Abstract
Cells have developed ways to sense and control the size of their organelles. Size sensing mechanisms range from direct measurements provided by dedicated reporters to indirect functional readouts, and they are used to modify organelle size both under normal and stress conditions. Organelle size can also be controlled in the absence of an identifiable size sensor. Studies on flagella have dissected principles of size sensing and control, and it will be exciting to see how these principles apply to other organelles.
Why cells care about organelle size
Organelles represent a scale of organization just below that of the cell itself, and their composition is tailored to their functions. Organelles are specialized to perform different biochemical, regulatory, and motile processes, and they display distinct morphologies that range from the unitary nucleus to the reticulated networks of the endoplasmic reticulum (ER) and mitochondria. These morphologies are constructed and maintained by the cell, and the mechanisms by which they are established remain a vital area of research. The fundamental principles of how cells can sense and control the size of their organelles remain unclear.
Why do cells care about the size of their organelles? At a basic level, the cell occupies a finite volume. Cellular reactions and pathways are divided among the individual organelles and cytoplasm, all of which have some size. Thus, at a minimum the cell must in some way ensure that the size of its organelles is greater than zero. In principle, larger organelles can contain larger quantities of metabolic enzymes and thus mediate higher fluxes through their biochemical pathways. Ideally, organelle size should be suited to cellular needs including functional, distribution and transport requirements, or other factors (1).
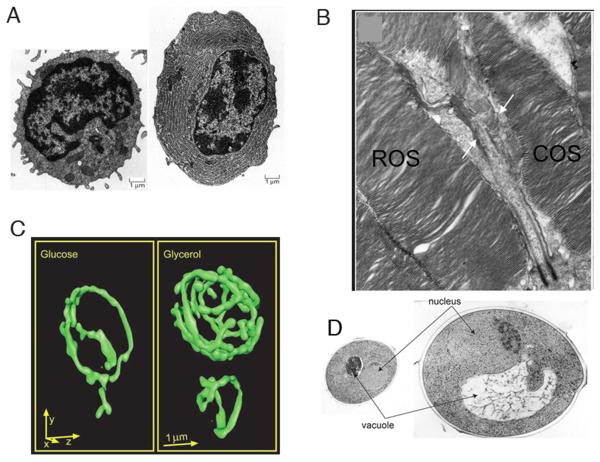
Organelle size optimization is evident in cells with specialized roles, such as in secretory cells with greatly expanded ER (Fig. 1a, 2) and in rod and cone photoreceptors whose outer segment size correlates to their light sensitivity (Fig. 1b, 3). Furthermore, defects in organelle size result in improper cell function. The enlarged vacuole in fab1 mutants of budding yeast leads to improper karyogamy and fitness defects (4). Mitotic spindle length defects can result in faulty chromosome separation (5). Cilia and flagella that are too long or short result in defective motility (6, 7). Thus, maintaining organelle size is critical for cell function.
Figure 1.
Examples of organelle size control. (A) Cell specialization-The ER expands greatly from an undifferentiated cell (left) to a professional secretory cell (right). (Image from (43)) (B) Cell specialization-The outer segment in cone (COS label) and rod (ROS label) cells differ in shape and size. (C) Functional demand-Mitochondria ramify in a yeast cell grown in respiratory (right) vs. fermentative (left) media. (D) Organelle/cell size scaling-The nucleus in the larger cell (right) is larger than in the smaller cell (left).
A key question is how organelle size is detected or sensed by the cell. This problem is complicated by the stochastic nature of the molecular tools available to the cell. In this review, we discuss fundamental principles and illustrative examples of the ways in which cells sense organelle size, as well as the reasons why such sensing is performed.
How do cells sense organelle size?
Though simple on its face, this question is complicated by what it would mean for a cell to sense size. At its simplest, sensing involves a measurement or observation. While humans can communicate that such an observation has been made, determining whether a cell has made such a measurement is non-trivial. We can infer that a cell meaningfully senses size based on the following criteria: [1] the identification of a readout mechanism that correlates with organelle size; [2] observing a change in cellular behavior or state as a consequence of that readout.
