Abstract
Mitochondria from persons with Alzheimer’s disease (AD) differ from those of age-matched, control subjects. Differences in mitochondrial morphology and function are well-documented, and are not brain-limited. Some of these differences are present during all stages of AD, and are even seen in individuals who are without AD symptoms and signs but who have an increased risk of developing AD. This chapter considers the status of mitochondria in AD subjects, the potential basis for AD subject mitochondrial perturbations, and the implications of these perturbations. Data from multiple lines of investigation, including epidemiologic, biochemical, molecular, and cytoplasmic hybrid studies are reviewed. The possibility that mitochondria could potentially constitute a reasonable AD therapeutic target is discussed, as are several potential mitochondrial medicine treatment strategies.
Keywords: aging, Alzheimer’s disease, bioenergetics, cybrids, mitochondria, therapeutics
I. Introduction
Alzheimer’s disease (AD) is the most prevalent form of dementia. In the United States, it is estimated that one out of every eight persons over the age of 65 suffers from AD, and almost half of those over the age of 85 are affected (Evans et al., 1989; Thies & Bleiler, 2011). It has also been recognized for some time, as Alois Alzheimer’s first reports were presented and published at the start of the 20th century (Alzheimer, 1907, 1911; Alzheimer et al., 1995). Many academic clinicians and scientists focus on AD, and industry maintains active AD drug development and testing programs.
All this helps create the false impression that we truly understand what AD is, what causes it, and how to effectively treat it. On the contrary, how we even define the disease is somewhat arbitrary, and this really has been the case since the term “Alzheimer’s disease” was first proposed.
By the late 19thcentury it was recognized that with advancing age, the brain cortex of some animal species develop extracellular protein accumulations called plaques (Blocq & Marinesco, 1892). During the first decade of the 20thcentury this phenomenon was also noted to occur in the brains of elderly humans, and that this histological change often associated with dementia, a clinical syndrome characterized by declining cognitive function (Fischer, 1907; Redlich, 1898). At this same time Alois Alzheimer reported the brains of several relatively young, or “presenile”, demented individuals also developed plaque deposits (Alzheimer, 1907, 1911). Alzheimer further described intracellular protein aggregations which he called tangles. Because dementia was relatively common in those reaching old age, affected persons were not felt to have an actual disease, even when plaques and tangles were present (Kraepelin, 1910). Such persons were simply felt to have a senile dementia syndrome that frequently accompanies old age. It was only those with presenile dementia, plaques, and tangles who actually qualified for an AD diagnosis.
Over the next 100 years, much was learned about the structural basis of the plaques and tangles. The major protein in the plaques is folded in an amyloid configuration (Divry, 1927), and is called beta amyloid (Aβ) (Glenner & Wong, 1984). Aβ arises as a degradation product of a larger protein called the amyloid precursor protein (APP) (Kang et al., 1987). The tangles contain aggregated assemblies of a protein called tau, and tau protein in tangles is heavily phosphorylated (Grundke-Iqbal et al., 1986).
During the 2nd half of the 20 th century the clinical definition underwent significant revision. The distinction between when a demented person with plaques and tangles was young enough to have AD or old enough to have age-associated senile dementia had always been somewhat arbitrary (Swerdlow, 2007a). To minimize the impact of this distinction (Katzman, 1976), the original AD subjects were stated to have presenile dementia of the Alzheimer’s type, while the elderly subjects were said to have senile dementia of the Alzheimer’s type. However, the age boundary between the presenile and senile conditions was still arbitrary, and most reverted to simply calling the clinical syndrome AD, regardless of age.
In the early 1990’s it was shown that mutations in the APP gene, which resides on chromosome 21, cause brain disease in general and can also cause an AD presentation characterized by progressive dementia, plaques, and tangles (Goate et al., 1991; Levy et al., 1990). This discovery gave rise to a hypothesis, the amyloid cascade hypothesis, that posited AD was itself induced by the presence of Aβ-containing amyloid plaques (Hardy & Allsop, 1991).
It was subsequently discovered that mutations in two other genes, the presenilin 1 (PS1) and presenilin 2 (PS2) genes, caused an AD presentation and that the presenilin proteins contributed to APP processing (Kimberly et al., 2000; Levy-Lahad et al., 1995; Sherrington et al., 1995; Wolfe et al., 1999). Aβ was found to be toxic under cell culture conditions (Yankner et al., 1989), and although belief that plaques drove AD neurodysfunction and neurodegeneration gradually fell out of favor, modified versions of the amyloid cascade hypothesis in which different pre-plaque Aβ configurations were deemed the critical toxic moiety increasingly came to dominate the field (Hardy & Selkoe, 2002; Walsh & Selkoe, 2007). Consistent with this view, transgenic mouse models that developed cortical plaques were created and became the mainstay of preclinical AD research (Hsiao et al., 1996).
Along the way, clinically-based AD concepts began to clash with the amyloid cascade hypothesis. The most important discrepancy arose from the fact that plaques are often observed in the brains of the non-demented elderly, a finding not entirely consistent with the idea that Aβ is the primary disease mediator (Swerdlow, 2011a). Recently, this has been administratively addressed by expanding the definition of AD to include anyone with brain plaques, regardless of clinical status. Those with plaques and dementia are now said to have AD, while those with plaques and no clinical signs can be diagnosed with “preclinical AD” (Sperling et al., 2011).
So, despite the fact that many people are diagnosed with it, many investigators study it, and much has been written about it, what we now call AD remains a somewhat arbitrary construct whose definition is subject to change. In essence, the same controversies that were identified over 100 years ago remain. We still don’t know whether AD is a single homogeneous entity or a collection of clinically and histologically overlapping conditions. The relationship between brain aging and AD is unclear. Whether Aβ truly induces a disease-driving cascade in all or even some patients remains unproven. To date, a number of therapeutic interventions that benefit AD transgenic mice have been shown not to benefit affected patients, which raises the question of how well this model models human AD (Holmes et al., 2008; Swerdlow, 2007a). With this in mind, this chapter will now address the role of mitochondria in AD and the possibility that mitochondria might offer a potential AD therapeutic target.
II. Mitochondrial Function in AD
AD is usually thought of as a disease of the brain. Biochemical changes, though, are certainly not brain-limited (Swerdlow, 2011b). Systemic mitochondrial changes between the mitochondria of AD and age-matched control subjects have been observed.
A. Brain
1. Morphology
Many of the changes typical of compromised mitochondria are seen in the AD brain. For example, disruption of mitochondrial cristae and intra-mitochondrial accumulations of osmiophilic material are prevalent in AD brains compared to controls (Baloyannis, 2006, 2011; Saraiva et al., 1985). There is an increased range of mitochondrial sizes, with more enlarged mitochondria but also elevated numbers of exceptionally small mitochondria (Hirai et al., 2001). Overall, the average size of AD neuron mitochondria is smaller than it is in control brain neurons (Baloyannis, 2006; Hirai et al., 2001).
2. Mass
How mitochondrial mass changes in AD brains is not straightforward. Using PCR-based approaches to quantify mitochondrial DNA (mtDNA) reveals that AD subject brain cortices contain lesser amounts of amplifiable mtDNA (Brown et al., 2001; de la Monte et al., 2000). The simplest explanation for this is that AD brains have reduced amounts of mtDNA, and by extension a reduced mitochondrial mass. However, in a study in which a labeled oligonucleotide probe was used to detect mtDNA in hippocampal neurons, a complex picture emerged (Hirai et al., 2001). When only mtDNA within normal-appearing mitochondria were considered, less mtDNA was revealed. A large number of mitochondria were also found within phagosomes, and these degrading mitochondria also hybridized the mtDNA oligonucleotide probe. When this additional mtDNA was taken into account, the AD hippocampal neurons actually contained more mtDNA.
Mitochondrial mass has been assessed using alternative approaches, including an immunochemical quantification of mitochondrial-localized proteins. In one study, an antibody probe to an mtDNA-encoded cytochrome oxidase (COX) subunit, COX1, was found to be increased in AD brain hippocampal neurons (Hirai et al., 2001). In a different study, tangle-free hippocampal neurons showed more COX1 and COX4 staining, while staining was markedly reduced in tangle-bearing neurons (Nagy et al., 1999). Other authors reported cytochrome COX protein in general was reduced in AD brain homogenates (Kish et al., 1999).
Although it could only be used as a very indirect index of mitochondrial mass, mtDNA expression in the form of mitochondrial RNA has also been evaluated. Northern blot-based studies found some, but not all, mitochondrial RNA transcripts were reduced (Chandrasekaran et al., 1994). Nuclear-encoded oxidative phosphorylation subunit expression similarly is reduced (Liang et al., 2008). Findings from other studies, though, suggest a more complex picture. For example, Manczak et al. reported COX subunit expression was actually increased in at least some AD brain neurons (Manczak et al., 2004). The authors concluded this upregulation might represent a compensatory response to perturbed COX function.
Electron microscopy (EM) has been used to quantify AD neuron mitochondria. Several studies have reported the number of normal appearing mitochondria was decreased (Baloyannis, 2006; Hirai et al., 2001). In one study there was a concomitant increase in mitochondria located within phagosomes (Hirai et al., 2001).
The aggregate of these studies suggest that within the brain the number of normal-appearing mitochondria is diminished. Whether this reflects increased turnover, decreased synthesis, or both is not entirely clear. Potentially pertinent to this question, two relatively recent studies measured protein levels of peroxisome-proliferator activated receptor gamma coactivator 1α(PGC1a), a transcriptional co-activator that serves as a master regulator of mitochondrial mass (Qin et al., 2009; Sheng et al., 2012). Both of these studies found PGC1a levels were reduced in AD brains.
3. Enzymes
In the AD brain, particular enzymes that mediate glucose metabolism may have either altered amounts or altered Vmax activities. For example, the neuronal enolase is more highly expressed, and also shows a high degree of oxidative modification (Butterfield & Lange, 2009).
Within the mitochondria themselves, the measured activities of pyruvate dehydrogenase complex (PDHC) and the Krebs cycle enzyme α-ketoglutarate dehydrogenase complex (KDHC), are reduced (Gibson et al., 1998). The KDHC activity reduction is likely a consequence of post-translational modification due to oxidative stress (Shi et al., 2011). The activity of isocitrate dehydrogenase, another proximal Krebs cycle enzyme, is reduced. Activities of enzymes in the distal Krebs cycle, including succinate dehydrogenase and malate dehydrogenase, are increased (Gibson et al., 2010).
Regarding the electron transport chain (ETC), COX activity has consistently been observed to be lower in AD subject brains than it is in control subject brains (Swerdlow, 2011b; Swerdlow & Kish, 2002). Histochemical approaches reveal AD brain hippocampi contain higher numbers of COX-activity deficient neurons (Cottrell et al., 2001). Some studies have suggested neuroanatomically limited reductions and are consistent with the possibility that the COX activity deficit is due to reduced synaptic activity (Simonian & Hyman, 1993). Other studies have utilized spectrophotometric Vmax measurements from brain homogenates (Swerdlow & Kish, 2002). In one study, dividing the COX Vmax to the density of a COX protein subunit on a Western blot rendered the activity comparable to that of the corrected control group activity (Kish et al., 1999). The authors concluded reduced COX in AD brains is a consequence of reduced COX enzyme. Another study, though, found that COX activity, when divided by the amount of spectrally determined COX was still low (Parker & Parks, 1995). It was further demonstrated that COX kinetics were altered, and that the holoenzyme’s low Km binding site was absent. These data argue that COX is structurally altered in the AD brain.
4. mtDNA
As discussed under the mitochondrial mass section, the case has been alternatively made that the amount of mtDNA in AD neurons is decreased or increased. These findings are not as diametrically opposed as it may seem. The amount of mtDNA may vary from neuron to neuron, and may further associate with the health of the neuron. Healthier neurons may have an increased amount of mtDNA, while more affected neurons may have decreased amounts. Certainly, in AD brain hippocampi the number of neurons that show succinate dehydrogenase activity but which lack COX activity is increased (Cottrell et al., 2001). Because succinate dehydrogenase is entirely encoded by nuclear genes while COX contains mtDNA-encoded subunits, this suggests AD neurons have abnormally high levels of perturbed or mutated mtDNA, or else a severe state of mtDNA depletion.
In addition to changes in the quantity of mtDNA, differences in the quality of mtDNA have been reported. A probe specific to mtDNA containing the 5 kDa common deletion found AD brain hippocampal neurons contained markedly increased amounts of this deletion (Hirai et al., 2001). Other studies using different approaches have also found that relative to control brains, AD brains contain increased amounts of the 5 kDa deletion (Corral-Debrinski et al., 1994; Hamblet & Castora, 1997).
Oxidative modifications of the mtDNA are increased in AD brains, as evidenced by higher levels of 8-hydroxy-2-deoxyguanosine (2DG) (Mecocci et al., 1994). Oxidation-related nucleotide modifications can induce replication errors and an accumulation of somatic mutations. Some AD brain studies that surveyed mtDNA protein coding genes have reported a quantitative increase in the number of these mutations, although others have not (Chang et al., 2000; Lin et al., 2002). Another study determined levels of low-abundance heteroplasmic mutations in the mtDNA D-loop control region. Specific mutations were found in AD brains that were not present in control brains, and the overall burden of control region mutations was markedly increased in the AD brains (Coskun et al., 2004). This study also reported reductions in an mtDNA-derived transcript, as well as a decreased mtDNA to nuclear DNA ratio.
