Abstract
Mitochondrial DNA (mtDNA) depletion syndromes (MDS) are a genetically and clinically heterogeneous group of autosomal recessive disorders that are characterized by a severe reduction in mtDNA content leading to impaired energy production in affected tissues and organs. MDS are due to defects in mtDNA maintenance caused by mutations in nuclear genes that function in either mitochondrial nucleotide synthesis (TK2, SUCLA2, SUCLG1, RRM2B, DGUOK, and TYMP) or mtDNA replication (POLG and C10orf2). MDS are phenotypically heterogeneous and usually classified as myopathic, encephalomyopathic, hepatocerebral or neurogastrointestinal. Myopathic MDS, caused by mutations in TK2, usually present before the age of 2 years with hypotonia and muscle weakness. Encephalomyopathic MDS, caused by mutations in SUCLA2, SUCLG1, or RRM2B, typically present during infancy with hypotonia and pronounced neurological features. Hepatocerebral MDS, caused by mutations in DGUOK, MPV17, POLG, or C10orf2, commonly have an early-onset liver dysfunction and neurological involvement. Finally, TYMP mutations have been associated with mitochondrial neurogastrointestinal encephalopathy (MNGIE) disease that typically presents before the age of 20 years with progressive gastrointestinal dysmotility and peripheral neuropathy. Overall, MDS are severe disorders with poor prognosis in the majority of affected individuals. No efficacious therapy is available for any of these disorders. Affected individuals should have a comprehensive evaluation to assess the degree of involvement of different systems. Treatment is directed mainly toward providing symptomatic management. Nutritional modulation and cofactor supplementation may be beneficial. Liver transplantation remains controversial. Finally, stem cell transplantation in MNGIE disease shows promising results.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0177-6) contains supplementary material, which is available to authorized users.
Keywords: Mitochondrial myopathy, Mitochondrial encephalomyopathy, Hepatocerebral syndrome, Mitochondrial neurogastrointestinal (MNGIE) disease, Alpers-Huttenlocher syndrome
Introduction
Mitochondrial DNA (mtDNA) depletion syndromes (MDS) are autosomal recessive disorders with a broad genetic and clinical spectrum that are characterized by a severe reduction in mtDNA content in affected tissues and organs. An adequate amount of mtDNA is required for the production of key subunits of mitochondrial respiratory chain complexes and therefore for energy production. Therefore, mtDNA depletion results in organ dysfunction that is likely due to insufficient synthesis of respiratory chain components needed for adequate energy production [1–3].
MDS are associated with defects in mtDNA maintenance caused by mutations in nuclear genes that function in either mitochondrial deoxyribonucleoside triphosphate (dNTP) synthesis or mtDNA replication. TK2 (thymidine kinase 2), SUCLA2 [adenosine diphosphate (ADP)-forming succinyl CoA ligase beta subunit], SUCLG1 [guanosine diphosphate (GDP)-forming succinyl CoA ligase alpha subunit], RRM2B (ribonucleotide reductase M2 B subunit), DGUOK (deoxyguanosine kinase), and TYMP (thymidine phosphorylase) encode proteins that maintain the mitochondrial dNTP pool; therefore, mutations in any of these genes result in depleting the mitochondria from DNA building blocks and, subsequently, mtDNA depletion. POLG (DNA polymerase gamma) and C10orf2 (Twinkle) are essential for mtDNA replication; therefore, mutations in these genes result in insufficient mtDNA synthesis to keep up with mtDNA turnover and segregation to daughter cells during cell divisions resulting in reduction of mtDNA content [4, 5].
MDS are phenotypically heterogeneous and may affect either a specific organ or a combination of organs, including muscle, liver, brain, and kidney. Clinically, MDS are usually classified as 1 of 4 forms: a myopathic form associated with mutations in TK2; an encephalomyopathic form associated with mutations in SUCLA2, SUCLG1, or RRM2B; a hepatocerebral form associated with mutations in DGUOK, MPV17, POLG, or C10orf2; and a neurogastrointestinal form associated with mutations in TYMP [4, 5].
In this review we discuss the genetic basis, clinical manifestations, and therapeutic options for MDS.
Genetic Basis of mtDNA Depletion Syndromes
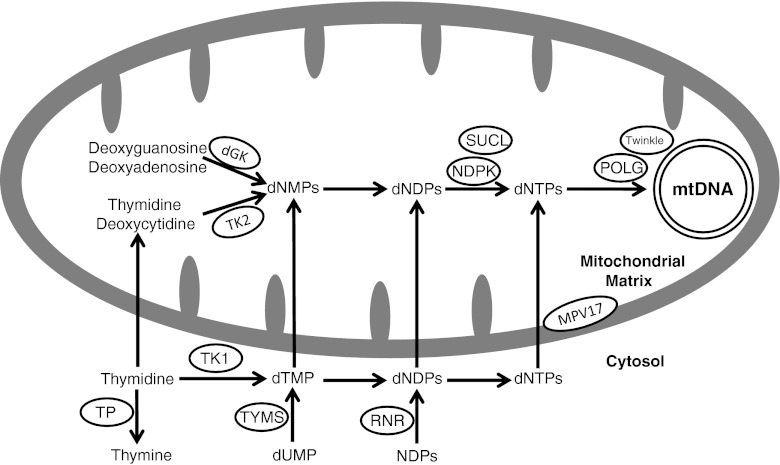
MDS are due to defects in mtDNA maintenance caused by mutations in nuclear genes, which function in either maintaining the mitochondrial nucleotide pool (TK2, DGUOK, SUCLA2, SUCLG1, RRM2B, and TYMP) or by mutations in genes associated with mtDNA replication (POLG and C10orf2). The function of the MPV17 gene remains unclear (Fig. 1).
Fig. 1.
Schematic presentation of protein involved in mitochondrial nucleotide pools maintenance and mitochondrial DNA replication. TK2 = mitochondrial thymidine kinase 2 (encoded by TK2 gene); dGK = mitochondrial deoxyguanosine kinase (encoded by the DGUOK gene); SUCL = succinyl CoA ligase (SUCL is composed of an alpha subunit, encoded by SUCLG1 and a beta subunit, encoded by either SUCLA2 or SUCLG2); NDPK = nucleoside diphosphate kinase; POLG = DNA polymerase gamma (POLG is a heterotrimer enzyme composed of one catalytic subunit encoded by POLG and two accessory subunits encoded by POLG2); TP = thymidine phosphorylase (encoded by TYMP gene); RNR = ribonucleotide reductase (RRM2B encodes the p53-inducible small subunit (p53R2) of the RNR); dNMP = deoxynucleoside monophosphate; dNDP = deoxynucleoside diphosphate; dNTP = deoxynucleoside triphosphate; NDP = nucleoside diphosphate; dTMP = deoxythymidine monophosphate; TK1 = cytosolic thymidine kinase 1; TYMS = thymidylate synthase. The twinkle protein is encoded by C10orf2 and the MPV17 by the MPV17 gene
Defects in Maintaining Mitochondrial Nucleotide Pool
Unlike nuclear DNA, which replicates with each cell division, mtDNA replicates continuously and independently of cell division. dNTPs can be synthesized via either the de novo pathway, which is cell cycle-regulated, thereby operative only in S-phase cells or the salvage pathway in which dNTPs are produced by utilizing pre-existing deoxynucleosides to synthesize DNA precursors. As mtDNA synthesis is continuous throughout the cell cycle, the salvage pathway becomes essential for mtDNA maintenance. TK2, DGUOK, SUCLA2, SUCLG1, RRM2B, and TYMP encode proteins that maintain the mitochondrial dNTP pool mainly through salvage pathways; therefore, mutations in any of these genes result in depleting the mitochondria from DNA building blocks with subsequent mtDNA depletion.
Mitochondrial thymidine kinase 2 (TK2) is encoded by the nuclear gene TK2 and plays an essential role in the pyrimidine nucleoside salvage pathway [6]. It mediates the first, and rate-limiting, step in the phosphorylation of pyrimidine nucleosides in the mitochondrial matrix. Mitochondrial deoxyguanosine kinase is encoded by the nuclear gene DGUOK and is essential for the purine nucleoside salvage pathway as it mediates the first step in the phosphorylation of purine nucleosides in the mitochondrial matrix [7]. Mutations in TK2 or DGUOK result in impaired synthesis of mitochondrial dNTPs, the building blocks for mtDNA, leading to decreased mtDNA amount and mtDNA depletion.
SUCLA2 and SUCLG1 encode subunits of succinyl CoA ligase (SUCL). SUCL is a mitochondrial tricarboxylic acid cycle enzyme that catalyzes the reversible conversion of succinyl-CoA and ADP or GDP to succinate and adenosine triphosphate or guanosine triphosphate. SUCL is composed of an alpha subunit, encoded by SUCLG1 and a beta subunit, encoded by either SUCLA2 or SUCLG2. The alpha subunit forms a heterodimer with either of its beta subunits, resulting in an ADP-forming SUCL and a GDP-forming SUCL, respectively. SUCL also forms a complex with the mitochondrial nucleoside diphosphate kinase, and the lack of this complex formation in SUCL deficiency has been suggested to disturb the kinase function, resulting in decreased mtDNA synthesis leading to mtDNA depletion [8].
