Abstract
Objective
First-degree relatives of persons with an autism spectrum disorder (ASD) are at increased risk for ASD-related characteristics. As little is known about the early expression of these characteristics, this study characterizes the non-ASD outcomes of 3-year-old high-risk (HR) siblings of children with ASD.
Method
Two groups of children without ASD participated: 507 HR siblings and 324 low-risk (LR) control subjects (no known relatives with ASD). Children were enrolled at a mean age of 8 months, and outcomes were assessed at 3 years. Outcome measures were Autism Diagnostic Observation Schedule (ADOS) calibrated severity scores, and Mullen Verbal and Non-Verbal Developmental Quotients (DQ).
Results
At 3 years, HR siblings without an ASD outcome exhibited higher mean ADOS severity scores and lower verbal and non-verbal DQs than LR controls. HR siblings were over-represented (21% HR versus 7% LR) in latent classes characterized by elevated ADOS severity and/or low to low-average DQs. The remaining HR siblings without ASD outcomes (79%) belonged to classes in which they were not differentially represented with respect to LR siblings.
Conclusions
Having removed a previously identified 18.7% of HR siblings with ASD outcomes from all analyses, HR siblings nevertheless exhibited higher mean levels of ASD severity and lower levels of developmental functioning than LR children. However, the latent class membership of four-fifths of the HR siblings was not significantly different from that of LR control subjects. One-fifth of HR siblings belonged to classes characterized by higher ASD severity and/or lower levels of developmental functioning. This empirically derived characterization of an early-emerging pattern of difficulties in a minority of 3-year-old HR siblings suggests the importance of developmental surveillance and early intervention for these children.
Keywords: ASD, high-risk siblings, outcome, broad autism phenotype
Autism spectrum disorders (ASD) are characterized by disturbances in social interaction and communication, the presence of restricted and repetitive behaviors, and variable levels of cognitive ability. ASDs are among the most common neurodevelopmental disorders, with recent surveillance efforts indicating that more than one in 100 children are affected.1 ASD characteristics emerge at different rates in different infants, with relatively stable diagnosis and characterization available at 3 years of age.2,3
Prospective studies indicate that high-risk (HR) siblings—the younger brothers and sisters of a child diagnosed with an ASD—are at increased risk both for ASD outcomes and for subclinical manifestations of ASD-related behavioral characteristics. A recent report4 from the Baby Siblings Research Consortium (BSRC) indicated that 18.7% of 3-year-old HR siblings will themselves develop an ASD. However, relatively little is known about the early developmental expression of ASD-related characteristics among HR siblings who do not have ASD outcomes. The manifestation of subclinical ASD-related behavioral characteristics in the first-degree relatives of persons with an ASD is referred to as the broad autism phenotype (BAP).5–7
Through 3 years of age, HR infants exhibit higher levels of ASD-related characteristics than controls on ASD screeners, but differences based on examiner-administered assessments designed to quantify these characteristics are less consistent.2,8–10 Likewise, HR siblings exhibit lower levels of receptive language and other communication skills than comparison infants,2,8,9,11 but direct comparisons of intellectual functioning have not yielded consistent results.8,9,11,12 From a categorical perspective, approximately 20% of HR siblings at 3 years both exhibit difficulties with language development on standardized assessment11 and satisfy clinical criteria of social or language delay.13 In addition to small sample sizes and inconsistent exclusion of those HR siblings who went on to an ASD diagnosis, heterogeneity in functioning among HR siblings is a likely source of variation in findings.13,14 In fact, there is, as yet, no large-scale investigation of the proportion of HR siblings affected by ASD-related characteristics and the proportion exhibiting typical developmental outcomes at 3 years.2,11,15–17
Family multiplex status—more than one older sibling with an ASD—and male sex of the HR sibling are risk factors for ASD recurrence.18 Complementing the over-representation of ASD among males, higher levels of ASD-related characteristics are reported among male than among female HR siblings without an ASD outcome.12,19 Likewise, there is accumulating evidence that HR siblings from multiplex families exhibit higher levels of ASD-related characteristics17,19,20 than do HR siblings who have a single older sibling with an ASD. It is not clear, however, how HR status, sex, and multiplex status are individually and jointly associated with developmental outcomes among HR siblings without ASD outcomes.
The current study compares a large sample of HR siblings without an ASD outcome to low-risk (LR) control subjects from families with no history of ASD.4 Participants were drawn from children in the BSRC recurrence sample who did not have an ASD outcome.21 Children were recruited early in development and followed up through 3 years of age, when standardized developmental and diagnostic assessments were administered by expert clinical researchers. The first aim of the study was to characterize HR siblings as a group. To do so, mean levels of ASD severity and verbal and nonverbal functioning were modeled with respect to risk group, infant sex, and family multiplex status. The second aim was to characterize the heterogeneity of outcomes among HR siblings through data-driven identification of subgroups of HR siblings with similar multidimensional profiles of ASD severity and developmental functioning. To do so, ASD severity and developmental quotients were incorporated in a latent class analysis, and class membership was analyzed with respect to risk, sex, and multiplex status.
