATP binds and precipitates myosin detachment from actin.
Fig. 1.
Scheme illustrating a four-step cross-bridge model of contraction. Major transitions are labeled. Dark arrows indicate transitions that we hypothesize to be enhanced when dATP is used as a substrate for contraction rather than ATP.
We (5–8) and others (9–17) have reported that striated muscle myosin can use most naturally occurring nucleotides to support cross-bridge cycling and contraction to varying degrees. Although most are not as effective as ATP, we found that 2-deoxy-ATP (dATP) is more effective than ATP as a substrate for contraction of demembranated cardiac muscle, augmenting both force and shortening at all levels of Ca2+-mediated contractile activation (5–8). Our detailed biochemical and mechanical analysis suggests that dATP increases the rates of both myosin binding and product release (steps 2 and 3 in Fig. 1) (6). More recently, we reported that adenoviral overexpression of ribonucleotide reductase (RR), the enzyme that converts ADP to dADP (which is rapidly phosphorylated to become dATP), increases the dATP content in cultured adult or neonatal rat cardiomyocytes ∼10-fold. This significantly enhanced the magnitude and rate of shortening and increased the rate of relaxation, without affecting the magnitude of intracellular Ca2+ transients (18). Interestingly, the 10-fold increase in cellular dATP content still represents <1–2% of the adenosine triphosphate nucleotide pool, and we also showed that this amount was sufficient to significantly increase force production of demembranated cardiac muscle at levels of Ca2+ measured during cardiac twitch contractions (18).
Although we demonstrated that acute increases in [dATP] (via RR overexpression) positively impacted contractility of cardiomyocytes in vitro, it was not clear whether this elevated function would occur in vivo. Additionally, it is not known whether chronically elevated dATP would have negative effects on myocardial energetics and/or cardiac function. Here, we report an animal model with enhanced cardiomyocyte cross-bridge chemomechanical cycling via manipulation of the adenosine triphosphate nucleotide pool of healthy animals. We studied a transgenic mouse line that globally overexpresses both the large (ribonucleotide reductase M1, Rrm1) and small (ribonucleotide reductase M2, Rrm2) subunits of RR (TgRR), compared with their wild-type (WT) littermates (named Rrm1Tg + Rrm2Tg in ref. 19). In this study, TgRR and WT animals were characterized for in vivo and in vitro cardiac function, myocardial energetics, and cardiomyocyte contractile properties to assess the effect of chronic, global overexpression of Rrm1 and Rrm2 (and thus increased dATP) on cardiac function. We found that left ventricular (LV) function is elevated in TgRR mice vs. WT under basal conditions but is similar during β-adrenergic challenge. The enhanced cardiac function was associated with an increased magnitude and rate of contraction and relaxation of isolated cardiomyocytes, without an increase in Ca2+ transient amplitudes. Furthermore, the improved cardiac function occurred at a normal energetic cost and with no effect on smooth muscle contraction. These data suggest that manipulation of the adenosine triphosphate nucleotide pool to increase cardiomyocyte intracellular dATP concentration merits further investigation as a potential therapeutic option for treatment of systolic heart failure.
Results
Heart Size and Histology.
At 3–5 mo of age, body weight (BW), heart weight (HW), and HW relative to BW (HW/BW) were obtained for TgRR mice and littermate controls (WT). No significant differences were noted in these measures (Table 1). The similar HW and HW/BW values suggest there is no overt hypertrophy (at least to 3–5 mo of age) resulting from the chronic inotropic state induced by elevated dATP. Histological assessment of TgRR and WT hearts showed no appreciable difference in myocyte size, organization, or fibrosis between groups at both 3 mo and 12 mo of age, which further demonstrates that TgRR hearts are not morphologically different from controls (Fig. S1).
Table 1.
Descriptive statistics of animal phenotype
| Measurement | WT | TgRR |
| BW, g | 27.91 ± 0.87 | 26.02 ± 1.11 |
| HW, mg | 115.32 ± 8.52 | 106.00 ± 3.64 |
| HW/BW, mg/g | 4.19 ± 0.19 | 4.36 ± 0.15 |
BW, HW, and HW/BW ratio from WT and TgRR animals between 3–5 mo of age. Data are presented as means ± SEM (WT, n = 6; TgRR, n = 6).
Echocardiography.
In vivo cardiac function was assessed via echocardiography for both WT and TgRR mice (Table 2). TgRR mice had significantly increased fractional shortening (FS) (from 34.6 ± 2.2% to 43.5 ± 1.4%; P < 0.05), ejection fraction (EF) (70.1 ± 2.8% to 80.7 ± 1.4%; P < 0.05), and cardiac output (from 30.0 ± 3.2 mL/min to 41.0 ± 2.8 mL/min; P < 0.05) at similar heart rates. During diastole, neither LV internal dimension (LVID;d) nor LV posterior wall thickness (LVPW;d) was altered in the transgenic animals (Table 2). These data suggest that increased cellular concentration of RR and dATP can result in a beat-to-beat elevation of cardiac performance in vivo. Combined with the similar HW/BW ratio for TgRR and WT mice, these data support the idea that enhanced ventricular function did not result in cardiac hypertrophy. Importantly, there were no significant differences in diastolic dimensions, suggesting no evidence of LV dilation. This indicates that increased cardiac output is achieved by increased contractile force and not attributable to changes in LV filling volume. These findings, combined with the HW/BW and histological observations described above, demonstrate that there is little or no compensatory adverse cardiac remodeling in TgRR mice.
Table 2.
