Abstract
Proper localization of various ion channels is fundamental to neuronal functions, including postsynaptic potential plasticity, dendritic integration, action potential initiation and propagation, and neurotransmitter release. Microtubule-based forward transport mediated by kinesin motors plays a key role in placing ion channel proteins to correct subcellular compartments. PDZ- and coiled-coil-domain proteins function as adaptor proteins linking ionotropic glutamate and GABA receptors to various kinesin motors, respectively. Recent studies show that several voltage-gated ion channel/transporter proteins directly bind to kinesins during forward transport. Three major regulatory mechanisms underlying intracellular transport of ion channels are also revealed. These studies contribute to understanding how mechanical forces are coupled to electrical signaling and illuminating pathogenic mechanisms in neurodegenerative diseases.
Keywords: ion channel, kinesin, adaptor protein, intracellular transport
Introduction
Electrical and chemical signaling of neurons depends on concerted actions of various types of voltage- and ligandgated ion channels that are properly placed at distinct neuronal compartments. A fully differentiated neuron usually has multiple dendrites with elaborate branches and a long axon, responsible for inputs and outputs of neuronal electrical signals, respectively. Dendritic ion channels, including ionotropic neurotransmitter receptors, are involved in postsynaptic potential generation in response to neurotransmitters, dendritic integration, and back-propagation of action potentials, whereas axonal ion channels, including voltage-gated K+ (Kv) and Na+ (Nav) channels, are required for an action potential’s initiation, propagation, frequency, and waveform. Voltage-gated Ca2+ channels, together with soluble N-ethylmaleimide-sensitive factor activating proteins, are required for Ca2+-dependent neurotransmitter release at axonal terminals.
A neuron’s genetic material, genomic DNA, is constrained within its nucleus in the cell body, or soma. Although local translation in dendrites or axons does occur for some proteins, most proteins are synthesized in the soma, including ion channel proteins. Ion channel genes, of which there are more than 250, constitute approximately 1.2% of all known protein-coding genes in the human genome. Because of alternative splicing of channel subunit genes and multimerization of channel protein complexes, thousands of different channel protein complexes can be generated in the nervous system in total. How different channel complexes are precisely placed into correct neuronal compartments appears to be a great challenge to a neuron.
Intracellular active transport by molecular motors plays a major role in ion channel placement. There are three groups of molecular motors, kinesin, myosin, and dynein (see recent reviews for detailed information regarding the three motors—Hammer and Sellers 2012; Hirokawa and others 2009; Kardon and Vale 2009). In this review, we focus on the kinesin motors, since kinesin-mediated transport of ion channels is relatively better understood, compared with myosin- or dynein-mediated transport of ion channels, which is beyond the scope of this review. The kinesin family consists of 45 members that can be divided into three groups depending on the location of the motor domain: either N-terminal, middle or C-terminal (see a review for details, Hirokawa and Noda 2008). The four kinesins that will be discussed in this review are KIF1/kinesin-3, KIF3/kinesin-2, KIF5/kinesin-1, and KIF17/kinesin-2. They are all N-terminal kinesins that move toward the plus end of microtubules. Most N-terminal kinesin motor heavy chains function as a dimer with each containing a motor domain, a coiled-coil domain for dimerization, and a tail domain that binds cargoes. KIF1 is an exception and it is a monomeric motor. The N-terminal kinesin motor “walks” down microtubules over long distances by use of ATP hydrolysis (Yang and others 1990).
How a limited number of kinesin motors selectively transport thousands of ion channel complexes is an intriguing question. The prevailing theme was that various ion channels are linked to kinesin motors through specific adaptor proteins. However, recent studies suggest that some ion channels or transporters may directly bind to kinesin motors without any adaptor protein. In this review, we focus on adaptor proteins and the physical link between ion channels and kinesins. We start our discussion with the transport of ligand-gated ion channels, including glutamate and GABAA receptors. These receptors are involved in fast synaptic transmission and have been extensively studied. Different adaptor proteins have been identified so that the same channel can be transported by different motors. Next, we discuss the direct binding of several voltage-regulated ion channels and transporters to kinesin motors. For most ion channels, the adaptor proteins and/or kinesins remain unknown. Finally, we discuss three regulatory mechanisms underlying ion channel transport and their potential roles in neurological diseases.
Transport of Ion Channels via Adaptor Proteins
AMPA Receptors
AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionate) receptors are ligand-gated glutamate receptor ion channels that function in rapid excitatory synaptic transmission (Hollmann and Heinemann 1994; Seeburg 1993). This non-selective cation channel allows for the influx of sodium, potassium, or calcium ions. Most AMPA receptors are heterotetrameric channels that can be made up of various combinations of four subunits, consisting of symmetric “dimer of dimers” of GluR1 through GluR4 subunits. GluR subunits share a similar structure of 4 transmembrane (TM) segments with a pore loop between TM2 and TM3, an extracellular N-terminal domain, and an intracellular C-terminal domain (Fig. 1A). One interesting note is that if the channel contains the GluR2 subunit then it no longer permits calcium influx (Hollmann and others 1991). AMPA receptor trafficking has been extensively studied. Multiple kinesin motors and adaptor proteins have been identified.
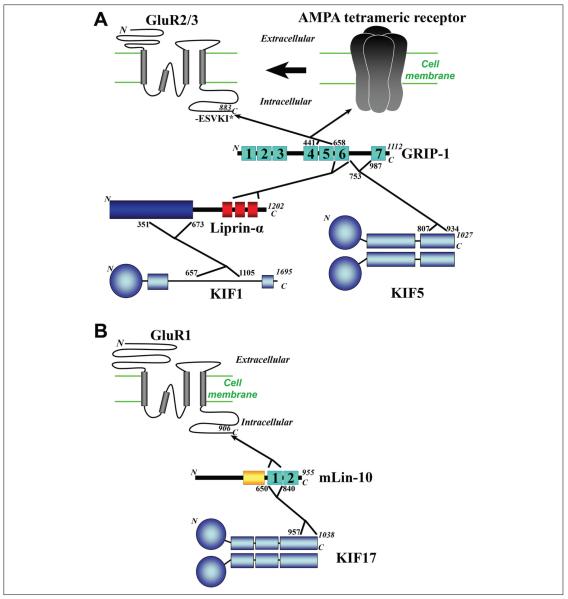
Figure 1.
Major binding proteins in the intracellular transport of AMPA receptors. (A) The GluR2/3 subunits of AMPA receptors interact with glutamate receptor interacting protein-1 (GRIP-1), which binds to either KIF5 or KIF1. The diagram of a tetrameric AMPA receptor/channel is on the upper right. The diagram of a single GluR subunit is on the upper left, which contains four TM segments (gray bars), extracellular N-terminal domain, and intracellular C-terminal domain. The GluR2/3 C-termini bind to the PDZ5 domain (aa. 441–658) of GRIP-1. The residue number and the last five residues (-ESVKI*) are from GluR2. The cyan boxes numbered from 1 to 7 indicate seven PDZ domains. The region between PDZ6 and PDZ7 of GRIP-1 (aa. 753–987) interacts with the KIF5 C-terminal cargo binding region (aa. 807–934 from KIF5B). The PDZ6 domain (aa. 658–753) of GRIP-1 interacts with the C-terminal region of Liprin-α, which contains sterile α motifs (red boxes). The C-terminal half of the coiled-coil domain (the dark blue box) in the Liprin-α N-terminus (aa. 351–673) binds to the C-terminal region of the KIF1A motor (aa. 657–1105). Blue balls: N-terminal motor domains of kinesins. “N,” the N-terminus of the protein; “C,” the C-terminus of the protein; the number near the C-terminus; the total residue number. The residue numbers of protein binding sites are given whenever they are available. (B) The GluR1 subunit of AMPA receptors interacts with KIF17 through mLin-10. The PDZ1 domain of mLin-10 (aa. 650–840) can interact with both the GluR1 C-terminus and the KIF17 C-terminal region (aa. 957–1038). Yellow box: phosphotyrosine binding domain.