METHOD
Participants
Participant data were obtained from nine member sites whose recruitment procedures and common assessment measures allowed for data pooling. Institutional review board approval was obtained to collect and analyze deidentified data from all sites. Infants were enrolled at a mean age of 8.04 months (SD = 4.00 months) and were administered complete clinical evaluations at a mean age of 37.10 months (SD = 2.32 months). All children with an ASD, that is, children who met both clinical judgment and Autism Diagnostic Observation Schedule (ADOS) criteria, were removed from the final sample, leaving two groups of 3-year-olds at outcome: a LR group (n = 324) with no known family history of ASD; and an HR group (n = 507) composed of later-born biological siblings of a proband with an ASD (autistic disorder, Asperger disorder, or pervasive developmental disorder not otherwise specified). The HR group overlaps with the ASD recurrence study sample4 absent those identified with ASD and those without data relevant to the current analyses (n = 157). All sites verified proband diagnoses in the high-risk group, typically using the ADOS22 and/or parent interviews such as the Autism Diagnostic Interview–Revised23 or the Social Communication Questionnaire.24 HR siblings were recruited by identifying affected older siblings through clinics and agencies serving individuals with ASD, community events, and other research studies. LR children were recruited by mailings, flyers, media announcements, and word-of-mouth. Exclusion criteria included identified neurologic or genetic condition in the infant or proband (e.g., fragile X syndrome, tuberous sclerosis).
Measures
Autism Diagnostic Observation Schedule (ADOS)
The ADOS is a semi-structured, standardized assessment of communication, social interaction, play, and repetitive behaviors that are diagnostic of ASD.22 Different ADOS modules are administered based on child language level. In the current sample, 86% (n = 719) of children received module 2 (phrase speech) at outcome, whereas 14% (n = 112) received module 1 (no words or single words only). To statistically pool ADOS scores across modules, total scores were converted to ASD-calibrated severity scores,25 which range from 1 to 10 and reflect the overall severity of ASD-related behavioral characteristics across the social interaction, communication, and repetitive behavior domains.
Mullen Scales of Early Learning (MSEL)
26 The MSEL is a standardized developmental test of children between birth and 68 months that yields age-equivalent scores on four cognitive subscales: Receptive Language, Expressive Language, Fine Motor, and Visual Reception. For the present analyses, developmental quotients (DQs) were calculated by dividing the subscale age-equivalent score by the child’s chronological age and multiplying by 100. This procedure avoided possible floor and ceiling effects while providing a familiar IQ metric, as recommended in the literature.27,28 Verbal domain (mean of Receptive and Expressive Language) and nonverbal domain (mean of Fine Motor and Visual Reception) DQs were used in analyses.
Analytic Strategy
Before performing outcome analyses, we examined associations between outcome measures within and between risk groups. The first phase of outcome analyses involved modeling mean ADOS severity scores, verbal DQs, and nonverbal DQs. These models simultaneously tested for risk group and child sex effects. Within the HR group, separate models were used to test for effects of multiplex status while controlling for child sex. Interaction effects between child sex and risk status (or multiplex status) were included in final models when significant. Covariates—for example, assessment site, race, age of assessment, and maternal education—with significant associations with a given outcome were included in final models or, if full covariate data were not available, in supplemental models.
In the second phase, latent class analysis (LCA) was used to identify latent groups within the full sample. The “flexmix” package29 in R 2.10 was used, which allowed for different distributional parameterization of each variable—ASD severity scores and verbal and nonverbal DQs—in the LCA. To determine the best class number solution, LCA models were run with two to eight latent classes. Each class number was run separately five times with randomly generated starting values to minimize problems with local maxima. The most parsimonious model was initially judged to be the first point at which an increasing number of classes resulted in little change in Bayesian information criterion (BIC) in a scree plot.30 In addition, Jeffreys’ Scale of Evidence31 was used to quantify model fit using 2*log(B), where B is the Bayes factor approximated as e([BIC1-BIC2]/−2), where BIC1 and BIC2 represent BIC values for models 1 and 2, respectively. When 2*log(B) > 10, there is very strong evidence for preferring the more complex model, and we used this cutoff to distinguish between solutions.
For each child, class membership was defined as the class having the highest posterior probability for that child. Multinomial logistic regression was used to simultaneously investigate risk group and sex as predictors of class membership. A separate logistic regression was used to investigate multiplex status as a predictor of class membership among the HR siblings.