Echocardiography measurements
| Measurement | WT | TgRR |
| LVPW;d, mm | 0.70 ± 0.12 | 0.81 ± 0.04 |
| LVID;d, mm | 3.22 ± 0.11 | 3.30 ± 0.14 |
| LVPW;s, mm | 1.04 ± 0.07 | 1.21 ± 0.04* |
| LVID;s, mm | 2.10 ± 0.09 | 1.72 ± 0.10* |
| FS, % | 34.58 ± 2.17 | 43.54 ± 1.43* |
| EF, % | 70.65 ± 2.75 | 80.68 ± 1.45* |
| HR, bpm | 454.20 ± 28.57 | 497.10 ± 21.67 |
| CO, mL/min | 30.00 ± 3.16 | 41.00 ± 2.77* |
Echocardiographic measures obtained in WT and TgRR animals. Data are presented as means ± SEM (WT, n = 5; TgRR, n = 10). CO, cardiac output; LVID;d, LVID in diastole; LVID;s, LVID in systole; LVPW;d, LVPW in diastole; LVPW;s, LVPW in systole.
*P < 0.05.
Ex Vivo Cardiac Function with 31P NMR Spectroscopy.
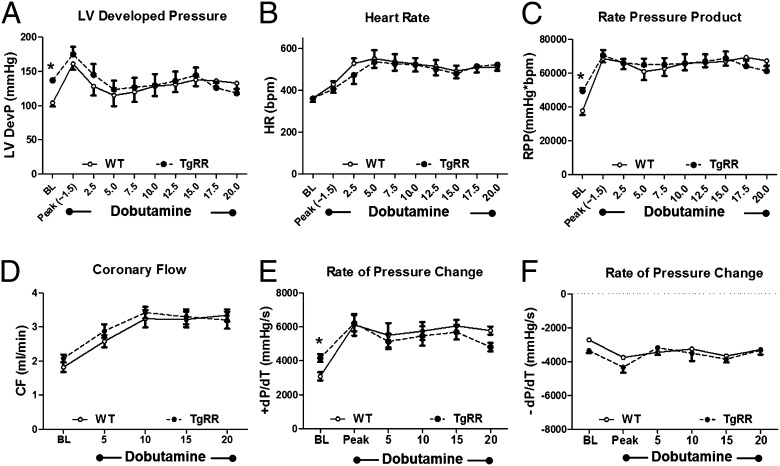
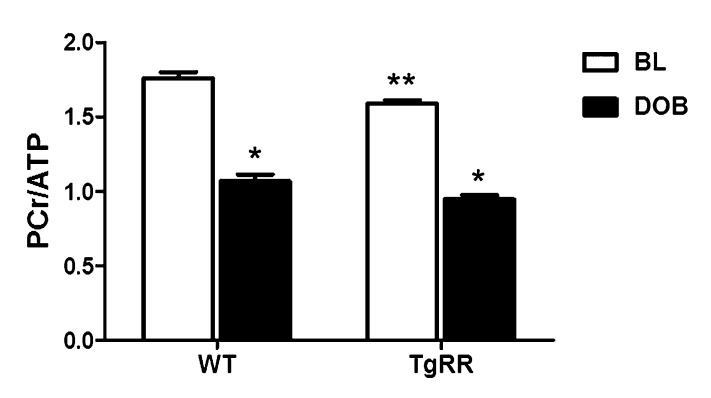
To quantitatively assess the effects of increased Rrm1 and Rrm2 (RR) and dATP on myocardial energetics and cardiac work, we measured high-energy phosphate content with 31P NMR spectroscopy in Langendorff-isolated heart preparations. Consistent with in vivo echocardiography data, TgRR hearts demonstrated a ∼30% increase in LV developed pressure (LVDevP) (P < 0.05, Fig. 2A and Fig. S2A) at a similar heart rate (HR) at baseline (Fig. 2B and Fig. S2B). Overall myocardial performance (or work) was assessed by the rate–pressure product (RPP), the product of LVDevP and HR. This was also significantly elevated in TgRR compared with WT under normal workload conditions (Fig. 2C and Fig. S2C) with equivalent coronary flows (Fig. 2D and Fig. S2D). A marker of the rate of contractility, the positive maximum LV pressure wave (+dP/dt), was ∼35% higher in TgRR mice (P < 0.05 vs. WT at BL; Fig. 2E and Fig. S2E), whereas the negative maximum LV pressure wave (–dP/dt), a marker of ventricular relaxation rate, appeared faster but was not significantly different (Fig. 2F and Fig. S2F). This enhanced cardiac performance at baseline resulted in a ∼10% decrease in levels of the energy reserve compound phosphocreatine (PCr) in TgRR mice (P < 0.05 vs. WT; Fig. 3 and Fig. S3A). Even though this is a statistically significant reduction, the energetic reserves are still quite considerable [pathologic conditions are associated with 40–50% decreases in PCr (20–22)]. There was no significant difference in ATP content (Fig. S3B), but Pi was significantly greater in TgRR hearts (P < 0.05 vs. WT; Fig. S3C) with no difference in intracellular pH (Fig. S3D). Even though elevation of Pi has not been demonstrated to significantly reduce function in the heart as it does in skeletal muscle, the higher Pi concentration (2.98 ± 0.37 vs. 4.29 ± 0.37 mM, respectively) could reduce LVDevP via Pi-mediated inhibition of force production (23–25). If so, the potential for increased LV function by elevated cardiomyocyte dATP levels may be even greater than in Table 2 under in vivo conditions where cardiac tissue Pi buffering occurs. Combined, these results suggest that increased cardiac contractility in TgRR hearts causes a mild reduction in high-energy phosphate reserves, without compromising cellular ATP concentration under normal workload (basal) conditions.
Fig. 2.