RRM2B encodes the p53-inducible small subunit (p53R2) of ribonucleotide reductase, a cytosolic enzyme that catalyzes the terminal step of de novo synthesis of deoxyribonucleoside by direct reduction of ribonucleoside diphosphates to their corresponding deoxyribonucleoside diphosphates. The p53R2 is expressed in post-mitotic cells and therefore has a key function in the maintenance of dNTP pools for mtDNA synthesis [9].
TYMP encodes thymidine phosphorylase (TP), which is a cytosolic enzyme that catalyzes the conversion of thymidine to thymine and deoxyuridine to uracil, and is therefore essential for the nucleotide salvage pathway. Low TP activity results in the accumulation of thymidine and deoxyuridine, leading to an imbalance of cytosolic dNTP pools. Because the mitochondrial dNTP pool relies, in part, on dNTP imported from the cytosol, an imbalanced cytosolic dNTP pool can lead to an imbalanced mitochondrial dNTP pool that can impair mtDNA synthesis [4].
Defects in mtDNA Replication
POLG encodes the catalytic subunit of DNA polymerase gamma, which is a heterotrimer enzyme composed of one catalytic subunit encoded by POLG and two accessory subunits encoded by POLG2 that assist in binding and processing the synthesized DNA. DNA polymerase gamma is required for mtDNA synthesis as it is the only DNA polymerase in humans that allows for replication and repair of mtDNA [10]. The twinkle protein, encoded by C10orf2, serves the important function of a DNA helicase that is required for DNA replication [11]. Therefore, POLG and C10orf2 are essential for mtDNA replication and mutations in these genes result in insufficient mtDNA synthesis to keep up with mtDNA turnover and segregation to daughter cells during cell divisions, resulting in a reduction of mtDNA content and mtDNA depletion.
mtDNA Depletion Caused by Defects in a Protein of Unknown Function
MPV17 encodes the MPV17 protein, an inner mitochondrial membrane protein whose function and role in the pathogenesis of mtDNA depletion are as yet unknown. It has been suggested that MPV17 plays a role in controlling mtDNA maintenance and oxidative phosphorylation activity in mammals and yeast [12]. A dysfunctional MPV17 protein caused by MPV17 mutations impairs mtDNA maintenance and can cause mtDNA depletion.
Clinical Manifestations of MDS
MDS are classified as 1 of 4 clinical forms: a myopathic form associated with mutations in TK2, an encephalomyopathic form associated with mutations in SUCLA2, SUCLG1, or RRM2B, a hepatocerebral form associated with mutations in DGUOK, MPV17, POLG, or C10orf2, and a neurogastrointestinal form associated with mutations in TYMP (Table 1).
Table 1.
Clinical phenotypes of different mitochondrial DNA depletion syndromes
| Mitochondrial DNA depletion syndromes | Age of onset | Common clinical features |
|---|---|---|
| Myopathic | ||
| TK2-related | Infancy—early childhood | Hypotonia and muscle weakness, facial weakness, bulbar weakness (dysarthria and dysphagia), elevated serum creatine phosphokinase |
| Encephalomyopathic | ||
| SUCLA2- and SUCLG1-related | Infancy | Hypotonia and muscle weakness, psychomotor delay, scoliosis/kyphosis, abnormal movement disorders (dystonia, athetoid, or choreiform), sensorineural hearing impairment, epilepsy, growth retardation, lactic acidosis, elevated methylmalonic acid in urine and plasma, cortical atrophy and basal ganglia involvement in neuroimaging |
| RRM2B-related | Neonatal—infancy | Hypotonia and muscle weakness, psychomotor delay, microcephaly, sensorineural hearing loss, failure to thrive, lactic acidosis |
| Hepatocerebral | ||
| DGUOK-related | Neonatal | Hepatic dysfunction, psychomotor delay, hypotonia, rotary nystagmus developing into opsoclonus, lactic acidosis, hypoglycemia |
| MPV17-related | Infantile—childhood | Hepatic dysfunction, psychomotor delay, hypotonia, peripheral neuropathy, lactic acidosis, hypoglycemia, leukoencephalopathy in neuroimaging |
| POLG-related | Early childhood | Hepatic dysfunction, epilepsy, psychomotor delay, ataxia, neuropathy, hyporeflexia and hypotonia evolving into spastic paraparesis, stroke or stroke-like episodes, myoclonus, choreoathetosis, parkinsonism, nystagmus, somnolence, irritability, cortical visual loss, and sensorineural hearing impairment, generalized brain atrophy in neuroimaging |
| C10orf2-related | Neonatal—infancy | Hepatic dysfunction, psychomotor delay, epilepsy, peripheral neuropathy, hypotonia, ophthalmoplegia, nystagmus, athetosis, ataxia, sensorineural hearing impairment, lactic acidosis, cerebellar cortical atrophy in neuroimaging |
| Neurogastrointestinal | ||
| TYMP-related | Late childhood—adolescence | Gastrointestinal dysmotility, weight loss, peripheral neuropathy, ptosis, ophthalmoplegia, elevated thymidine and deoxyuridine in plasma, leukoencephalopathy in neuroimaging |
TK2-Related Myopathic MDS
To date, approximately 50 affected individuals have been reported with TK2-related MDS. The clinical presentation of TK2-related MDS is variable, with a broad phenotype. Initial development is typically normal and the majority of affected children present before the age of 2 years with gradual onset of hypotonia, generalized fatigue, decreased physical stamina, proximal muscle weakness, and feeding difficulty. Some patients develop facial weakness and bulbar weakness, including dysarthria and dysphagia. Hypotonia and weakness is observed in all patients and previously acquired motor skills are lost. However, cognitive function is typically spared [13–23].
Although TK2-related MDS has been thought to be associated with a purely myopathic form, other organ system involvements have been reported, including an encephalomyopathic presentation with hypotonia, weakness, epilepsy, and microcephaly [20], and hepatic involvement with hepatomegaly and elevated transaminases accompanied by mtDNA depletion in muscle and liver [19]. Other, less common, presentations include spinal muscular atrophy-like presentation [13] and chronic progressive external ophthalmoplegia with proximal muscle weakness [23]. Milder presentations have been reported and include late onset proximal muscle weakness [15], adult-onset progressive myopathy [22], and sensorineural hearing loss [21].
Serum creatine phosphokinase concentration is usually elevated and electromyography (EMG) usually shows non-specific myopathic changes. Histopathological findings on skeletal muscle include prominent variance in fiber size, sarcoplasmic vacuoles, and increased connective tissue. Ragged red fibers are present. Succinate dehydrogenase activity is increased, whereas cytochrome c oxidase activity is low, or absent. Electron microscopy shows abnormal mitochondria with circular cristae. mtDNA content is typically severely reduced in muscle tissue. Electron transport chain (ETC) activity assays in skeletal muscle typically show decreased activity of multiple complexes with complex I, I + III, and IV being the most affected [13–23].
Typically, muscle weakness rapidly progresses leading to respiratory failure and death within a few years of onset. The most common cause of death is pulmonary infection. Only a few patients have survived to late childhood and adolescence.
SUCLA2 and SUCLG1-Related Encephalomyopathic MDS
Nearly 20 individuals have been reported with SUCLA2-related MDS. Mutations in SUCLG1 have been reported less frequently with a similar phenotype to that observed in SUCLA2-related MDS [4, 24–30]. Affected infants present with hypotonia typically before the age of 6 months. All affected children develop hypotonia, muscle atrophy, and psychomotor delay. Other frequent manifestations include progressive scoliosis or kyphosis, abnormal movements, including dystonia and athetoid or choreiform movements, feeding difficulty, gastroesophageal reflux, sensorineural hearing impairment, postnatal growth retardation, and respiratory insufficiency that can result in frequent pulmonary infections. Other, less common, manifestations include hyperhidrosis, strabismus, ptosis, and epilepsy presenting with either infantile spasms or generalized convulsions. Urine organic acids analysis consistently shows elevated methylmalonic acid. Similarly, plasma methylmalonic acid concentration is elevated. Lactate is elevated in both plasma and cerebrospinal fluid (CSF) in most affected individuals. EMG may reveal findings suggestive of motor neuron involvement, whereas neuroimaging may show cortical atrophy, bilateral basal ganglia involvement, and delayed myelination. Histopathological findings on skeletal muscle include increased fiber variability, increased number of mitochondria, and extensive intracellular fat accumulation. ETC activity assays in muscle typically show a combined deficiency of respiratory complex I, III, and IV, with normal complex II activity. Quantitation of mtDNA shows a decreased mtDNA content in muscle. Prognosis is poor, with most affected children dying in childhood, most commonly from an intercurrent infection [24–30].
RRM2B-Related Encephalomyopathic MDS
To date, RRM2B mutations have been reported in about 15 infants with severe encephalomyopathic MDS that is associated with early-onset (neonatal or infantile), multi-organ presentation, and mortality during infancy. Affected individuals typically present during the first months of life with hypotonia, lactic acidosis, failure to thrive, tubulopathy, microcephaly, psychomotor delay, sensorineural hearing loss, and profound mtDNA depletion in muscle. The disease progresses rapidly, leading to death in few months [4, 31–34].
RRM2B mutations have also been reported to cause a mitochondrial neurogastrointestinal encephalopathy (MNGIE)-like phenotype with mtDNA depletion [35] and autosomal-dominant progressive external ophthalmoplegia (PEO) with multiple mtDNA deletions [36, 37].