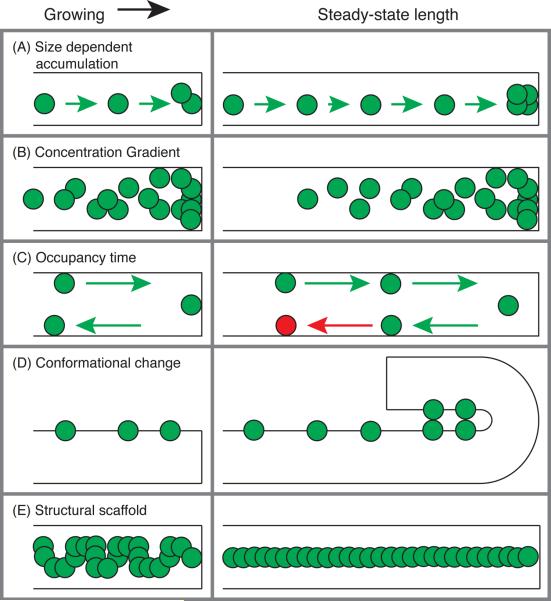
At an extreme, we might expect that cells have a dedicated reporter molecule whose chemical state gives some readout of organelle size. There are several ways in which a reporter molecule could convey such a measurement within the cell (Fig. 2):
-
(A)
Size dependent accumulation – If reporter molecules bind to the organelle with some affinity, or are inserted into the organelle at a fixed density, there will be more the larger the organelle is. Downstream responses would be dependent on the extent of accumulation of the reporter.
-
(B)
Concentration gradient – If reporter molecules are synthesized in or delivered to a certain part of the organelle at some rate, then transport or diffusion of the molecule would create a concentration gradient whose properties depend on the size of the structure.
-
(C)
Occupancy time – If reporter molecules are synthesized in or delivered to a certain part of the organelle, then residency time within the organelle will depend on the size of the organelle. The kinetics of phosphorylation or some other modification could give a readout of the residency time and thus organelle size.
-
(D)
Conformational change – The reporter molecule might change or induce a change in conformation or structure depending on organelle size.
-
(E)
Structural scaffold – The size of some organelles is shaped by a molecular scaffold. The scaffold's size is itself dependent on the self-assembly and conformation of the constituent proteins.
Figure 2.
Cartoons illustrating possible models for size sensing in a one-dimensional organelle, where size-sensing molecules are represented by green and red circles. Examples are shown for when the organelle (black rectangle) is growing (left column) and has reached a steady-state length (right column). The organelle grows until: (A) enough sensing molecules accumulate on the organelle; (B) the concentration gradient of the sensing molecule achieves the right profile; (C) the sensing molecule's occupancy time is long enough to induce a state change (green to red circle); (D) a conformational change in the organelle is induced by accumulated sensing molecules; (E) its size conforms to a structural scaffold set by the sensing molecule.
The reporter can affect cell processes in several ways, including signaling, transcriptional regulation, or directly (de)activating growth mechanisms. The effects of cellular size measurements include changes in cell state as in the case of the size checkpoint of budding yeast. More commonly, though, they are used to regulate the size of the structure or organelle being measured.
Multiple mechanisms for size sensing can work in concert. For example, neurite length sensing has been proposed to be based on the anterograde transport and accumulation of the protein shootin1 to the tip balanced by the retrograde diffusion of shootin1 back to the cell body. As a result, the concentration of shootin1 accumulated at the tip depends on the length of the neurite, making it a readout of size. Then, shootin1 participates in a positive feedback signaling loop to amplify growth in longer neurites. This cycle eventually leads to the symmetry breaking and the focused growth of a single axon (8).
Telomere length control depends on the accumulation of Rap1p in budding yeast (9) and TRF1 & TRF2 in human cells (10). These proteins bind to tandem repeat sequences and recruit a number of other factors; longer telomeres have more protein bound. At a certain telomere length, enough protein accumulates to induce a conformational change in the entire protein-DNA complex. At this point, the telomere enters a capped state that is inaccessible to further lengthening by telomerase.