Glutamate receptor interacting protein-1 (GRIP-1) is a potential major adaptor protein linking AMPA receptors to kinesin motors. GRIP-1 was identified as a binding partner of the C-terminus of GluR2 with the yeast two-hybrid method (Dong and others 1997). The GRIP family proteins (GRIP-1 and GRIP-2) contain six or seven PDZ domains (Fig. 1A). The PDZ domain was named due to the first three proteins it was discovered in; mammalian PSD-95, drosophila Discs-large, and mammalian ZO-1 (Kennedy 1995). Two alternative splicing variants of GRIP-1, GRIP-1a and GRIP-1b, differ in their N-termini (Yamazaki and others 2001). The fifth PDZ domain of GRIP-1 and GRIP-2 binds to the C-terminal sequence of GluR2/3 subunits of the AMPA receptor (Dong and others 1997; Srivastava and others 1998; Wyszynski and others 1999). In particular, the region of GRIP-1 (aa ~441–658, PDZ5) was shown to directly interact with the C-terminal of GluR2 (-ESVKI; Fig. 1A), and deletion of the last seven residues of GluR2 eliminates this interaction (Dong and others 1997). The GRIP-1/KIF5 interaction was later mapped to the region between the sixth and seventh PDZ domains of GRIP-1 and the C-terminal tail of KIF5 (aa. 807–934, adjacent to the KLC-binding site, aa. 771–813; Setou and others 2002). The KIF5-binding site in GRIP-1 is not present in GRIP-2. The GRIP-1-binding site is present in all KIF5 isoforms (KIF5A, KIF5B, and KIF5C), but not in other KIFs (Setou and others 2002). This interaction leads to KIF5 being directed to the dendrites, where it functions as a motor to transport AMPA receptors (Fig. 1A; Setou and others 2002).
Liprin-α is another potential adaptor protein that links GluR2/3 receptors to a different kinesin motor, KIF1A. The Liprin-α family of proteins (α1 to α4) was originally found to bind to the LAR family of receptor proteintyrosine phosphatases (Pulido and others 1995; Serra-Pages and others 1995; Serra-Pages and others 1998). Liprin-α has an N-terminal coiled-coil domain and C-terminal sterile α motifs (Fig. 1A). The Liprin-α C-terminal region was shown to directly interact with the GRIP-1 PDZ6 domain using yeast two-hybrid assay (Fig. 1A; Wyszynski and others 2002). This is intriguing because this binding site is right next to the GluR2 binding site. Therefore, the two binding site may interact with each other. This seems to be the case, since interfering with the Liprin-α/GRIP-1 interaction led to reductions in GluR2 clustering along dendrites and in AMPA receptor surface expression (Wyszynski and others 2002). The N-terminal region of Liprin-α was also shown to bind to KIF1A C-terminus (Fig 1A; Shin and others 2003). This allows for transport of Liprin-α-associated proteins via KIF1A, including AMPA receptors, through Liprin-α’s interaction with GRIP-1 (Fig. 1A).
LIN-10, another PDZ-domain protein, initially discovered in Caenorhabditis elegans, play an important role in trafficking GLR-1 the C. elegans homolog of the mammalian GluR1 subunit (Kaech and others 1998; Rongo and others 1998). The mammalian ortholog, mLin-10, has two isoforms Mint-1 and Mint-2 (Okamoto and Sudhof 1997). mLin-10 contains a phosphotyrosine binding domain, and two PDZ domains (PDZ1-2) at its C-terminal region (Fig. 1B; Kaech and others 1998; Okamoto and Sudhof 1997). Both GST-pulldown and co-immunoprecipitation (co-IP) experiments showed a direct interaction between mLin-10 and GluR1. The first PDZ domain of mLin-10 can interact with the C-terminal tail of GluR1, and perhaps the tail of GluR2 as well (Fig. 1B). Exogenous expression of mLin-10 with a point mutation in the PDZ1 domain leads to an increase in surface expression of exogenous GluR1 (Stricker and Huganir 2003). However, the motor that transports the AMPA receptor via the mLin-10 adaptor is unknown, but it is most likely to be KIF17, the same motor to transport NMDA receptors.
The binding of different subunits of AMPA receptors to different adaptor proteins that are linked to different kinesin motors, reveals a complex transport system. Since AMPA receptors are tetramers, the composition of the AMPA tetramer complex may determine the transport system involved. Also it becomes possible that one AMPA tetramer receptor can be linked to two different kinesin motors, resulting in a “tug-of-war.” Furthermore, there is a common feature for all three potential adaptor proteins of AMPA receptors, their interaction with the AMPA receptor through PDZ domains (Fig. 1). This may lead to the discovery of more adaptor proteins by examining other PDZ domain containing proteins.
NMDA Receptors
NMDA (N-methyl-D-aspartate) receptors are ionotropic glutamate receptors that are non-selective ligand-gated cation channels with a high permeability for calcium. These receptors play a critical role in synaptic plasticity via calcium signaling and are key devices in learning and memory (Tang and others 1999; Tsien and others 1996). This channel is a heterotetramer made of NR1, NR2, and, occasionally, NR3 subunits (Carroll and Zukin 2002; Dingledine and others 1999; Laube and others 1998). These subunits contain a similar structure of four TM domains with a pore loop between TM2 and TM3 (Fig. 2). NMDA receptors are unique since they are gated by both ligand (requiring the binding of both glycine and glutamate) and voltage (Kleckner and Dingledine 1988). NMDA receptors are also special because of their voltage-sensitive block by extracellular Mg2+ (Nowak and others 1984). In adult hippocampus, NR2A and NR2B are the predominant NR2 subunits, and they determine many biophysical and pharmacological properties of NMDA receptors (Lau and Zukin 2007; Monyer and others 1994).
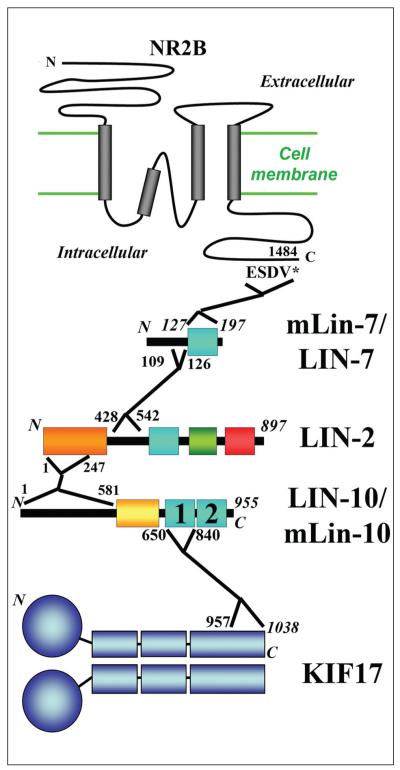
Figure 2.