DGUOK-Related Hepatocerebral MDS
Approximately 100 individuals have been reported with DGUOK-related MDS, which can present in two forms: multi-organ disease in neonates and isolated hepatic disease later in infancy or childhood [38]. The majority of affected individuals have a neonatal-onset multi-organ illness that presents with lactic acidosis and hypoglycemia in the first week of life. Within weeks of birth, all infants develop hepatic disease and neurologic dysfunction. Severe myopathy, developmental regression, and typical rotary nystagmus developing into opsoclonus are also seen. Cholestasis is prominent early in the clinical course. Liver involvement may cause neonatal- or infantile-onset liver failure that is generally progressive with ascites, edema, and coagulopathy. A minority of affected individuals present initially in infancy or childhood with isolated hepatic disease, occasionally following a viral illness. Affected individuals with this form may develop mild hypotonia and renal involvement manifesting as proteinuria and aminoaciduria [2, 38–55]. More recently, DGUOK mutations have been reported in a neonate with clinical and autopsy findings consistent with neonatal hemochromatosis and mtDNA depletion [56], and in individuals with adult-onset mitochondrial myopathy and mtDNA multiple deletions in skeletal muscle [57].
The majority of affected newborns with the multi-organ form of the disease show elevated serum concentration of tyrosine or phenylalanine on newborn screening [54, 55]. Findings of intrahepatic cholestasis typically include elevations in serum concentrations of liver transaminases, gamma-glutamyltransferase (GGT), and conjugated hyperbilirubinemia. Increased serum concentration of ferritin is observed in a large number of affected infants. mtDNA content is reduced in liver and muscle [54, 55]. ETC activity in liver typically shows a combined deficiency of complexes I, III, and IV [49]. Liver histopathology typically reveals microvesicular cholestasis, but may show bridging fibrosis, giant cell hepatitis, or cirrhosis. Liver electron microscopy may reveal an increase in the number of mitochondria and is commonly associated with abnormal cristae [40, 45, 54, 55, 58].
Hepatic dysfunction is progressive in the majority of individuals with both forms of DGUOK-related MDS and is the most common cause of death. Hepatocellular carcinoma has also been reported in 1 patient. For children with the multi-organ form, liver transplantation provides no survival benefit [53].
MPV17-Related Hepatocerebral MDS
MPV17-related hepatocerebral MDS, an infantile-onset disorder, can present with a spectrum of combined hepatic, neurologic, and metabolic manifestations. Approximately 30 affected individuals have been reported with MPV17-related hepatocerebral MDS [59–68]. Of note, among those confirmed cases are individuals with Navajo neurohepatopathy who were found to have homozygous p.Arg50Gln mutations in MPV17. Navajo neurohepatopathy, a disorder prevalent in the Native American Navajo population, has the manifestations of MPV17-related hepatocerebral MDS, as well as painless fractures, acral mutilation, and corneal anesthesia, ulceration, and scarring [60].
Affected individuals typically present with manifestations of liver dysfunction, including jaundice, cholestasis, and coagulopathy. Infancy is the typical age of onset; however, individuals homozygous for the p.Arg50Gln mutation may present later in childhood [60]. In the vast majority of affected individuals, liver disease progresses to liver failure typically during infancy or early childhood. Hepatomegaly and liver cirrhosis occur in some affected individuals. Hepatocellular carcinoma has also been reported in 2 affected individuals. The vast majority of affected individuals exhibited neurologic manifestations, including developmental delay, hypotonia, muscle weakness, and motor and sensory peripheral neuropathy. Some affected individuals presented with psychomotor delays during early infancy, while others had normal development early in life followed by loss of motor and cognitive abilities later in infancy or early childhood. Less frequent neurologic manifestations include epilepsy, ataxia, dystonia, microcephaly, cerebrovascular infarction, and subdural hematoma. Failure to thrive is one of the common manifestations, although some children have normal growth, especially early in the course of the disease. The vast majority of affected individuals have metabolic derangements, including lactic acidosis and hypoglycemia, which typically presents during the first 6 months of life. Less frequent manifestations include renal tubulopathy, hypoparathyroidism, and gastrointestinal dysmotility that manifests as gastroesophageal reflux, cyclic vomiting, and diarrhea. Corneal anesthesia and ulcers were reported in individuals homozygous for the mutation p.Arg50Gln [59–68].
More recently, MPV17 mutations have been reported in adult presentation of neuropathy and leukoencephalopathy with multiple mtDNA deletions in muscle indicating that MPV17 mutations are associated with an evolving broader phenotype [69]
Affected infants demonstrate elevated transaminases and GGT, and hyperbilirubinemia. Liver histopathology may show cholestasis and cirrhosis. Neuroimaging may show white matter abnormalities (leukoencephalopathy). mtDNA content is severely and consistently reduced in liver tissue, and can also be reduced in muscle tissue. ETC activity assays in liver and muscle tissue typically show decreased activity of multiple complexes with complex I or I + III being the most affected [66].
Liver disease typically progresses to liver failure in affected children and liver transplantation remains the only treatment option for liver failure. Approximately half of affected children reported did not undergo liver transplantation and died because of progressive liver failure—the majority during infancy or early childhood. A few children were reported to survive without liver transplantation [66].
POLG-Related Hepatocerebral MDS
POLG-related disorders present a continuum of broad and overlapping phenotypes presenting from early childhood to late adulthood. The clinical phenotypes of POLG-related disorders include autosomal recessive and dominant adult-onset PEO [70–73], myoclonic epilepsy, myopathy, sensory ataxia (MEMSA) syndrome [74, 75], ataxia-neuropathy spectrum including mitochondrial recessive ataxia syndrome (MIRAS), and sensory ataxia, neuropathy, dysarthria, ophthalmoplegia (SANDO) syndrome [76–79], and hepatocerebral MDS (Alpers-Huttenlocher syndrome) [80–90]. More recently, POLG mutations were identified in individuals with clinical features of MNGIE, but no leukoencephalopathy [91].
The incidence of Alpers-Huttenlocher syndrome has been estimated to be ~1:50,000 [92]. It is the most severe phenotype associated with POLG mutations and characterized by a progressive encephalopathy with intractable epilepsy and psychomotor delay, neuropathy, and hepatic failure. Affected individuals usually present between the age of 2 and 4 years with seizures (focal, generalized, myoclonic, epilepsia partialis continua, or status epilepticus), headaches that are typically associated with visual sensations or visual auras, hypotonia, and psychomotor regression. Early in the disease course areflexia and hypotonia are present and later followed by spastic paraparesis that evolves over months to years, leading to psychomotor regression. All affected individuals develop neuropathy, ataxia, and loss of cognitive function, including concentration, language skills, and memory. Affected individuals may also develop stroke and stroke-like episodes, myoclonus, choreoathetosis, parkinsonism, nystagmus, somnolence, irritability, loss of normal emotional responses, depression, cortical visual loss, and sensorineural hearing loss. Neurologic signs and symptoms may worsen during infections or other stressful situations. Affected individuals develop liver dysfunction with elevated transaminases, hypoalbuminemia, coagulopathy, hypoglycemia, and hyperammonemia. Liver involvement can progress rapidly to end-stage liver failure within a few months. CSF protein is generally elevated. Neuroimaging may show gliosis and generalized brain atrophy. Liver histology may demonstrate macro- and microvesicular steatosis, centrilobular necrosis, fibrosis, cirrhosis, bile duct proliferation, and mitochondrial proliferation. mtDNA content is reduced in liver. Disease progression is variable, with life expectancy from onset of symptoms ranging from 3 months to 12 years [80–90].
C10orf2-Related Hepatocerebral MDS
Mutations in C10orf2 have been associated with variable phenotypes, including infantile-onset spinocerebellar ataxia [11, 93–97], autosomal dominant PEO [98–100], and hepatocerebral MDS [101, 102]. Mutations in C10orf2 are a rare cause of early-onset hepatocerebral MDS that has been reported in 5 children from 2 unrelated families. Affected individuals typically present in the neonatal or infantile period with lactic acidosis, hepatomegaly, hypotonia, and psychomotor delay. The neurologic involvement progresses to include hyporeflexia, muscular atrophy, ophthalmoplegia, nystagmus, athetosis, ataxia, epilepsy, sensory neuropathy, sensorineural hearing impairment, psychomotor regression. Liver involvement includes cholestasis, increased transaminases, and coagulopathy. Affected infants may also have feeding difficulties and growth retardation. Lactate is increased in plasma and CSF. Neuroimaging can show cerebellar cortical atrophy. ETC activity assays show reduced activities of complexes I, III, and IV. mtDNA content is severely reduced in liver tissue. Prognosis is poor with 3 of the reported affected children dying between 2 and 3 years of age [101, 102].
MNGIE Disease
Mutations in TYMP have been reported in about 70 individuals with MNGIE disease [103]. Affected individuals usually present clinical manifestations between the first and fifth decades with the majority starting with symptoms before age 20 years. All affected individuals develop weight loss and progressive gastrointestinal dysmotility manifesting as early satiety, nausea, dysphagia, gastroesophageal reflux, postprandial emesis, episodic abdominal pain and distention, and diarrhea. In addition, all affected individuals have peripheral demyelinating motor and sensory neuropathy that may be accompanied by axonal neuropathy in some cases. The neuropathy typically presents with distal weakness and paresthesias occurring in a symmetric stocking-glove distribution. Ptosis and ophthalmoplegia are common findings. Intellectual disability occurs in some individuals. Other variable manifestations include hepatic cirrhosis with increased liver enzymes and macrovesicular steatosis, anemia, sensorineural hearing loss, short stature, autonomic nervous system dysfunction (usually orthostatic hypotension), bladder dysfunction, ventricular hypertrophy, and diverticulosis [103–119].