RESULTS
Preliminary Analyses
Descriptive Statistics
There were no group differences in race (white versus other) or sex (Table 1). There was a significant group difference for maternal education (X2 = 6.21, df 1, p < .05), with a higher proportion of mothers in the LR group having college or graduate degrees. HR siblings were enrolled slightly later than children in the LR group (F1,830 = 36.92, p < .001) , and were more likely to have received module 1 rather than module 2 of the ADOS at outcome (χ2 = 10.65, df = 1, p < .01).
TABLE 1.
Sample Characteristics
| Variable | Low-Risk (n = 324) | High-Risk (n = 507) |
|---|---|---|
| Sex (% male) | 53.7 | 49.9 |
| Race (% minority) | 10.6 | 11.4 |
| Maternal education (% college or graduate degree) | 87.0 | 77.4 |
| Age at enrollment (mo), mean (SD) | 7.01 (2.87) | 8.70 (4.45) |
| Age at outcome (mo), mean (SD) | 37.04 (2.63) | 37.24 (3.25) |
| ADOS module (% module 2) | 91.4 | 83.4 |
| ASD symptom severity score, mean (SD) | 1.64 (1.22) | 2.19 (1.76) |
| Verbal DQ, mean (SD) | 110.76 (15.79) | 104.43 (17.47) |
| Nonverbal DQ, mean (SD) | 111.61 (15.43) | 107.58 (16.89) |
Note: ADOS = Autism Diagnostic Observation Scale; ASD = autism spectrum disorder; DQ = Developmental Quotient.
Associations between Study Outcomes
As shown in Table 2, ADOS severity was negatively correlated with verbal and nonverbal DQs (range, −0.22 to 0.08), whereas verbal and nonverbal DQs showed substantial positive correlations (0.54 to 0.67).32
TABLE 2.
Correlations Between Autism Diagnostic Observation Scale (ADOS) Severity, Verbal Developmental Quotient (DQ), and Nonverbal DQ
| Group | ADOS Severity | Verbal DQ |
|---|---|---|
| High-risk | ||
| Verbal DQ | −0.14** | |
| Nonverbal DQ | −0.22*** | 0.67*** |
| Low-risk | ||
| Verbal DQ | −0.08 | |
| Nonverbal DQ | −0.15* | 0.54*** |
| Total sample | ||
| Verbal DQ | −0.15*** | |
| Nonverbal DQ | −0.22*** | 0.64*** |
Note: p<.05;
p<.01;
p<.001.
Analyses of Means
ADOS Calibrated Severity Scores
The distribution of ADOS severity scores was significantly positively skewed, with a peak frequency at 1. Consequently, the severity scores were modeled as a negative binomial distribution by subtracting 1 from each score, yielding a range from 0 to 9. Preliminary analyses revealed no significant differences in ASD severity scores as a function of maternal education, race, ADOS module, age at enrollment, or maternal education. There were significant site effects (p < .001), and site was retained as a covariate in final models.
Risk group had a significant effect on ADOS severity scores (Wald z = 4.02, p < .001). Parameter estimates indicated that the high-risk group had significantly higher severity scores than the low-risk group by an estimated 0.41 point on the 10-point scale (95% confidence interval [CI] = 0.18–0.70, d = 0.26). Sex also had a significant effect on severity scores (Wald z = 4.82, p < .001), with male children having higher scores than female children by an estimated 0.43 point (CI = 0.23–0.68; d = 0.27). There was no interaction between sex and risk group as a predictor of severity (Wald z = 1.29, p = .20).
Multiplex status was next examined as a predictor of ASD severity scores above and beyond site and sex for the 477 HR siblings with available data. There were 19 HR siblings in the multiplex group and 458 in the simplex group. The analysis did not reveal a significant effect of multiplex status (Wald z = −1.67, p = .10). A significant sex term indicated = that male children scored higher than female children (Wald z = 2.79, p = .001). The multiplex-by-sex interaction term was not significant (Wald z = 1.92, p = .05).
MSEL DQ Scores
Analysis of MSEL verbal and nonverbal DQ scores revealed no significant effects for race or age at enrollment. There were significant effects of maternal education on both verbal (t = 3.91, p < .001) and nonverbal (t 2.30, p < .05) DQ scores, with children of mothers having less than a college degree exhibiting lower DQ scores. As maternal education was available for only 53% of the sample (n = 445), it was not included in final models. Inclusion of maternal education in models did not alter study findings (see Table S1, available online). There were significant site effects on verbal DQ (p < .001) and non-verbal DQ (p < .01), and site was retained as a covariate in final models.
There were significant risk group effects on verbal DQ (t = −5.43, p < .001), with the HR group scoring an estimated 7.76 points lower than the LR group (CI = 4.96–10.56, d = 0.46). There were also significant sex effects on verbal DQ (t = −3.05, p < .01), with males scoring an average of 8.34 (CI = 5.82–10.87, d = 0.50) points lower than females. There was not a significant sex-by–risk group interaction effect.