Response of LV function during 20 min of acute physiological demand. (A–C) LVDevP, HR, and RPP, the product of LVDevP and HR, measured in isolated hearts perfused at baseline (BL) and dobutamine infusion (n = 5 each group). (D) Coronary flow, estimated by collecting the perfusate effluent over a 2-min period, in Langendorff heart preparations during normal workload and dobutamine infusion (n = 5 each group). (E and F) Rate of pressure change calculated by the first derivative of the LV pressure wave (dP/dt) in Langendorff heart preparations at baseline and during dobutamine infusion. The positive maximum (+dP/dt) is an index of the rate of LV pressure development. The negative maximum (−dP/dt) is an index of the rate of ventricular relaxation. *P < 0.05 vs. WT at baseline (n = 5 each group).
Fig. 3.
Energetic response to dobutamine challenge. Ratio of PCr and ATP, as measured by 31P NMR spectroscopy in isolated perfused hearts at baseline (BL) and at the end of 300 nM dobutamine infusion (DOB). *P < 0.05 vs. WT at baseline (n = 5 each group).
To test whether overexpression of the RR transgene alters myocardial responsiveness to β-adrenergic stimulation, we infused dobutamine in isolated perfused hearts from TgRR and WT mice. During the initial 90 s of dobutamine infusion, LVDevP, +dP/dt, and –dP/dt were significantly increased in both WT and TgRR hearts, demonstrating expected peak β-adrenergic responsiveness (Fig. 2 A, E, and F). However, as the HR approached 500 beats per minute (bpm) after 5 min of dobutamine infusion (Fig. 2B), LVDevP, +dP/dt, and –dP/dt declined in both groups (Fig. 2 A, E, and F), consistent with the negative treppe effect (i.e., force–frequency relationship) in mouse myocytes (26). Over the final 15 min of the experimental period, HR remained significantly elevated to a comparable degree in both TgRR and WT mice, demonstrating normal adrenergic reserve (Fig. 2B and Fig. S2B). Interestingly, even though LVDevP at the end of dobutamine infusion in WT hearts was similar to baseline values in TgRR (Fig. S2A), dobutamine infusion did statistically increase RPP over the entire infusion time course and remained statistically elevated over baseline levels in both groups at the end of the protocol (Fig. 2C and Fig. S2C). Thus, the TgRR hearts generate higher baseline force and respond to adrenergic challenge principally with increased rate. The normal rate response suggests that much of β-adrenergic signaling is intact, neither blunted nor sensitized, in the TgRR heart. Both TgRR and control hearts experienced similar decreases in PCr (∼40%) and ATP (∼20%) in response to acute demand (Fig. 3 and Fig. S3) with associated increases in Pi (Fig. S3C). Intracellular pH remained stable in hearts for both groups (Fig. S3D). In total, these data indicate that TgRR hearts responded to the acute physiological challenge with a similar work output to controls with comparable changes of high energy phosphate content.
dATP Does Not Affect Cardiac Smooth Muscle Function.
Although we (5–8) and others (9–17) have characterized the effect of dATP on striated (skeletal and cardiac) muscle, its effect on smooth muscle is unknown. To determine whether vascular smooth muscle responds differently than striated muscle, we measured contraction of nontransgenic mouse aortic muscle strips with complete replacement of ATP for dATP. Fig. 4 demonstrates that there was no significant difference in the mechanical and biochemical parameters measured for skinned aortic smooth muscle activated with 5 mM ATP vs. 5 mM dATP. When smooth muscle was activated, force rapidly increased to a steady state with both nucleotides. Maximal Ca2+-activated force was 25.7 ± 4.8 mN/mm2 (n = 8) vs. 25.4 ± 5.5 mN/mm2 (n = 7) for ATP vs. dATP, respectively. Because myosin light chain (LC20) phosphorylation regulates smooth muscle cross-bridge cycling, we quantified the level of phosphorylation in smooth muscle preparations with solutions containing ATP vs. dATP. For ATP, LC20 phosphorylation was 2 ± 0.5% at rest [negative logarithm of calcium ion concentration (pCa) 9] and increased to 33 ± 2% at pCa 4 (n = 3), whereas for dATP, LC20 phosphorylation increased from 3 ± 1% to 34 ± 5% (n = 3). Because LC20 phosphorylation did not differ between preparations with ATP vs. dATP, these data suggest that dATP does not alter the regulation of smooth muscle. Importantly, this indicates that even though increased [dATP] significantly enhances striated muscle contraction, smooth muscle contraction is unchanged by dATP.
Fig. 4.
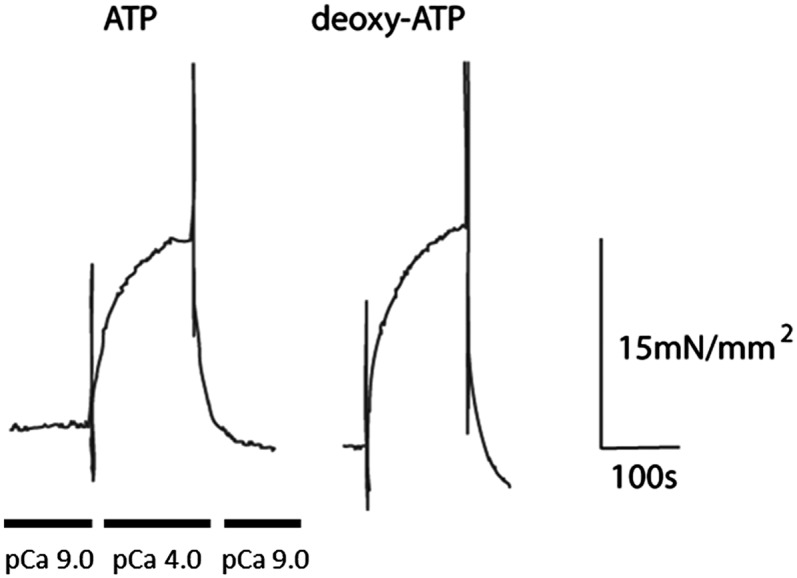
Smooth muscle contraction is not disrupted in WT mice with full exchange of dATP for ATP. An example trace from smooth muscle contraction assay is shown. Maximum force was not different between two groups: WT mouse smooth muscle strips with either 5 mM ATP or 5 mM dATP. Vertical lines before force development and immediately following plateau are noise resulting from switching calcium solutions.