Affected individuals can have elevated CSF protein and plasma lactate. Thymidine and deoxyuridine are increased in plasma. In affected individuals, thymidine phosphorylase enzyme activity in leukocytes is usually less than 10 % of the control mean [107, 112]. EMG and nerve conduction velocity show decreased motor and sensory nerve conduction velocities, and myopathic changes. Neuroimaging typically demonstrates diffuse white matter abnormalities (leukoencephalopathy) [103, 117]. mtDNA depletion, mitochondrial proliferation, and smooth cell atrophy are observed in the external layer of the muscularis propria in the stomach and in the small intestine. Skeletal muscle generally shows histologic abnormalities of a mitochondrial myopathy including ragged-red fibers and defects in single or multiple ETC complexes with the most common defect in complex IV. However, MNGIE has been reported without skeletal muscle involvement at the morphological, enzymatic, or mtDNA content level [120].
MNGIE is a progressive disease with mean age of death is approximately 40 years (ranging from 25–60 years) [108].
Therapeutic Options for MDS
Although MDS are severe disorders with poor prognosis in the majority of affected individuals, no curative therapy is available for any of these disorders. MDS are multi-organ disorders; therefore, affected individuals should have a comprehensive evaluation to assess the degree of involvement of different systems. Management of MDS should involve a multidisciplinary team, including different specialists and aims to provide supportive care and symptomatic treatment for complications associated with these disorders. Other treatment options for some MDS include dietary modulation, cofactor supplementation, liver transplantation, and stem cell transplantation.
Assessment of the Extent of MDS
Affected individuals with MDS should have an extensive evaluation to understand the involvement of different organs, including the neuromuscular, hepatic, gastrointestinal, cardiac, and renal systems.
Almost all affected individuals with MSD show neuromuscular manifestations; therefore, a neurology consultation with comprehensive neurologic examination and developmental/cognitive assessment are mandatory. The following diagnostic modalities can be used to assess the degree of neurological involvement: neuroimaging (mainly brain magnetic resonance imaging) to establish the degree of central nervous system, nerve conduction velocity to establish the degree of the peripheral nervous system involvement, EMG to assess myopathy, and electroencecephalography if seizures are suspected. A thorough ophthalmologic and hearing evaluation is also required.
The degree of liver involvement in the hepatocerebral forms of MDS can be assessed by liver function tests, including liver transaminases, GGT, albumin, fasting blood glucose, ammonia, and coagulation profile; ultrasound examination to assess liver size and texture, and for the presence of masses; alpha fetoprotein (AFP) to screen for hepatocellular carcinoma; and hepatology/liver transplantation consultation.
Gastrointestinal evaluation in MNGIE disease may depend on the symptoms and can include the following: gastrointestinal consultation, abdominal imaging (X-ray and computed tomography), upper gastrointestinal contrast radiography, esophagogastroduodenoscopy, sigmoidoscopy, liquid phase scintigraphy, and antroduodenal manometry. These studies may show hypoperistalsis, gastroparesis, dilated duodenum, and diverticulosis. Small bowel manometry shows reduced amplitude of contractions [103].
Echocardiogram and electrocardiogram are needed to determine cardiac involvement. Pulmonary function tests and assessment of blood gases are needed for patients with myopathy to assess for respiratory insufficiency. Nutritional evaluation and swallowing assessment are needed in those with feeding difficulty and growth retardation. Urine analysis, urine electrolytes, and urine amino acids can be performed to assess renal tubulopathy.
Symptomatic Management for MDS
Seizures are common features in MDS with neurological involvement. Seizure control with antiepileptic medications is the goal of treatment; however, refractory epilepsy may be very difficult to control. The use of high-dose anticonvulsants and/or treatment with more than 1 medication often becomes necessary to control refractory seizures. It is very important to avoid valproic acid (Depakene®) and sodium divalproate (divalproex) (Depakote®) in treating seizures in MDS, particularly POLG-related disorders, because of the risk of precipitating and/or accelerating liver disease [121, 122].
Physical therapy can help maintain muscle function and prevent joint contractures. Feeding difficulties and failure to thrive may require nutritional support by experienced dietitian, occupational therapy to improve oromotor functions, and the use of a nasogastric tube or gastrostomy tube feedings.
Respiratory insufficiency can benefit from chest physiotherapy, aggressive antibiotic treatment of chest infections, and artificial ventilation that could include assisted nasal ventilation or intubation, and the use of a tracheostomy and ventilator.
Other treatment options include bracing to treat scoliosis or kyphosis, surgery for ptosis, and cochlear implantation for sensorineural hearing loss.
Nutritional Modulation in MDS
Formulas with an enriched medium-chain triglyceride content may provide better nutritional support for infants with cholestasis than formulas with predominantly long-chain triglycerides [123].
Prevention of hypoglycemia requires avoidance of fasting by frequent or continuous feeding. In addition, uncooked cornstarch may reduce symptomatic hypoglycemia in individuals with DGUOK and MPV17-related hepatocerebral MDS [54, 65]. Furthermore, cornstarch use may slow the progression of the liver disease in MPV17-related hepatocerebral MDS [63, 65].
Cofactor Use in MDS
Succinate and ubiquinone were reported to slow the progression of liver impairment in MPV17-related MDS [64].
Elevated CSF inflammatory cytokines and blocking folate receptor autoantibodies associated with reduced CSF folate were reported in a child with Alpers-Huttenlocher syndrome. Treatment with oral folinic acid (leucovorine) resulted in improvement of CSF folate level and seizure frequency, and communicative abilities improved [124]. Therefore, CSF folate may be deficient in disorders that lead to mtDNA depletion. It has been suggested that testing for CSF folate deficiency with treatment offered to those with deficiency can be one option; the other option can be empiric therapy with folinic acid [90].
Levocarnitine, creatine monohydrate, coenzyme Q10, B vitamins, and antioxidants, such as alpha lipoic acid, vitamin E, and vitamin C, have been used as mitochondrial supplements. These cofactors have been used in MDS; however, there is very limited evidence for their effectiveness [125, 126].
More recently, enteral administration of sodium pyruvate to a child with myopathic MDS has been reported to improve muscle strength and quality of life score using used the Newcastle Pediatric Mitochondrial Disease Scale. No significant change of the blood lactate level or lactate-to-pyruvate ratio were noticed [127]. Further evaluation is need before reaching conclusions about the effectiveness of such therapy.
Studying myotube cells of individuals with MDS have demonstrated that the application of variable combinations of deoxynucleoside monophosphates in different types of MDS result in near normalization of mtDNA content in many cases. Therefore, the use of deoxynucleoside monophosphate combinations may be a possible therapeutic approach for individuals with MDS [128]. Further clinical investigation is needed to investigate this approach.
Liver Transplantation in MDS
Although liver transplantation remains the only treatment option for liver failure in hepatocerebral MDS, liver transplantation in mitochondrial hepatopathy is controversial, largely because of the multi-organ involvement.
Liver transplantation has been performed in about a third of affected individuals with MPV17-related MDS; the outcome has not been satisfactory, with half of the transplanted children dying in the post-transplantation period because of multi-organ failure and/or sepsis [66].
For children with multi-organ DGUOK-related MDS, liver transplantation provides no survival benefit. However, several children with isolated hepatic disease have had excellent 10-year survival with liver transplantation and, thus, it is a potential therapeutic option. However, this option warrants careful discussion with parents because at least 1 child with isolated liver disease developed neurologic features after liver transplantation [53].
Liver transplantation is not advised in children with Alpers-Huttenlocher syndrome because transplanting the liver does not alter the rapid progression of the neurological complications [129]. However, liver transplantation in adults who have an acceptable quality of life may be of benefit. Two affected individuals with POLG-related disorders were reported to survive after liver transplantation [79, 88].
Thymidine Reduction in MNGIE Disease
In MNGIE, a correlation between plasma thymidine levels and the severity of the phenotype has been observed [130]. Therefore, it has been proposed that the reduction in circulating thymidine levels can result in disease improvement. Peritoneal dialysis has been used to reduce the thymidine levels leading to an improvement of the symptoms in affected individuals with MNGIE disease [131]. Enzyme replacement therapy via infusion of platelets from healthy donors to individuals with MNGIE resulted in reduction of circulating thymidine and partially restored TP activity [132].
Allogeneic hematopoietic stem cell transplantation (HSCT) offers the possibility of sustained correction of enzyme deficiency and has become an established treatment for many different storage diseases. More than 10 individuals with MNGIE disease have so far been treated with allogeneic HSCT [133–136]. Allogeneic HSCT has been shown to restore TP activity, lowering thymidine levels and improving the gastrointestinal dysmotility [132–135]. However, neurological assessments remained unchanged [136]. Although HSCT corrects biochemical abnormalities and improves gastrointestinal symptoms, the procedure can be risky in patients already in poor medical condition, as are many MNGIE patients and several affected patients were reported to die in the post-transplantation period [136]. As transplant-related morbidity and mortality increase with the progression of the disease and the number of associated comorbidities, it has been suggested that individuals with MNGIE should be referred to HSCT when they are still relatively healthy in order to minimize the complications of the procedure [136].