Adaptor and motor proteins in the intracellular transport of NMDA receptors. During the intracellular transport of NMDA receptors, KIF17 interacts with the NR2B subunit through three PDZ-domain proteins. The NR2B C-terminus binds to the only PDZ domain of mLin-7 (aa. 127–197). The last residue number (1484) and the last four residues (-ESDV*) of NR2B are indicated. A small region right before the PDZ domain of LIN-7 (aa. 109–126) interacts with a region in the middle of LIN-2 (aa. 428–524). An N-terminal region of LIN-2 (aa. 1–247) interacts with the N-terminal region of LIN-10 (aa. 1–581). The PDZ1 of mLin-10 (aa. 650-840) binds to the C-terminal tail of KIF17 (aa. 957–1038). In the PDZ-domain proteins, cyan boxes, PDZ domains; yellow box in LIN-10/mLin-10, phosphotyrosine binding domain; orange box in LIN-2, N-terminal CaM kinase domain; green box in LIN-2, SH3 domain; red box in LIN-2, guanylate kinase domain.
Although mLin-10 may act as a potential adaptor protein for AMPA receptors containing GluR1, it certainly plays an important role in transporting NMDA receptors containing NR2B (Fig. 2). LIN-10 and other two PDZ-domain-containing proteins, LIN-2 and LIN-7, were initially implicated in polarized receptor localization to the basolateral membranes of vulval precursor cells in C. elegans (Simske and others 1996). LIN-2 is a MAGUK protein similar to mammalian CASK or mLin-2 (Hata and others 1996; Hoskins and others 1996). LIN-2 contains an N-terminal CaM kinase domain, a PDZ domain, a Src homology 3 (SH3) domain and a C-terminal guanylate kinase (GuK) domain (Kaech and others 1998). Using a yeast two-hybrid assay LIN-2 was shown to interact with both LIN-7 and LIN-10 via aa 428–542 and aa 1–247, respectively. Conversely, LIN-7 and LIN-10 interact with LIN-2 via aa 109–126 and aa 1–581 (Kaech and others 1998). LIN-7 is a small protein containing little more than a single PDZ domain, similar to mammalian MALS/Velis (or mLin-7), which directly binds to NR2B C-terminus (Jo and others 1999). LIN-10 contains two PDZ domains similar to mammalian Mint1 (or mLin-10; Kaech and others 1998; Okamoto and Sudhof 1997). The first PDZ domain within the C-terminal of mLin-10 was found to interact with the C-terminal of KIF17, and the interaction sites were mapped to aa. 650–840 and 957–1038, respectively (Fig. 2; Setou and others 2000). This interaction allows transport of a large protein complex containing the NR2B subunit. This interaction could be disrupted by mutating the PDZ1 domain of mLin-10 or deleting the last residue of the KIF17 tail. In living neurons, KIF17 transports vesicles containing NR2B in the dendrites without entering the postsynaptic regions directly, and knockdown or up-regulation of the channel or motor shows the possibility of a shared mechanism of regulation (Guillaud and others 2003). Disruption of the murine KIF17 gene inhibits NR2B transport, but not NR2A transport, suggesting that NR2A subunit is likely to be transported by a different molecular motor (Yin and others 2011). Reduced level of NR2 receptors in KIF17 knockout mice likely resulted in attenuation of NMDA receptor-mediated synaptic current, early and late long-term potentiation, and long-term depression (Yin and others 2011). Taken together, the link between ionotrophic glutamate receptors (including both AMPA and NMDA receptors) and kinesin motors appears to rely on various PDZ-domain-containing proteins (Figs. 1 and 2).
GABAA Receptors
GABAA (γ-aminobutyric acid type A) receptors are ligand-gated chloride channels, which are the major inhibitory neurotransmitter receptors in the central nervous system (Whiting 1999). These channels are pentameric channels made up of a combination of α, β, γ, δ, ε, θ, and π subunits (Bonnert and others 1999). The most common structure is a pentamer of 2α, 2β, and 1γ subunits, which is expressed at inhibitory synapses (Tretter and others 1997). Each subunit contains an extracellular N-terminal domain, four TM, domains and an extracellular C-terminal region (Fig. 2B).
The β2 subunit of GABAA receptors is the most abundant β subunit in the adult brain (Li and De Blas 1997; Sur and others 2001). Using this subunit as a bait, a yeast two-hybrid screen uncovered a new protein called GRIF-1 (GABAA receptor interacting factor 1; Beck and others 2002). GRIF-1 is an adaptor protein that contains two coiled-coil domains at the N terminal region (Fig. 3). GRIF-1 is the orthologue of the Drosophila protein Milton, a kinesin-binding protein involved in the transport of mitochondria (Stowers and others 2002). The first coil of the coiled-coil domain of GRIF-1 interacts with the GABAA receptor β2 subunit intracellular loop (Fig. 3; Beck and others 2002). KIF5C was shown to also directly interact with GRIF-1 via the first coiled-coil domain of GRIF-1 (Brickley and others 2005). This is intriguing because of the fact that this is the same region that interacts with GABAA β2. The GRIF-1/KIF5C interaction was further narrowed to the non-motor domain of KIF5C (Pozo and Stephenson 2006) before being specified to the cargo binding domain (aa. 827–957) of KIF5C (Fig. 3; Smith and others 2006).
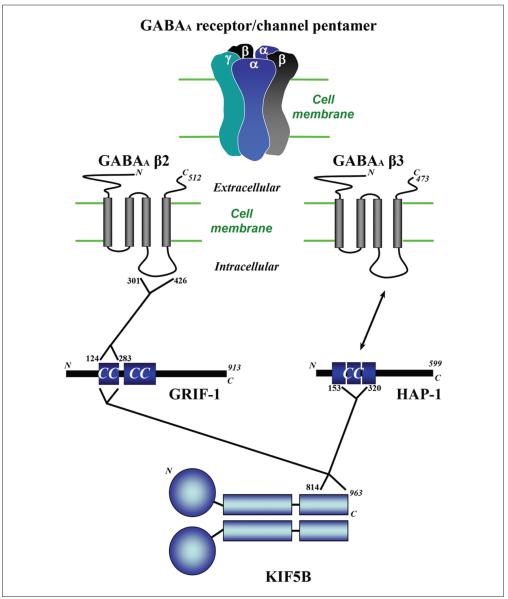
Figure 3.
Different adaptor proteins mediate the interaction between GABAA receptors and KIF5 motors. Pentameric structural diagram of the GABAA receptor is shown on the top. The first coiled-coil domain of GRIF-1 (aa. 124–283) can bind to either the second intracellular loop of GABAA β2 (aa. 301–426) or the C-terminal tail region of KIF5B (aa. 827–957). The coiled-coil domains of huntingtin-associated protein-1 (HAP-1; aa. 153-320) interact with C-terminal of KIF5B (aa. 814–963). Whether and how HAP-1 interacts with GABAA β3 directly remains unknown at this time. Blue boxes: coiled-coil domains.
Although GRIF-1 was shown to play an important role in anterograde trafficking of GABAA receptors, how these receptors reach the synapses was unknown at that time. Twelvetrees and others (2010) showed that GABAA trafficking to the synapse was regulated by another adaptor protein, huntingtin-associated protein 1 (HAP1). HAP1 contains three coiled-coil domains in its N-terminal region (Fig. 3). HAP1 was previously shown to directly interact with GABAA receptors via the β3 subunit (Fig. 3; Kittler and others 2004). HAP1 was co-IPed with KIF5C or GABAA receptor β3 subunits. GST pulldown with purified GST-HAP1 and both KIF5A-C heavy or kinesin light chain showed all three isoforms of KIF5 can interact directly with HAP1. The site of interaction was identified using GST-HAP1 fragments to pull down GFP-KIF5 fragments, to the coiled-coil domain of HAP1 (aa. 153–320) and the C-terminal tail of KIF5B (aa. 814–963). Interfering with the HAP1–KIF5 interaction, via dominant negative KIF5-HAP1 binding domain or HAP1 RNAi, reduces the surface expression of GABAA receptors (Twelvetrees and others 2010).