The mitotic spindle provides excellent examples of size dependent accumulation and concentration gradient mechanisms. Spindle microtubules elongate with some basal kinetics, but they recruit motors which act to compress the spindle (11, 12) as well as microtubule depolymerizing (Kinesin-8, (13)) and severing factors (katanin (14)) that reduce microtubule length (15). Longer microtubules are able to recruit more of these shortening factors, thus larger spindles will grow more slowly until a steady-state is reached. Changes in activity of microtubule severing enzymes between various species is apparently one cause of specific differences in spindle length (16). In these cases, the recruited molecules are themselves effectors that change length, so there is no need for an intervening reporter. One molecule that clearly has a role in spindle length is the small GTPase Ran. RanGTP is produced at the center of the spindle and diffuses out. The resulting concentration gradient's properties depend on spindle length (17), thus providing a length “sensor”. It remains to be determined how strongly this gradient influences length in comparison to the other molecules already discussed. Finally, there is evidence that the spindle poles may contain a molecular switch that responds to length-dependent changes in mechanical pressure and modulates microtubule depolymerization accordingly (18). Such a switch uses mechanical properties as a readout of spindle size.
A classic example of scaffold-based size control is the clathrin coat, which sterically constrains the size and curvature of endocytic vesicles as it assembles around the lipid membrane. Many infectious bacteria also rely on a structural scaffold to control the length of their injection needle projections (19). In Yersinia pestis and Y. enterocolitica cells, the ends of the protein YscP are anchored in the proximal and distal ends of the needles. Then, when the needle has reached the length of the YscP “ruler”, extension of the YscP protein acts to discontinue the export of the major structural subunit of the needle, stopping growth. Similar proteins have been found to determine the length of T4 and Lambda bacteriophage tails (20). Remarkably, changing the length of the “ruler” subunits via protein engineering leads to predictable and systematic decreases in the length of the associated structure.
Indirect size sensing
Size may be sensed indirectly, without a molecule whose specific role is to measure size. In the case of organelles, size affects their functional capacity and their distribution around the cell. If an organelle is unable to meet functional demand, that could lead to a build-up of stress agents or other changes in cellular environment, triggering a subsequent response to increase the size of that organelle to relieve that stress. In these cases, there are no specific measures of absolute organelle size. Rather, size sensing is both indirect (the cell infers organelle size based on the accumulation of other molecules) and relative (the organelle is too small to meet the functional demand). However, the end effect is still regulation of organelle size.
An example of such sensing is found in the relation of the ER to the unfolded protein response (UPR). The accumulation of unfolded protein triggers oligomerization of the transmembrane protein Ire1p, leading to phosphorylation of its cytosolic kinase domain and eventually to increased expression of UPR response genes. Among these are lipid biosynthesis genes, and the increase in lipid mass results in growth of the ER, in part to increase ER lumenal capacity to address the unfolded protein stress (21, 22). In another example, exposure of plant and animal cells to oxidative stress induces the expression of Peroxin 1, leading to expansion and proliferation of peroxisomes to deal with increased levels of reactive oxygen species (23). Also, mitochondria in budding yeast ramify upon transfer from fermentative to respiratory media (Figure 1c, 24).
Size control in the absence of size sensing
In many cases, size control occurs without an obvious size sensor. For example, many organelles including the nucleus (Figure 1d, 25, 26), nucleolus (27), and vacuoles (28, 29) show a scaling behavior with cell size, that is larger cells have larger organelles. However, to our knowledge, no specific size sensor of these organelles has been identified. Thus, size control might arise even in the absence of specific size sensors.
One way to achieve size control is to have stereotyped growth, by which both organelle and cell grow according to a preset plan. This seems to be the case with centrosome number control because the organelle's duplication cycle is coupled to the cell cycle. In a simple case of stereotyped growth, both cell and organelle would grow at constant rates. Another way to achieve scaling is allometric growth in which organelle growth is some constant fraction of cell growth, in which case the organelle will inherently be some proportion of cell size. This could arise if both organelle and cell growth were dependent on overall metabolism.
The cell can also control size by synthesizing a limited pool of precursor (30). Assuming that an organelle grows until the pool is depleted, size would be readily modulated by changing the pool size. Fixed precursor recruitment has been proposed as a size control model for the bacterial flagellar hook, in which it is suggested that the C-ring structure of the basal body would act as “measuring cup” to bind a fixed quantity of hook precursor and then release this fixed precursor set to allow assembly (31).