Conclusion
MDS are a genetically and clinically heterogeneous group characterized by a severe reduction in mtDNA content in affected tissues. MDS are due to a defect in mtDNA maintenance caused by mutations in nuclear genes which function in either mitochondrial dNTP synthesis (TK2, SUCLA2, SUCLG1, RRM2B, DGUOK, and TYMP) or mtDNA replication (POLG and C10orf2). The function of MPV17 is not yet known. According to the organ majorly involved, MDS are classified as the myopathic form associated with mutations in TK2, the encephalomyopathic form associated with mutations in SUCLA2, SUCLG1, or RRM2B, the hepatocerebral form associated with mutations in DGUOK, MPV17, POLG, or C10orf2, and the neurogastrointestinal form associated with mutations in TYMP. Although mutations in these different genes have been traditionally assigned to these phenotypes, the phenotypic associations have been expanding with the current advancement in the molecular testing. Although MDS are severe disorders with poor prognosis in the majority of affected individuals, no curative therapy is available for any of these disorders. Affected individuals should have a comprehensive evaluation to assess the degree of involvement of different systems. Treatment is directed mainly toward providing symptomatic management. Nutritional modulation and cofactor supplementation may be beneficial. Liver transplantation remains controversial. Finally, stem cell transplantation in MNGIE disease shows promising results.
Electronic Supplementary Material
(PDF 583 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Moraes CT, Shanske S, Tritschler HJ, et al. MtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991;48:492–501. [PMC free article] [PubMed] [Google Scholar]
- 2.Sarzi E, Bourdon A, Chrétien D, et al. Mitochondrial DNA depletion is a prevalent cause of multiple respiratory chain deficiency in childhood. J Pediatr. 2007;150:531–534. doi: 10.1016/j.jpeds.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Spinazzola A, Invernizzi F, Carrara F, et al. Clinical and molecular features of mitochondrial DNA depletion syndromes. J Inherit Metab Dis. 2009;32:143–158. doi: 10.1007/s10545-008-1038-z. [DOI] [PubMed] [Google Scholar]
- 4.Suomalainen A, Isohanni P. Mitochondrial DNA depletion syndromes—many genes, common mechanisms. Neuromuscul Disord. 2010;20:429–437. doi: 10.1016/j.nmd.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Spinazzola A. Mitochondrial DNA mutations and depletion in pediatric medicine. Semin Fetal Neonatal Med. 2011;16:190–196. doi: 10.1016/j.siny.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Johansson M, Karlsson A. Cloning of the cDNA and chromosome localization of the gene for human thymidine kinase 2. J Biol Chem. 1997;272:8454–8458. doi: 10.1074/jbc.272.29.17961. [DOI] [PubMed] [Google Scholar]
- 7.Johansson M, Karlsson A. Cloning and expression of human deoxyguanosine kinase cDNA. Proc Nat Acad Sci. 1996;93:7258–7262. doi: 10.1073/pnas.93.14.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowluru A, Tannous M, Chen HQ. Localization and characterization of the mitochondrial isoform of the nucleoside diphosphate kinase in the pancreatic beta cell: evidence for its complexation with mitochondrial succinyl-CoA synthetase. Arch Biochem Biophys. 2002;398:160–169. doi: 10.1006/abbi.2001.2710. [DOI] [PubMed] [Google Scholar]
- 9.Pontarin G, Fijolek A, Pizzo P, et al. Ribonucleotide reduction is a cytosolic process in mammalian cells independently of DNA damage. Proc Natl Acad Sci. 2008;105:17801–17806. doi: 10.1073/pnas.0808198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecrenier N, van der Bruggen P, Foury F. Mitochondrial DNA polymerases from yeast to man: a new family of polymerases. Gene. 1997;185:147–152. doi: 10.1016/S0378-1119(96)00663-4. [DOI] [PubMed] [Google Scholar]
- 11.Spelbrink JN, Li FY, Tiranti V, et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet. 2001;28:223–231. doi: 10.1038/90058. [DOI] [PubMed] [Google Scholar]
- 12.Dallabona C, Marsano RM, et al. Sym1, the yeast ortholog of the MPV17 human disease protein, is a stress-induced bioenergetic and morphogenetic mitochondrial modulator. Hum Mol Genet. 2010;19:1098–1107. doi: 10.1093/hmg/ddp581. [DOI] [PubMed] [Google Scholar]
- 13.Pons R, Andreetta F, Wang CH, et al. Mitochondrial myopathy simulating spinal muscular atrophy. Pediatr Neurol. 1996;15:153–158. doi: 10.1016/0887-8994(96)00118-X. [DOI] [PubMed] [Google Scholar]
- 14.Galbiati S, Bordoni A, Papadimitriou D, et al. New mutation in TK2 gene associated with mitochondrial DNA depletion. Pediatr Neurol. 2006;34:177–185. doi: 10.1016/j.pediatrneurol.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Oskoui M, Davidzon G, Pascual J, et al. Clinical spectrum of mitochondrial DNA depletion due to mutation in the thymidine kinase 2 gene. Arch Neurol. 2006;63:1122–1126. doi: 10.1001/archneur.63.8.1122. [DOI] [PubMed] [Google Scholar]
- 16.Blakely E, He L, Gardner JL, et al. Novel mutationin the TK2 gene associated with fatal mitochondrial DNA depletion myopathy. Neuromuscul Disord. 2008;18:557–560. doi: 10.1016/j.nmd.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Gotz A, Isohanni P, Pihko H, et al. Thymidine kinase 3 defects can cause multi-tissue mtDNA depletion syndrome. Brain. 2008;131:2841–2850. doi: 10.1093/brain/awn236. [DOI] [PubMed] [Google Scholar]
- 18.Collins J, Bove KE, Dimmock D, Morehart P, Wong LJ, Wong B. Progressive myofiber loss with extensive fibro-fatty replacement in a child with mitochondrial DNA depletion syndrome and novel thymidine kinase 2 gene mutations. Neuromuscul Disord. 2009;19:784–787. doi: 10.1016/j.nmd.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Li FY, Bass HN, et al. Application of oligonucleotide array CGH to the simultaneous detection of a deletion in the nuclear TK2 gene and mtDNA depletion. Mol Genet Metab. 2010;99:53–57. doi: 10.1016/j.ymgme.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Lesko N, Naess K, Wibom R, et al. Two novel mutations in thymidine kinase-2 cause early onset fatal encephalomyopathy and severe mtDNA depletion. Neuromuscul Disord. 2010;20:198–203. doi: 10.1016/j.nmd.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Martí R, Nascimento A, Colomer J, et al. Hearing loss in a patient with the myopathic form of mitochondrial DNA depletion syndrome and novel mutation in the TK2 gene. Pediatr Res. 2010;68:151–154. doi: 10.1203/PDR.0b013e3181e33bbe. [DOI] [PubMed] [Google Scholar]
- 22.Behim A, Jardel C, Claeys KG, et al. Adult cases of mitochondrial DNA depletion due to TK2 defect: an expanding spectrum. Neurology. 2012;78:644–648. doi: 10.1212/WNL.0b013e318248df2b. [DOI] [PubMed] [Google Scholar]
- 23.Tyynismaa H, Sun R, Ahola-Erkkilä S, et al. Thymidine kinase 2 mutations in autosomal recessive progressive external ophthalmoplegia with multiple mitochondrial DNA deletions. Hum Mol Genet. 2012;21:66–75. doi: 10.1093/hmg/ddr438. [DOI] [PubMed] [Google Scholar]
- 24.Elpeleg O, Miller C, Hershkovitz E, et al. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am J Hum Genet. 2005;76:1081–1086. doi: 10.1086/430843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrozzo R, Dionisi-Vici C, Steuerwald U, et al. SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain. 2007;130:862–874. doi: 10.1093/brain/awl389. [DOI] [PubMed] [Google Scholar]
- 26.Ostergaard E, Hansen FJ, Sorensen N, et al. Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain. 2007;130:853–561. doi: 10.1093/brain/awl383. [DOI] [PubMed] [Google Scholar]
- 27.Ostergaard E, Christensen E, Kristensen E, et al. Deficiency of the alpha subunit of succinate-coenzyme A ligase causes fatal infantile lactic acidosis with mitochondrial DNA depletion. Am J Hum Genet. 2007;81:383–387. doi: 10.1086/519222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morava E, Steuerwald U, Carrozzo R, et al. Dystonia and deafness due to SUCLA2 defect; Clinical course and biochemical markers in 16 children. Mitochondrion. 2009;9:438–442. doi: 10.1016/j.mito.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Ostergaard E. SUCLA2-related mitochondrial DNA depletion syndrome, encephalomyopathic form, with mild methylmalonic aciduria. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK6803/. Updated 26 May 2009. Accessed 14 Nov 2012. [PubMed]
- 30.Ostergaard E, Schwartz M, Batbayli M, et al. A novel missense mutation in SUCLG1 associated with mitochondrial DNA depletion, encephalomyopathic form, with methylmalonic aciduria. Eur J Pediatr. 2010;169:201–205. doi: 10.1007/s00431-009-1007-z. [DOI] [PubMed] [Google Scholar]
- 31.Bourdon A, Minai L, Serre V, et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet. 2007;39:776–780. doi: 10.1038/ng2040. [DOI] [PubMed] [Google Scholar]
- 32.Bornstein B, Area E, Flanigan KM, et al. Mitochondrial DNA depletion syndrome due to mutations in the RRM2B gene. Neuromuscul Disord. 2008;18:453–459. doi: 10.1016/j.nmd.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acham-Roschitz B, Plecko B, Lindbichler F, et al. A novel mutation of the RRM2B gene in an infant with early fatal encephalomyopathy, central hypomyelination, and tubulopathy. Mol Genet Metab. 2009;98:300–304. doi: 10.1016/j.ymgme.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Kollberg G, Darin N, Benan K, et al. A novel homozygous RRM2B missense mutation in association with severe mtDNA depletion. Neuromuscul Disord. 2009;19:147–150. doi: 10.1016/j.nmd.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Shaibani A, Shchelochkov OA, Zhang S, et al. Mitochondrial neurogastrointestinal encephalopathy due to mutations in RRM2B. Arch Neurol. 2009;66:1028–1032. doi: 10.1001/archneurol.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyynismaa H, Ylikallio E, Patel M, Molnar MJ, Haller RG, Suomalainen A. A heterozygous truncating mutation in RRM2B causes autosomal-dominant progressive external ophthalmoplegia with multiple mtDNA deletions. Am J Hum Genet. 2009;85:290–295. doi: 10.1016/j.ajhg.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fratter C, Raman P, Alston CL, et al. RRM2B mutations are frequent in familial PEO with multiple mtDNA deletions. Neurology. 2011;76:2032–2034. doi: 10.1212/WNL.0b013e31821e558b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaglia F, Dimmock D, Wong LJ. DGUOK-related mitochondrial DNA depletion syndrome, hepatocerebral form. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK7040/. Updated 18 Jun 2009. Accessed 14 Nov 2012.