While some have focused on characterizing presumably somatic, acquired mutations, others have probed whether inherited mtDNA sequences and mutations are present. To date, particular homoplasmic mtDNA mutations have been reported in AD subjects, but the causality of these mutations has been virtually impossible to prove (Swerdlow, 2011b). In any event, inherited homoplasmic mtDNA mutations are at most an extremely rare cause of AD.
MtDNA sequences between individuals are extremely variable to begin with (Lu et al., 2010), and series of linked sequence deviations that can vary between populations and members of populations have been used to define mtDNA haplogroups (Torroni et al., 1996). Haplogroup sequence changes present in blood are also present in the brains of those who carry them, and haplogroups are amenable to association studies. A number of mtDNA hapolgroup studies have reported associations between particular mtDNA haplogroups and AD (Swerdlow, 2011b). Some studies have found certain haplogroups associate with an increased risk of AD, while other studies have found that other haplogroups associate with a decreased risk of AD. Although findings from some populations have also been found in others, the results of haplogroup association studies in AD have generally been inconsistent. To summarize, the presence of at least a small effect of mtDNA haplogroups on AD risk remains a possibility.
5. Fission/Fusion
Impaired mitochondrial dynamics have been widely implicated in neurodegenerative disorders such as AD (Chan, 2006a; Su et al., 2010). Mitochondria can undergo consecutive cycles of fusion, in which physically separate mitochondria link to produce a single organelle. This is counterbalanced by the process of fission, which features the breakdown of a single mitochondrion into smaller mitochondria (Detmer & Chan, 2007). These phenomena rely on a large group of conserved proteins, the dynamin-related GTPases.
Mitochondrial fission is crucial for mitochondrial renewal, redistribution, and proliferation within synapses, whereas mitochondrial fusion facilitates mitochondrial movement and distribution across axons and to the synapses themselves (Chen et al., 2007; Hoppins et al., 2007; Santos et al., 2010). A balance between these two events is crucial to maintain mitochondrial functional integrity, especially in neurons where mitochondrial fission and fusion are mandatory for the formation of synapses and dendritic spines (Arduino et al., 2011). Fusion is orchestrated by the mitofusins Mfn1 and Mfn2, which are responsible for outer membrane fusion, and Opa1, which participates in the fusion of outer and inner membranes. Fission requires Drp1 in mitochondria, and membrane constriction is facilitated by Fis1 (Chan, 2006b; Yoon et al., 2003).
Analyses of AD brains show down regulation of the Mnf1, Mnf2, and Opa1 fusion genes and increased expression of the Fis1 fission gene (Manczak et al., 2011a; Wang et al., 2009). The status of the other fission-mediating protein, Drp1, is less clear as levels have been reported to be both reduced and increased (Manczak et al., 2011b; Wang et al., 2009). Regardless, Drp1 activity is inactivated by S-nitrosylation (SNO-Drp1), and Cho and colleagues reported high levels of SNO-Drp1 in brain biopsies from AD subjects (Cho et al., 2009). Perturbed Drp1 function could lead to the production of functionally impaired mitochondria, and ultimately reduce synapse energy supplies (Barsoum et al., 2006).
Drp1, Opa1, Mfn1, Mfn2, andFis1 proteins re -distribute so that they accumulate in the cell soma. Neuronal processes are therefore depleted of these fission-fusion proteins (Wang et al., 2009). The authors of this study also manipulated mitochondrial fission-fusion proteins in M17 cells and hippocampal primary neurons, and found this affected intracellular mitochondrial distributions. They concluded that altered mitochondrial fission-fusion protein dynamics may play an important role in mitochondrial distribution and, consequently, synaptic dysfunction in AD neurons.
When primary cortical neuron cultures are exposed to S-nitrosocysteine (SNOC), a nitric oxide (NO)donor, uncontrolled fission occurs and this appears to represent an upstream and early event in neuronal cell death (Barsoum et al., 2006). Further, when mitochondrial fission is blocked by expression of the dominant negative Drp1K38A, cell death is reduced. In a similar type of study, when cultured cerebrocortical neurons were exposed to Aβ, S-nitrosylation of Drp1 occurred and resulted in the formation of SNO-Drp1 dimers (Westermann, 2009). This pattern is similar to what is found in the brains of human AD patients (Cho et al., 2009).
6. Transport
The delivery of mitochondria to regions of the neuron with high bioenergetic demand is required for proper neuron function (Hollenbeck & Saxton, 2005; Li et al., 2004; MacAskill et al., 2010; Mattson et al., 2008). This mitochondrial transport is impaired in a number of neurodegenerative disorders. For example, it has been reported that mutant huntingtin protein in Huntington’s disease (HD) enhances mitochondrial Drp1 activity, disrupts mitochondrial trafficking, and induces mitochondrial fission (Reddy & Shirendeb, 2011; Shirendeb et al., 2012). Anterograde mitochondrial transport is significantly reduced in neuronal cultures of SOD1-mutant mice, suggesting that impaired mitochondrial trafficking is an early event in amyotrophic lateral sclerosis (ALS) (De Vos et al., 2007). Additionally, mitochondrial transport is impaired in dopaminergic neurons from a Parkinson’s disease (PD) mouse model (Sterky et al., 2011). Given the prevalence of altered mitochondrial transport in other neurodegenerative diseases, it is likely that this critical phenomenon is also impaired in AD.
Indeed, mitochondrial distribution is abnormal in AD brains (Wang et al., 2009). One study showed that mitochondrial transport in AD patient brains is significantly decreased compared to control brains (Dai et al., 2002). In an elegant study by Trimmer and Borland, fluorescently-labeled mitochondria in differentiated cytoplasmic hybrid (cybrid) cell lines generated from AD patients displayed reduced trafficking to neurite-like processes compared to control cybrid lines (Trimmer & Borland, 2005). This study provides further evidence that mitochondrial transport may be impaired in AD. Additionally, this study suggests that impaired mitochondrial transport in AD is mediated by mitochondrial function itself and ultimately by mtDNA. Other studies suggest that mitochondrial transport is altered in cell cultures treated with Aβ (Calkins & Reddy, 2011; Wang et al., 2009) and in mouse models of AD (Calkins et al., 2011; Massaad et al., 2010; Pigino et al., 2003).
In general, it appears that Drp1 abnormalities may impair mitochondrial transport by perturbing a functional relationship that exists between Drp1 and the dynein-dynactin transport complex (Ishihara et al., 2009; Varadi et al., 2004; Wang et al., 2009). For example, in AD subject fibroblasts Drp1 expression is significantly lower than in control fibroblasts (Wang et al., 2008a). This Drp1 down-regulation occurs in conjunction with an abnormal mitochondrial distribution, and restoring normal Drp1 levels to AD fibroblasts repairs their mitochondrial transport defect. Therefore, although mitochondrial transport is certainly difficult to study in the autopsy brain, data suggest that in AD perturbed mitochondrial fission-fusion dynamics may contribute to the apparent presence of impaired mitochondrial transport.
7. Oxidative stress
Reactive oxygen species (ROS) are a frequent by-product of electron leakage from the inner mitochondrial membrane during mitochondrial oxidative phosphorylation. It is estimated that up to 4% of O2used by mitochondria is converted to superoxide radical (Hansford et al., 1997; Inoue et al., 2003; Markesbery & Lovell, 1998; Morten et al., 2006; Turrens & Boveris, 1980), and that approximately 109to 1011 ROS are produced per cell per day (Bonda et al., 2010; Feinendegen, 2002; Ji, 1999; Petersen et al., 2007). Under normal conditions, ROS are rapidly cleared to increasingly lesser reactive species by enzymes such as SOD1, SOD2, catalase, and glutathione peroxidase (GPx). When mitochondria are perturbed, however, ROS production may exceed the cell’s ability to neutralize them, resulting in oxidative damage to the cell (Smith et al., 2000). Aging itself is associated with elevated ROS production by mitochondria (Ames et al., 1995; Shigenaga et al., 1994), and accumulation of oxidative damage over time may contribute to the noted association between advancing age and AD.
Oxidative stress is thought to be an early manifestation of AD(Nunomura et al., 2001). Studies of post-mortem AD brains indicate widespread oxidative damage. Four-hydroxynonenal and acrolein, which are aldehydes produced by lipid peroxidation, and isoprostanes, which are pro-inflammatory products of arachidonic acid peroxidation, are significantly elevated in hippocampi from AD brains (Markesbery& Lovell, 1998; Pratico et al., 1998; Sayre et al., 1997; Singh et al., 2010). This indicates that excessive lipid oxidation occurs in the AD brain. In AD brains both nuclear and mitochondrial DNA and RNA also display evidence of oxidative damage (Gabbita et al., 1998; Mecocci et al., 1994; Nunomura et al., 1999). Brains from individuals affected with AD further display increased protein oxidation, as evidenced by carbonyl-alterations of specific proteins (Castegna et al., 2002a; Castegna et al., 2002b; Smith et al., 1991; Sultana et al., 2010).
Many studies suggest that oxidative damage is also present in individuals with mild cognitive impairment (MCI), a syndromic state that in many cases represents a very early AD clinical stage (Aluise et al., 2011; Butterfield et al., 2006b; Butterfield et al., 2007; Keller et al., 2005; Lovell & Markesbery, 2008; Markesbery & Lovell, 2007; Pratico et al., 2002). In fact, studies suggest that levels of oxidative markers directly correlate with severity of cognitive impairment as well as symptomatic progression from MCI to AD (Ansari & Scheff, 2010; Keller et al., 2005).
Extensive oxidative damage in AD brains likely has significant consequences for neurons, as oxidative modification of proteins and other molecular components can alter cell function (Butterfield et al., 1997; Lauderback et al., 2001; Subramaniam et al., 1997; Sultana & Butterfield, 2009). As a major source of ROS production, mitochondria are themselves at risk of acquiring oxidative damage. As discussed earlier, the activities of certain mitochondrial enzymes including isocitrate dehydrogenase, PDHC, KDHC, and COX are significantly reduced in the AD brain (Aksenov et al., 1999; Bubber et al., 2005; Butterfield et al., 2006a; Gibson et al., 1998; Manczak et al., 2004; Yates et al., 1990). These enzyme impairments may represent a consequence or cause of ROS production, or both. For instance, COX dysfunction might further elevate ROS production by stalling electron transfer (Barrett et al., 2004; Skulachev, 1996; Sullivan & Brown, 2005; Sullivan et al., 2004). Thus, dysfunctional mitochondria in AD may give rise to and perpetuate a vicious cycle of oxidant production in which impairment of one mitochondrial enzyme elevates ROS production, which in turn impairs the function of other mitochondrial enzymes, which in turn further increases ROS production (Bonda et al., 2010; Zhu et al., 2004).
8. Apoptosis
AD brains experience significant neuron loss, which likely contributes to an affected person’s cognitive decline (Shimohama, 2000; Terry et al., 1991). While some neuron loss is due to necrosis, the rest is likely due to or else invokes aspects of apoptosis, a tightly-regulated form of programmed cell death (Barinaga, 1998).
DNA fragmentation, as assessed by TUNEL staining, is a common hallmark of apoptosis. Neurons in AD brains display increased DNA fragmentation compared to control brains (Anderson et al., 1996; Broe et al., 2001; Colurso et al., 2003; Lassmann et al., 1995; Li et al., 1997; Smale et al., 1995; Su et al., 1994; Troncoso et al., 1996). Many of these studies also reveal morphologic changes associated with apoptosis including abnormal chromatin, an absence of nucleoli, and shrunken or irregular cell shapes (Shimohama, 2000). Other studies note an increased proportion of apoptotic to normal neurons (Broe et al., 2001). In AD cell death surveys, DNA fragmentation also associates with expression of c-Jun, which is typical of apoptotic neurons (Behl, 2000), and caspase proteins (Masliah et al., 1998). These studies suggest apoptosis pathways are activated in the AD brain.
Correspondingly, AD brains express significantly higher levels of the pro-apoptotic proteins Bak and Bad (Kitamura et al., 1998; Shimohama, 2000). Other studies suggest that AD brains display elevated pro-apoptotic Bax (Su et al., 1997). Caspases 3 and 6, which are apoptosis “executioner” caspases, are increased in AD brains (Avila, 2010; Guo et al., 2004; Masliah et al., 1998; Rohn et al., 2001b; Selznick et al., 1999; Stadelmann et al., 1999), as are the initiator caspases 8 and 9 (Albrecht et al., 2007; Rohn & Head, 2009; Rohn et al., 2001a; Rohn et al., 2002).
Further evidence that apoptotic events are more frequent in AD brains than in age -matched controls comes from experiments evaluating the presence of the cytoskeletal spectrin protein fodrin, which is cleaved early in the apoptotic cascade by caspases (Cribbs et al., 2004). Brains from AD patients display increased amounts of fodrin cleavage products (Ayala-Grosso et al., 2006; Masliah et al., 1991; Masliah et al., 1990).