To determine if these models apply, it is useful to monitor the growth of the organelle along with that of the cell. Stereotyped growth is demonstrated if the growth trajectories fit to standard growth models (i.e. constant or exponential). Otherwise, a consistent pattern of growth among different individuals or in successive cell divisions might also indicate a preexisting plan. In the case of allometric growth, there would be a correlation between instantaneous cell and organelle growth rates. Although these simple models of growth result in inherent organelle-to-cell size scaling, they are sensitive to perturbation as they do not include mechanisms for recovery. To achieve that, growth of either the cell or organelle must be tunable in some way with respect to the relative size of the organelle.
Flagellar length control and the balance-point model
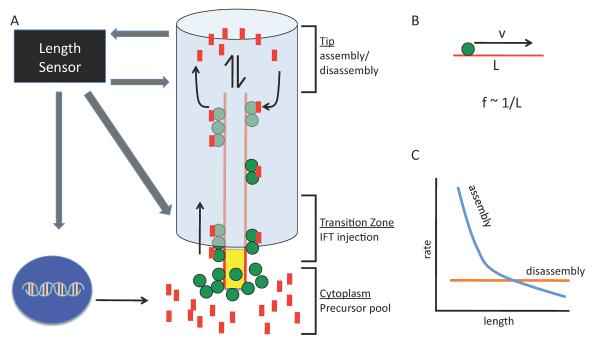
The eukaryotic flagellum has been studied in detail as a model system for organelle size control (Fig. 3A). Flagellar length is dependent on competing processes of assembly and disassembly, both of which occur at the distal flagellar tip and rely on the microtubule motor based transport of structural material, or intraflagellar transport (IFT) (32) which carries tubulin to the growing flagellar tip (33). When flagella are severed, they regenerate with decelerating kinetics. The rapidity of growth back to the original size argues against a stereotyped growth mechanism for length control, whereas decreased growth rate as the flagella reach their final length is suggestive of feedback control. In the biflagellate green alga Chlamydomonas, when one flagellum is severed, during its regeneration the other, intact flagellum shortens until the two flagella reach equal lengths, at which point they resume growth together (34). This equalization of lengths seems to indicate that the cell “knows” how long both its flagella are.
Figure 3.
Illustration of the balance-point model of flagellar length control. (A) IFT particles (green) associated with the flagellar basal body bind precursor molecules from cytoplasmic pool (red) and are injected into the flagellum where they travel to the distal tip to deliver precursor for flagellar assembly. Flagella assembly rate depends on the rate of anterograde IFT, and the disassembly rate is constant. A length sensor may act at the flagellar tip or base or both, and it can affect gene regulation & precursor synthesis, injection of IFT, or assembly or disassembly rates to create feedback loop for length control. (B) Assuming a constant IFT speed, v, the delivery frequency of IFT particles, f, will vary inversely with flagellum length, L. (C) In the balance-point model, flagellar length reaches a steady state where the assembly rate (blue) and disassembly rate (orange) curves intersect.
As further evidence of flagellar length sensing, the frequency of injection of IFT material into the flagellum from the cytoplasm changes as a function of flagellar length (35). The motors and associated proteins that drive IFT associate into linear arrays known as trains, and as a regenerating flagellum becomes longer, the trains become smaller but more frequent. The net effect is that the total number of individual IFT subunits is roughly independent of length, but this is only achieved by having the frequency and size of the trains vary with length. So how does the IFT system know how long the flagellum is?
As discussed above, one way for the cell to “know” organelle size is having a dedicated reporter molecule whose state is sensitive to organelle size. The phosphorylation state of an aurora-like kinase depends on flagellar length (36), so it could act as a length sensor. On the other hand, depletion of this kinase by RNAi has no effect on flagellar length (37), raising the question of whether the cell utilizes the information on length encoded by the kinase. A complete feedback loop, in which length regulates the state of a kinase whose function then modulates assembly or disassembly, remains to be found.
One likely output of any length reporter molecule would be regulation of IFT, a critical pathway for maintaining flagellar length. The total quantity of IFT material per flagellum is roughly constant (32, 35). Consequently, the transport rate should be a decreasing function of length, because in longer flagella it takes longer for the motors to reach the tip of the flagellum and deliver their cargo (Fig. 3B). Furthermore, disassembly rate is length-independent (38), mediated by microtubule depolymerizing kinesins (39). Combined with the length-dependent assembly rate, this constant disassembly gives a unique steady-state solution for length (Fig. 3C). The maintenance of constant total IFT protein per flagellum is critical for this model, and it appears to be the result of length-dependent changes in the size and frequency of IFT train import into the flagellum. A major goal now is to identify the molecules that mediate these length dependent processes.