A significant effect for risk group was also found for nonverbal DQ (t = −3.05, p < .01), with the high-risk group scoring an estimated 4.20 points lower than the low-risk group (CI = 1.50–6.89, d = 0.26). There were also significant sex effects on nonverbal DQ scores (t = 7.27, p < .01), with males scoring an average of 9.01 (CI = 6.58–11.44, d = 0.57) points lower than females. There was not a significant sex-by–risk group interaction effect.
Within the high-risk sample, siblings from multiplex families scored an average of 8.06 (CI = 0.21–15.91, d = 0.49) points lower on verbal DQ (t = −2.01, p < .05), and an average of 10.98 (CI = 3.34–18.61, d = 0.68) points lower on nonverbal DQ than participants from simplex families (t = −2.82, p < .01). There were significant sex differences favoring males on both the verbal DQ (t = 5.16, p < .001) and nonverbal DQ (t = 5.72, p < .001). There were no sex-by–multiplex status interaction effects.
Latent Class Analysis
Results of the LCA yielded a five-class solution as the best fitting model. Visual inspection of the scree plot indicated leveling off of BIC values at the five-class solution, and Jeffreys’ Scale of Evidence provided very strong evidence supporting a five-class solution over a four-class solution (2*log[B] = 19.41) or a six-class solution (2*log[B] = 5.79) (Figure 1 and see Table S2, available online). In addition, the mean of individual posterior probabilities of class membership were relatively high within each of the five classes, ranging from 0.77 to 0.89. Minimum individual probabilities within each class were 0.35 or higher, well above the randomly expected value of 0.20.
FIGURE 1.
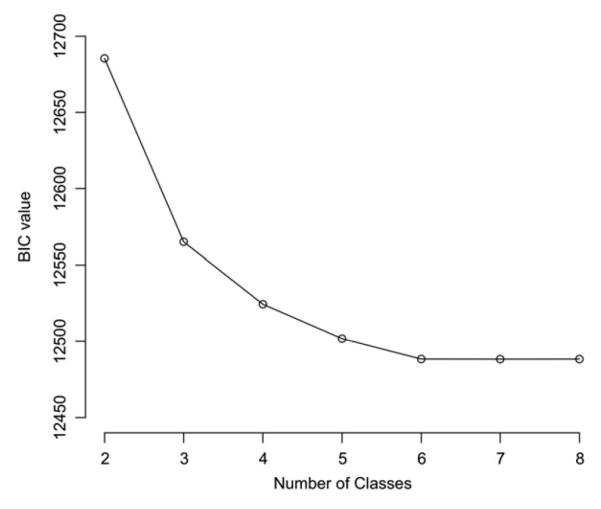
Scree plot of Bayesian information criterion (BIC) by solutions of various class numbers.
ASD severity and MSEL DQ characterizing the five latent classes identified by the LCA are presented in Table 3 (see Table S3, available online, for MSEL domain t scores). Labels describe class means and are not clinical characterizations of all children in a given class. Table 4 displays the percentage of children in each class as a function of risk group (HR and LR, none of whom had ASD outcomes) and sex. Classes 1 and 2 were characterized by low ASD severity scores and, respectively, high and average DQs. Together, these classes contained 65% of HR siblings. Class 3, characterized by elevated ASD severity scores and high DQ, and contained 14% of HR siblings. The remaining 21% of HR siblings were contained in class 4, characterized by low ASD severity scores and low DQ, and in class 5, characterized by elevated ASD severity scores and low-average DQ. A multinomial logistic regression analysis of class membership revealed significant main effects for both risk group (χ2 = 34.20, df = 4, p < .001) and sex (χ2 = 42.86, df = 4, p < .001), but no interaction between risk group and sex. Odds ratios reflect the relative odds of a HR sibling—none of whom had an ASD outcome—belonging to a given class rather than class 2 (low ASD severity/average DQ), which functioned as a comparison class (Table 4). HR siblings were 3.3 times more likely than LR controls to belong to class 4 (low ASD severity/low DQ), and 4.6 times more likely than LR controls to belong to class 5 (elevated ASD severity/low-average DQ). Odds ratios for other classes were not significant.
TABLE 3.
Outcome Scores for Latent Classes
| Measure, Mean (SD) |
|||
|---|---|---|---|
| Latent Class | ASD Severity | Verbal DQ | Nonverbal DQ |
| 1. Low ASD Severity / High DQ | 1.23 (0.46) | 119.31 (13.45) | 122.32 (10.17) |
| 2. Low ASD Severity / Average DQ | 1.26 (0.44) | 99.00 (7.61) | 101.95 (9.13) |
| 3. Elevated ASD Severity / High DQ | 4.29 (1.57) | 112.52 (10.68) | 113.04 (8.51) |
| 4. Low ASD Severity / Low DQ | 1.33 (0.48) | 77.29 (11.12) | 81.64 (12.95) |
| 5. Elevated ASD Severity / Low-Average DQ | 5.06 (1.47) | 89.28 (10.78) | 89.15 (10.49) |
Note: ASD = autism spectrum disorder; DQ = developmental quotient.