Since one channel complex often has multiple adaptor proteins, the potential interactions among these adaptors may play an important role in precisely transporting the channel complex to the correct location. For instance, one adaptor may carry the channel to a certain location, where it is picked up by another adaptor protein. Or maybe specific neuronal cell types contain only specific adaptor proteins. These are interesting questions for future investigation.
Transport of Voltage-Gated Ion Channel/Transporter Proteins via Direct Binding to Kinesin Motors
Kv3 (Shaw) Voltage-Gated Potassium (Kv) Channels and Kinesin 1
Although adaptor proteins are needed to link ionotrophic neurotransmitter receptors to kinesin motors, recent studies show that some other ion channels can interact directly with their kinesin motors. Kv channels play critical roles in regulating neuronal excitability and synaptic transmission. Members of the Kv channel superfamily display not only a variety of biophysical and pharmacological properties but also distinct expression and subcellular localization patterns (Gu and Barry 2011; Vacher and others 2008). Each Kv channel contains four voltage-sensing and pore-forming subunits. Each subunit consists of six TM domains and intracellular N- and C-terminal domains (Fig. 4A). N-terminal T1 domains form tetramers, responsible for the proper tetramerization of Kv channel subunits within a Kv channel subfamily (Choe 2002; Li and others 1992; Xu and others 1995). Zn2+-binding is required for assembly of T1 tetramers from Kv2, Kv3 and Kv4, but not Kv1 channels (Bixby and others 1999; Choe 2002; Jahng and others 2002).
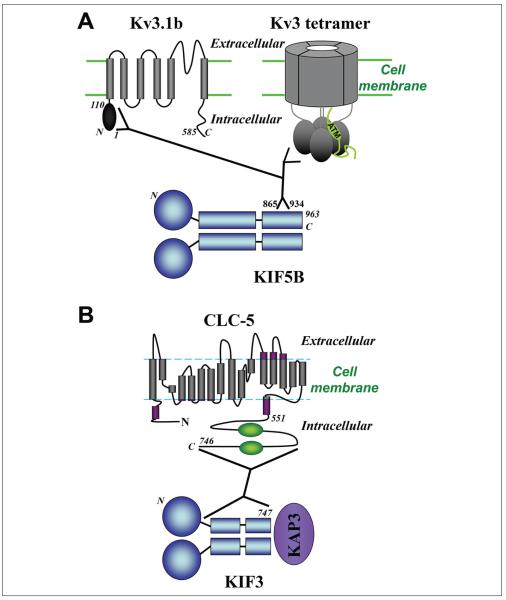
Figure 4.
Direct binding between ion channel/transport and kinesins. (A) Structural diagrams of Kv3.1b subunit (left) and tetrameric channel complex of Kv3 (right). T1 domain of Kv3.1 (aa. 1–110) interacts with the C-terminal region of KIF5B (aa. 865–934). Gray bars, transmembrane domains; black ovals, T1 domains. Importantly, only properly tetramerized T1 domains, but not T1 monomers, bind to KIF5 tail domains. In the diagram for a channel tetramer complex (upper right), for clarification, only the C-terminus of one subunit is shown in green, containing the ATM (axonal targeting motif). (B) Diagrams of CLC-5 subunit structure and KIF3. CLC-5 C-terminal domain interacts with the non-motor region (coiled-coil and cargo binding domains) of KIF3. Gray bars, transmembrane domains in CLC-5; purple bars, extra-/intracellular domains; green ovals, CBS (cystathionine beta synthase) domain. KAP3 = kinesin-associated protein 3.
Kv3 channels are involved in fast spiking of some neurons, because of their unique biophysical properties, high activation threshold (about −20 mV), and rapid deactivation kinetics (Bean 2007; Rudy and McBain 2001). Kv3 subfamily contains four members (Kv3.1 to Kv3.4). Kv3.1 has two variants because of alternative splicing at the C-terminus, Kv3.1a and Kv3.1b, which have different polarized axon-dendrite targeting patterns (Xu and others 2007). Both the biophysical properties and axonal localization of Kv3.1 are required for maximal spiking frequency (Gu and others 2012). The polarized targeting of Kv3.1 channels is governed by a C-terminal axonal targeting motif, which binds to both the N-terminal T1 domain and ankryin G at the axon initial segment (Xu and others 2007).
The intracellular transport of Kv3 channels is mediated by kinesin 1/KIF5, which binds to the N-terminal T1 domain of Kv3 channels (Xu and others 2010). A pulldown from rat brain showed the N-terminal T1 domain (required for proper tetramerization) of Kv3.1 can interact with KIF5, specifically at residues 865–934 of the C-terminal cargo binding domain of KIF5 (Fig. 4A; Xu and others 2010). The direct binding between Kv3.1 and KIF5 is supported by both domain mapping and in vitro pulldown assays with purified proteins (Xu and others 2010). The T1 domains within the Kv3 subfamily are highly homologous. The binding between the Kv3 T1 domain and the 70 residue region in KIF5 tail is evolutionarily conserved (Xu and others 2010). Kv3 T1 domains are highly conserved from fruit fly to human. The binding site, the 70 residue region in KIF5 tail, also shares >75% identity from fruit fly to human (Xu and others 2010). Our data further shows that the Kv3.1–KIF5 interaction requires proper T1 tetramerization of Kv3.1 channels (Xu and others 2010). When the Zn2+ binding site within the T1 domain (which is necessary for proper T1 tetramerization) is mutated, KIF5 no longer binds. This Kv3.1–KIF5 interaction is necessary for transporting Kv3.1 channel proteins into the axon. This study is important because this is the first paper to demonstrate direct binding between an ion channel and kinesin motor, in which no adaptor protein is required.
Chloride/Proton Antiporter CLC-5 and Kinesin 2
CLC-5 is a member of the CLC family of voltage-gated chloride channels and chloride/proton antiporters, including nine members, CLC-1 to CLC-7, CLC-Ka, and CLC-Kb. CLC-5 has been shown to directly interact with KIF3B/kinesin 2 (Reed and others 2010). CLC-5 is a chloride/proton antiporter that is activated by depolarization of the membrane potential and it contains 18 helices with intracellular N- and C-termini (Fig. 4B; Dutzler 2006). CLC-5 plays an important role in renal tubular reabsorption. Malfunction of CLC-5 caused by mutations can lead to Dent’s disease, an X-linked renal tubular disorder (Devuyst and others 1999; Gunther and others 1998). A yeast two-hybrid screen using the C-terminal of CLC-5 uncovered KIF3B as an interacting partner (Reed and others 2010). GST pulldown and co-IP experiments showed that the C-terminus of CLC-5 interacts with both the coiled-coil and cargo-binding domain of KIF3B, but does not interact with the motor domain at all (Fig. 4B; Reed and others 2010). KIF3B siRNA was used to show that surface expression of CLC-5 is reduced when KIF3B expression is decreased. Although this interaction takes place in kidney cells, this channel directly binds to its motor in forward transport.