Interestingly, evidence suggests that the pro-apoptotic shifts seen in AD subjects are not brain-limited. One study found that lymphocytes from AD patients were pre-disposed to apoptosis (Eckert et al., 2001). Another study reported increased fodrin cleavage in fibroblasts from AD patients (Peterson et al., 1991).
In summary, substantial data suggest that apoptosis is elevated in AD. This is not surprising given the other molecular and biochemical perturbations observed in this disease. For example, oxidative stress can predispose cells to apoptosis (Buttke & Sandstrom, 1994; Ray et al., 2012; Sandstrom et al., 1994). The prolific oxidative damage present in AD may, therefore, contribute to increased apoptosis.
B. Systemic
1. Enzymes
Reduced platelet COX activity is observed in subjects with AD and with MCI (Cardoso et al., 2004; Parker et al., 1990; Valla et al., 2006). MCI is considered a transitional state between normal aging and AD (Morris et al., 2001; Padurariu et al., 2010). The platelet COX activity reduction is apparent in the setting of preserved COX subunit levels (Cardoso et al., 2004). One study has also reported COX activity is reduced in AD subject fibroblasts (Curti et al., 1997).
KDHC catalyses a critical reaction in the Krebs cycle and is also important in glutamate metabolism (Blass et al., 1997). Its activity is reduced in AD brains. Cultured skin fibroblasts from sporadic AD subjects and AD subjects with PS1 mutations also show reduced KDHC activity (Blass et al., 1997; Sheu et al., 1994). In contradistinction to this, despite the fact that PDHC activity is reduced in AD brains, non-brain tissues do not show reduced activity (Gibson et al., 1998).
ROS can modify the structure and function of cell proteins, lipids, and DNA (Facchinetti et al., 1998). ROS levels are controlled through the action of antioxidant enzymes, such as SOD1, SOD2, GPx, and catalase. It has been reported that MCI and AD subjects have lower plasma SOD and GPx activities than control subjects (Padurariu et al., 2010; Rinaldi et al., 2003). In erythrocytes from AD subjects, however, catalase and GPx activity were elevated. The activity of another antioxidant enzyme, glutathione reductase (GR), was reduced in both MCI and AD subjects(Torres et al., 2011).
2. mtDNA
Excess deletion mutations have not been demonstrated in AD subject peripheral tissues. However, it is important to note that in general, deletions are uncommon in some peripheral tissues, such as blood, which are commonly studied because they are easy to collect. An increase in control region point mutations was reported in AD subject lymphocytes (Coskun et al., 2010). Interestingly, mtDNA haplogroup studies have utilized mtDNA from blood samples, and many of these studies have reported associations between haplogroups (Swerdlow, 2011b).
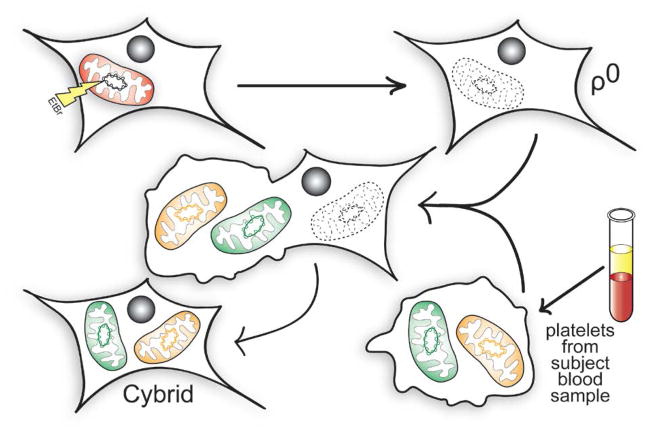
Studies using cybrid cell lines consistently suggest that if mtDNA does in fact differ between AD and control subjects, then these differences are not brain-limited (Swerdlow, 2011b). Cybrid studies are performed by transferring mitochondria from a particular cell population to cell lines depleted of endogenous mtDNA. These mtDNA-depleted cell lines, referred to as ρ0 cell lines, do not have a functional oxidative phosphorylation apparatus because they lack 13 crucial mtDNA-encoded proteins (7 from complex I, 1 from complex III, 3 from complex IV, and 2 from complex V). MtDNA contained within the transferred mitochondria populates the cell lines and restores their aerobic competence. The resulting unique cell lines are true cytoplasmic hybrids because they contain cytosolic components from two sources, the original ρ0 cell line and the cells that provided their functional mitochondria (Figure 1). Biochemical differences, although often subtle, are demonstrable between cybrid cell lines whose mtDNA is reconstituted from different sources. Because different cybrid cell lines prepared using the same parent ρ0 line have identical nuclear DNA genes, and because cell lines are expanded and maintained under identical conditions, these biochemical differences presumably reflect and arise from differences in their mtDNA.
FIGURE 1. Cytoplasmic hybrids.
ρ0 cell lines are generated by chronically treating an established cell line with ethidium bromide (EtBr). This blocks mtDNA replication and leads to its total depletion from the EtBr-treated cells. Due to a lack of mtDNA-encoded oxidative phosphorylation complex subunits, the resultant cell lines are unable to complete electron transport chain transport and oxidative phosphorylation. The ρ0 cells can then be fused with platelets isolated from patient blood samples to generate cytoplasmic hybrid (cybrid) cell lines. Cybrid lines contain mtDNA from the platelet donors, and nuclear DNA from the ρ0 cell line. This relationship lets investigators study how specific mtDNA sequences affect cell bioenergetics, and how these effects influence downstream cell biochemical, molecular, and physiologic parameters.
When COX activities between AD cybrid line series are compared to those of control cybrid cell line series, although considerable overlap between individual lines from each group are seen the mean COX activity is characteristically lower in the AD cybrid cell lines (Swerdlow, 2007b, 2011b). This has been reported in multiple studies that have used ρ0 cells prepared from SH-SY5Y neuroblastoma and NT2 teratocarcinoma cell lines. In these studies, the mtDNA used to restore mtDNA to the ρ0 cells came from platelets obtained from human AD and control subjects. If these mean differences in fact derive from mtDNA, it would indicate that mtDNA between age-matched individuals with and without AD does differ. It would further indicate that this mtDNA difference is not brain-limited, and extends at least to platelets and the megakaryocytes from which it derives.
Additional indirect support for systemic mtDNA differences between AD and non-AD individuals comes from AD endophenotype studies. An endophenotype is a partial or limited manifestation of a condition that is not sufficient to render a diagnosis of that condition. The presence of an endophenotype state does not necessarily indicate the affected individual will acquire the condition, although it does infer the carrier individual has an increased risk of developing the condition.
In recent years numerous studies have reported AD-consistent endophenotypes can be demonstrated in the asymptomatic, middle-aged children of AD subjects. Interestingly, these endophenotypes are more profound in the children of AD mothers than they are in the children of AD fathers (Swerdlow, 2011b). This suggests that although both parents contribute to AD risk, AD mothers contribute to a greater extent. This, in turn, implies that a maternally -inherited genetic factor influences the development of AD (Mosconi et al., 2010a).
These AD endophenotype studies have been conducted using metabolic, structural, and biochemical approaches. 2-deoxy-2 [F-18]fluoro -D-glucose positron emission tomography (FDG PET) studies show the children of AD mothers, but not the children of AD fathers, have patterns of reduced glucose utilization that resembles patterns observed in AD subjects themselves (Mosconi et al., 2007; Mosconi et al., 2009). MRI studies show increased brain atrophy and rates of brain atrophy in the children of AD mothers as compared to the children of AD fathers (Berti et al., 2011; Honea et al., 2011; Honea et al., 2010). Oxidative stress and Aβ changes can be observed in the children of AD mothers (Mosconi et al., 2010b). As they age, the children of AD mothers accumulate greater amounts of Aβ in their brain parenchyma than do the children of AD fathers (Mosconi et al., 2010c). In persons at risk for mid-life cognitive softening due to the possession of an APOE4 allele, those with an AD mother perform less well on memory tests (Debette et al., 2009). Finally, platelet COX activity is lower in the children of AD mothers than it is in the children of AD fathers (Mosconi et al., 2011). Collectively, these findings suggest a maternally-inherited genetic factor influences AD risk, and that this maternally-inherited genetic factor is more likely to be mtDNA than an epigenetic or sex-linked factor (Table 1).
Table 1.
Effect of maternal influence on non-demented subject AD endophenotypes
| Endophenotype Parameter | Evaluated by | Change |
|---|---|---|
| Brain glucose utilization | FDG PET | Reduced glucose utilization and more rapid glucose utilization decline rate in regions commonly affected in AD subjects |
| Brain Volume | MRI with voxel-based morphometry | More atrophy and higher rates of atrophy in AD-affected regions |
| Brain Aβ | PET PIB | Decreased brain parenchyma Aβ levels |
| CSF Aβ | CSF ELISA | Aβ42/Aβ40 ratio decreased |
| CSF Isoprostanes | Mass spectrometry | Isoprostanes elevated |
| Memory performance | Cognitive evaluation | Among APOE4 carriers, lower memory test scores |
| COX activity | Platelet mitochondria COX Vmax assay | Reduced COX activity |
3. Fission/Fusion
Fission/fusion dynamics are also altered in at least one non-brain tissue of AD patients (Wang et al., 2008a). Potential mechanisms that may underlie this phenomenon have been proposed and evaluated.
In one experiment, fibroblasts from non-AD subjects were treated with hydrogen peroxide (H2O2) in order to simulate a state of oxidative stress. This caused levels of the mitochondrial fission-promoting Drp1 protein to fall. Drp1 protein reduction, in turn, associated with a redistribution of mitochondria, and recapitulated changes observed in sporadic AD fibroblasts (Wang et al., 2008a).
M17 cell lines that over-express APP show mitochondrial fragmentation and redistribution of their mitochondria (Wang et al., 2008b). Similarly, fibroblasts that over-express wild type or mutant APP show a reduction in Drp1 and altered mitochondrial trafficking (Wang et al., 2008b). In both sporadic AD fibroblasts and in M17 cells over-expressing APP, mitochondria cluster in the perinuclear region while the number of mitochondria in the cell periphery falls. Whether perturbed mitochondrial fission and fusion dynamics in sporadic AD subject fibroblasts is truly caused by Aβ overproduction is unclear, but the simple fact that mitochondrial fusion-fission dynamics are altered outside the brains of sporadic AD subjects contributes to the increasing realization that at biochemical and molecular levels, AD is not a brain-limited disease.
4. Oxidative stress
As previously discussed, brains from individuals with AD undergo extensive oxidative damage throughout the disease process. Significant evidence suggests that oxidative damage in AD is not brain-limited, but is also present systemically in AD patients (Burns et al., 2009).
One study evaluated the presence of oxidative stress in platelets and erythrocytes from normal controls and AD patients. This study found elevated oxidative stress markers in AD patients in the form of thiobarbituric acid-reactive substances, nitric oxide synthase activity, and Na, K-ATPase activity, suggesting that oxidative stress is present systemically in AD (Kawamoto et al., 2005). Another study found that reactive oxygen species are elevated in circulating neutrophils from AD patients (Vitte et al., 2004). Plasma from AD subjects shows significantly decreased levels of the antioxidants lycopene, lutein, and carotene when compared to plasma from control subjects, and leukocytes from AD patients display elevated levels of oxidized DNA (Mecocci et al., 2002; Mecocci et al., 1998; Migliore et al., 2005; Morocz et al., 2002).
Oxidative stress is also ubiquitous in patients with MCI, suggesting that the oxidative damage seen in AD is a continuation of the stress that is also present during MCI. Interestingly, many studies further suggest that between MCI and AD subjects, no major differences in oxidative stress markers such as malondialdehyde and oxidized glutathione exist (Baldeiras et al., 2008; Bermejo et al., 2008; Padurariu et al., 2010). Rather, these studies propose that the primary biochemical differences between MCI and AD lie in the levels and activity of antioxidants such as superoxide dismutase, glutathione peroxidase, and vitamin E. This suggests that a loss of one’s ability to compensate for oxidative stress may underlie or else serve as a marker of MCI-to-AD progression.
Additional evidence suggests that oxidative stress markers may correlate with disease progression and severity in AD patients. Torres et al. recently found that plasma levels of malondialdehyde, a lipid peroxidation product, directly associate with impaired cognitive function in AD patients. The authors also found that the ratio of GR activity to GPx activity, which provides an indication of a cell’s antioxidant capacity, associates with cognitive function (Torres et al., 2011). It has also been reported that in individual AD subjects, serum levels of vitamin E, a dietary antioxidant, relate to cognitive status (Baldeiras et al., 2008; Panza et al., 2010).
Data such as these have encouraged investigators to attempt to develop peripheral AD diagnostic and biomarker tests (Burns et al., 2009; Pratico, 2005). While a definitive biomarker with adequate sensitivity and specificity remains to be identified, a plethora of data suggests that at least on a biochemical and molecular level, AD is a systemic disorder.