TABLE 4.
Risk Group and Sex by Latent Class
| Risk Group |
Sex |
|||||
|---|---|---|---|---|---|---|
| Latent Class | LR (n = 197), n (%) |
HR (n = 447), n (%) |
HR:LR Odds Ratio (95% CI) |
Female (n = 316), n (%) |
Male (n = 327), n (%) |
M:F Odds Ratio (95% CI) |
| 1. Low ASD severity/ high DQ |
97 (49) | 158 (35) | 0.74 (0.50–1.10) | 154 (49) | 100 (31) | 0.57 (0.39–0.83) |
| 2. Low ASD severity/ average DQ |
66 (34) | 134 (30) | — | 95 (30) | 105 (32) | — |
| 3. Elevated ASD severity/high DQ |
21 (11) | 61 (14) | 1.44 (0.80–2.57) | 39 (12) | 43 (13) | 1.04 (0.62–1.74) |
| 4. Low ASD severity/ low DQ |
6 (3) | 36 (8) | 3.31 (1.32–8.29) | 11 (4) | 31 (10) | 2.82 (1.33–5.94) |
| 5. Elevated ASD severity/ low-averageDQ |
7 (4) | 58 (13) | 4.57 (1.97–10.64) | 17 (5) | 48 (15) | 2.87 (1.53–5.36) |
Note: Odds ratios for risk group and sex are calculated simultaneously with reference to Class 2 (low autism spectrum disorder [ASD] severity/average developmental quotient [DQ]). Boldface values are significant (p<.05). HR = high-risk; LR = low-risk.
Odds ratios reflecting the main effect of sex calculated with reference to comparison class 2 are shown in Table 4. Males were 2.8 times more likely to be classified in class 4 (low ASD severity/low DQ), and 2.9 times more likely than females to be classified in class 5 (elevated ASD severity/low-average DQ). These were the same two classes that were significantly more likely to contain HR than LR children. In addition, males were 0.6 times less likely than females to be classified in class 1 (low ASD severity/high DQ). Finally, a logistic regression analysis of class by multiplex status among the HR siblings was significant (χ2 = 10.03, df = 4, p < .05). Examination of parameter effects revealed that children from simplex families were 10.29 (CI = 1.26–82.61) times more likely than children from multiplex families to belong to class 1 (low ASD severity/high DQ) rather than comparison class 2.
DISCUSSION
This is the first large-scale examination of ASD behavioral characteristics and developmental functioning in HR siblings without an ASD outcome. Objective, standardized assessments indicated that, as a group, HR siblings had slightly higher ASD severity scores, lower levels of verbal functioning, and slightly lower levels of nonverbal functioning than LR comparison children. A latent class analysis distinguished groups of HR and LR children with similar 3-year outcomes. Although HR siblings were overrepresented in latent classes characterized by elevated ASD severity and/or lower levels of developmental functioning, the majority of HR siblings belonged to classes characterized by low levels of ASD severity and typical levels of developmental functioning.
Overall, HR siblings had slightly higher ASD severity scores than LR comparison children on the ADOS. Differences in severity scores were small, approximately half a point on a 10-point scale. In addition, the mean ASD severity score for HR siblings was well below the cut-off of 4 points that indexes an ASD classification.33 Findings of slightly elevated levels of ASD-related characteristics in HR siblings without ASD outcomes extends previous reports of risk group differences, suggesting that overall differences in social, communication, and behavioral characteristics between HR and LR children are relatively subtle.8,10,11,34
Independent of risk group, boys exhibited higher ASD severity scores than girls at 3 years, which extends similar findings based on parent report19 and parallels the higher rate of ASD diagnosis among boys in epidemiologic and prospective high-risk samples.4,35 Within the HR group, there was no independent effect of multiplex status; instead, male children exhibited particularly elevated ASD severity scores. However, the limited number of children from multiplex families suggests caution in interpreting even negative findings related to multiplex status.
Overall, HR siblings evidenced lower verbal and nonverbal DQs than LR children. Previous research has indicated reduced language abilities among HR siblings without ASD,8,19 but this appears to be the first report of subtle reductions in nonverbal intellectual functioning.15 Verbal differences yielded medium effect sizes, whereas nonverbal differences yielded small effect sizes. In both the verbal and nonverbal domains, mean DQs for the HR group were above the sample standardization mean of 100,26 suggesting that average differences are not of substantial clinical significance.
Independent of risk group, male children exhibited lower levels of verbal and nonverbal functioning than female children.36,37 These findings are similar to DQ advantages reported for girls in other risk samples,38 and to overall IQ population advantages for girls at 3 years.36 Within the HR group, siblings from multiplex families exhibited substantially lower verbal and nonverbal functioning than siblings from simplex families, an effect that did not differ by infant sex. Together, risk group effects and the additional, independent effects associated with being male and being a sibling from a multiplex family were clinically significant. By way of example, a male HR sibling from a multiplex family was estimated to have verbal and nonverbal DQs more than 20 points below those of a child with none of these characteristics.