Moreover, a recent study identified a direct interaction between Arabidopsis kinesin-like protein 1 (KP1) and voltage-dependent anion channel 3 (VDAC3) residing in mitochondria outer membranes with a yeast two-hybrid screen (Yang and others 2011). Both KP1 and VDAC3 regulate aerobic respiration during seed germination at low temperature (Yang and others 2011). Finally, another recent study revealed the direct binding between K+-dependent Na+/Ca2+ exchanger 2 (NCKX2) and KIF21 also with a yeast two-hybrid screen (Lee and others 2012). The binding is critical for axonal targeting of NCKX2 (Lee and others 2012). Therefore, kinesin transporting ion channel/transporters via direct binding is likely a major mechanism underlying the placement of these membrane proteins that are involved in cellular electrical signaling.
Kinesin-Mediated Transport of Ion Channels via Unknown Adaptors
Despite the progress of identifying the kinesin motor and adaptor proteins for some ion channels, mechanisms underlying the intracellular transport of most ion channels remain unclear. In this section, we discuss several ion channels whose kinesin motors have been identified but adaptor proteins remain unknown.
Kv1 (Shaker) and Kv3 channels are two major Kv channel subfamilies that operate in axons (Gu and Barry 2011). Our previous studies have revealed distinct mechanisms underlying their targeting into axons (Gu and Barry 2011; Gu and Gu 2011; Gu and others 2003; Gu and others 2006; Xu and others 2007; Xu and others 2010). The axonal targeting domain is the N-terminal T1 domain of Kv1 channels, which is evolutionarily conserved and highly homologous among the Kv1 subfamily members. When fused to either the C-terminus of CD4 or the N-terminus of the transferin receptor, T1 domains of several Kv1 channels can target these reporter membrane proteins to axonal membranes (Gu and others 2003). The Kv1 auxiliary subunit, Kvβ2 binds to Kv1 T1 domains and may mediate Kv1 axonal targeting, since YFP-tagged Kvβ2 is concentrated in axons (Gu and others 2003). Our studies further suggest that Kvβ2 binds to EB1, a microtubule plus-end tracking protein, and KIF3A/kinesin 2, which is required for Kv1.2/Kvβ2 axonal targeting (Gu and others 2006). This result is further confirmed with the live cell imaging experiments, indicating that KIF3/kinesin 2 is the major motor to anterogradely transport Kv1 channels along axons (Gu and Gu 2010). A recent study further shows that cyclin-dependent kinase phosphorylates Kvβ2 to regulate the EB1-Kvβ2 interaction to control the axonal targeting of Kv1 channels (Vacher and others 2011). However, it remains unknown how Kvβ2 interacts with KIF3, directly or indirectly. It is possible that Kvβ2 is the adaptor protein to link Kv1 to KIF3. Since Kvβ2, by itself or when binding to Kv1 α subunits, also forms tetramers (Gulbis and others 1999), it will be interesting to determine whether Kvβ2 tetramerization is required for binding to KIF3. Another study shows that Kv1.3 channels can be targeted to the axon via their T1 domain’s interaction with KIF5B (Rivera and others 2007). Despite the difference, tetramerization or channel assembly appears to play a critical role in the interaction of both Kv1 and Kv3 channels with kinesin motors (Gu and Barry 2011).
Kv4.2 (Shal) is a dendritic A-type Kv channel (Rivera and others 2003). KIF17/kinesin 2, the major kinesin motor transporting NMDA receptors, is implicated in the dendritic transport of Kv4.2 (Chu and others 2006). To uncover the kinesin required for dendritic transport of Kv4.2, dominant-negative forms of different kinesins were examined on their effects of dendritic localization of myc-tagged Kv4.2. Only KIF17 mutant caused Kv4.2 to be confined to the soma. All other KIF constructs had no effect on the dendritic targeting of Kv4.2. KIF17 and Kv4.2 interact through the very end of the C-terminus of Kv4.2 (Chu and others 2006). Whether an adaptor protein is needed or not remains unclear. Furthermore, a recent study shows that the level of Kv4.2 in KIF17 knockout mice remains unchanged, suggesting that other motor proteins may be involved in compensation (Yin and others 2011).
Cyclic nucleotide-gated (CNG) ion channels are targeted to the non-motile cilia on olfactory sensory neurons. These tetrameric channels normally contain three different subunit isoforms, CNGA2, CNGA4, and CNGB1b (Bonigk and others 1999). CNGB1b has a ciliary targeting motif that CNGA2 and CNGA4 lack (Jenkins and others 2006). Furthermore, KIF17 was implicated in transport CNG channels by the result that a KIF17 dominant-negative construct blocked YFP-tagged CNG channels from entering the cilia (Jenkins and others 2006). However, whether this interaction is through direct binding or indirectly through an adaptor protein remains unknown. Alternatively, ion channel proteins residing within an intracellular organelle may be transported along axons indirectly via organelle’s transporting machinery. Mitochondria is a good example and its axonal transport has been extensively studied (Sheng and Cai 2012).
Ankyrin G and spectrin play critical roles in clustering ion channels and transporters though actin cytoskeleton at axon initial segments and nodes of Ranvier (see a review for details, Bennett and Healy 2009). Among the various Nav, Kv, and Ca2+ channels, and transporters, that interact with ankyrin G or spectrin, Kv3.1 channel so far is only one that is known to be specifically transported by a kinesin motor (Xu and others 2010).
Regulation of the Channel and Kinesin Interaction
Regulation of the channel and kinesin interaction is essential for the proper localization of ion channel proteins. To be correctly transported, an ion channel has to associate or disassociate with its motor at the right time in the right location. Furthermore, altered ion channel transport affects neuronal excitability and synaptic transmission. The three major types of regulation that will be discussed are the adaptor proteins regulated by other binding proteins, posttranscriptional modifications, including phosphorylation by protein kinases, and oligomerization of channel subunits during assembly.
Multiple Binding Partners of the Adaptor Protein
Besides the ion channel, an adaptor protein may have many other binding partners, which can profoundly regulate the channel transport (Fig. 5A). One of the adaptor proteins for AMPA receptors, GRIP-1, binds to many other proteins through various domains, including EphB2 and EphA7 receptor tyrosine kinases, and ephrinB ligands (Bruckner and others 1999; Lin and others 1999; Torres and others 1998), as well as GRASP-1, a Ras guaninenucleotide exchange factors (Ye and others 2000; Fig. 5A). Moreover, protein interacting with C-kinase-1 (PICK1) binds to the linker II region in GRIP-1 to regulate AMPA receptor trafficking (Lu and Ziff 2005), whereas Phosphoinositide 3-kinase enhancer binds to its 4th PDZ domain (Chan and others 2011) and a neuronal early endosomal protein, NEEP21, binds to its C-terminus (Steiner and others 2005; Fig. 5A). These binding proteins may affect the function of GRIP in interaction with AMPA receptors and/or KIF5 motors to alter the transport of the channel proteins. The Liprin-α/GIT1 interaction is required for AMPA receptor targeting. Interfering with the interaction leads to a decrease in the dendritic and surface clustering of AMPA receptors (Ko and others 2003). A recent study shows that mutated HAP1 can alter AMPA receptor trafficking by competing with GRIP-1 for the overlapping binding sites in KIF5 C-terminal region (Mandal and others 2011). Therefore, an adaptor protein may be responsible for multiple different types of cargos of the kinesin motor. Binding proteins of the adaptor protein will profoundly change the transport of ion channels.
Figure 5.