III. Mitochondria as a Therapeutic Target in AD
Accumulating data suggest mitochondrial function, if not changes in cell bioenergetics or the pathways that regulate cell bioenergetics, are perturbed early in the course of AD. In this respect it is possible that at the commencement of AD itself mitochondria are altered by a more upstream process. If so, then treating mitochondrial abnormalities may benefit affected patients to some degree. It may also be the case that mitochondrial or bioenergetic dysfunction may actually constitute the primary, etiologic cause of AD (Swerdlow et al., 2010). If so, then therapies directed towards the mitochondria or cell bioenergetics could, should they target and remediate the primary problem, prove clinically transformative (Swerdlow, 2009; Swerdlow, 2011c). To date, several clinical trials have evaluated agents intended to target mitochondria or the consequences of mitochondrial dysfunction.
A. Overview of Mitochondrial Medicine
1. Historical perspective
Mitochondrial medicine can be defined as any therapeutic intervention that specifically targets mitochondria themselves or specific consequences of mitochondrial dysfunction (Swerdlow, 2009). Mitochondrial medicine approaches were pioneered in rare diseases characterized by mitochondrial dysfunction and, in some cases, in rare diseases arising from identified mtDNA mutations (Luft, 1994).
For example, it has seemed obvious for some time that enhancing overall mitochondrial function might benefit patients with mtDNA diseases (Swerdlow, 2007c). Small studies have evaluated the effects of supplementing electron acceptor and donor molecules, such as coenzyme Q and menadione. The intent of such treatments has been to increase the passage of electrons through the ETC, or increase the COX-mediated delivery of electrons to molecular oxygen by bypassing upstream bottlenecks. Others have attempted to increase the flow of pyruvate-derived carbon into the Krebs cycle by activating PDHC. To accomplish this, investigators have administered drugs such as dichloroacetate that inhibit the PDHC kinase, which is an upstream inhibitor of PDHC. Other approaches have included raising the levels of PDHC cofactors, such as thiamine and lipoic acid (Swerdlow; Swerdlow, 2007c; Swerdlow, 2009; Swerdlow, 2011c).
Other classic mitochondrial medicine approaches intended to help maintain cell ATP levels have been tried in various degenerative mitochondriopathies. Creatine within cells binds high energy phosphate groups, initially generated by the mitochondrial ATP synthase (complex V), to form phosphocreatine. It has been postulated that increasing cell creatine levels will increase levels of cell phosphocreatine, and that in the event that cell ATP levels are expended then the high-energy phosphocreatine phosphate group might be used to regenerate ATP. This has been tried in some neurodegenerative diseases including HD, PD, and ALS but these trials have failed to show a meaningful benefit (Swerdlow, 2007c; Swerdlow, 2009; Swerdlow, 2011c).
Some mitochondrial medicine approaches have targeted the replacement of specific missing or depleted mitochondrial molecules, to some cases with great effect. Carnitine supplementation can prove transformative in cases of carnitine deficiency, just as coenzyme Q supplementation can greatly improve the clinical status of persons with coenzyme Q deficiency (Quinzii et al., 2007).
Although it is often not considered a “mitochondrial medicine” approach per se, interventions that target potential downstream effects of mitochondrial dysfunction have been tried in a variety of conditions. The most common of these targets has included the reduction of oxidative stress. While antioxidant clinical trials to date have not proved transformative in any trial, some trials have concluded particular antioxidant interventions may possibly confer, in some cases, a very limited therapeutic effect (Swerdlow, 2007c).
2. Newer strategies
Recently, attempts have been made to make relatively non-specific interventions more specific. For example, it has been posited that the failure of antioxidant therapies to truly benefit persons with mitochondrial disorders may relate to the fact that most antioxidants do not target free radicals within mitochondria themselves. This has justified the creation of mitochondrially-targeted antioxidant molecules (Reddy, 2008; Reddy et al., 2011).
Other recently promoted strategies have sought to take advantage of drugs that have more general effects on mitochondrial physiology. Mitochondrial “stabilization” is one such effect. Under in vitro conditions, mitochondrial stabilization is typically defined as an induced perpetuation of the mitochondrial membrane potential under stress conditions, or as a preservation of mitochondrial size under stress conditions. Mitochondrial stabilization has been attempted in ALS. Development of the mitochondrial stabilizer minocycline, which interferes with mitochondrial permeability pore function, was terminated after trial results indicated accelerated decline in treated subjects (Gordon et al., 2007). Another mitochondrial stabilizer, R-pramipexole, suggested a therapeutic benefit and more definitive trials are now underway (Cudkowicz et al., 2011).
For cases in which mtDNA may initiate dysfunction, attempts have been made to manipulate mtDNA itself. Although protein nucleic acids (PNAs) were reported years ago to have the ability to strategically influence mtDNA replication under in vitro conditions (Taylor et al., 1997), this approach has had problems under more physiologic conditions. The feasibility of delivering mitochondrial targeted restriction enzymes that degrade specific mtDNA sequences has also been shown in a number of studies (Wenz et al., 2010). One group has been working towards the development of mtDNA delivery systems that can deposit exogenous mtDNA payloads to mitochondrial matrices (Khan & Bennett, 2004).
Induction of mitochondrial biogenesis has been proposed for the enhancement of mitochondria function in conditions in which mitochondrial mass is reduced (Ghosh et al., 2007; Swerdlow, 2007c). Advocated strategies include increasing activity or levels of the transcription factor of the mitochondria (TFAM) or PGC1a (Swerdlow, 2009; Swerdlow, 2011c).
B. Track Record of Mitochondrial Medicine for AD
1. Oxidative stress
Perturbed mitochondrial function can be associated with increased ROS production. Electrons that enter the ETC, when not added to molecular oxygen by COX to form water, can react with molecular oxygen in a less controlled fashion to produce the superoxide anion. The superoxide anion, in turn, can be converted to H2O2 and from there into other ROS species (Balaban et al., 2005; Fukui & Moraes, 2008). Some degree of physiologic ROS production occurs as a byproduct of cell respiration, and in AD the rate of ROS production appears to be increased(Lin & Beal, 2006; Shi et al., 2008). Because of this, a number of investigators have proposed using approaches intended to decrease oxidative stress, such as the administration of antioxidant compounds (Aliev et al., 2009; Moreira et al., 2009).
Although several antioxidants have been tested in AD subjects, none have shown a robust effect (Swerdlow, 2011c). Discouragingly, successes obtained in studies of animal models tend not to be reflected in human trials. For example, administering vitamin E to Tg2576 mice before Aβ plaque deposition occurred suppressed brain lipid peroxidation and Aβ plaque deposition (Sung et al., 2004), which suggests vitamin E supplementation could potentially benefit AD pathology. Although a large human AD study that evaluated Vitamin E at doses of 2000 IU per day did report a possible slowing of clinical progression, this was only evident after the data were mathematically adjusted to account for the fact that at the start of the study the treatment and placebo groups were not identical in terms of their cognitive abilities (Sano et al., 1997). Without this correction, no difference was observed. Although for some time after this trial high dose vitamin E was commonly offered to AD patients, a re-assessment of vitamin E therapy concluded the adverse effects of this approach might outweigh its very limited (if any) benefits (Bjelakovic et al., 2007; Miller et al., 2005). Subsequently, it has become uncommon to prescribe high doses of vitamin E to AD patients.
Lipoic acid (LA), in addition to serving as a coenzyme for the mitochondrial pyruvate and α-ketoglutarate dehydrogenase complexes, has robust antioxidant properties (Moreira et al., 2009). Some clinical studies have reported AD subjects treated with LA showed a slowed rate of decline (Hager et al., 2007; Hager et al., 2001), but this finding has yet to be replicated in a large-scale trial.
Idebenone, a water-soluble synthetic analogue of CoQ10, has been evaluated in AD subjects (Chaturvedi & Beal, 2008; Senin et al., 1992). A 6 month placebo-controlled trial reported idebenone-treated subjects showed less decline on the Alzheimer’s Disease Assessment Scale (ADAS), a test of cognitive performance (Weyer et al., 1997). In another study performed by the Alzheimer’s Disease Cooperative Study (ADCS) group, idebenone appeared to slow decline on the ADAS, but did not meaningfully benefit global function (Thal et al., 2003). The ADCS group idebenone trial was therefore interpreted as a negative trial.
The failure of antioxidants to clearly benefit AD patients may be due to several factors. One possibility is that in the human clinical trials, treatment was initiated too late in the course of the disease (Conte et al., 2004; Kamat et al., 2008; Sung et al., 2004). Other possibilities are that the compounds tested to date may have had limited brain penetration, or may have failed to reach mitochondria, the likely source of increased ROS production in AD (Manczak et al., 2010). To circumvent this, mitochondria-targeted antioxidants have now been developed. The most studied mitochondria-targeted antioxidant is MitoQ, which is generated through the covalent binding of ubiquinone, an antioxidant, to the triphenylphosphonium (TPP +) cation. This compound rapidly crosses the blood-brain barrier (BBB) and accesses neuron mitochondria (Bolognesi et al., 2009; McManus et al., 2011). In animal models MitoQ has been shown to effectively reduce Aβ-induced oxidative stress and Aβ toxicity in neuron cultures, where it promotes neurite outgrowth and synaptic connectivity (Manczak et al., 2010). It has been shown to prevent cognitive decline in 3xTg-AD mice(McManus et al., 2011). MitoQ has been evaluated in a phase II trial of another neurodegenerative disease, PD, but the results of that trial were not encouraging.
Of course, another potential explanation for the failure of antioxidants thus far to demonstrate efficacy in AD is that oxidative stress may not be a major driver of neurodysfunction and degeneration in AD. If oxidative stress is a downstream consequence of mitochondrial dysfunction, removing it might do little to repair the underlying, more critical mitochondrial lesion.
2. Electron transport
Small studies have evaluated the effects of thiamine and lipoic acid in AD subjects. In some cases these cofactors have been used as part of a “cocktail” with other cofactors and vitamins (Blass & Gibson, 2006). Encouraging preliminary results have been reported using this strategy, but results from more conclusive studies have yet to appear. Some agents that may serve as ETC-associated electron donors and acceptors have undergone early-stage testing in humans, such as a methylene blue derivative (Wischik et al., 2008). In developing methylene blue for the treatment of AD the responsible investigators have not identified the mitochondria as a potential target, but nevertheless under in vitro conditions methylene blue does appear to affect ETC electron transport (Atamna & Kumar, 2010; Atamna et al., 2008; Callaway et al., 2004).
Another approach for enhancing mitochondrial oxidative phosphorylation includes using ketone bodies such as beta-hydroxybutyrate (Swerdlow et al., 1989). The rationale underlying this approach is based on the observations that glucose utilization is reduced in the AD brain, and that ketone bodies constitute an alternative carbon source that can be used to support oxidative phosphorylation (Swerdlow, 2011c). More recently, a medium chain triglyceride (MCT) supplement has been marketed for the treatment of AD. As is the case with other MCTs, it is converted by the liver to beta-hydroxybutyrate and this elevates plasma beta-hydoxybutyrate levels. This strategy has been tested in humans with AD. While not conclusive, reported data do not exclude the possibility that some subjects benefit from this treatment (Henderson et al., 2009).
3. Membrane stabilization
Latrepirdine (dimebon) is an antihistamine that was subsequently shown, under in vitro conditions, to have mitochondrial membrane stabilization properties (Bachurin et al., 2003; Zhang et al., 2010). Dimebon was studied in AD subjects. Although a phase II trial reported promising results (Doody et al., 2008), follow-up phase III studies did not replicate that finding and dimebon development efforts were terminated. Very recently, another mitochondrial membrane stabilizing agent, R-pramipexole, entered into an AD clinical trial.
C. Anticipated Mitochondrial Medicine Strategies
Some of the more recent mitochondrial medicine approaches listed above have been advocated and, in some cases, even attempted. Other very unique mitochondrial medicine approaches have also been proposed and are being evaluated under pre-clinical conditions (Figure 2).
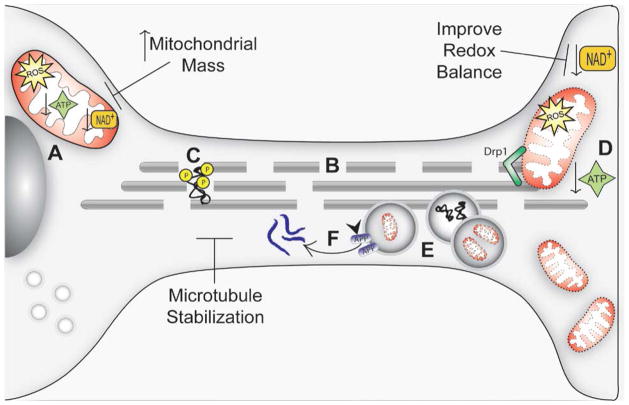
FIGURE 2. Current and anticipated mitochondrial medicine strategies.
Dysfunctional mitochondria in AD result in reduced ATP availability (A), which promotes microtubule network breakdown (B), jeopardizing the transport of molecules and organelles along the cell. Further, it promotes tau dissociation from microtubules and its consequent hyperphosphorylation (C). These events compromise synapse energy supplies (D), and the transport of AVs towards the cell body (where lysosomes are located) is impeded (E). This promotes accumulation of Aβ aggregates, which may be formed by APP cleavage at AV membranes (F). Microtubule network stabilization may be improve ALP function, promote transport along axons, and reduce Aβ production. Increasing mitochondrial mass may compensate for declines in mitochondrial function and their overall functional capacity. Shifting the cell redox balance to a more oxidized state may cause the cell bioenergetic infrastructure to function more efficiently, promote mitochondrial biogenesis, and activate pathways that allow cells to tolerate stress conditions.