TgRR Mice Have Enhanced Cardiomyocyte Contractility.
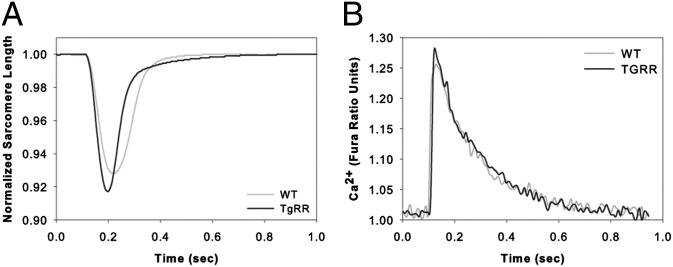
Video imaging of sarcomere length (IonOptix) was used to compare the rate and extent of shortening and relengthening (relaxation) in single ventricular cells from both TgRR and WT mice. Simultaneous measurements of Ca2+ transients were made using Fura-2 fluorescence. Representative shortening traces and Ca2+ transients can be seen in Fig. 5 A and B, respectively. Cardiomyocytes from the TgRR mice had enhanced contractility relative to control (WT) cells, measured by a number of parameters (Table 3). The rate and magnitude of shortening were increased by 15.4% and 21.5%, respectively, and rate of relaxation was increased 28.4% (Fig. 5A and Table 3). These increases in contraction and relaxation properties are similar, but smaller in magnitude, to those we recently reported for adult and neonatal rat cardiomyocytes transduced with an adenoviral vector to elevate cellular RR levels and dATP content (AV-RR) (18). These cardiomyocyte contractility data support our in vivo observations of increased FS and EF, as well as our in vitro observations of increased LV rate and magnitude of pressure development. Interestingly, the increased cardiomyocyte contractility occurred with no change in Ca2+ transient amplitude (Fig. 5B and Table 3); thus, myofilament responsiveness to Ca2+ is increased in TgRR animals (Table 3). This also agrees with our previous report of AV-RR–transduced adult rat cardiomyocytes (18) and, together, suggests that increasing dATP concentration acts primarily to increase myofilament cross-bridge cycling at similar levels of Ca2+.
Fig. 5.
Representative sarcomere length (A) and calcium (B) traces from TgRR and WT single isolated ventricular myocytes. (A) Representative sarcomere length traces obtained using video microscopy (IonOptix). WT shortening is shown in gray and TgRR is shown in black. (B) Representative Fura-2 fluorescence traces obtained during shortening. No observable difference in calcium handling was noted. WT is shown in gray and TgRR in black. Quantification of these traces (and others) can be found in Table 2.
Table 3.
Sarcomere length and calcium transient values at 1-Hz stimulation
| Measurement | WT | TgRR |
| FS, % | 7.26 ± 0.36 | 8.40 ± 0.23* |
| Rate of shortening, μm/s | −2.93 ± 0.18 | −3.56 ± 0.12* |
| Time to Peak, ms | 99.95 ± 3.23 | 92.92 ± 2.61 |
| Rate of Relaxation, μm/s | 1.76 ± 0.15 | 2.26 ± 0.11* |
| Magnitude of Ca2+ release, % | 38.14 ± 1.89 | 39.08 ± 1.69 |
| Rate of Ca2+ release, fluorescent ratio units per second | 24.90 ± 1.18 | 25.71 ± 1.08 |
| Rate of Ca2+ reuptake, fluorescent ratio units per second | −1.98 ± 0.09 | −1.95 ± 0.10 |
Measurements of sarcomere length and calcium transients were obtained in isolated cardiomyocytes from WT and TgRR at 3–5 mo of age. Cardiomyocytes were stimulated at 1 Hz. Data are presented as means ± SEM (WT, n = 73 cells; TgRR, n = 75 cells).
*P < 0.05.
Discussion
The purpose of this study was to characterize the cardiac function of transgenic animals that overexpress RR, resulting in increased cardiomyocyte [dATP]. The data presented here demonstrate that increased rate and magnitude of cardiomyocyte contraction and increased cardiac performance resulting from elevated cardiomyocyte [dATP] persist into adulthood. We further show that this occurs with minimal effects on cardiac energetic reserves. Additionally, β-adrenergic rate responsiveness to increase cardiac output is maintained for TgRR mice, although the absolute magnitude of increased cardiac output is somewhat reduced because of significantly increased basal pressure development. Importantly, with β-adrenergic stimulation maximal performance is maintained and myocardial energetics are comparable to WT hearts. Also, important is that enhancement in contractile function does not result from, or result in, hypertrophy or altered diastolic dimensions, at least out to 3–5 mo of age in mice. To further assess changes in the expression of genes related to myosin isoforms and cardiac hypertrophy, we measured expression levels of myh6 (α-myosin), myh7 (β-myosin), anp (atrial natriuretic peptide), and bnp (brain natriuretic peptide) via RT-PCR (Fig. S4). Both myh6 and myh7 were significantly down-regulated to a similar degree in TgRR vs. WT (Fig. S4 A and B). Because up-regulation of the fetal isoform myh7 is associated with cardiac pathologies in mice, these data suggest that chronic elevation of dATP did not cause pathological-associated changes in the myosin isoforms. Consistent with this, up-regulation of anp or bnp was not observed in TgRR hearts. This elevation of baseline cardiac function may persist through life, because we have observed elevation of LV function with no signs of hypertrophy in 8–10 mo TgRR mice.