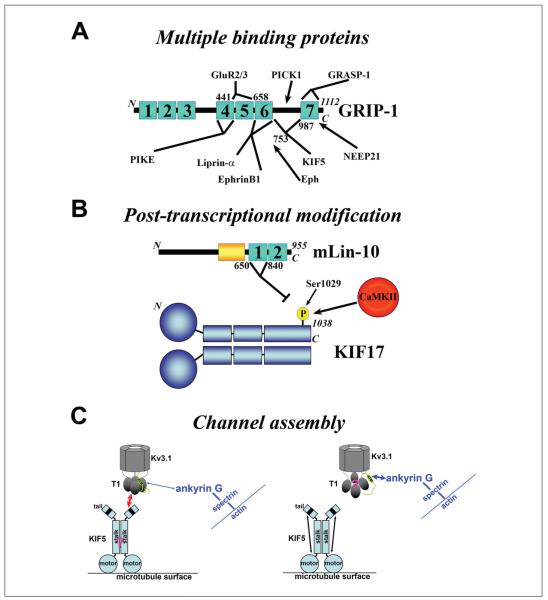
Regulation of ion channel protein loading to kinesin motors. (A) Multiple binding proteins of GRIP-1. Cyan boxes, PDZ domains. (B) Phosphorylation of KIF17 at aa. Ser1029 by CaM kinase II causes disassociation from mLin-10. (C) Kv3.1 tetramerization is required for the interaction with KIF5 motor [Source. Adapted from Xu and others (2010)].
Posttranscriptional Modification
The physical link of channel-adaptor-kinesin or channel-kinesin can be regulated by posttranscriptional modification. Protein phosphorylation mediated by protein kinases is a major form. Phosphorylation can take place in kinesins to alter the strength of the cargo binding. For instance, phosphorylation of Ser1029 in the C-terminal of KIF17 disrupts the interaction between KIF17 and mLin-10 (Guillaud and others 2008). This phosphorylation event is caused by CaM kinase II. Disruption the KIF17/mLin-10 interaction leads to a release of the kinesin’s cargo (Fig. 5B).
Many examples are also available that phosphorylation takes place in adaptor proteins or ion channels. Phosphorylation of GRIP-1, at Ser 917, by an unknown kinase regulates the surface expression of GluR2, the AMPA receptor subunit (Kulangara and others 2007). Phosphorylated GRIP-1 interferes with trafficking mechanism for surface expression of GluR2, whereas the unphosphorylated GRIP-1 does not. Persistent phosphorylation of GRIP-1 by PKC and Src tyrosine kinase reduces the AMPAR-GRIP-1 interaction, as a result, AMPA receptor targeting to synapses is reduced in cocaine-induced long-term depression (Bakshi and others 2009). PICK1 is a protein kinase C (PKC) α adaptor protein that interacts with GRIP-1. If this interaction is disrupted the S880 phosphorylation of GluR2 reduces surface expression of GluR2 (Lu and Ziff 2005). Therefore the PICK1/GRIP-1 interaction may be a key regulator in AMPA receptor trafficking.
Other types of posttranscriptional modifications may also be involved. Palmitoylated GRIP-1 is targeted into trafficking endosomes in dendrites and links endosomes to kinesin motors, thereby enhancing AMPA receptor recycling (Thomas and others 2012).
Channel Assembly and Transport by Kinesin Motors
Channel assembly through oligomerization is the third important strategy to regulate the transport of channel protein complexes by kinesin motors. One prominent example is the interaction between Kv3 channels and KIF5 motors (Xu and others 2010). We have demonstrated that only properly formed Kv3.1 T1 tetramers, but not monomers, interact with KIF5 motors (Fig. 5C; Xu and others 2010). This assembly-dependent transport mechanism ensures that only the functioning channel complexes to be transported into axons or cell membranes. On the other hand, heteromultimerization of channel protein complex may play an important role in determining the specificity of kinesin motor loading. For instance, GluR2/3 binds to KIF5 via GRIP-1, whereas GluR1 binds to KIF17 via mLin-10. This may have significant physiological consequences, given that the presence of GluR2 eliminates the Ca2+ permeability. Indeed, in cerebellar synapses, repetitive synaptic activity triggers loss of synaptic GluR2-lacking AMPA receptors by selectively disrupting their interaction with GRIP-1 and that PICK1 drives activity-dependent delivery of GluR2-containing receptors (Liu and Cull-Candy 2005). Therefore, it is possible that KIF5 transports AMPA receptors that are not permeable to Ca2+, whereas KIF17 transports Ca2+-permeable glutamate receptors, including both AMPA and NMDA receptors.
Future Perspectives
Despite the progress in determine how ion channels are transported by different molecular motors, it remains unclear for most ion channel proteins regarding how they are transported intracellularly, and whether or not an adaptor protein is needed. In particular, much less is known about how ion channel proteins are transported retrogradely along microtubules by dynein motors. It is shown that Kv1.5 interacts with dynein complex through its N-terminal SH3-binding domain during endocytosis (Choi and others 2005), but the systematical study regarding the channel–dynein interaction is lacking. Furthermore, ion channel proteins can also be transported by myosins that move along actin filaments. Therefore, an immediate question in this research field is to understand how the cargos containing ion channel proteins are specifically loaded to different microtubule- or actin-based transport machinery. Using unbiased screening approach and/or candidate approach, to address the question should be highly feasible, since the number of kinesin, dynein and myosin genes is limited.
To understand the physiological and pathological consequences of the regulation of ion channel transport also represents an important research topic for future studies. Activity-dependent up-regulation of kinesin heavy chain in the neurons of the Gill-withdrawal reflex of Aplysia results in enhanced anterograde transport machinery (Puthanveettil and others 2008). In fact, the up-regulation is both necessary and sufficient for the induction, but not for the persistence, of long-term facilitation by repeated application of serotonin (Puthanveettil and others 2008). In diseases, as discussed earlier, HAP1 is an adaptor which affects GABAA receptor expression when its interaction with KIF5C is perturbed. A recent article, however, examined the role HAP1 and mutant huntingtin play on AMPA receptor trafficking. When mutant huntingtin interacts with HAP1 this disrupts the GluR2/HAP1/KIF5 complex which leads to impaired trafficking of AMPA receptors along dendrites (Mandal and others 2011).
To ensure the precise transport of different ion channel proteins into proper subcellular compartments, a neuron has a variety of strategies to specifically load cargos to kinesin motors, via different adaptor proteins or direct interaction. The loading is also a highly regulated process. Taken together, this research field is to address a fundamental question in neuroscience, how mechanical forces provided by molecular motors are coupled to electrical signaling provided by various ion channels. This research also contributes to understanding the pathogenic mechanisms in different neurodegenerative diseases, in which axonal transport deficits are involved to ultimately alter the electrical and chemical functions of axons.
Acknowledgments
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article:
This work was supported by a grant from National Institute of Neurological Disorders and Stroke/National Institutes of Health (R01NS062720) to CG.