- 39.Ducluzeau PH, Lachaux A, Bouvier R, Streichenberger N, Stepien G, Mousson B. Depletion of mitochondrial DNA associated with infantile cholestasis and progressive liver fibrosis. J Hepatol. 1999;30:149–155. doi: 10.1016/S0168-8278(99)80019-1. [DOI] [PubMed] [Google Scholar]
- 40.Mandel H, Szargel R, Labay V, et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat Genet. 2001;29:337–341. doi: 10.1038/ng746. [DOI] [PubMed] [Google Scholar]
- 41.Salviati L, Sacconi S, Mancuso M, et al. Mitochondrial DNA depletion and dGK gene mutations. Ann Neurol. 2002;52:311–317. doi: 10.1002/ana.10284. [DOI] [PubMed] [Google Scholar]
- 42.Taanman JW, Kateeb I, Muntau AC, Jaksch M, Cohen N, Mandel H. A novel mutation in the deoxyguanosine kinase gene causing depletion of mitochondrial DNA. Ann Neurol. 2002;52:237–239. doi: 10.1002/ana.10247. [DOI] [PubMed] [Google Scholar]
- 43.Filosto M, Mancuso M, Tomelleri G, et al. Hepato-cerebral syndrome: genetic and pathological studies in an infant with a dGK mutation. Acta Neuropathol. 2004;108:168–171. doi: 10.1007/s00401-004-0872-9. [DOI] [PubMed] [Google Scholar]
- 44.Rabinowitz SS, Gelfond D, Chen CK, et al. Hepatocerebral mitochondrial DNA depletion syndrome: clinical and morphologic features of a nuclear gene mutation. J Pediatr Gastroenterol Nutr. 2004;38:216–220. doi: 10.1097/00005176-200402000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Labarthe F, Dobbelaere D, Devisme L, et al. Clinical, biochemical and morphological features of hepatocerebral syndrome with mitochondrial DNA depletion due to deoxyguanosine kinase deficiency. J Hepatol. 2005;43:333–341. doi: 10.1016/j.jhep.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 46.Mancuso M, Ferraris S, Pancrudo J, et al. New DGK gene mutations in the hepatocerebral form of mitochondrial DNA depletion syndrome. Arch Neurol. 2005;62:745–747. doi: 10.1001/archneur.62.5.745. [DOI] [PubMed] [Google Scholar]
- 47.Slama A, Giurgea I, Debrey D, et al. Deoxyguanosine kinase mutations and combined deficiencies of the mitochondrial respiratory chain in patients with hepatic involvement. Mol Genet Metab. 2005;86:462–465. doi: 10.1016/j.ymgme.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Tadiboyina VT, Rupar A, Atkison P, et al. Novel mutation in DGUOK in hepatocerebral mitochondrial DNA depletion syndrome associated with cystathioninuria. Am J Med Genet A. 2005;135:289–291. doi: 10.1002/ajmg.a.30748. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Limongelli A, Vila MR, Carrara F, Zeviani M, Eriksson S. Molecular insight into mitochondrial DNA depletion syndrome in two patients with novel mutations in the deoxyguanosine kinase and thymidine kinase 2 genes. Mol Genet Metab. 2005;84:75–82. doi: 10.1016/j.ymgme.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Freisinger P, Fütterer N, Lankes E, et al. Hepatocerebral mitochondrial DNA depletion syndrome caused by deoxyguanosine kinase (DGUOK) mutations. Arch Neurol. 2006;63:1129–1134. doi: 10.1001/archneur.63.8.1129. [DOI] [PubMed] [Google Scholar]
- 51.Alberio S, Mineri R, Tiranti V, Zeviani M. Depletion of mtDNA: syndromes and genes. Mitochondrion. 2007;7:6–12. doi: 10.1016/j.mito.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Mousson de Camaret B, Taanman JW, Padet S, et al. Kinetic properties of mutant deoxyguanosine kinase in a case of reversible hepatic mtDNA depletion. Biochem J. 2007;402:377–385. doi: 10.1042/BJ20060705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dimmock DP, Dunn JK, Feigenbaum A, et al. Abnormal neurological features predict poor survival and should preclude liver transplantation in patients with deoxyguanosine kinase deficiency. Liver Transpl. 2008;14:1480–1485. doi: 10.1002/lt.21556. [DOI] [PubMed] [Google Scholar]
- 54.Dimmock DP, Zhang Q, Dionisi-Vici C, et al. Clinical and molecular features of mitochondrial DNA depletion due to mutations in deoxyguanosine kinase. Hum Mutat. 2008;29:330–331. doi: 10.1002/humu.9519. [DOI] [PubMed] [Google Scholar]
- 55.Lee NC, Dimmock D, Hwu WL, et al. Simultaneous detection of mitochondrial DNA depletion and single-exon deletion in the deoxyguanosine gene using array-based comparative genomic hybridisation. Arch Dis Child. 2009;94:55–58. doi: 10.1136/adc.2008.139584. [DOI] [PubMed] [Google Scholar]
- 56.Hanchard NA, Shchelochkov OA, Roy A, et al. Deoxyguanosine kinase deficiency presenting as neonatal hemochromatosis. Mol Genet Metab. 2011;103:262–267. doi: 10.1016/j.ymgme.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Ronchi D, Garone C, Bordoni A, et al. Next-generation sequencing reveals DGUOK mutations in adult patients with mitochondrial DNA multiple deletions. Brain. 2012;135:3404–3415. doi: 10.1093/brain/aws258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandel H, Hartman C, Berkowitz D, Elpeleg ON, Manov I, Iancu TC. The hepatic mitochondrial DNA depletion syndrome: ultrastructural changes in liver biopsies. Hepatology. 2001;34:776–784. doi: 10.1053/jhep.2001.27664. [DOI] [PubMed] [Google Scholar]
- 59.Spinazzola A, Viscomi C, Fernandez-Vizarra E, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. 2006;38:570–575. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 60.Karadimas CL, Vu TH, Holve SA, et al. Navajo neurohepatopathy is caused by a mutation in the MPV17 gene. Am J Hum Genet. 2006;79:544–548. doi: 10.1086/506913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong LJ, Brunetti-Pierri N, Zhang Q, et al. Mutations in the MPV17 gene are responsible for rapidly progressive liver failure in infancy. Hepatology. 2007;46:1218–1227. doi: 10.1002/hep.21799. [DOI] [PubMed] [Google Scholar]
- 62.Navarro-Sastre A, Martín-Hernández E, Campos Y, et al. Lethal hepatopathy and leukodystrophy caused by a novel mutation in MPV17 gene: description of an alternative MPV17 spliced form. Mol Genet Metab. 2008;94:234–239. doi: 10.1016/j.ymgme.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Spinazzola A, Santer R, Akman OH, et al. Hepatocerebral form of mitochondrial DNA depletion syndrome: novel MPV17 mutations. Arch Neurol. 2008;65:1108–1113. doi: 10.1001/archneur.65.8.1108. [DOI] [PubMed] [Google Scholar]
- 64.Kaji S, Murayama K, Nagata I, et al. Fluctuating liver functions in siblings with MPV17 mutations and possible improvement associated with dietary and pharmaceutical treatments targeting respiratory chain complex II. Mol Genet Metab. 2009;97:292–296. doi: 10.1016/j.ymgme.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Parini R, Furlan F, Notarangelo L, et al. Glucose metabolism and diet-based prevention of liver dysfunction in MPV17 mutant patients. J Hepatol. 2009;50:215–221. doi: 10.1016/j.jhep.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 66.El-Hattab AW, Li FY, Schmitt E, Zhang S, Craigen WJ, Wong LJ. MPV17-associated hepatocerebral mitochondrial DNA depletion syndrome: new patients and novel mutations. Mol Genet Metab. 2010;99:300–308. doi: 10.1016/j.ymgme.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Merkle AN, Nascene DR, McKinney AM. MR imaging findings in the reticular formation in siblings with MPV17-related mitochondrial depletion syndrome. AJNR Am J Neuroradiol. 2012;33:E34–35. doi: 10.3174/ajnr.A2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Hattab AW, Scaglia F, Craigen WJ, Wong LJ. MPV17-related hepatocerebral mitochondrial DNA depletion syndrome. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK92947/. Updated 17 May 2012. Accessed 14 Nov 2012.