Flagellar length is also regulated, to some degree, by a limiting precusor mechanism. Flagellar assembly is accompanied by massive increase in transcription of virtually all genes encoding flagellar proteins (40). In the absence of protein synthesis, flagella can only regenerate to about half of their normal length (34). Therefore, control of precursor quantity is a key element of length control and represents a potential target for signaling molecules in a length sensing pathway.
In contrast to eukaryotic flagella, bacterial flagella grow at a constant rate and it has been proposed that their length is determined by a balance between constant growth and random breakage (41).
Distinguishing mechanisms of size control
An ongoing challenge in studying organelle size control is that it can be difficult to make measurements on morphologically complex subcellular structures. Organelles typically use their lumen and membrane for distinct functions, raising the question of how the cell controls the size of both for a single organelle. Measurements of organelle volume and surface area require three-dimensional analysis. Recent advances in imaging (24), tomography (28) and analysis methodology (29), three-dimensional size measurements are crucial to understanding mechanisms of organelle size control.
The systems that contain dedicated size sensing pathways tend to be ones for which size plays an obvious part in function. Flagellar length is important for cells to create the appropriate waveform to allow movement. Consequently, length control mutations in Chlamydomonas often show severe motility defects, and homologs to these genes are implicated in several ciliopathies (40). Mitotic spindle defects can result in improper chromosome segregation, and telomere length is implicated in aging. Bacterial injection needles with altered lengths can show less virulence during infection (42).
However, the effect of size in the case of other organelles is less clear. Although the nucleus shows a size scaling to cytoplasmic volume, it does not seem to be related to the amount of genomic DNA, as might be expected from a functional perspective (25, 26). Changes to organelle size under stress conditions can be readily explained, but absent such stress, organelle size may be less tightly regulated. To test for this possibility, it is useful to refer to deflagellation and recovery experiments, which illustrate that direct manipulation of the size of organelles can be a powerful tool to establish the principles of size sensing and control. Although deflagellation can be induced by various methods, less is known about how to change other organelles' sizes. Laser ablation can remove centrosomes and sever mitotic spindles. Reversible drug treatment or other manipulations may also be effective.
Monitoring the response after perturbing organelle size will reveal whether there is recovery to the proper size scaling. If the organelle is too small, recovery may involve upregulation of assembly pathways or synthesis of precursor components or both. On the other hand, an organelle which is too large could restore scaling by undergoing disassembly, autophagy, or by reducing or eliminating its own growth and allowing the cell to grow larger. An absence of recovery would indicate a lack of size sensing and regulation. This could indicate that the organelle size falls within an acceptable range, or that there is a passive growth mechanism without size sensing.
Concluding thoughts
As we have shown, there are several ways in which cells can sense and control the size of their organelles. A number of follow-up questions arise (Box 1) including: How is the size sensor calibrated, or how is the size set-point determined? For example, how does a bacterial cell ensure that its molecular ruler is the appropriate length? Or how have the rates in Chlamydomonas flagellar assembly and disassembly been determined so that the flagella are the correct lengths? Ultimately, the answers to these questions are likely to involve evolutionary tuning of these mechanisms so that organelle size is optimized to some function. As experiments on organelle size sensing and control continue, it will also be necessary to study the functional implications of organelle size.
Box 1.
Outstanding issues in Size Sensing.
What determines the size set-point for a given organelle?
What selective pressures have influenced organelle sizes?
How does size impact the biochemical pathways the organelle encapsulates?
How do cells respond to perturbations in organelle size?
What feedback loops connect size sensing and control mechanisms?
Do distinct mechanisms regulate organelle surface area and volume?
Do the size control pathways for different organelles interact?