We used a latent class analysis of ASD severity scores and verbal and nonverbal DQs to identify outcome configurations among HR siblings without an ASD and controls. HR siblings were more than 3 times more likely than LR children to occupy class 4, which was characterized by low severity scores and low levels of developmental functioning, particularly in the verbal domain. In addition, HR siblings were more than four times more likely than LR children to occupy class 5, which was characterized by both elevated ASD severity and low-average developmental functioning. The overrepresentation of HR siblings among latent classes characterized by subtly disturbed levels of functioning is consonant with genetic models of ASD that encompass the transmission of subclinical manifestations of the autistic syndrome in a subset of first-degree relatives, on the basis of either incomplete penetrance or additive (multigenic) models of inheritance.39–41 These results suggest that an early manifestation of a BAP—characterized by ASD-related behaviors and/or lower levels of developmental functioning—was present in approximately one in five HR siblings without an ASD outcome.
Differences between HR siblings and LR controls in class membership were paralleled by independent effects of sex on class membership. Males were almost three times more likely than females to belong both to class 4 and to class 5. These sex differences in class membership highlight male susceptibility to ASD-related behavioral characteristics and to delays in developmental functioning.19,42 Sex effects did not vary by risk group, but their presence in the HR group is clinically significant. Although half of HR siblings were male, approximately three quarters of the HR siblings in classes 4 and 5 were male.
Among HR siblings without an ASD outcome, there were no significant risk group differences in the composition of class 3 (14% HR siblings, 11% controls), which was characterized by elevated ASD severity and high levels of verbal and nonverbal functioning. The composition of this class is consistent with previous research indicating that a range of ASD-related behavioral features is present in low-risk as well as high-risk children.20,43,44 There were not significant risk group differences in the composition of classes 1 and 2 (65% of HR siblings and 83% of controls). This indicates that HR siblings without an ASD outcome are not less likely than controls to be characterized by average and above average levels of functioning at 3 years of age.13
This study has several limitations. The study relied on an ADOS-based measure of ASD severity that was originally designed to distinguish among children with an ASD.22,25 However, the severity measure has subsequently been used to distinguish among HR siblings without ASD diagnoses.45 Although the severity measure did not allow comparisons of specific ADOS domain scores (e.g., social interaction) across modules, there is currently no common metric available for doing so. More generally, although the study relied on an observational measure of ASD severity, parent report measures of severity appear to yield congruent results.12 Although data on maternal educational attainment data were not available for the entire sample, study results were unchanged in analyses controlling for maternal education. It is possible that exposure to community interventions ameliorated outcomes for some children studied. An additional limitation of the study is the strictness of the ASD exclusion criteria, which required both meeting the ADOS cut-off and a clinical diagnosis. In addition, outcome was assessed relatively early, at 3 years of age. It is possible that some of the children studied will meet criteria for ASD at later ages. Assessing the stability of both early manifestations of ASD-related behavioral features and differences in verbal and nonverbal functioning will require continued longitudinal follow-up.
A previous report indicated that close to one in five of HR siblings (18.7%) will have an ASD outcome.4 The current study provides a large-scale, empirically derived characterization of the 3-year outcomes of the remaining majority of HR siblings without an ASD. Four-fifths of these HR siblings were not significantly less likely than control subjects to fall into classes characterized by a range of typical outcomes. However, one-fifth of HR siblings, the majority of them male, disproportionately fell into classes exhibiting higher levels of ASD severity and/or lower levels of developmental functioning. This appears to suggest an early BAP encompassing both ASD-related characteristics and decrements in developmental functioning. The study, then, adds to growing body of evidence suggesting challenges faced by a minority of HR siblings without an ASD.13,17,19 Early plasticity and the emergence of developmentally appropriate, efficacious intervention models46,47 suggest important opportunities to support the long-term outcomes of those HR children manifesting features of a BAP.
Supplementary Material
TABLE S1 Mullen Scales of Early Learning (MSEL) Developmental Quotient (DQ) by Risk Group and Sex, Controlling for Maternal Education
TABLE S2 Latent Class Analysis (LCA) Model Fit Statistics
TABLE S3 Mullen Scales of Early Learning (MSEL) T-Scores for Latent Classes
Clinical Guidance.
High-risk siblings who do not have an ASD diagnosis nevertheless exhibit slightly higher ASD-related severity scores on the ADOS and slightly lower verbal and nonverbal developmental functioning on the Mullen than low-risk control children at age 3.
Among high-risk siblings who do not themselves have ASD outcomes, the majority (65%) occupy classes that appear typical with respect to mean ASD severity and developmental functioning.