1. Mitochondrial mass manipulation
Increasing mitochondrial mass for the treatment of AD was first proposed in 2007 (Ghosh et al., 2007; Swerdlow, 2007c). A rudimentary attempt to increase mitochondrial mass was previously attempted using the thiazolidinedione drugs rosiglitazone and pioglitazone. Although these drugs were originally considered for AD treatment based largely on their demonstrated anti-inflammatory effects, these drugs, which are used to treat type II diabetes, were subsequently shown to activate mitochondrial biogenesis signaling under pre-clinical testing paradigms. However, it is doubtful that they can reach levels within the brain that are high enough to activate mitochondrial biogenesis. Although the thiazolidinedione clinical trial data to date have not been uniformly negative, the overall impression these data give is that pioglitazone and rosiglitazone will provide no measurable benefit or, at best, an extremely small benefit (Geldmacher et al., 2011; Gold et al., 2010; Risner et al., 2006). In the meantime, other ways to manipulate mitochondrial mass are in various stages of development.
2. Redox state manipulation
In this section, redox state refers to a cell’s electron balance as defined by ratios of electron donor and acceptor molecule pairs. In this respect, an important indicator of a cell’s bioenergetic state is the ratio defined by amounts of nicotinamide adenine dinucleotide’s oxidized (NAD+) and reduced (NADH) derivatives.
While bioenergetics help determine a cell’s redox state, a cell’s redox state can also influence its bioenergetic function. Data demonstrating this latter point comes from multiple lines of investigation. Caloric restriction shifts the liver’s redox balance towards a more oxidized state, and this is associated with mitochondrial biogenesis at least in the liver (Civitarese et al., 2007; Lambert et al., 2004; Lopez-Lluch et al., 2006)and also perhaps in the brain (Nisoli et al., 2005). Physical exercise, which should shift the muscle redox balance towards a more oxidized state, induces muscle mitochondrial biogenesis (Baar et al., 2002; Holloszy & Coyle, 1984; Hood, 2009).
Theoretically, enzymes that depend on NAD+ levels, such as SIRT1, should activate in the setting of increased NAD+ (Guarente, 2007; Haigis & Guarente, 2006). SIRT1 activation has been advocated for the treatment of several diseases, including AD (Anekonda & Reddy, 2006; Guarente, 2007; Haigis & Guarente, 2006). Polyphenol compounds are believed to work as sirtuin activators (Baur, 2010; Lagouge et al., 2006). An AD clinical that will evaluate the effects of resveratrol, a polyphenol, on AD clinical status is scheduled to be performed.
3. Cytoskeletal manipulation
Mitochondrial dysfunction can induce cytoskeletal perturbations (Cardoso et al., 2010; Moreira et al., 2006). In the AD brain, reduced ATP levels may deregulate cytoskeleton homeostasis and microtubule integrity. Due to several unique neuron morphologic features such as extended axons, branched dendritic arbors, and synaptic connections both communication and continuity between a neuron’s cell body and its distal regions must be adequately maintained. In this regard it is well known that neurons are highly sensitive to disturbances in microtubule -dependent transport (Trimmer & Borland, 2005).
AD neurons show cytoskeletal changes. Compared to brains from control subjects, microtubule assemblies are reduced (Cash et al., 2003; Santa-Maria et al., 2005). Surveys of AD brain pyramidal neurons suggest that changes in microtubule homeostasis precede neurofibrillary tangle (NFT) formation (Cash et al., 2003). NFTs consist of the microtubule-associated protein (MAP) tau, which plays a role in microtubule function, neurite growth, and cytoskeleton maintenance (Stamer et al., 2002). Although a number of tau residues are phosphorylated under physiological conditions, in AD tau phosphorylation increases 3 to 4-fold. Other studies show that tau over-expression alters cell shape, leads to a loss of polarization, and slows cell growth. This is accompanied by a change in mitochondrial distribution ; this change is characterized by organelle clustering (Ebneth et al., 1998).
The associated microtubule perturbations are accompanied by changes in the autophagic-lysosomal pathway (ALP), or autophagy. Autophagy is a tightly regulated process that plays an important role in cellular maintenance. It ensures adequate levels of essential cell intermediates are maintained (Cuervo, 2004). The process begins with the regulated formation of a cytosolic membrane that encapsulates a region of the cytoplasm and its organelles within a double membrane called an autophagic vacuole (AV), or autophagosome(Levine & Kroemer, 2008). APP, which is a transmembrane protein, can be processed by the endosomal-lysosomal pathway which initiates when material internalized by endocytosis or pinocytosis is sorted into endosomes (Nixon, 2007). Data suggest that a change in the rate of autophagy, or the factors which cause autophagic vacuoles to accumulate, may contribute to Aβ over production in AD (Levine & Kroemer, 2008; Yu et al., 2004). Indeed, evidence from AD subject brains shows massive AV accumulation within dystrophic neurites occurs (Nixon et al., 2005; Yu et al., 2005).
Autophagy may therefore be simultaneously impaired and induced in AD. One potential explanation for this is that mitochondrial dysfunction may disrupt the microtubule cytoskeleton, and lead to impaired AV retrograde transport towards the cell body where lysosomes are located. In support of this, it has been shown that microtubule depolymerizing agents disrupt vesicular transport and induce rapid AV accumulation(Kochl et al., 2006). Vinblastine, which inhibits microtubule assembly, leads to microtubule depolymerization and prevents AV-lysosome fusion (Boland et al., 2008; Xie et al., 2010).
Recently, Miyasaka and colleagues (Miyasaka et al., 2010)reported that tau hyperphosphorylation is more likely a consequence, as opposed to a cause, of microtubule disruption. This is consistent with the view that when the microtubule network is disrupted, tau dissociates from microtubules and becomes accessible to the kinases that promote its hyperphosphorylation (Silva et al., 2011a). For this reason, it was postulated that microtubule stabilizing agents could potentially reduce neuronal dystrophy (Lee et al., 1994; Silva et al., 2011b).
Data consistent with this view come from experiments using taxol (paclitaxel), a microtubule polymerizing agent that has been shown to mitigate AD-associated pathology in AD model systems. In rats, taxol was found to protect cortical neurons from Aβ25–35 toxicity, decrease calpain activation, and decrease cdk5/p25 complex formation (Li et al., 2003). In other models, taxol pretreatment prevented tau hyperphosphorylation and reduced Aβ -induced apoptosis (Michaelis et al., 2002). In a hippocampal slice model of lysosomal dysfunction. it was found that pretreatment with TX67, an analogue of taxol, restored pre and post synaptic proteins levels and reduced synapse damage (Butler et al., 2007).
Recently, Silva and co-workers demonstrated that in SH-SY5Y cells exposed to Aβ1–42, the resultant mitochondrial dysfunction perturbs AV transport via a microtubule-dependent mechanism(Silva et al., 2011a). Taxol prevents Aβ1–42-induced disorganization of the tubulin cytoskeleton, which secondarily reduces both cytosolic and mitochondrial Aβ content by enhancing ALP function.
Taxol, though, does not robustly access the central nervous system (CNS) (Liu et al., 2002). A drug with taxol-like properties, NAP (davunetide), an eight amino acid peptide derived from the activity-dependent neuroprotective protein (ADNP), was recently shown to cross the blood brain barrier after systemic or intranasal administration(Gozes et al., 2005). Although NAP was first described as an antioxidant, it is now recognized that following cell internalization NAP interacts with the microtubule cytoskeleton (Divinski et al., 2004; Gozes & Divinski, 2007). NAP has been shown to reduce tau hyperphosphorylation and Aβ accumulation in both in vitro and in vivo AD models, and also benefit cognitive test performance in some of these models (Gozes & Divinski, 2004; Matsuoka et al., 2007; Matsuoka et al., 2008; Shiryaev et al., 2009; Vulih-Shultzman et al., 2007). Although its biological effects remain to be fully investigated (Shiryaev et al., 2011), based on encouraging preclinical data and an apparent lack of toxicity NAP is now being tested in persons with AD and other disorders of the CNS (Greggio et al., 2011; Idan-Feldman et al., 2011; Javitt et al., 2011). A recent phase IIa clinical study reported that intranasal NAP improved memory performance in patients with an amnestic MCI syndrome, a frequent an AD precursor state (Gozes et al., 2009).
IV. Conclusion
Many key questions about AD remain unresolved. There is no uniform agreement over whether AD is a homogeneous or a heterogeneous entity, how it relates to brain aging, or even what causes most of the cases. It is clear that from a population perspective, mitochondria and mitochondria-related phenomena differ between those who do and do not have this disease. The importance of these mitochondrial and bioenergetic differences to AD, however it is defined, has been variably considered to be irrelevant or epiphenomenal, a mediator of disease pathology, a major mediator of disease pathology, or the actual initiating cause of the disease. This latter view is based on observations that distinct mitochondrial parameters are observed in subjects at all stages of AD, in persons at increased risk for developing AD, and that these differences are not brain-limited.
Unless mitochondrial changes turn out to be so far downstream of another critical disease-driving process that they are inconsequential to the condition, mitochondria and cell bioenergetics should constitute reasonable therapeutic targets. Some rudimentary attempts at mitochondrial medicine have been attempted in AD, with clinical results to date showing either no or perhaps minor beneficial effects. In the meantime, a more sophisticated and integrated view of how mitochondrial function, maintenance, and biogenesis play out in cells and relate to other aspects of cell function is emerging. Advances along this line will help to guide and refine the development of future mitochondrial medicine approaches. In coming years or perhaps decades, it will be interesting to see how mitochondria and cell bioenergetics-targeted interventions affect persons with AD.
Acknowledgments
The authors receive support from the University of Kansas Alzheimer’s Center (NIA P30AG035982), the Frank and Evangeline Thompson Alzheimer’s Disease Therapeutic Development Fund, and the Portugal Institute of Science and Technology.