The assessment of contractile function presented in this study strongly indicates that the primary effect of dATP is on the myofilament level, because Ca2+ transients are not affected. This conclusion is supported by previously published biochemical and mechanical studies suggesting that dATP binds to the myosin head and results in increased chemomechanical cycling. In skeletal muscle, the forward rate of cross-bridge attachment and the power stroke (transition 2 in Fig. 1: A + M⋅ADP⋅Pi → AM*ADP ± Pi; where A is actin, M is myosin, and AM is actin– myosin complex) and the rate of ADP release (transition 3 in Fig. 1: AM*ADP → AM*) are increased when dATP is used as a substrate for contraction (vs. ATP) (6). The increase in skeletal muscle contraction is relatively small compared with what we have reported for cardiac muscle containing either α- or β-myosin (5, 7, 8). Indeed, with only 2% dATP (98% ATP; 5 mM total NTP), there is significant augmentation of force in demembranated cardiac muscle at (submaximal) Ca2+ concentrations that occur during a cardiac twitch (18). Because dATP-binding affinity is similar to that for ATP (18), we hypothesize that contractile augmentation may occur because the few myosin cross-bridges using dATP may bind and cycle more rapidly to actin than myosins using ATP. Indeed, recent evidence suggests that small changes in the minority population of rat ventricular β-myosin content or the minority content of rabbit and human ventricular α-myosin content have a significant effect on the magnitude and rate of cardiac tissue contraction and relaxation (27–29). It may be that the initial, rapid binding of a few myosins can augment thin filament activation, which is a highly cooperative process where initial Ca2+ binding allows subsequent myosin binding, and this facilitates tropomyosin movement and stabilization (30, 31) and additional Ca2+ binding (32, 33). Both of these promote additional myosin cross-bridge binding in a highly cooperative manner for cardiac muscle (30, 31, 34).
Interestingly, dATP appears to be an effective substrate for contraction in all three muscle types (cardiac, skeletal, and smooth muscle), but its ability to enhance maximal force is restricted to cardiac muscle, where the augmentation of submaximal force is also most prevalent. Our studies have shown that it is an effective substrate for myosins from fast and slow twitch skeletal muscle (5–7), α- and β-cardiac muscle (8, 18, 35), vascular smooth muscle (this study), and human fetal skeletal muscle.
Importantly, dATP may also be used by other cellular ATPases. It has been reported that sarcoplasmic reticulum pump [sarcoplasmic reticulum calcium ATPase (SERCA)2a] activity is enhanced by dATP (36). Here, we observed similar Ca2+ transient-decay kinetics in cardiomyocytes from TgRR vs. WT mice, suggesting the level of dATP in the TgRR mice had little or no effect on SERCA2a function. However, we previously reported a small (albeit significant) increase in the rate of Ca2+ transient decay for cultured adult rat cardiomyocytes transduced with AV-RR to increase cellular [dATP] (18). Thus, it is reasonable to hypothesize that other cardiomyocyte ATPases could use dATP for function, although it is unknown whether the affinities of these ATPases is similar or dissimilar for dATP. However, given the millimolar levels of competing ATP in the cardiomyocyte and our inability to see the dATP signal by solution NMR (Fig. S3), it is unlikely that the levels of dATP attained in the TgRR mice (or through viral-mediated transgenesis of RR) would have a dominant effect, unless there is a highly cooperative interaction with other proteins, as occurs with cardiac thin filament activation. Future studies will be required to specifically determine the global influence of dATP on cardiomyocyte function.
Previous work has shown that TgRR animals significantly overexpress both Rrm1 and Rrm2 in both cardiac and skeletal muscle compared with their WT littermates and that this was associated with and ∼10-fold increase in cellular dATP concentration (19). This is similar to the level of elevation we recently reported for cultured adult cardiomyocytes transduced with adenoviral vectors to elevate Rrm1 and Rrm2 (18). Thus, small but significant increases in dATP content appear to be able to increase contractility at the cell, tissue (in vitro), and organ (in vivo) levels via enhancement of both the magnitude and kinetics of myofilament contraction and relaxation. Importantly, here we demonstrate that even chronic overexpression of Rrm1 and Rrm2 (and, thus, chronic increases in [dATP]) do not significantly deplete energetic reserves or eliminate adrenergic responsiveness. Also important for ischemic conditions is that dATP does not increase vascular smooth muscle contraction, suggesting it does not precipitate vasoconstriction. This is important because adequate coronary flow is required for long-term enhancement of cardiac performance.
Implications for Translational Research.
Although this study was primarily focused on characterizing healthy animals with increased dATP concentration, the results compel us to consider enhancing cardiac dATP concentration as a therapy to increase the basal contractility of the heart in cases where systolic function is reduced. Heart disease is the leading cause of mortality and morbidity in the United States and has been rising dramatically around the world (37). Many cardiopathologies, as well as ischemia–reperfusion injury and myocardial infarct, result in reduced systolic function (1–4). Whether heart failure results from infarction or other diseases that reduce systolic function, the most common treatment strategies are pharmacological therapies and/or surgery. However, these strategies have significant limitations, including multiple off-target drug effects, unintended drug activity within the target myocardium, and complications from surgery and recovery. Additionally, they ultimately only serve as short-term treatments that only slow progression of heart failure. Thus, novel strategies focused on recovery of function are desirable. A potential strategy is to improve basal cardiac function via direct enhancement of myofilament contractile capacity by altering substrate conditions for cross-bridge cycling. Here, we have reported considerable in vitro and in vivo evidence that increasing cardiomyocyte [dATP] increases contractility in muscle via increased (actin–myosin) cross-bridge binding and cycling, which elevates basal cardiac function. This elevation persists over time in transgenic mice, the hearts of which appear nonpathogenic, respond to a high workload challenge, and maintain normal myocardial energetics. Thus, further studies to test the potential of the elevated cardiac dATP approach for treatment of systolic failure are merited. Such studies could involve cardiac-targeted treatment to avoid potential organ-specific or systemic effects of elevated Rrm1 and Rrm2 overexpression and/or dATP.