Another 14% occupy a class characterized by high ASD severity but high levels of developmental functioning.
The final 21% of high risk siblings without an ASD outcome are overrepresented in two classes characterized by high levels of ASD severity in the presence of low-average developmental functioning, and by low levels of ASD severity in the presence of lower developmental functioning.
High-risk siblings without ASD outcomes are nevertheless at high risk for the emergence of ASD-related behavioral characteristics and developmental difficulties, reinforcing the importance of developmental surveillance.
Acknowledgments
Autism Speaks provided funding for the creation of a Baby Siblings Research Consortium (BSRC) database and data analysis (G.S.Y.). Data collection was supported by the National Institutes of Health (NIH) grants MH068398 (S.O., S.J.R., G.S.Y.), NIH DC10290 (A.C.), NIH HD047417 (D.M.), NIH HD057284 (W.L.S., D.M.), NIH HD042541 (J.N.C.), HD052804 (K.D., L.J.C.), HD54979 (J.M.I.), NIH U54-MH066417 and MH059630 (R.J.L.), NIH U54-MH068172 (M.S., T.H.), and NIH HD043292 (W.L.S.); the United States–Israel Binational Science Foundation 94-66/3 and 97-00073 (M.S.); and the Canadian Institute for Health Research 62924 and 102665 (L.Z., S.B.).
Dr. Gregory S. Young served as the statistical expert for this research.
The authors thank Alycia Halladay, Ph.D., of Autism Speaks, for tremendous organization support, and Batya Elbaum, Ph.D., of the University of Miami, for editorial assistance.
Dr. Carter has received royalties from the Infant Toddler Social Emotional Assessment (ITSEA). Dr. Charman has received grant or research support from the UK Medical Research Council, the European Science Foundation, Autistica, Research Autism, the Autism Education Trust, and Autism Speaks. He has received royalties from Guilford Press and Sage. Dr. Constantino has received grant or research support from NIH, the National Institute of Child Health and Human Development, the National Institute of Mental Health, the Department of Health and Human Services, Autism Speaks, and the Centers for Disease Control and Prevention. He has received royalties for the authorship of assessment tools from Western Psychological Services (SRS). Dr. Rogers is an author of Early Start Denver Model for Young Children With Autism, published by Guilford Press, from which she has received royalties. Dr. Sigman is deceased.
Footnotes
Disclosure: Drs. Messinger, Young, Ozonoff, Dobkins, Zwaigenbaum, Landa, Stone, Hutman, Carver, Bryson, Iverson, and Strauss report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Autism and Developmental Disabilities Monitoring (ADDM) Network Prevalence of Autism Spectrum Disorders—Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Morbid Mortal Wkly Rep (MMWR) 2012;61:1–19. [PubMed] [Google Scholar]
- 2.Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis autism spectrum disorders. Arch Gen Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- 3.Shumway S, Thurm A, Swedo SE, et al. Brief report: Symptom onset patterns and functional outcomes in young children with autism spectrum disorders. J Autism Developmental Disorders. 2011;41:1727–1732. doi: 10.1007/s10803-011-1203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozonoff S, Young GS, Carter A, et al. [Accepted December 18, 2012];Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011 128:15. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piven J. Genetic liability for autism: the behavioural expression in relatives. Int Rev Psychiatry. 1999;11:299–308. [Google Scholar]
- 6.Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 7.Pickles A, Starr E, Kazak S, et al. Variable expression of the autism broader phenotype: findings from extended pedigrees. J Child Psychol Psychiatry Allied Discipl. 2000;41:491–502. [PubMed] [Google Scholar]
- 8.Toth K, Dawson G, Meltzoff A, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. J Autism Dev Disord. 2007;37:145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone W, McMahon CR, Yoder PJ, Walden TA. Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161:384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- 10.Garon N, Bryson SE, Zwaigenbaum L, et al. Temperament and its relationship to autistic symptoms in a high-risk infant sib cohort. J Abnorm Child Psychol. 2009;37:59–78. doi: 10.1007/s10802-008-9258-0. [DOI] [PubMed] [Google Scholar]
- 11.Yirmiya N, Gamliel I, Shaked M, Sigman M. Cognitive and verbal abilities of 24- to 36-month-old siblings of children with autism. J Autism Dev Disord. 2007;37:218–229. doi: 10.1007/s10803-006-0163-5. [DOI] [PubMed] [Google Scholar]
- 12.Zwaigenbaum L, Bryson SE, Szatmari P, et al. Sex differences in children with autism spectrum disorder identified within a high-risk infant cohort. J Autism Dev Disord. 2012;28:28. doi: 10.1007/s10803-012-1515-y. [DOI] [PubMed] [Google Scholar]