Non-standard abbreviations
- 2DG
8-hydroxy-2-deoxyguanosine
- Aβ
beta amyloid
- AD
Alzheimer’s disease
- ADAS
Alzheimer’s Disease Assessment Scale
- ADCS
Alzheimer’s Disease Cooperative Study
- ADNP
activity dependent neuroprotective protein
- ALP
autophagic lysosomal pathway
- ALS
amyotrophic lateral sclerosis
- APOE
apolipoprotein E gene
- APP
amyloid precursor protein
- AV
autophagic vacuole
- BBB
blood-brain barrier
- CSF
cerebrospinal fluid
- CNS
central nervous system
- CoQ
coenzyme Q
- COX
cytochrome oxidase
- Cybrid
cytoplasmic hybrid
- Drp
dynamin related protein
- ELISA
enzyme-linked immunosorbent assay
- EM
electron microscopy
- EtBr
ethidium bromide
- ETC
electron transport chain
- FDG PET
2-deoxy-2 [F-18]fluoro -D-glucose positron emission tomography
- Fis1
fission 1
- GPx
glutathione peroxidase
- GR
glutathione reductase
- H2O2
hydrogen peroxide
- HD
Huntinton’s disease
- KDHC
α-ketoglutarate dehydrogenase complex
- LA
lipoic acid
- MAP
microtubule associated protein
- MCI
mild cognitive impairment
- MCT
medium chain triglyceride
- Mfn
mitofusin
- mtDNA
mitochondrial DNA
- NAD
nicotinamide adenine dinucleotide
- NFT
neurofibrillary tangle
- NO
nitric oxide
- Opa1
optic atrophy 1
- PD
Parkinson’s disease
- PDHC
pyruvate dehydrogenase complex
- PIB
Pittsburgh Compound B
- PGC1a
peroxisome proliferator activated receptor gamma coactivator 1α
- PNA
protein nucleic acid
- PS
presenilin
- ROS
reactive oxygen species
- SIRT1
silent mating type information regulation 2 homolog1
- SNO
S-nitrosylation
- SNOC
S-nitrosocysteine
- SOD
Superoxide dismutase
- TFAM
transcription factor A of the mitochondria
- TPP+
triphenylphosphonium cation
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
References
- Aksenov MY, Tucker HM, Nair P, Aksenova MV, Butterfield DA, Estus S, et al. The expression of several mitochondrial and nuclear genes encoding the subunits of electron transport chain enzyme complexes, cytochrome c oxidase, and NADH dehydrogenase, in different brain regions in Alzheimer’s disease. Neurochem Res. 1999;24(6):767–774. doi: 10.1023/a:1020783614031. [DOI] [PubMed] [Google Scholar]
- Albrecht S, Bourdeau M, Bennett D, Mufson EJ, Bhattacharjee M, LeBlanc AC. Activation of caspase-6 in aging and mild cognitive impairment. Am J Pathol. 2007;170(4):1200–1209. doi: 10.2353/ajpath.2007.060974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev G, Palacios HH, Walrafen B, Lipsitt AE, Obrenovich ME, Morales L. Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int J Biochem Cell Biol. 2009;41(10):1989–2004. doi: 10.1016/j.biocel.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Aluise CD, Robinson RA, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer’s disease: insights into memory loss in MCI. J Alzheimers Dis. 2011;23(2):257–269. doi: 10.3233/JAD-2010-101083. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiat Psych-Gerichtl Med. 1907;64:146–148. [Google Scholar]
- Alzheimer A. Uber eigenartige Krankheitsfalle des spateren Alters. Z die Gesamte Neurologie Pscyhiatrie. 1911;4:456–485. [Google Scholar]
- Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8(6):429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271(1):165–170. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- Anderson AJ, Su JH, Cotman CW. DNA damage and apoptosis in Alzheimer’s disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J Neurosci. 1996;16(5):1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer’s disease. J Neurochem. 2006;96(2):305–313. doi: 10.1111/j.1471-4159.2005.03492.x. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69(2):155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduino DM, Esteves AR, Cardoso SM. Mitochondrial fusion/fission, transport and autophagy in Parkinson’s disease: when mitochondria get nasty. Parkinsons Dis. 2011;2011:767230. doi: 10.4061/2011/767230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamna H, Kumar R. Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis. 2010;20(Suppl 2):S439–452. doi: 10.3233/JAD-2010-100414. [DOI] [PubMed] [Google Scholar]
- Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, et al. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22(3):703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- Avila J. Alzheimer disease: caspases first. Nat Rev Neurol. 2010;6(11):587–588. doi: 10.1038/nrneurol.2010.157. [DOI] [PubMed] [Google Scholar]
- Ayala-Grosso C, Tam J, Roy S, Xanthoudakis S, Da Costa D, Nicholson DW, et al. Caspase-3 cleaved spectrin colocalizes with neurofilament-immunoreactive neurons in Alzheimer’s disease. Neuroscience. 2006;141(2):863–874. doi: 10.1016/j.neuroscience.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16(14):1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. discussion 345–339. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Baldeiras I, Santana I, Proenca MT, Garrucho MH, Pascoal R, Rodrigues A, et al. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis. 2008;15(1):117–128. doi: 10.3233/jad-2008-15110. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ. Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis. 2006;9(2):119–126. doi: 10.3233/jad-2006-9204. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ. Mitochondria are related to synaptic pathology in Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:305395. doi: 10.4061/2011/305395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinaga M. Is apoptosis key in Alzheimer’s disease? Science. 1998;281(5381):1303–1304. doi: 10.1126/science.281.5381.1303. [DOI] [PubMed] [Google Scholar]
- Barrett MJ, Alones V, Wang KX, Phan L, Swerdlow RH. Mitochondria-derived oxidative stress induces a heat shock protein response. J Neurosci Res. 2004;78(3):420–429. doi: 10.1002/jnr.20249. [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25(16):3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA. Biochemical effects of SIRT1 activators. Biochim Biophys Acta. 2010;1804(8):1626–1634. doi: 10.1016/j.bbapap.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C. Apoptosis and Alzheimer’s disease. J Neural Transm. 2000;107(11):1325–1344. doi: 10.1007/s007020070021. [DOI] [PubMed] [Google Scholar]
- Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, et al. Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from Mild Cognitive Impairment. Free Radic Res. 2008;42(2):162–170. doi: 10.1080/10715760701861373. [DOI] [PubMed] [Google Scholar]
- Berti V, Mosconi L, Glodzik L, Li Y, Murray J, De Santi S, et al. Structural brain changes in normal individuals with a maternal history of Alzheimer’s. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Blass JP, Gibson GE. Correlations of disability and biologic alterations in Alzheimer brain and test of significance by a therapeutic trial in humans. J Alzheimers Dis. 2006;9(2):207–218. doi: 10.3233/jad-2006-9212. [DOI] [PubMed] [Google Scholar]
- Blass JP, Sheu KF, Piacentini S, Sorbi S. Inherent abnormalities in oxidative metabolism in Alzheimer’s disease: interaction with vascular abnormalities. Ann N Y Acad Sci. 1997;826:382–385. doi: 10.1111/j.1749-6632.1997.tb48488.x. [DOI] [PubMed] [Google Scholar]
- Blocq P, Marinesco G. Sur les lesions et la pathogenie de l’epilepsie dite essentielle. La Semaine Medicale. 1892;12:445–446. [Google Scholar]
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28(27):6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi ML, Matera R, Minarini A, Rosini M, Melchiorre C. Alzheimer’s disease: new approaches to drug discovery. Curr Opin Chem Biol. 2009;13(3):303–308. doi: 10.1016/j.cbpa.2009.04.619. [DOI] [PubMed] [Google Scholar]
- Bonda DJ, Wang X, Perry G, Nunomura A, Tabaton M, Zhu X, et al. Oxidative stress in Alzheimer disease: a possibility for prevention. Neuropharmacology. 2010;59(4–5):290–294. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Broe M, Shepherd CE, Milward EA, Halliday GM. Relationship between DNA fragmentation, morphological changes and neuronal loss in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2001;101(6):616–624. doi: 10.1007/s004010000337. [DOI] [PubMed] [Google Scholar]
- Brown AM, Sheu RK, Mohs R, Haroutunian V, Blass JP. Correlation of the clinical severity of Alzheimer’s disease with an aberration in mitochondrial DNA (mtDNA) J Mol Neurosci. 2001;16(1):41–48. doi: 10.1385/JMN:16:1:41. [DOI] [PubMed] [Google Scholar]
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57(5):695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- Burns DH, Rosendahl S, Bandilla D, Maes OC, Chertkow HM, Schipper HM. Near-infrared spectroscopy of blood plasma for diagnosis of sporadic Alzheimer’s disease. J Alzheimers Dis. 2009;17(2):391–397. doi: 10.3233/JAD-2009-1053. [DOI] [PubMed] [Google Scholar]
- Butler D, Bendiske J, Michaelis ML, Karanian DA, Bahr BA. Microtubule-stabilizing agent prevents protein accumulation-induced loss of synaptic markers. Eur J Pharmacol. 2007;562(1–2):20–27. doi: 10.1016/j.ejphar.2007.01.053. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Cole P, Subramaniam R, Aksenov M, Aksenova M, et al. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer’s disease. J Neurochem. 1997;68(6):2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Lange ML. Multifunctional roles of enolase in Alzheimer’s disease brain: beyond altered glucose metabolism. J Neurochem. 2009;111(4):915–933. doi: 10.1111/j.1471-4159.2009.06397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, et al. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis. 2006a;22(2):223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, et al. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci Lett. 2006b;397(3):170–173. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, et al. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007;1148:243–248. doi: 10.1016/j.brainres.2007.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15(1):7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20(23):4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Reddy PH. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim Biophys Acta. 2011;1812(4):507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav. 2004;77(1):175–181. doi: 10.1016/j.pbb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Pereira CF, Moreira PI, Arduino DM, Esteves AR, Oliveira CR. Mitochondrial control of autophagic lysosomal pathway in Alzheimer’s disease. Exp Neurol. 2010;223(2):294–298. doi: 10.1016/j.expneurol.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol Aging. 2004;25(1):105–110. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, et al. Microtubule reduction in Alzheimer’s disease and aging is independent of tau filament formation. Am J Pathol. 2003;162(5):1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, et al. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002a;33(4):562–571. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, et al. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002b;82(6):1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006a;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006b;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Giordano T, Brady DR, Stoll J, Martin LJ, Rapoport SI. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res Mol Brain Res. 1994;24(1–4):336–340. doi: 10.1016/0169-328x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Chang SW, Zhang D, Chung HD, Zassenhaus HP. The frequency of point mutations in mitochondrial DNA is elevated in the Alzheimer’s brain. Biochem Biophys Res Commun. 2000;273(1):203–208. doi: 10.1006/bbrc.2000.2885. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann N Y Acad Sci. 2008;1147:395–412. doi: 10.1196/annals.1427.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130(3):548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324(5923):102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Smith SR, Ravussin E. Diet, energy metabolism and mitochondrial biogenesis. Curr Opin Clin Nutr Metab Care. 2007;10(6):679–687. doi: 10.1097/MCO.0b013e3282f0ecd2. [DOI] [PubMed] [Google Scholar]
- Colurso GJ, Nilson JE, Vervoort LG. Quantitative assessment of DNA fragmentation and beta-amyloid deposition in insular cortex and midfrontal gyrus from patients with Alzheimer’s disease. Life Sci. 2003;73(14):1795–1803. doi: 10.1016/s0024-3205(03)00512-5. [DOI] [PubMed] [Google Scholar]
- Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, et al. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004;90(3):758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, McKee AC, Beal MF, et al. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics. 1994;23(2):471–476. doi: 10.1006/geno.1994.1525. [DOI] [PubMed] [Google Scholar]
- Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101(29):10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, et al. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J Alzheimers Dis. 2010;20(Suppl 2):S293–310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell DA, Blakely EL, Johnson MA, Ince PG, Turnbull DM. Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology. 2001;57(2):260–264. doi: 10.1212/wnl.57.2.260. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Poon WW, Rissman RA, Blurton-Jones M. Caspase-mediated degeneration in Alzheimer’s disease. Am J Pathol. 2004;165(2):353–355. doi: 10.1016/S0002-9440(10)63302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz M, Bozik ME, Ingersoll EW, Miller R, Mitsumoto H, Shefner J, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med. 2011;17(12):1652–1656. doi: 10.1038/nm.2579. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004;263(1–2):55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- Curti D, Rognoni F, Gasparini L, Cattaneo A, Paolillo M, Racchi M, et al. Oxidative metabolism in cultured fibroblasts derived from sporadic Alzheimer’s disease (AD) patients. Neurosci Lett. 1997;236(1):13–16. doi: 10.1016/s0304-3940(97)00741-6. [DOI] [PubMed] [Google Scholar]
- Dai J, Buijs RM, Kamphorst W, Swaab DF. Impaired axonal transport of cortical neurons in Alzheimer’s disease is associated with neuropathological changes. Brain Res. 2002;948(1–2):138–144. doi: 10.1016/s0006-8993(02)03152-9. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Luong T, Neely TR, Robinson D, Wands JR. Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer’s disease. Lab Invest. 2000;80(8):1323–1335. doi: 10.1038/labinvest.3780140. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16(22):2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Wolf PA, Beiser A, Au R, Himali JJ, Pikula A, et al. Association of parental dementia with cognitive and brain MRI measures in middle-aged adults. Neurology. 2009;73(24):2071–2078. doi: 10.1212/WNL.0b013e3181c67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8(11):870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Divinski I, Mittelman L, Gozes I. A femtomolar acting octapeptide interacts with tubulin and protects astrocytes against zinc intoxication. J Biol Chem. 2004;279(27):28531–28538. doi: 10.1074/jbc.M403197200. [DOI] [PubMed] [Google Scholar]
- Divry P. Etude histochimique des plaques seniles. J Belg Neurol Psychiatry. 1927;9:643–657. [Google Scholar]
- Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372(9634):207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol. 1998;143(3):777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A, Oster M, Zerfass R, Hennerici M, Muller WE. Elevated levels of fragmented DNA nucleosomes in native and activated lymphocytes indicate an enhanced sensitivity to apoptosis in sporadic Alzheimer’s disease. Specific differences to vascular dementia. Dement Geriatr Cogn Disord. 2001;12(2):98–105. doi: 10.1159/000051242. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18(6):667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinendegen LE. Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol. 2002;21(2):85–90. doi: 10.1191/0960327102ht216oa. [DOI] [PubMed] [Google Scholar]