Materials and Methods
Animal Model.
Transgenic mice that overexpress both the Rrm1 and Rrm2 subunits of ribonucleotide reductase via the chicken β-actin promoter and cytomegalovirus enhancer (referred to as TgRR mice hereafter) have been described previously (38). WT mice used in this study were transgenic animals bred on the same background (FVB/N) as TgRR mice. All animal experiments were approved by the University of Washington (UW) Animal Care Committee. Animals were cared for in accordance with US National Institutes of Health Policy on Humane Care and Use of Laboratory Animals in the Department of Comparative Medicine at UW.
Histology.
Adult mice were euthanized by carbon dioxide asphyxiation. Tissues for histological analysis were excised, fixed overnight at room temperature in 10% (vol/vol) buffered formalin, processed, and sectioned at 5μm thickness before staining with hematoxylin and eosin (H&E) or Masson’s trichrome. Sample dehydration, embedding, sectioning, and staining were conducted by the Histology Core Facility in the College of Veterinary Medicine at Cornell University.
Echocardiography.
Echocardiography was carried out on mice 3–5 mo old. LV end-diastolic (LVEDD) and LV end-systolic (LVESD) dimensions were determined and FS was calculated from these data using the formula: (LVEDD − LVESD)/LVEDD × 100. Additional information on echocardiographic measurements is provided in SI Materials and Methods.
RNA Isolation and Real-Time PCR.
Total RNA was isolated from frozen heart tissue using the RNeasy Mini Kit (Qiagen), and cDNA was synthesized using the Omniscript RT kit (Qiagen) according to the manufacturer’s guidelines. Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad) with the following primer sequences: myh6 forward (F), GGCAAAGTCACTGCGGAAACTGAA; myh6 reverse (R), TCTTGTCGAACTTGGGTGGGTTCT; myh7 F, GCAGCTGTGCATCAACTTCACCAA; myh7 R, TCCACTCAATGCCCTCCTTCTTGT; anp F, ATTGACAGGATTGGAGCCCAGAGT; anp R, TGACACACCACAAGGGCTTAGGAT; bnp F, GCCAGTCTCCAGAGCAATTCA; and bnp R, GGGCCATTTCCTCCGACTT.
Smooth Muscle Contractile Studies.
All solutions and the methods for tissue preparation have been described in detail (39–42) (SI Materials and Methods). Briefly, strips of mouse aorta were mounted between a force transducer and a motor, stretched, and skinned. They were then maximally activated with calcium. The effects of dATP were investigated in solutions containing either 5 mM ATP or 5 mM dATP.
LC20 Phosphorylation.
LC20 phosphorylation was determined as described previously (39–42) (SI Materials and Methods). Briefly, skinned tissue strips were denatured, washed, and dried. LC20 was solubilized in PAGE sample buffer, resolved on a polyacrylamide gel, and transferred to a nitrocellulose membrane. Phosphorylated and unphosphorylated LC20 were identified by immunoblotting using a LC20 mAb (Sigma).
Isolated Perfused Mouse Heart Preparation and 31P NMR Spectroscopy.
Myocardial energetics and LV function were measured in Langendorff isolated heart preparations combined with 31P NMR spectroscopy as described previously (43–45) (SI Materials and Methods) (43–45). In brief, excised mouse hearts were perfused at a constant pressure of 80 mmHg with a modified Krebs–Henseleit buffer at 37.5 °C. After equilibration, baseline function was monitored at a fixed end diastolic pressure of 8–10 mmHg. After baseline, dobutamine was infused. Dynamic changes in high-energy phosphate content (PCr, ATP, Pi) were monitored using 31P NMR spectroscopy simultaneously with continuous recording of LV function.
Adult Mouse Cell Isolation and Contractile Assessment.
Adult mouse cardiomyocytes were isolated by enzymatic digestion from 3- to 5-mo-old mice as described previously (46) in accordance with the Alliance for Cellular Signaling protocol (SI Materials and Methods). Briefly, the hearts were rapidly excised, cannulated, and perfused. Myocytes were dissociated via enzymatic digestion; following which, the ventricles were removed, minced, and, after reintroduction to calcium, resuspended in 37 °C standard media.
Contractile assessment of cells was carried out on the same day as isolation. Cells were treated with Fura-2–acetoxymethyl ester to measure calcium transients, and cell shortening and relengthening were recorded and measured using IonOptix SarcLen system video microscopy.
Statistical Analysis.
Statistical differences for echocardiography, sarcomere length, and calcium transients were performed using Student’s t test (SPSS Version 18). Summary ex vivo function data (Fig. 3 and Fig. S2) were assessed using a two-way ANOVA with a Bonferroni post hoc analysis. Figs. S2 and S3 data were analyzed using two-way ANOVA with repeated measures with a Bonferroni post hoc analysis (Graph Pad/Prism Version 5.01). P values < 0.05 were considered significant. Data are displayed as means ± SEM.