- 13.Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby siblings of children with autism. J Child Psychol Psychiatry. 2012;10:1469–7610. doi: 10.1111/j.1469-7610.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamliel I, Yirmiya N, Jaffe DH, Manor O, Sigman M. Developmental trajectories in siblings of children with autism: cognition and language from 4 months to 7 years. J Autism Dev Disord. 2009;39:1131–1144. doi: 10.1007/s10803-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Schwichtenberg AJ, Young GS, Sigman M, Hutman T, Ozonoff S. Can family affectedness inform infant sibling outcomes of autism spectrum disorders? J Child Psychol Psychiatry. 2010;51:1021–1030. doi: 10.1111/j.1469-7610.2010.02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozonoff S, Losif A-M, Baguio F, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256–266. [PMC free article] [PubMed] [Google Scholar]
- 19.Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167:1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantino J, Lajonchere C, Lutz M, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163:294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 21.Hallmayer J, Cleveland S, Torres A, et al. Genetic Heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;2011(68):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lord, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule–Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 23.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 24.Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- 25.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullen EM. Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- 27.Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 28.Munson J, Dawson G, Sterling L, et al. Evidence for latent classes of IQ in young children with autism spectrum disorder. Am J Ment Retard. 2008;113:439–452. doi: 10.1352/2008.113:439-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leisch F. FlexMix: a general framework for finite mixture models and latent class regression in R. J Stat Software. 2004;11:1–18. [Google Scholar]
- 30.Nylund KL, Asparouhov T, Muthen B. Deciding on the number of classes in latent class analysis and growth mixture modeling. A Monte Carlo simulation study. Struct Equat Model. 2007;14:535–569. [Google Scholar]
- 31.Kass RE, Raftery AE. Bayes factors. J Am Stat Assoc. 1995;90:773–795. [Google Scholar]
- 32.Shumway S, Farmer C, Thurm A, Joseph L, Black D, Golden C. The ADOS calibrated severity score: relationship to phenotypic variables and stability over time. Autism Res. 2012;5:267–276. doi: 10.1002/aur.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 34.Chapman HA, Kim DA, Susskind JM, Anderson AK. In bad taste: evidence for the oral origins of moral disgust. Science. 2009;323:1222–1226. doi: 10.1126/science.1165565. [DOI] [PubMed] [Google Scholar]
- 35.Sumi STH, Miyachi T, Tanemura M. Sibling risk of pervasive developmental disorder estimated by means of an epidemiologic survey in Nagoya, Japan. J Hum Genet. 2006;51:518–522. doi: 10.1007/s10038-006-0392-7. [DOI] [PubMed] [Google Scholar]
- 36.Arden R, Plomin R. Sex differences in variance of intelligence across childhood. Personal Indiv Diff. 2006;41:39–48. [Google Scholar]
- 37.Dezoete JA, MacArthur BA, Tuck B. Prediction of Bayley and Stanford-Binet scores with a group of very low birthweight children. Child. 2003;29:367–372. doi: 10.1046/j.1365-2214.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 38.Spinillo A, Montanari L, Gardella B, Roccio M, Stronati M, Fazzi E. Infant sex, obstetric risk factors, and 2-year neurodevelopmental outcome among preterm infants. Dev Med Child Neurol. 2009;51:518–525. doi: 10.1111/j.1469-8749.2009.03273.x. [DOI] [PubMed] [Google Scholar]
- 39.Eapen V. Genetic basis of autism: is there a way forward? Curr Opin Psychiatry. 2011;24:226–236. doi: 10.1097/YCO.0b013e328345927e. [DOI] [PubMed] [Google Scholar]
- 40.Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Constantino JN, Todorov A, Hilton C, et al. Autism recurrence in half siblings: strong support for genetic mechanisms of transmission in ASD [published online February 28, 2012] Mol Psychiatry. 2012 doi: 10.1038/mp.2012.9. doi:10.1038/mp.2012.9. [DOI] [PubMed] [Google Scholar]
- 42.Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011;9:e1001081. doi: 10.1371/journal.pbio.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Constantino J, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:5824–5530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Yizhak N, Yirmiya N, Seidman I, Alon R, Lord C, Sigman M. Pragmatic language and school related linguistic abilities in siblings of children with autism. J Autism Dev Disord. 2011;41:750–760. doi: 10.1007/s10803-010-1096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Georgiades S, SPZL, et al. A prospective study of autistic-like traits in unaffected siblings of probands with autism spectrum disorder. Arch Gen Psychiatry. 2012:1–7. doi: 10.1001/2013.jamapsychiatry.1. [DOI] [PubMed] [Google Scholar]
- 46.Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zwaigenbaum L, Byson S, Lord C, et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics. 2009;123:1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Mullen Scales of Early Learning (MSEL) Developmental Quotient (DQ) by Risk Group and Sex, Controlling for Maternal Education
TABLE S2 Latent Class Analysis (LCA) Model Fit Statistics
TABLE S3 Mullen Scales of Early Learning (MSEL) T-Scores for Latent Classes