- Fischer O. Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eine regelmabige Veranderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiatr Neurol. 1907;22:361–372. [Google Scholar]
- Fukui H, Moraes CT. The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci. 2008;31(5):251–256. doi: 10.1016/j.tins.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbita SP, Lovell MA, Markesbery WR. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J Neurochem. 1998;71(5):2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68(1):45–50. doi: 10.1001/archneurol.2010.229. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Patel N, Rahn D, McAllister J, Sadeghi S, Horwitz G, et al. The thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Mol Pharmacol. 2007;71(6):1695–1702. doi: 10.1124/mol.106.033845. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105(8–9):855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Starkov A, Blass JP, Ratan RR, Beal MF. Cause and consequence: mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim Biophys Acta. 2010;1802(1):122–134. doi: 10.1016/j.bbadis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30(2):131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6(12):1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Gozes I, Divinski I. The femtomolar-acting NAP interacts with microtubules: Novel aspects of astrocyte protection. J Alzheimers Dis. 2004;6(6 Suppl):S37–41. doi: 10.3233/jad-2004-6s605. [DOI] [PubMed] [Google Scholar]
- Gozes I, Divinski I. NAP, a neuroprotective drug candidate in clinical trials, stimulates microtubule assembly in the living cell. Curr Alzheimer Res. 2007;4(5):507–509. doi: 10.2174/156720507783018208. [DOI] [PubMed] [Google Scholar]
- Gozes I, Morimoto BH, Tiong J, Fox A, Sutherland K, Dangoor D, et al. NAP: research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP) CNS Drug Rev. 2005;11(4):353–368. doi: 10.1111/j.1527-3458.2005.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Stewart A, Morimoto B, Fox A, Sutherland K, Schmeche D. Addressing Alzheimer’s disease tangles: from NAP to AL-108. Curr Alzheimer Res. 2009;6(5):455–460. doi: 10.2174/156720509789207895. [DOI] [PubMed] [Google Scholar]
- Greggio S, de Paula S, de Oliveira IM, Trindade C, Rosa RM, Henriques JA, et al. NAP prevents acute cerebral oxidative stress and protects against long-term brain injury and cognitive impairment in a model of neonatal hypoxia-ischemia. Neurobiol Dis. 2011;44(1):152–159. doi: 10.1016/j.nbd.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC. Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol. 2004;165(2):523–531. doi: 10.1016/S0002-9440(10)63317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager K, Kenklies M, McAfoose J, Engel J, Munch G. Alpha-lipoic acid as a new treatment option for Alzheimer’s disease--a 48 months follow-up analysis. J Neural Transm Suppl. 2007;(72):189–193. doi: 10.1007/978-3-211-73574-9_24. [DOI] [PubMed] [Google Scholar]
- Hager K, Marahrens A, Kenklies M, Riederer P, Munch G. Alpha-lipoic acid as a new treatment option for Alzheimer [corrected] type dementia. Arch Gerontol Geriatr. 2001;32(3):275–282. doi: 10.1016/s0167-4943(01)00104-2. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Castora FJ. Elevated levels of the Kearns -Sayre syndrome mitochondrial DNA deletion in temporal cortex of Alzheimer’s patients. Mutat Res. 1997;379(2):253–262. doi: 10.1016/s0027-5107(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29(1):89–95. doi: 10.1023/a:1022420007908. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) 2009;6:31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118(Pt 23):5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56(4):831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Honea RA, Swerdlow RH, Vidoni E, Burns JM. Progressive regional atrophy in normaladults with a maternal history of Alzheimer disease. Neurology. 2011;76:822–829. doi: 10.1212/WNL.0b013e31820e7b74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Swerdlow RH, Vidoni ED, Goodwin J, Burns JM. Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology. 2010;74(2):113–120. doi: 10.1212/WNL.0b013e3181c918cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34(3):465–472. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Idan-Feldman A, Schirer Y, Polyzoidou E, Touloumi O, Lagoudaki R, Grigoriadis NC, et al. Davunetide (NAP) as a preventative treatment for central nervous system complications in a diabetes rat model. Neurobiol Dis. 2011;44(3):327–339. doi: 10.1016/j.nbd.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, et al. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10(23):2495–2505. doi: 10.2174/0929867033456477. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11(8):958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF, et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res. 2011 doi: 10.1016/j.schres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222(3):283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15(3):473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Katzman R. Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Arch Neurol. 1976;33(4):217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
- Kawamoto EM, Munhoz CD, Glezer I, Bahia VS, Caramelli P, Nitrini R, et al. Oxidative state in platelets and erythrocytes in aging and Alzheimer’s disease. Neurobiol Aging. 2005;26(6):857–864. doi: 10.1016/j.neurobiolaging.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64(7):1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Khan SM, Bennett JP., Jr Development of mitochondrial gene replacement therapy. J Bioenerg Biomembr. 2004;36(4):387–393. doi: 10.1023/B:JOBB.0000041773.20072.9e. [DOI] [PubMed] [Google Scholar]
- Kimberly WT, Xia W, Rahmati T, Wolfe MS, Selkoe DJ. The transmembrane aspartates in presenilin 1 and 2 are obligatory for gamma-secretase activity and amyloid beta-protein generation. J Biol Chem. 2000;275(5):3173–3178. doi: 10.1074/jbc.275.5.3173. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Mastrogiacomo F, Guttman M, Furukawa Y, Taanman JW, Dozic S, et al. Decreased brain protein levels of cytochrome oxidase subunits in Alzheimer’s disease and in hereditary spinocerebellar ataxia disorders: a nonspecific change? J Neurochem. 1999;72(2):700–707. doi: 10.1046/j.1471-4159.1999.0720700.x. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Shimohama S, Kamoshima W, Ota T, Matsuoka Y, Nomura Y, et al. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 1998;780(2):260–269. doi: 10.1016/s0006-8993(97)01202-x. [DOI] [PubMed] [Google Scholar]
- Kochl R, Hu XW, Chan EY, Tooze SA. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic. 2006;7(2):129–145. doi: 10.1111/j.1600-0854.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Klinishce Psychiatrie. Verlag Johann Ambrosius Barth; Lepzig: 1910. Psychiatrie. Ein Lehrbuch fur Studierende und Arzte. [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Wang B, Yardley J, Edwards J, Merry BJ. The effect of aging and caloric restriction on mitochondrial protein density and oxygen consumption. Exp Gerontol. 2004;39(3):289–295. doi: 10.1016/j.exger.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Bancher C, Breitschopf H, Wegiel J, Bobinski M, Jellinger K, et al. Cell death in Alzheimer’s disease evaluated by DNA fragmentation in situ. Acta Neuropathol. 1995;89(1):35–41. doi: 10.1007/BF00294257. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, et al. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1–42. J Neurochem. 2001;78(2):413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- Lee VM, Daughenbaugh R, Trojanowski JQ. Microtubule stabilizing drugs for the treatment of Alzheimer’s disease. Neurobiol Aging. 1994;15(Suppl 2):S87–89. doi: 10.1016/0197-4580(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, et al. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Li G, Faibushevich A, Turunen BJ, Yoon SO, Georg G, Michaelis ML, et al. Stabilization of the cyclin-dependent kinase 5 activator, p35, by paclitaxel decreases beta-amyloid toxicity in cortical neurons. J Neurochem. 2003;84(2):347–362. doi: 10.1046/j.1471-4159.2003.01526.x. [DOI] [PubMed] [Google Scholar]
- Li WP, Chan WY, Lai HW, Yew DT. Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J Mol Neurosci. 1997;8(2):75–82. doi: 10.1007/BF02736774. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci U S A. 2008;105(11):4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet. 2002;11(2):133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ali SM, Boge TC, Georg GI, Victory S, Zygmunt J, et al. A systematic SAR study of C10 modified paclitaxel analogues using a combinatorial approach. Comb Chem High Throughput Screen. 2002;5(1):39–48. doi: 10.2174/1386207023330615. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103(6):1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidatively modified RNA in mild cognitive impairment. Neurobiol Dis. 2008;29(2):169–175. doi: 10.1016/j.nbd.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wang K, Rodova M, Esteves R, Berry D, EL, et al. Polymorphic variation in cytochrome oxidase subunit genes. J Alzheimers Dis. 2010;21(1):141–154. doi: 10.3233/JAD-2010-100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci U S A. 1994;91(19):8731–8738. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Atkin TA, Kittler JT. Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur J Neurosci. 2010;32(2):231–240. doi: 10.1111/j.1460-9568.2010.07345.x. [DOI] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011a;20(13):2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011b doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, et al. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20(Suppl 2):S609–631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5(2):147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. Four -hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging. 1998;19(1):33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch Neurol. 2007;64(7):954–956. doi: 10.1001/archneur.64.7.954. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Mallory M, Albright T, Terry RD. Abnormal brain spectrin immunoreactivity in sprouting neurons in Alzheimer disease. Neurosci Lett. 1991;129(1):1–5. doi: 10.1016/0304-3940(91)90707-z. [DOI] [PubMed] [Google Scholar]
- Masliah E, Iimoto DS, Saitoh T, Hansen LA, Terry RD. Increased immunoreactivity of brain spectrin in Alzheimer disease: a marker for synapse loss? Brain Res. 1990;531(1–2):36–44. doi: 10.1016/0006-8993(90)90755-z. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, Tanaka S, Hansen LA. Caspase dependent DNA fragmentation might be associated with excitotoxicity in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57(11):1041–1052. doi: 10.1097/00005072-199811000-00007. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Amin SK, Hu L, Mei Y, Klann E, Pautler RG. Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer’s disease. PLoS One. 2010;5(5):e10561. doi: 10.1371/journal.pone.0010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Gray AJ, Hirata-Fukae C, Minami SS, Waterhouse EG, Mattson MP, et al. Intranasal NAP administration reduces accumulation of amyloid peptide and tau hyperphosphorylation in a transgenic mouse model of Alzheimer’s disease at early pathological stage. J Mol Neurosci. 2007;31(2):165–170. doi: 10.1385/jmn/31:02:165. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF, et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2008;325(1):146–153. doi: 10.1124/jpet.107.130526. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60(5):748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2011;31(44):15703–15715. doi: 10.1523/JNEUROSCI.0552-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol. 1994;36(5):747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Polidori MC, Cherubini A, Ingegni T, Mattioli P, Catani M, et al. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch Neurol. 2002;59(5):794–798. doi: 10.1001/archneur.59.5.794. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Polidori MC, Ingegni T, Cherubini A, Chionne F, Cecchetti R, et al. Oxidative damage to DNA in lymphocytes from AD patients. Neurology. 1998;51(4):1014–1017. doi: 10.1212/wnl.51.4.1014. [DOI] [PubMed] [Google Scholar]
- Michaelis ML, Dobrowsky RT, Li G. Tau neurofibrillary pathology and microtubule stability. J Mol Neurosci. 2002;19(3):289–293. doi: 10.1385/JMN:19:3:289. [DOI] [PubMed] [Google Scholar]
- Migliore L, Fontana I, Trippi F, Colognato R, Coppede F, Tognoni G, et al. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging. 2005;26(5):567–573. doi: 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- Miyasaka T, Sato S, Tatebayashi Y, Takashima A. Microtubule destruction induces tau liberation and its subsequent phosphorylation. FEBS Lett. 2010;584(14):3227–3232. doi: 10.1016/j.febslet.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Cardoso SM, Pereira CM, Santos MS, Oliveira CR. Mitochondria as a therapeutic target in Alzheimer’s disease and diabetes. CNS Neurol Disord Drug Targets. 2009;8(6):492–511. doi: 10.2174/187152709789824651. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer’s disease. J Alzheimers Dis. 2006;9(2):101–110. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- Morocz M, Kalman J, Juhasz A, Sinko I, McGlynn AP, Downes CS, et al. Elevated levels of oxidative DNA damage in lymphocytes from patients with Alzheimer’s disease. Neurobiol Aging. 2002;23(1):47–53. doi: 10.1016/s0197-4580(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Morten KJ, Ackrell BA, Melov S. Mitochondrial reactive oxygen species in mice lacking superoxide dismutase 2: attenuation via antioxidant treatment. J Biol Chem. 2006;281(6):3354–3359. doi: 10.1074/jbc.M509261200. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, de Leon M. Maternal transmission of Alzheimer’s disease: prodromal metabolic phenotype and the search for genes. Hum Genomics. 2010a;4(3):170–193. doi: 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, et al. Maternal family history of Alzheimer’s disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A. 2007;104(48):19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, de Leon M, Murray J, EL, Lu J, Javier E, et al. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer’s disease. J Alzheimers Dis. 2011;27(3):483–490. doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Glodzik L, Mistur R, McHugh P, Rich KE, Javier E, et al. Oxidative stress and amyloid-beta pathology in normal individuals with a maternal history of Alzheimer’s. Biol Psychiatry. 2010b;68(10):913–921. doi: 10.1016/j.biopsych.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, et al. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009;72(6):513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Rinne JO, Tsui WH, Berti V, Li Y, Wang H, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci U S A. 2010c;107(13):5949–5954. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, LeGris M, Matthews PM. Mitochondrial enzyme expression in the hippocampus in relation to Alzheimer-type pathology. Acta Neuropathol. 1999;97(4):346–354. doi: 10.1007/s004010050997. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(Pt 23):4081–4091. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64(2):113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, et al. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19(6):1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2010;469(1):6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Pilotto A, Frisardi V. Peripheral antioxidant markers in mild cognitive impairment and its progression to dementia. J Alzheimers Dis. 2010;21(4):1179–1183. doi: 10.3233/jad-2010-101224. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Parks JK. Cytochrome c oxidase in Alzheimer’s disease brain: purification and characterization. Neurology. 1995;45(3 Pt 1):482–486. doi: 10.1212/wnl.45.3.482. [DOI] [PubMed] [Google Scholar]