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
References and Notes
- 1.Chan Y-HM, Marshall WF. Scaling properties of cell and organelle size. Organogenesis. 2010;6:88–96. doi: 10.4161/org.6.2.11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Federovitch CM, Ron D, Hampton RY. The dynamic ER: experimental approaches and current questions. Curr Opin Cell Biol. 2005;17:409–414. doi: 10.1016/j.ceb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Avasthi P, Watt CB, Williams DS, Le YZ, Li S, et al. Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric Kinesin-II. Neurobiol Dis. 2009;29:14287–14298. doi: 10.1523/JNEUROSCI.3976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, et al. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell. 1995;6:525–539. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goshima G, Saitoh S, Yanagida M. Proper metaphase spindle length is determined by centromere proteins Mis12 and Mis6 required for faithful chromosome segregation. Genes & Dev. 1999;13:1664–1677. doi: 10.1101/gad.13.13.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuchka MR, Jarvik JW. Short-flagella mutants of Chlamydomonas reinhardtii. Genetics. 1987;115:685–91. doi: 10.1093/genetics/115.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barsel SE, Wexler DE, Lefebvre PA. Genetic analysis of long-flagella mutants of Chlamydomonas reinhardtii. Genetics. 1988;118:637–648. doi: 10.1093/genetics/118.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toriyama M, Shimada T, Kim KB, Mitsuba M, Nomura E, et al. Shootin1: a protein involved in the organization of an asymmetric signal for neuronal polarization. J Cell Biol. 2006;175:147–157. doi: 10.1083/jcb.200604160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcand S, Gilson E, Shore D. A protein-counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 10.Smogorzewska A, Steensel B. v., Bianchi A, Oelmann S, Schaefer MR, et al. Control of Human Telomere Length by TRF1 and TRF2. Mol Cell Biol. 2000;20:1659–166. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchison TJ, Maddox P, Gaetz J, Groen A, Shirasu M, et al. Roles of polymerization dynamics, opposed motors, and a tensile element in governing the length of Xenopus extract meiotic spindles. Mol Biol Cell. 2005;16:3064–3076. doi: 10.1091/mbc.E05-02-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brust-Mascher I, Sommi P, Cheerambathur DK, Scholey JM. Kinesin-5-dependent poleward flux and spindle length control in Drosophila embryo mitosis. Mol Biol Cell. 2009;20:1749–1762. doi: 10.1091/mbc.E08-10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga V, Helenius J, Tanaka K, Hyman AA, Tanaka TU, et al. Yeast kinesin-8 depolymerizes microtubules in a length-dependent manner. Nat Cell Biol. 2006;8:957–962. doi: 10.1038/ncb1462. [DOI] [PubMed] [Google Scholar]
- 14.McNally K, Audhya A, Oegema K, McNally FJ. Katanin controls mitotic and meiotic spindle length. J Cell Biol. 2006;175:881–891. doi: 10.1083/jcb.200608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver LN, Ems-McClung SC, Stout JR, LeBlanc C, Shaw SL, et al. Kif18A uses a microtubule binding site in the tail for plus-end localization and spindle length regulation. Curr Biol. 2011;21:1500–1506. doi: 10.1016/j.cub.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loughlin R, Wilbur JD, McNally FJ, Nedelec FJ, Heald R. Katanin contributes to interspecies spindle length scaling in Xenopus. Cell. 2011;147:1397–1407. doi: 10.1016/j.cell.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastiaens P, Caudron M, Niethammer P, Karsenti E. Gradients in the self-organization of the mitotic spindle. Trends Cell Biol. 2006;16:125–134. doi: 10.1016/j.tcb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Dumont S, Mitchison TJ. Compression regulates mitotic spindle lenth by a mechanochemical switch at the poles. Curr Biol. 2009;19:1086–1095. doi: 10.1016/j.cub.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Journet L, Agrain C, Broz P, Cornelis GR. The needle length of bacterial injectisomes is determined by a molecular ruler. Science. 2003;302:1757–1760. doi: 10.1126/science.1091422. [DOI] [PubMed] [Google Scholar]
- 20.Katsura I. Determination of bacteriophage λ tail length by a protein ruler. Nature. 1987;327:73–75. doi: 10.1038/327073a0. [DOI] [PubMed] [Google Scholar]
- 21.Welihinda AA, Tirasophon W, Kaufman RJ. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expr. 1999;7:293–300. [PMC free article] [PubMed] [Google Scholar]