Supplementary Material
Acknowledgments
We thank Sarah Dupras and Dr. Elina Minami for assistance with mouse echocardiography measurements and analysis. This work was supported by National Institutes of Health (NIH) Grants R01 HL111197 and R01 HL65497 (to M.R.), R01 HL084642 and P01 HL094374 (to C.E.M.), R01 HL64387 and R21 HL091368 (to M.R. and C.E.M.), and HL064137 (to F.B.); the National Science Foundation Graduate Research Fellowship Program and NIH Training Grant T32EB001650 (to S.G.N.); NIH Grant R01 HL110349-02 (to R.T.); and NIH Grant F32 HL0962842 (to S.C.K.). M.R. is an Established Investigator of the American Heart Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220693110/-/DCSupplemental.
References
- 1.Alpert NR, Mulieri LA, Warshaw D. The failing human heart. Cardiovasc Res. 2002;54(1):1–10. doi: 10.1016/s0008-6363(02)00248-1. [DOI] [PubMed] [Google Scholar]
- 2.Maughan DW. Kinetics and energetics of the crossbridge cycle. Heart Fail Rev. 2005;10(3):175–185. doi: 10.1007/s10741-005-5248-2. [DOI] [PubMed] [Google Scholar]
- 3.Moss RL, Razumova M, Fitzsimons DP. Myosin crossbridge activation of cardiac thin filaments: Implications for myocardial function in health and disease. Circ Res. 2004;94(10):1290–1300. doi: 10.1161/01.RES.0000127125.61647.4F. [DOI] [PubMed] [Google Scholar]
- 4.Schaub MC, Hefti MA, Zuellig RA, Morano I. Modulation of contractility in human cardiac hypertrophy by myosin essential light chain isoforms. Cardiovasc Res. 1998;37(2):381–404. doi: 10.1016/s0008-6363(97)00258-7. [DOI] [PubMed] [Google Scholar]
- 5.Regnier M, Martyn DA, Chase PB. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys J. 1998;74(4):2005–2015. doi: 10.1016/S0006-3495(98)77907-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regnier M, Homsher E. The effect of ATP analogs on posthydrolytic and force development steps in skinned skeletal muscle fibers. Biophys J. 1998;74(6):3059–3071. doi: 10.1016/S0006-3495(98)78013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regnier M, Lee DM, Homsher E. ATP analogs and muscle contraction: Mechanics and kinetics of nucleoside triphosphate binding and hydrolysis. Biophys J. 1998;74(6):3044–3058. doi: 10.1016/S0006-3495(98)78012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regnier M, Rivera AJ, Chen Y, Chase PB. 2-Deoxy-ATP enhances contractility of rat cardiac muscle. Circ Res. 2000;86(12):1211–1217. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- 9.Schoffstall B, Chase PB. Increased intracellular [dATP] enhances cardiac contraction in embryonic chick cardiomyocytes. J Cell Biochem. 2008;104(6):2217–2227. doi: 10.1002/jcb.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoffstall B, Clark A, Chase PB. Positive inotropic effects of low dATP/ATP ratios on mechanics and kinetics of porcine cardiac muscle. Biophys J. 2006;91(6):2216–2226. doi: 10.1529/biophysj.105.079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SH, Fuchs F. Length dependence of cardiac myofilament Ca(2+) sensitivity in the presence of substitute nucleoside triphosphates. J Mol Cell Cardiol. 2002;34(5):547–554. doi: 10.1006/jmcc.2002.1537. [DOI] [PubMed] [Google Scholar]
- 12.Takenaka H, Ikehara M, Tonomura Y. Interaction between actomyosin and 8-substituted ATP analogs. Proc Natl Acad Sci USA. 1978;75(9):4229–4233. doi: 10.1073/pnas.75.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber A. Parallel response of myofibrillar contraction and relaxation to four different nucleoside triphophates. J Gen Physiol. 1969;53(6):781–791. doi: 10.1085/jgp.53.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pate E, Nakamaye KL, Franks-Skiba K, Yount RG, Cooke R. Mechanics of glycerinated muscle fibers using nonnucleoside triphosphate substrates. Biophys J. 1991;59(3):598–605. doi: 10.1016/S0006-3495(91)82275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pate E, Franks-Skiba K, White H, Cooke R. The use of differing nucleotides to investigate cross-bridge kinetics. J Biol Chem. 1993;268(14):10046–10053. [PubMed] [Google Scholar]
- 16.White HD, Belknap B, Jiang W. Kinetics of binding and hydrolysis of a series of nucleoside triphosphates by actomyosin-S1. Relationship between solution rate constants and properties of muscle fibers. J Biol Chem. 1993;268(14):10039–10045. [PubMed] [Google Scholar]
- 17.Shimizu T, et al. Nucleotide specificity of the enzymatic and motile activities of dynein, kinesin, and heavy meromyosin. J Cell Biol. 1991;112(6):1189–1197. doi: 10.1083/jcb.112.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korte FS, et al. Upregulation of cardiomyocyte ribonucleotide reductase increases intracellular 2 deoxy-ATP, contractility, and relaxation. J Mol Cell Cardiol. 2011;51(6):894–901. doi: 10.1016/j.yjmcc.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ylikallio E, et al. Ribonucleotide reductase is not limiting for mitochondrial DNA copy number in mice. Nucleic Acids Res. 2010;38(22):8208–8218. doi: 10.1093/nar/gkq735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottomley PA, et al. Reduced myocardial creatine kinase flux in human myocardial infarction: An in vivo phosphorus magnetic resonance spectroscopy study. Circulation. 2009;119(14):1918–1924. doi: 10.1161/CIRCULATIONAHA.108.823187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Chacko VP, Schär M, Akki A, Weiss RG. Impaired ATP kinetics in failing in vivo mouse heart. Circ Cardiovasc Imaging. 2011;4(1):42–50. doi: 10.1161/CIRCIMAGING.110.959320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao R, Nascimben L, Friedrich J, Gwathmey JK, Ingwall JS. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ Res. 1996;78(5):893–902. doi: 10.1161/01.res.78.5.893. [DOI] [PubMed] [Google Scholar]