- 69.Blakely EL, Butterworth A, Hadden RD, et al. MPV17 mutation causes neuropathy and leukoencephalopathy with multiple mtDNA deletions in muscle. Neuromuscul Disord. 2012;22:587–591. doi: 10.1016/j.nmd.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Goethem G, Dermaut B, Lofgren A, Martin JJ, Van Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet. 2001;28:211–212. doi: 10.1038/90034. [DOI] [PubMed] [Google Scholar]
- 71.Lamantea E, Tiranti V, Bordoni A, et al. Mutations of mitochondrial DNA polymerase gammaA are a frequent cause of autosomal dominant or recessive progressive external ophthalmoplegia. Ann Neurol. 2002;52:211–219. doi: 10.1002/ana.10278. [DOI] [PubMed] [Google Scholar]
- 72.Van Goethem G, Martin JJ, Dermaut B, et al. Recessive POLG mutations presenting with sensory and ataxic neuropathy in compound heterozygote patients with progressive external ophthalmoplegia. Neuromuscul Disord. 2003;13:133–142. doi: 10.1016/S0960-8966(02)00216-X. [DOI] [PubMed] [Google Scholar]
- 73.Filosto M, Mancuso M, Nishigaki Y, et al. Clinical and genetic heterogeneity in progressive external ophthalmoplegia due to mutations in polymerase gamma. Arch Neurol. 2003;60:1279–1284. doi: 10.1001/archneur.60.9.1279. [DOI] [PubMed] [Google Scholar]
- 74.Finsterer J, Zarrouk MS. Epilepsy in mitochondrial disorders. Seizure. 2012;21:316–321. doi: 10.1016/j.seizure.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Rahman S. Mitochondrial disease and epilepsy. Dev Med Child Neurol. 2012;54:397–406. doi: 10.1111/j.1469-8749.2011.04214.x. [DOI] [PubMed] [Google Scholar]
- 76.Fadic R, Russell JA, Vedanarayanan VV, Lehar M, Kuncl RW, Johns DR. Sensory ataxic neuropathy as the presenting feature of a novel mitochondrial disease. Neurology. 1997;49:239–245. doi: 10.1212/WNL.49.1.239. [DOI] [PubMed] [Google Scholar]
- 77.Winterthun S, Ferrari G, He L, et al. Autosomal recessive mitochondrial ataxic syndrome due to mitochondrial polymerase gamma mutations. Neurology. 2005;64:1204–1208. doi: 10.1212/01.WNL.0000156516.77696.5A. [DOI] [PubMed] [Google Scholar]
- 78.Hakonen AH, Heiskanen S, Juvonen V, et al. Mitochondrial DNA polymerase W748S mutation: a common cause of autosomal recessive ataxia with ancient European origin. Am J Hum Genet. 2005;77:430–441. doi: 10.1086/444548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tzoulis C, Engelsen BA, Telstad W, et al. The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain. 2006;129:1685–1692. doi: 10.1093/brain/awl097. [DOI] [PubMed] [Google Scholar]
- 80.Worle H, Kohler B, Schlote W, Winkler P, Bastanier CK. Progressive cerebral degeneration of childhood with liver disease (Alpers-Huttenlocher disease) with cytochrome oxidase deficiency presenting with epilepsia partialis continua as the first clinical manifestation. Clin Neuropathol. 1998;17:63–68. [PubMed] [Google Scholar]
- 81.Naviaux RK, Nyhan WL, Barshop BA, Poulton J, Karpinski NC, Haas RH. Mitochondrial DNA polymerase gamma deficiency and mtDNA depletion in a child with Alpers syndrome. Ann Neurol. 1999;45:54–58. doi: 10.1002/1531-8249(199901)45:1<54::AID-ART10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 82.Gauthier-Villars M, Landrieu P, Cormier-Daire V, et al. Respiratory chain deficiency in Alpers syndrome. Neuropediatrics. 2001;32:150–152. doi: 10.1055/s-2001-16614. [DOI] [PubMed] [Google Scholar]
- 83.Naviaux RK, Nguyen KV. POLG mutations associated with Alpers’ syndrome and mitochondrial DNA depletion. Ann Neurol. 2004;55:706–712. doi: 10.1002/ana.20079. [DOI] [PubMed] [Google Scholar]
- 84.Ferrari G, Lamantea E, Donati A, et al. Infantile hepatocerebral syndromes associated with mutations in the mitochondrial DNA polymerase-gammaA. Brain. 2005;128:723–731. doi: 10.1093/brain/awh410. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen KV, Østergaard E, Ravn SH, et al. POLG mutations in Alpers syndrome. Neurology. 2005;65:1493–1495. doi: 10.1212/01.wnl.0000182814.55361.70. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen KV, Sharief FS, Chan SS, Copeland WC, Naviaux RK. Molecular diagnosis of Alpers syndrome. J Hepatol. 2006;45:108–116. doi: 10.1016/j.jhep.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 87.Horvath R, Hudson G, Ferrari G, et al. Phenotypic spectrum associated with mutations of the mitochondrial polymerase γ gene. Brain. 2006;129:1674–1684. doi: 10.1093/brain/awl088. [DOI] [PubMed] [Google Scholar]
- 88.Wong LJ, Naviaux RK, Brunetti-Pierri N, et al. Molecular and clinical genetics of mitochondrial diseases due to POLG mutations. Hum Mutat. 2008;29:E150–E172. doi: 10.1002/humu.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Milone M, Benarroch EE, Wong LJ. POLG-related disorders: defects of the nuclear and mitochondrial genome interaction. Neurology. 2011;77:1847–1852. doi: 10.1212/WNL.0b013e318238863a. [DOI] [PubMed] [Google Scholar]
- 90.Cohen BH, Chinnery PF, Copeland WC. POLG-related disorders. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK26471/. Updated 11 October 2012. Accessed 14 Nov 2012.
- 91.Tang S, Dimberg EL, Milone M, Wong LJ. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE)-like phenotype: an expanded clinical spectrum of POLG1 mutations. J Neurol. 2012;259:862–868. doi: 10.1007/s00415-011-6268-6. [DOI] [PubMed] [Google Scholar]
- 92.Darin N, Oldfors A, Moslemi AR, Holme E, Tulinius M. The incidence of mitochondrial encephalomyopathies in childhood: clinical features and morphological, biochemical, and DNA anbormalities. Ann Neurol. 2001;49:377–383. doi: 10.1002/ana.75. [DOI] [PubMed] [Google Scholar]
- 93.Koskinen T, Santavuori P, Sainio K, Lappi M, Kallio AK, Pihko H. Infantile onset spinocerebellar ataxia with sensory neuropathy: a new inherited disease. J Neurol Sci. 1994;121:50–56. doi: 10.1016/0022-510X(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 94.Koskinen T, Sainio K, Rapola J, Pihko H, Paetau A. Sensory neuropathy in infantile onset spinocerebellar ataxia (IOSCA) Muscle Nerve. 1994;17:509–515. doi: 10.1002/mus.880170507. [DOI] [PubMed] [Google Scholar]
- 95.Koskinen T, Pihko H, Voutilainen R. Primary hypogonadism in females with infantile onset spinocerebellar ataxia. Neuropediatrics. 1995;26:263–266. doi: 10.1055/s-2007-979769. [DOI] [PubMed] [Google Scholar]
- 96.Koskinen T, Valanne L, Ketonen LM, Pihko H. Infantile-onset spinocerebellar ataxia: MR and CT findings. AJNR Am J Neuroradiol. 1995;16:1427–1433. [PMC free article] [PubMed] [Google Scholar]
- 97.Hartley JN, Booth FA, Del Bigio MR, Mhanni AA. Novel autosomal recessive c10orf2 mutations causing infantile-onset spinocerebellar ataxia. Case Rep Pediatr. 2012;2012:303096. doi: 10.1155/2012/303096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kiechl S, Horváth R, Luoma P, et al. Two families with autosomal dominant progressive external ophthalmoplegia. J Neurol Neurosurg Psychiatry. 2004;75:1125–1128. doi: 10.1136/jnnp.2003.025890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jeppesen TD, Schwartz M, Colding-Jørgensen E, Krag T, Hauerslev S, Vissing J. Phenotype and clinical course in a family with a new de novo Twinkle gene mutation. Neuromuscul Disord. 2008;18:306–309. doi: 10.1016/j.nmd.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 100.Virgilio R, Ronchi D, Hadjigeorgiou GM, et al. Novel Twinkle (PEO1) gene mutations in mendelian progressive external ophthalmoplegia. J Neurol. 2008;255:1384–1391. doi: 10.1007/s00415-008-0926-3. [DOI] [PubMed] [Google Scholar]
- 101.Sarzi E, Goffart S, Serre V, et al. Twinkle helicase (PEO1) gene mutation causes mitochondrial DNA depletion. Ann Neurol. 2007;62:579–587. doi: 10.1002/ana.21207. [DOI] [PubMed] [Google Scholar]
- 102.Hakonen AH, Isohanni P, Paetau A, Herva R, Suomalainen A, Lönnqvist T. Recessive Twinkle mutations in early onset encephalopathy with mtDNA depletion. Brain. 2007;130:3032–3040. doi: 10.1093/brain/awm242. [DOI] [PubMed] [Google Scholar]
- 103.Shoffner JM. Mitochondrial neurogastrointestinal encephalopathy disease. In: GeneReviews™ [online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1179/. Updated 11 May 2010. Accessed 14 Nov 2012.