- 22.Schuck S, Prinz WA, Thorn KS, Voss C, Walter P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol. 2009;187:525–536. doi: 10.1083/jcb.200907074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Huertas E, Charlton WL, Johnson B, Graham IA, Baker A. Stress induces peroxisome biogenesis genes. EMBO J. 2000;19:6770–6777. doi: 10.1093/emboj/19.24.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egner A, Jakobs S, Hell SW. Fast 100-nm resolution three-dimensional microscope reveals structural plasticity of mitochondria in live yeast. Proc Natl Acad Sci USA. 2002;99:3370–3375. doi: 10.1073/pnas.052545099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgensen P, Edgington NP, Schneider BL, Rupeš I, Tyers M, et al. The Size of the Nucleus Increases as Yeast Cells Grow. Mol Biol Cell. 2007;18:3523–3532. doi: 10.1091/mbc.E06-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann FR, Nurse P. Nuclear size control in fission yeast. J Cell Biol. 2007;179:593–600. doi: 10.1083/jcb.200708054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berciano MT, Novell M, Villagra NT, Casafont I, Bengoechea R, et al. Cajal body number and nucleolar size correlate with the cell body mass in human sensory ganglia neurons. J Struct Biol. 2007;158:410–420. doi: 10.1016/j.jsb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Uchida M, Sun Y, McDermott G, Knoechel C, Le Gros MA, et al. Quantitative analysis of yeast internal architecture using soft X-ray tomography. Yeast. 2011;28:227–236. doi: 10.1002/yea.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan Y-HM, Marshall WF. Threshold-free method for three-dimensional segmentation of organelles. Proc. SPIE. 2012;8225:822529. [Google Scholar]
- 30.Goehring NW, Hyman AA. Organelle growth control through limiting pools of cytoplasmic components. Curr Biol. 2012;22:R330–339. doi: 10.1016/j.cub.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Makishima S, Komoriya K, Yamaguchi S, Aizawa S-I. Length of the flagellar hook and the capacity of the type III export apparatus. Science. 2001;291:2411–2413. doi: 10.1126/science.1058366. [DOI] [PubMed] [Google Scholar]
- 32.Marshall WF, Rosenbaum JL. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol. 2001;155:405–414. doi: 10.1083/jcb.200106141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat. Cell Biol. 2011;13:790–8. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum JL, Moulder JE, Ringo DL. Flagellar elongation and shortening in Chlamydomonas: The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969;41:600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engel BD, Ludington WB, Marshall WF. Intraflagellar transport particle size scales inversely with flagellar length: revisiting the balance-point length control model. J Cell Biol. 2009;187:81–89. doi: 10.1083/jcb.200812084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo M, Cao M, Kan Y, Li G, Snell W, et al. The phosphorylation state of an aurora-like kinase marks the length of growing flagella in Chlamydomonas. Curr Biol. 2011;21:586–591. doi: 10.1016/j.cub.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- 38.Marshall WF, Qin H, Brenni MR, Rosenbaum JL. Flagellar length control system: Testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell. 2005;16:270–278. doi: 10.1091/mbc.E04-07-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaineau C, Tessier M, Dubessay P, Tasse L, Crobu L, Pages M, Bastien P. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr. Biol. 2007;17:778–82. doi: 10.1016/j.cub.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 40.Stolc V, Samanta MP, Tongprasit W, Marshall WF. Genome-wide transcriptional analysis of flagellar regeneration in Chlamydomonas reinhardtii identifies orthologs of ciliary disease genes. Proc Natl Acad Sci USA. 2005;102:3703–3707. doi: 10.1073/pnas.0408358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner L, Stern AS, Berg HC. Growth of flagellar filaments of Escherichia coli is independent of filament length. J. Bacteriol. 2012;194:2437–42. doi: 10.1128/JB.06735-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mota LJ, Journet L, Sorg I, Agrain C, Cornelis G,R. Bacterial injectisomes: Needle length does matter. Science. 2005;307:1278. doi: 10.1126/science.1107679. [DOI] [PubMed] [Google Scholar]
- 43.Zucker-Franklin D. Atlas of Blood Cells: Function and Pathology. ed. 2 Lea & Febiger; Milan, Italy: 1998. [Google Scholar]
- 44.Y.-H. M. C. acknowledges the support of the Herbert Boyer Postdoctoral Fellowship and the NIH NRSA award 1F32GM090442-01A1. W.F.M. acknowledges the support of NIH grant R01 GM097017