- Petersen RB, Nunomura A, Lee HG, Casadesus G, Perry G, Smith MA, et al. Signal transduction cascades associated with oxidative stress in Alzheimer’s disease. J Alzheimers Dis. 2007;11(2):143–152. doi: 10.3233/jad-2007-11202. [DOI] [PubMed] [Google Scholar]
- Peterson C, Vanderklish P, Seubert P, Cotman C, Lynch G. Increased spectrin proteolysis in fibroblasts from aged and Alzheimer donors. Neurosci Lett. 1991;121(1–2):239–243. doi: 10.1016/0304-3940(91)90694-o. [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23(11):4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D. Peripheral biomarkers of oxidative damage in Alzheimer’s disease: the road ahead. Neurobiol Aging. 2005;26(5):581–583. doi: 10.1016/j.neurobiolaging.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59(6):972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Pratico D, VMYL, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12(15):1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, et al. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66(3):352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, DiMauro S, Hirano M. Human coenzyme Q10 deficiency. Neurochem Res. 2007;32(4–5):723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012 doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10(4):291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Shirendeb UP. Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington’s disease. Biochim Biophys Acta. 2011;1822(2):101–110. doi: 10.1016/j.bbadis.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: Implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich E. Über miliare Sklerosen der Hirnrinde bei seniler Atrophie. Jahrb Psychiat Neurol. 1898;17:208–216. [Google Scholar]
- Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24(7):915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6(4):246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Head E. Caspases as therapeutic targets in Alzheimer’s disease: is it time to “cut” to the chase? Int J Clin Exp Pathol. 2009;2(2):108–118. [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH. Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiol Dis. 2001a;8(6):1006–1016. doi: 10.1006/nbdi.2001.0449. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Head E, Su JH, Anderson AJ, Bahr BA, Cotman CW, et al. Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer’s disease. Am J Pathol. 2001b;158(1):189–198. doi: 10.1016/S0002-9440(10)63957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis. 2002;11(2):341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- Sandstrom PA, Tebbey PW, Van Cleave S, Buttke TM. Lipid hydroperoxides induce apoptosis in T cells displaying a HIV-associated glutathione peroxidase deficiency. J Biol Chem. 1994;269(2):798–801. [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997;336(17):1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Santa-Maria I, Smith MA, Perry G, Hernandez F, Avila J, Moreno FJ. Effect of quinones on microtubule polymerization: a link between oxidative stress and cytoskeletal alterations in Alzheimer’s disease. Biochim Biophys Acta. 2005;1740(3):472–480. doi: 10.1016/j.bbadis.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Santos RX, Correia SC, Wang X, Perry G, Smith MA, Moreira PI, et al. A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 2):S401–412. doi: 10.3233/JAD-2010-100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva AA, Borges MM, Madeira MD, Tavares MA, Paula-Barbosa MM. Mitochondrial abnormalities in cortical dendrites from patients with Alzheimer’s disease. J Submicrosc Cytol. 1985;17(3):459–464. [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68(5):2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Selznick LA, Holtzman DM, Han BH, Gokden M, Srinivasan AN, Johnson EM, Jr, et al. In situ immunodetection of neuronal caspase-3 activation in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(9):1020–1026. doi: 10.1097/00005072-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Senin U, Parnetti L, Barbagallo-Sangiorgi G, Bartorelli L, Bocola V, Capurso A, et al. Idebenone in senile dementia of Alzheimer type: a multicentre study. Arch Gerontol Geriatr. 1992;15(3):249–260. doi: 10.1016/0167-4943(92)90060-h. [DOI] [PubMed] [Google Scholar]
- Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. 2012;120(3):419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Sheu KF, Cooper AJ, Koike K, Koike M, Lindsay JG, Blass JP. Abnormality of the alpha-ketoglutarate dehydrogenase complex in fibroblasts from familial Alzheimer’s disease. Ann Neurol. 1994;35(3):312–318. doi: 10.1002/ana.410350311. [DOI] [PubMed] [Google Scholar]
- Shi C, Guo K, Yew DT, Yao Z, Forster EL, Wang H, et al. Effects of ageing and Alzheimer’s disease on mitochondrial function of human platelets. Exp Gerontol. 2008;43(6):589–594. doi: 10.1016/j.exger.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Shi Q, Xu H, Yu H, Zhang N, Ye Y, Estevez AG, et al. Inactivation and reactivation of the mitochondrial alpha-ketoglutarate dehydrogenase complex. J Biol Chem. 2011;286(20):17640–17648. doi: 10.1074/jbc.M110.203018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohama S. Apoptosis in Alzheimer’s disease--an update. Apoptosis. 2000;5(1):9–16. doi: 10.1023/a:1009625323388. [DOI] [PubMed] [Google Scholar]
- Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, et al. Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Hum Mol Genet. 2012;21(2):406–420. doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev N, Jouroukhin Y, Giladi E, Polyzoidou E, Grigoriadis NC, Rosenmann H, et al. NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis. 2009;34(2):381–388. doi: 10.1016/j.nbd.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Shiryaev N, Pikman R, Giladi E, Gozes I. Protection Against Tauopathy by the Drug Candidates NAP (Davunetide) and D-SAL: Biochemical, Cellular and Behavioral Aspects. Curr Pharm Des. 2011;17(25):2603–2612. doi: 10.2174/138161211797416093. [DOI] [PubMed] [Google Scholar]
- Silva DF, Esteves AR, Arduino DM, Oliveira CR, Cardoso SM. Amyloid-beta-induced mitochondrial dysfunction impairs the autophagic lysosomal pathway in a tubulin dependent pathway. J Alzheimers Dis. 2011a;26(3):565–581. doi: 10.3233/JAD-2011-110423. [DOI] [PubMed] [Google Scholar]
- Silva DF, Esteves AR, Oliveira CR, Cardoso SM. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer’s disease. Curr Alzheimer Res. 2011b;8(5):563–572. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Hyman BT. Functional alterations in Alzheimer’s disease: diminution of cytochrome oxidase in the hippocampal formation. J Neuropathol Exp Neurol. 1993;52(6):580–585. [PubMed] [Google Scholar]
- Singh M, Nam DT, Arseneault M, Ramassamy C. Role of by-products of lipid oxidation in Alzheimer’s disease brain: a focus on acrolein. J Alzheimers Dis. 2010;21(3):741–756. doi: 10.3233/JAD-2010-100405. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29(2):169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- Smale G, Nichols NR, Brady DR, Finch CE, Horton WE., Jr Evidence for apoptotic cell death in Alzheimer’s disease. Exp Neurol. 1995;133(2):225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer’s disease. Biochim Biophys Acta. 2000;1502(1):139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Bruck W, Jellinger K, et al. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Evidence for apoptotic cell death. Am J Pathol. 1999;155(5):1459–1466. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol. 2002;156(6):1051–1063. doi: 10.1083/jcb.200108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci U S A. 2011;108(31):12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wang X, Bonda D, Perry G, Smith M, Zhu X. Abnormal mitochondrial dynamics--a novel therapeutic target for Alzheimer’s disease? Mol Neurobiol. 2010;41(2–3):87–96. doi: 10.1007/s12035-009-8095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JH, Anderson AJ, Cummings BJ, Cotman CW. Immunohistochemical evidence for apoptosis in Alzheimer’s disease. Neuroreport. 1994;5(18):2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- Su JH, Deng G, Cotman CW. Bax protein expression is increased in Alzheimer’s brain: correlations with DNA damage, Bcl-2 expression, and brain pathology. J Neuropathol Exp Neurol. 1997;56(1):86–93. doi: 10.1097/00005072-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, et al. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J Neurochem. 1997;69(3):1161–1169. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Brown MR. Mitochondrial aging and dysfunction in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(3):407–410. doi: 10.1016/j.pnpbp.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55(4):576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Sultana R, Butterfield DA. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J Bioenerg Biomembr. 2009;41(5):441–446. doi: 10.1007/s10863-009-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Newman SF, Pierce WM, Cini C, Coccia R, et al. Redox proteomic analysis of carbonylated brain proteins in mild cognitive impairment and early Alzheimer’s disease. Antioxid Redox Signal. 2010;12(3):327–336. doi: 10.1089/ars.2009.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, et al. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18(2):323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- Swerdlow R, Marcus DM, Landman J, Harooni M, Freedman ML. Brain glucose and ketone body metabolism in patients with Alzheimer’s disease. Clin Res. 1989;37(2):461A. [Google Scholar]
- Swerdlow RH. Role and treatment of mitochondrial DNA-related mitochondrial dysfunction in sporadic neurodegenerative diseases. Curr Pharm Des. doi: 10.2174/138161211798072535. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Is aging part of Alzheimer’s disease, or is Alzheimer’s disease part of aging? Neurobiol Aging. 2007a;28(10):1465–1480. doi: 10.1016/j.neurobiolaging.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007b;85(15):3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Treating neurodegeneration by modifying mitochondria: potential solutions to a “complex” problem. Antioxid Redox Signal. 2007c;9(10):1591–1603. doi: 10.1089/ars.2007.1676. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondrial medicine and the neurodegenerative mitochondriopathies. Pharmaceuticals. 2009;2:150–167. doi: 10.3390/ph2030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Brain aging, Alzheimer’s disease, and mitochondria. Biochim Biophys Acta. 2011a;1812(12):1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria and Cell Bioenergetics: Increasingly Recognized Components and a Possible Etiologic Cause of Alzheimer’s Disease. Antioxid Redox Signal. 2011b doi: 10.1089/ars.2011.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Role and treatment of mitochondrial DNA-related mitochondrial dysfunction in sporadic neurodegenerative diseases. Curr Pharm Des. 2011c;17(31):3356–3373. doi: 10.2174/138161211798072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Kish SJ. Mitochondria in Alzheimer’s disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Chinnery PF, Turnbull DM, Lightowlers RN. Selective inhibition of mutant human mitochondrial DNA replication in vitro by peptide nucleic acids. Nat Genet. 1997;15(2):212–215. doi: 10.1038/ng0297-212. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thal LJ, Grundman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E, et al. Idebenone treatment fails to slow cognitive decline in Alzheimer’s disease. Neurology. 2003;61(11):1498–1502. doi: 10.1212/01.wnl.0000096376.03678.c1. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Torres LL, Quaglio NB, de Souza GT, Garcia RT, Dati LM, Moreira WL, et al. Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2011;26(1):59–68. doi: 10.3233/JAD-2011-110284. [DOI] [PubMed] [Google Scholar]
- Torroni A, Huoponen K, Francalacci P, Petrozzi M, Morelli L, Scozzari R, et al. Classification of European mtDNAs from an analysis of three European populations. Genetics. 1996;144(4):1835–1850. doi: 10.1093/genetics/144.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer PA, Borland MK. Differentiated Alzheimer’s disease transmitochondrial cybrid cell lines exhibit reduced organelle movement. Antioxid Redox Signal. 2005;7(9–10):1101–1109. doi: 10.1089/ars.2005.7.1101. [DOI] [PubMed] [Google Scholar]
- Troncoso JC, Sukhov RR, Kawas CH, Koliatsos VE. In situ labeling of dying cortical neurons in normal aging and in Alzheimer’s disease: correlations with senile plaques and disease progression. J Neuropathol Exp Neurol. 1996;55(11):1134–1142. doi: 10.1097/00005072-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, et al. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6(6):323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A, Johnson-Cadwell LI, Cirulli V, Yoon Y, Allan VJ, Rutter GA. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117(Pt 19):4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- Vitte J, Michel BF, Bongrand P, Gastaut JL. Oxidative stress level in circulating neutrophils is linked to neurodegenerative diseases. J Clin Immunol. 2004;24(6):683–692. doi: 10.1007/s10875-004-6243-4. [DOI] [PubMed] [Google Scholar]
- Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther. 2007;323(2):438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers -a decade of discovery. J Neurochem. 2007;101(5):1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008a;173(2):470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29(28):9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008b;105(49):19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T, Williams SL, Bacman SR, Moraes CT. Emerging therapeutic approaches to mitochondrial diseases. Dev Disabil Res Rev. 2010;16(2):219–229. doi: 10.1002/ddrr.109. [DOI] [PubMed] [Google Scholar]
- Westermann B. Nitric oxide links mitochondrial fission to Alzheimer’s disease. Sci Signal. 2009;2(69):pe29. doi: 10.1126/scisignal.269pe29. [DOI] [PubMed] [Google Scholar]
- Weyer G, Babej-Dolle RM, Hadler D, Hofmann S, Herrmann WM. A controlled study of 2 doses of idebenone in the treatment of Alzheimer’s disease. Neuropsychobiology. 1997;36(2):73–82. doi: 10.1159/000119366. [DOI] [PubMed] [Google Scholar]
- Wischik CM, Bentham P, Wischik DJ, Seng KM. Tau aggregation inhibitor (TAI) therapy with rember arrests disease progression in mild and moderate Alzheimer’s disease over 50 weeks. Azheimer’s and Dementia. 2008;4:T167. [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398(6727):513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Xie R, Nguyen S, McKeehan WL, Liu L. Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol. 2010;11:89. doi: 10.1186/1471-2121-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science. 1989;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- Yates CM, Butterworth J, Tennant MC, Gordon A. Enzyme activities in relation to pH and lactate in postmortem brain in Alzheimer-type and other dementias. J Neurochem. 1990;55(5):1624–1630. doi: 10.1111/j.1471-4159.1990.tb04948.x. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23(15):5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, et al. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171(1):87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Kumar A, Peterhoff C, Shapiro Kulnane L, Uchiyama Y, Lamb BT, et al. Autophagic vacuoles are enriched in amyloid precursor protein-secretase activities: implications for beta-amyloid peptide over-production and localization in Alzheimer’s disease. Int J Biochem Cell Biol. 2004;36(12):2531–2540. doi: 10.1016/j.biocel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hedskog L, Petersen CA, Winblad B, Ankarcrona M. Dimebon (latrepirdine) enhances mitochondrial function and protects neuronal cells from death. J Alzheimers Dis. 2010;21(2):389–402. doi: 10.3233/JAD-2010-100174. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 2004;3(4):219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]