- 23.Homsher E, Lacktis J, Regnier M. Strain-dependent modulation of phosphate transients in rabbit skeletal muscle fibers. Biophys J. 1997;72(4):1780–1791. doi: 10.1016/S0006-3495(97)78824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millar NC, Homsher E. Kinetics of force generation and phosphate release in skinned rabbit soleus muscle fibers. Am J Physiol. 1992;262(5 Pt 1):C1239–C1245. doi: 10.1152/ajpcell.1992.262.5.C1239. [DOI] [PubMed] [Google Scholar]
- 25.Tesi C, Colomo F, Piroddi N, Poggesi C. Characterization of the cross-bridge force-generating step using inorganic phosphate and BDM in myofibrils from rabbit skeletal muscles. J Physiol. 2002;541(Pt 1):187–199. doi: 10.1113/jphysiol.2001.013418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87(4):275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 27.Locher MR, Razumova MV, Stelzer JE, Norman HS, Moss RL. Effects of low-level α-myosin heavy chain expression on contractile kinetics in porcine myocardium. Am J Physiol Heart Circ Physiol. 2011;300(3):H869–H878. doi: 10.1152/ajpheart.00452.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herron TJ, Devaney EJ, Metzger JM. Modulation of cardiac performance by motor protein gene transfer. Ann N Y Acad Sci. 2008;1123:96–104. doi: 10.1196/annals.1420.011. [DOI] [PubMed] [Google Scholar]
- 29.Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90(11):1150–1152. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- 30.Gordon AM, Rivera AJ, Wang CK, Regnier M. Cooperative activation of skeletal and cardiac muscle. Adv Exp Med Biol. 2003;538:371–378. doi: 10.1007/978-1-4419-9029-7_34. [DOI] [PubMed] [Google Scholar]
- 31.Smith L, Tainter C, Regnier M, Martyn DA. Cooperative cross-bridge activation of thin filaments contributes to the Frank-Starling mechanism in cardiac muscle. Biophys J. 2009;96(9):3692–3702. doi: 10.1016/j.bpj.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martyn DA, Regnier M, Xu D, Gordon AM. Ca2+ - and cross-bridge-dependent changes in N- and C-terminal structure of troponin C in rat cardiac muscle. Biophys J. 2001;80(1):360–370. doi: 10.1016/S0006-3495(01)76020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YP, Fuchs F. Osmotic compression of skinned cardiac and skeletal muscle bundles: Effects on force generation, Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol. 1995;27(6):1235–1244. doi: 10.1016/s0022-2828(05)82385-5. [DOI] [PubMed] [Google Scholar]
- 34.Tanner BCW, Daniel TL, Regnier M. Sarcomere lattice geometry influences cooperative myosin binding in muscle. PLOS Comput Biol. 2007;3(7):e115. doi: 10.1371/journal.pcbi.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regnier M, et al. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys J. 2004;87(3):1815–1824. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trumble WR, Sutko JL, Reeves JP. Cardiac sarcolemmal and sarcoplasmic reticulum membrane vesicles exhibit distinctive (Ca-Mg)-ATPase substrate specificities. J Biol Chem. 1981;256(14):7101–7104. [PubMed] [Google Scholar]
- 37.WHO . Global Atlas on Cardiovascular Disease Prevention and Control. Geneva: WHO; 2011. [Google Scholar]
- 38.Xu X, et al. Broad overexpression of ribonucleotide reductase genes in mice specifically induces lung neoplasms. Cancer Res. 2008;68(8):2652–2660. doi: 10.1158/0008-5472.CAN-07-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogut O, Brozovich FV. Determinants of the contractile properties in the embryonic chicken gizzard and aorta. Am J Physiol Cell Physiol. 2000;279(6):C1722–C1732. doi: 10.1152/ajpcell.2000.279.6.C1722. [DOI] [PubMed] [Google Scholar]
- 40.Karagiannis P, Babu GJ, Periasamy M, Brozovich FV. The smooth muscle myosin seven amino acid heavy chain insert’s kinetic role in the crossbridge cycle for mouse bladder. J Physiol. 2003;547(Pt 2):463–473. doi: 10.1113/jphysiol.2002.035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karagiannis P, Babu GJ, Periasamy M, Brozovich FV. Myosin heavy chain isoform expression regulates shortening velocity in smooth muscle: Studies using an SMB KO mouse line. J Muscle Res Cell Motil. 2004;25(2):149–158. doi: 10.1023/b:jure.0000035879.87045.4b. [DOI] [PubMed] [Google Scholar]
- 42.Rhee AY, Ogut O, Brozovich FV. Nonmuscle myosin, force maintenance, and the tonic contractile phenotype in smooth muscle. Pflugers Arch. 2006;452(6):766–774. doi: 10.1007/s00424-006-0091-4. [DOI] [PubMed] [Google Scholar]
- 43.Kolwicz SC, Tian R. 2010. Assessment of cardiac function and energetics in isolated mouse hearts using 31P NMR spectroscopy. J Vis Exp (42):e2069.
- 44.Luptak I, et al. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116(8):901–909. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- 45.Yan J, et al. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119(21):2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, et al. Transcription factor CHF1/Hey2 regulates EC coupling and heart failure in mice through regulation of FKBP12.6. Am J Physiol Heart Circ Physiol. 2012;302(9):H1860–H1870. doi: 10.1152/ajpheart.00702.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.