- 104.Hirano M, Silvestri G, Blake DM, et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): clinical, biochemical, and genetic features of an autosomal recessive mitochondrial disorder. Neurology. 1994;44:721–727. doi: 10.1212/WNL.44.4.721. [DOI] [PubMed] [Google Scholar]
- 105.Papadimitriou A, Comi GP, Hadjigeorgiou GM, et al. Partial depletion and multiple deletions of muscle mtDNA in familial MNGIE syndrome. Neurology. 1998;51:1086–1092. doi: 10.1212/WNL.51.4.1086. [DOI] [PubMed] [Google Scholar]
- 106.Perez-Atayde AR, Fox V, Teitelbaum JE, et al. Mitochondrial neurogastrointestinal encephalomyopathy: diagnosis by rectal biopsy. Am J Surg Pathol. 1998;22:1141–1147. doi: 10.1097/00000478-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 107.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–692. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- 108.Nishino I, Spinazzola A, Papadimitriou A, et al. Mitochondrial neurogastrointestinal encephalomyopathy: an autosomal recessive disorder due to thymidine phosphorylase mutations. Ann Neurol. 2000;47:792–800. doi: 10.1002/1531-8249(200006)47:6<792::AID-ANA12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 109.Teitelbaum JE, Berde CB, Nurko S, Buonomo C, Perez-Atayde AR, Fox VL. Diagnosis and management of MNGIE syndrome in children: case report and review of the literature. J Pediatr Gastroenterol Nutr. 2002;35:377–383. doi: 10.1097/00005176-200209000-00029. [DOI] [PubMed] [Google Scholar]
- 110.Vissing J, Ravn K, Danielsen ER, et al. Multiple mtDNA deletions with features of MNGIE. Neurology. 2002;59:926–929. doi: 10.1212/WNL.59.6.926. [DOI] [PubMed] [Google Scholar]
- 111.Nishigaki Y, Martí R, Copeland WC, Hirano M. Site-specific somatic mitochondrial DNA point mutations in patients with thymidine phosphorylase deficiency. J Clin Invest. 2003;111:1913–1921. doi: 10.1172/JCI17828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marti R, Spinazzola A, Tadesse S, Nishino I, Nishigaki Y, Hirano M. Definitive diagnosis of mitochondrial neurogastrointestinal encephalomyopathy by biochemical assays. Clin Chem. 2004;50:120–124. doi: 10.1373/clinchem.2003.026179. [DOI] [PubMed] [Google Scholar]
- 113.Hirano M, Nishigaki Y, Marti R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a disease of two genomes. Neurologist. 2004;10:8–17. doi: 10.1097/01.nrl.0000106919.06469.04. [DOI] [PubMed] [Google Scholar]
- 114.Blondon H, Polivka M, Joly F, Flourie B, Mikol J, Messing B. Digestive smooth muscle mitochondrial myopathy in patients with mitochondrial-neuro-gastro-intestinal encephalomyopathy (MNGIE) Gastroenterol Clin Biol. 2005;29:773–778. doi: 10.1016/S0399-8320(05)86346-8. [DOI] [PubMed] [Google Scholar]
- 115.Giordano C, Sebastiani M, Plazzi G, et al. Mitochondrial neurogastrointestinal encephalomyopathy: evidence of mitochondrial DNA depletion in the small intestine. Gastroenterology. 2006;130:893–901. doi: 10.1053/j.gastro.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 116.Giordano C, Sebastiani M, De Giorgio R, et al. Gastrointestinal dysmotility in mitochondrial neurogastrointestinal encephalomyopathy is caused by mitochondrial DNA depletion. Am J Pathol. 2008;173:1120–1128. doi: 10.2353/ajpath.2008.080252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Finsterer J. Mitochondrial disorders, cognitive impairment and dementia. J Neurol Sci. 2009;283:143–148. doi: 10.1016/j.jns.2009.02.347. [DOI] [PubMed] [Google Scholar]
- 118.Finsterer J. Inherited mitochondrial neuropathies. J Neurol Sci. 2011;304:9–16. doi: 10.1016/j.jns.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Wong LJ. Mitochondrial syndromes with leukoencephalopathies. Semin Neurol. 2012;32:55–61. doi: 10.1055/s-0032-1306387. [DOI] [PubMed] [Google Scholar]
- 120.Szigeti K, Wong LJ, Perng CL, et al. MNGIE with lack of skeletal muscle involvement and a novel TP splice site mutation. J Med Genet. 2004;41:125–129. doi: 10.1136/jmg.2003.013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bicknese AR, May W, Hickey WF, Dodson WE. Early childhood hepatocerebral degeneration misdiagnosed as valproate hepatotoxicity. Ann Neurol. 1992;32:767–775. doi: 10.1002/ana.410320610. [DOI] [PubMed] [Google Scholar]
- 122.Saneto RP, Lee I-C, Koenig MK, et al. POLG DNA testing as an emerging standard of care before instituting valproic acid therapy for pediatric seizure disorders. Seizure. 2010;19:140–146. doi: 10.1016/j.seizure.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feranchak AP, Sokol RJ. Medical and nutritional management of cholestasis in infants and children. In: Suchy FJ, Sokol RJ, Balistreri WF, eds. Liver Disease in Children. 3 ed. New York, NY: Cambridge University Press; 2007: pp. 190–231.
- 124.Hasselmann O, Blau N, Ramaekers VT, Quadros EV, Sequeira JM, Weissert M. Cerebral folate deficiency and CNS inflammatory markers in Alpers disease. Mol Genet Metab. 2010;99:58–61. doi: 10.1016/j.ymgme.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Gold DR, Cohen BH. Treatment of mitochondrial cytopathies. Semin Neurol. 2001;21:309–325. doi: 10.1055/s-2001-17948. [DOI] [PubMed] [Google Scholar]
- 126.Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235–242. doi: 10.1002/mus.20688. [DOI] [PubMed] [Google Scholar]
- 127.Saito K, Kimura N, Oda N, et al. Pyruvate therapy for mitochondrial DNA depletion syndrome. Biochim Biophys Acta. 2012;1820:632–636. doi: 10.1016/j.bbagen.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 128.Bulst S, Holinski-Feder E, Payne B, et al. In vitro supplementation with deoxynucleoside monophosphates rescues mitochondrial DNA depletion. Mol Genet Metab. 2012;107:95–103. doi: 10.1016/j.ymgme.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kelly DA. Liver transplantation: to do or not to do? Pediatr Transplant. 2000;4:170–172. doi: 10.1034/j.1399-3046.2000.00125.x. [DOI] [PubMed] [Google Scholar]
- 130.Lara MC, Valentino ML, Torres-Torronteras J, Hirano M, Marti R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): biochemical features and therapeutic approaches. Biosci Rep. 2007;27:151–163. doi: 10.1007/s10540-007-9043-2. [DOI] [PubMed] [Google Scholar]
- 131.Yavuz H, Ozel A, Christensen M, et al. Treatment of mitochondrial neurogastrointestinal encephalomyopathy with dialysis. Arch Neurol. 2007;64:435–438. doi: 10.1001/archneur.64.3.435. [DOI] [PubMed] [Google Scholar]
- 132.Lara MC, Weiss B, Illa I, et al. Infusion of platelets transiently reduces nucleoside overload in MNGIE. Neurology. 2006;67:1461–1463. doi: 10.1212/01.wnl.0000239824.95411.52. [DOI] [PubMed] [Google Scholar]
- 133.Hirano M, Martí R, Casali C, et al. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology. 2006;67:1458–1460. doi: 10.1212/01.wnl.0000240853.97716.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rahman S, Hargreaves IP. Allogeneic stem cell transplantation corrects biochemical derangements in MNGIE. Neurology. 2007;68:1872. doi: 10.1212/01.wnl.0000265356.98295.be. [DOI] [PubMed] [Google Scholar]
- 135.Halter J, Schüpbach WM, Casali C, et al. Allogeneic hematopoietic SCT as treatment option for patients with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a consensus conference proposal for a standardized approach. Bone Marrow Transplant. 2011;46:330–337. doi: 10.1038/bmt.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Filosto M, Scarpelli M, Tonin P, et al. Course and management of allogeneic stem cell transplantation in patients with mitochondrial neurogastrointestinal encephalomyopathy. J Neurol. 2012;259:2699–2706. doi: 10.1007/s00415-012-6572-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 583 kb)