Footnotes
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Bakshi K, Gennaro S, Chan CY, Kosciuk M, Liu J, Stucky A. Prenatal cocaine reduces AMPA receptor synaptic expression through hyperphosphorylation of the synaptic anchoring protein GRIP. J Neurosci. 2009;29(19):6308–19. doi: 10.1523/JNEUROSCI.5485-08.2009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8(6):451–65. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL. Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem. 2002;277(33):30079–90. doi: 10.1074/jbc.M200438200. others. [DOI] [PubMed] [Google Scholar]
- Bennett V, Healy J. Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb Perspect Biol. 2009;1(6):a003012. doi: 10.1101/cshperspect.a003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby KA, Nanao MH, Shen NV, Kreusch A, Bellamy H, Pfaffinger PJ. Zn2+-binding and molecular determinants of tetramerization in voltage-gated K+ channels. Nat Struct Biol. 1999;6(1):38–43. doi: 10.1038/4911. others. [DOI] [PubMed] [Google Scholar]
- Bonigk W, Bradley J, Muller F, Sesti F, Boekhoff I, Ronnett GV. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J Neurosci. 1999;19(13):5332–47. doi: 10.1523/JNEUROSCI.19-13-05332.1999. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW. theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc Natl Acad Sci U S A. 1999;96(17):9891–6. doi: 10.1073/pnas.96.17.9891. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem. 2005;280(15):14723–32. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22(3):511–24. doi: 10.1016/s0896-6273(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25(11):571–7. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Chan CB, Chen Y, Liu X, Tang X, Lee CW, Mei L. PIKE-mediated PI3-kinase activity is required for AMPA receptor surface expression. EMBO J. 2011;30(20):4274–86. doi: 10.1038/emboj.2011.281. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S. Potassium channel structures. Nat Rev Neurosci. 2002;3(2):115–21. doi: 10.1038/nrn727. [DOI] [PubMed] [Google Scholar]
- Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97(4):363–71. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281(1):365–73. doi: 10.1074/jbc.M508897200. [DOI] [PubMed] [Google Scholar]
- Devuyst O, Christie PT, Courtoy PJ, Beauwens R, Thakker RV. Intra-renal and subcellular distribution of the human chloride channel, CLC-5, reveals a pathophysiological basis for Dent’s disease. Hum Mol Genet. 1999;8(2):247–57. doi: 10.1093/hmg/8.2.247. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386(6622):279–84. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- Dutzler R. The ClC family of chloride channels and transporters. Curr Opin Struct Biol. 2006;16(4):439–46. doi: 10.1016/j.sbi.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Gu C, Barry J. Function and mechanism of axonal targeting of voltage-sensitive potassium channels. Prog Neurobiol. 2011;94(2):115–32. doi: 10.1016/j.pneurobio.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Gu Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. J Biol Chem. 2011;286(29):25835–47. doi: 10.1074/jbc.M111.219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Jan YN, Jan LY. A conserved domain in axonal targeting of Kv1 (Shaker) voltage-gated potassium channels. Science. 2003;301(5633):646–9. doi: 10.1126/science.1086998. [DOI] [PubMed] [Google Scholar]
- Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN, Jan LY. The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron. 2006;52(5):803–16. doi: 10.1016/j.neuron.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Gu Y, Barry J, McDougel R, Terman D, Gu C. Alternative splicing regulates Kv3.1 polarized targeting to adjust maximal spiking frequency. J Biol Chem. 2012;287(3):1755–69. doi: 10.1074/jbc.M111.299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Gu C. Dynamics of Kv1 channel transport in axons. PLoS ONE. 2010;5(8):e11931. doi: 10.1371/journal.pone.0011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003;23(1):131–40. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10(1):19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- Gulbis JM, Mann S, MacKinnon R. Structure of a voltage-dependent K+ channel beta subunit. Cell. 1999;97(7):943–52. doi: 10.1016/s0092-8674(00)80805-3. [DOI] [PubMed] [Google Scholar]
- Gunther W, Luchow A, Cluzeaud F, Vandewalle A, Jentsch TJ. ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci U S A. 1998;95(14):8075–80. doi: 10.1073/pnas.95.14.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer JA, 3rd, Sellers JR. Walking to work: roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol. 2012;13(1):13–26. doi: 10.1038/nrm3248. [DOI] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16(8):2488–94. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev. 2008;88(3):1089–118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin super-family motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10(10):682–96. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991;252(5007):851–3. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hoskins R, Hajnal AF, Harp SA, Kim SK. The C. elegans vulval induction gene lin-2 encodes a member of the MAGUK family of cell junction proteins. Development. 1996;122(1):97–111. doi: 10.1242/dev.122.1.97. [DOI] [PubMed] [Google Scholar]
- Jahng AW, Strang C, Kaiser D, Pollard T, Pfaffinger P, Choe S. Zinc mediates assembly of the T1 domain of the voltage-gated K channel 4.2. J Biol Chem. 2002;277(49):47885–90. doi: 10.1074/jbc.M208416200. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol. 2006;16(12):1211–6. doi: 10.1016/j.cub.2006.04.034. others. [DOI] [PubMed] [Google Scholar]
- Jo K, Derin R, Li M, Bredt DS. Characterization of MALS/Velis-1, -2, and -3: a family of mammalian LIN-7 homologs enriched at brain synapses in association with the postsynaptic density-95/NMDA receptor postsynaptic complex. J Neurosci. 1999;19(11):4189–99. doi: 10.1523/JNEUROSCI.19-11-04189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Whitfield CW, Kim SK. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 1998;94(6):761–71. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10(12):854–65. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. Origin of PDZ (DHR, GLGF) domains. Trends Biochem Sci. 1995;20(9):350. doi: 10.1016/s0968-0004(00)89074-x. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A. 2004;101(34):12736–41. doi: 10.1073/pnas.0401860101. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241(4867):835–7. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Ko J, Kim S, Valtschanoff JG, Shin H, Lee JR, Sheng M. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J Neurosci. 2003;23(5):1667–77. doi: 10.1523/JNEUROSCI.23-05-01667.2003. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulangara K, Kropf M, Glauser L, Magnin S, Alberi S, Yersin A. Phosphorylation of glutamate receptor interacting protein 1 regulates surface expression of glutamate receptors. J Biol Chem. 2007;282(4):2395–404. doi: 10.1074/jbc.M606471200. others. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8(6):413–26. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18(8):2954–61. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Lee JS, Lee D, Seog DH, Lytton J, Ho WK. KIF21A-mediated axonal transport and selective endocytosis underlie the polarized targeting of NCKX2. J Neurosci. 2012;32(12):4102–17. doi: 10.1523/JNEUROSCI.6331-11.2012. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, De Blas AL. Coexistence of two beta subunit isoforms in the same gamma-aminobutyric acid type A receptor. J Biol Chem. 1997;272(26):16564–9. doi: 10.1074/jbc.272.26.16564. [DOI] [PubMed] [Google Scholar]
- Li M, Jan YN, Jan LY. Specification of subunit assembly by the hydrophilic amino-terminal domain of the Shaker potassium channel. Science. 1992;257(5074):1225–30. doi: 10.1126/science.1519059. [DOI] [PubMed] [Google Scholar]
- Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem. 1999;274(6):3726–33. doi: 10.1074/jbc.274.6.3726. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci. 2005;8(6):768–75. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]
- Lu W, Ziff EB. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47(3):407–21. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mandal M, Wei J, Zhong P, Chen J, Duffney LJ, Liu W. Impaired AMPA receptor trafficking and function by mutant Huntingtin. J Biol Chem. 2011;285:6101–8. doi: 10.1074/jbc.M111.236521. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307(5950):462–5. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Sudhof TC. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J Biol Chem. 1997;272(50):31459–64. doi: 10.1074/jbc.272.50.31459. [DOI] [PubMed] [Google Scholar]
- Pozo K, Stephenson FA. GRIF-1-kinesin-1 interactions: a confocal microscopy study. Biochem Soc Trans. 2006;34(Pt 1):48–50. doi: 10.1042/BST0340048. [DOI] [PubMed] [Google Scholar]
- Pulido R, Serra-Pages C, Tang M, Streuli M. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc Natl Acad Sci U S A. 1995;92(25):11686–90. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthanveettil SV, Monje FJ, Miniaci MC, Choi YB, Karl KA, Khandros E. A new component in synaptic plasticity: upregulation of kinesin in the neurons of the gill-withdrawal reflex. Cell. 2008;135(5):960–73. doi: 10.1016/j.cell.2008.11.003. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AA, Loh NY, Terryn S, Lippiat JD, Partridge C, Galvanovskis J. CLC-5 and KIF3B interact to facilitate CLC-5 plasma membrane expression, endocytosis, and microtubular transport: relevance to pathophysiology of Dent’s disease. Am J Physiol Renal Physiol. 2010;298(2):F365–80. doi: 10.1152/ajprenal.00038.2009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Chu PJ, Lewis TL, Jr, Arnold DB. The role of Kif5B in axonal localization of Kv1 K+ channels. Eur J Neurosci. 2007;25(1):136–46. doi: 10.1111/j.1460-9568.2006.05277.x. [DOI] [PubMed] [Google Scholar]
- Rivera JF, Ahmad S, Quick MW, Liman ER, Arnold DB. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat Neurosci. 2003;6(3):243–50. doi: 10.1038/nn1020. [DOI] [PubMed] [Google Scholar]
- Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998;94(6):751–9. doi: 10.1016/s0092-8674(00)81734-1. [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24(9):517–26. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TiPS/TINS lecture: the molecular biology of mammalian glutamate receptor channels. Trends Pharmacol Sci. 1993;14(8):297–303. doi: 10.1016/0165-6147(93)90047-N. [DOI] [PubMed] [Google Scholar]
- Serra-Pages C, Kedersha NL, Fazikas L, Medley Q, Debant A, Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein colocalize at focal adhesions. EMBO J. 1995;14(12):2827–38. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Pages C, Medley QG, Tang M, Hart A, Streuli M. Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins. J Biol Chem. 1998;273(25):15611–20. doi: 10.1074/jbc.273.25.15611. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288(5472):1796–802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417(6884):83–7. doi: 10.1038/nature743. others. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13(2):77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Wyszynski M, Huh KH, Valtschanoff JG, Lee JR, Ko J. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J Biol Chem. 2003;278(13):11393–401. doi: 10.1074/jbc.M211874200. others. [DOI] [PubMed] [Google Scholar]
- Simske JS, Kaech SM, Harp SA, Kim SK. LET-23 receptor localization by the cell junction protein LIN-7 during C. elegans vulval induction. Cell. 1996;85(2):195–204. doi: 10.1016/s0092-8674(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Pozo K, Brickley K, Stephenson FA. Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J Biol Chem. 2006;281(37):27216–28. doi: 10.1074/jbc.M600522200. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21(3):581–91. doi: 10.1016/s0896-6273(00)80568-1. others. [DOI] [PubMed] [Google Scholar]
- Steiner P, Alberi S, Kulangara K, Yersin A, Sarria JC, Regulier E. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO J. 2005;24(16):2873–84. doi: 10.1038/sj.emboj.7600755. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36(6):1063–77. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Stricker NL, Huganir RL. The PDZ domains of mLin-10 regulate its trans-Golgi network targeting and the surface expression of AMPA receptors. Neuropharmacology. 2003;45(6):837–48. doi: 10.1016/s0028-3908(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci. 2001;21(10):3409–18. doi: 10.1523/JNEUROSCI.21-10-03409.2001. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–9. doi: 10.1038/43432. others. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Hayashi T, Chiu SL, Chen CM, Huganir RL. Palmitoylation by DHHC5/8 Targets GRIP1 to Dendritic Endosomes to Regulate AMPA-R Trafficking. Neuron. 2012;73(3):482–96. doi: 10.1016/j.neuron.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres R, Firestein BL, Dong H, Staudinger J, Olson EN, Huganir RL. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21(6):1453–63. doi: 10.1016/s0896-6273(00)80663-7. others. [DOI] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17(8):2728–37. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87(7):1327–38. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65(1):53–65. doi: 10.1016/j.neuron.2009.12.007. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88(4):1407–47. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Yang JW, Cerda O, Autillo-Touati A, Dargent B, Trimmer JS. Cdk-mediated phosphorylation of the Kvbeta2 auxiliary subunit regulates Kv1 channel axonal targeting. J Cell Biol. 2011;192(5):813–24. doi: 10.1083/jcb.201007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ. The GABA-A receptor gene family: new targets for therapeutic intervention. Neurochem Int. 1999;34(5):387–90. doi: 10.1016/s0197-0186(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34(1):39–52. doi: 10.1016/s0896-6273(02)00640-2. others. [DOI] [PubMed] [Google Scholar]
- Wyszynski M, Valtschanoff JG, Naisbitt S, Dunah AW, Kim E, Standaert DG. Association of AMPA receptors with a subset of glutamate receptor-interacting protein in vivo. J Neurosci. 1999;19(15):6528–37. doi: 10.1523/JNEUROSCI.19-15-06528.1999. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yu W, Jan YN, Jan LY, Li M. Assembly of voltage-gated potassium channels. Conserved hydrophilic motifs determine subfamily-specific interactions between the alpha-subunits. J Biol Chem. 1995;270(42):24761–8. doi: 10.1074/jbc.270.42.24761. [DOI] [PubMed] [Google Scholar]
- Xu M, Cao R, Xiao R, Zhu MX, Gu C. The axon-dendrite targeting of Kv3 (Shaw) channels is determined by a targeting motif that associates with the T1 domain and ankyrin G. J Neurosci. 2007;27(51):14158–70. doi: 10.1523/JNEUROSCI.3675-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Gu Y, Barry J, Gu C. Kinesin I transports tetramerized Kv3 channels through the axon initial segment via direct binding. J Neurosci. 2010;30(47):15987–6001. doi: 10.1523/JNEUROSCI.3565-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Fukaya M, Abe M, Ikeno K, Kakizaki T, Watanabe M. Differential palmitoylation of two mouse glutamate receptor interacting protein 1 forms with different N-terminal sequences. Neurosci Lett. 2001;304(1-2):81–4. doi: 10.1016/s0304-3940(01)01766-9. others. [DOI] [PubMed] [Google Scholar]
- Yang JT, Saxton WM, Stewart RJ, Raff EC, Goldstein LS. Evidence that the head of kinesin is sufficient for force generation and motility in vitro. Science. 1990;249(4964):42–7. doi: 10.1126/science.2142332. [DOI] [PubMed] [Google Scholar]
- Yang XY, Chen ZW, Xu T, Qu Z, Pan XD, Qin XH. Arabidopsis kinesin KP1 specifically interacts with VDAC3, a mitochondrial protein, and regulates respiration during seed germination at low temperature. Plant Cell. 2011;23(3):1093–106. doi: 10.1105/tpc.110.082420. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Liao D, Zhang X, Zhang P, Dong H, Huganir RL. GRASP-1: a neuronal RasGEF associated with the AMPA receptor/GRIP complex. Neuron. 2000;26(3):603–17. doi: 10.1016/s0896-6273(00)81198-8. [DOI] [PubMed] [Google Scholar]
- Yin X, Takei Y, Kido MA, Hirokawa N. Molecular motor KIF17 is fundamental for memory and learning via differential support of synaptic NR2A/2B levels. Neuron. 2011;70(2):310–25. doi: 10.1016/j.neuron.2011.02.049. [DOI] [PubMed] [Google Scholar]