Abstract
Alzheimer’s disease (AD) is the major cause of late-life brain failure. In the past 25 years, autosomal dominant forms of AD were found to be primariy attributable to mutations in one of two presenilins, polytopic proteins that contain the catalytic site of the γ-secretase protease that releases the amyloid beta (Aβ) peptide. Some familial AD is also due to mutations in the amyloid precursor protein (APP), but recently a mutation in APP was discovered that reduces Aβ generation and is protective against AD, further implicating amyloid metabolism. Prion-like seeding of amyloid fibrils and neurofibrillary tangles has been invoked to explain the stereotypical spread of AD within the brain. Treatment trials with anti-Aβ antibodies have shown target engagement, if not significant treatment effects. Attention is increasingly focused on presymptomatic intervention, because Aβ mismetabolism begins up to 25 years before symptoms begin. AD trials deriving from new biological information involve extraordinary international collaboration and may hold the best hope for success in the fight against AD.
Keywords: dementia, neurodegeneration, cognitive decline, memory disorder, amyloidosis
ALZHEIMER’S DISEASE IS AN EPIDEMIC
The demographics of the post–World War II “baby boomer” generation and the increased incidence and prevalence of dementia in the seventh decade and beyond have made Alzheimer’s disease (AD) a major health care threat. As discussed below, research is now focusing on prevention as well as symptomatic treatment. In the United States, 40% of people ages 85 years and above are cognitively impaired (1); AD pathology probably contributes to 75%–80% of these cases. Thus, the discovery of compounds to slow the development and progression of AD is a major imperative for federal agencies and pharmaceutical companies.
In the 1970s, researchers in Great Britain discovered that the AD brain is deficient in acetylcholine (2), leading to the development of a strategy using cholinesterase inhibition. Although this strategy forms the basis for the main medications to treat AD today, it took almost 20 years for the first cholinesterase inhibitor drug to be approved. In the 1990s, the increased availability of neuroimaging and the advances in genetics led to discovery of pathogenic mutations in three genes that produce early-onset autosomal dominant familial AD (EOFAD) (3). The convergence of all three EOFAD gene products on the biosynthetic pathway underlying amyloid precursor protein (APP) metabolism affirmed the amyloid hypothesis of AD originally articulated by Glenner, Masters and Beyreuther (4, 5). These findings stimulated broad therapeutic efforts to suppress amyloid beta (Aβ) generation or to remove amyloid from the brain. Brain amyloid imaging (BAI), described first in 2004, confirmed that AD pathology emerges more than a decade prior to any clinical symptoms, offering a wide opportunity for interventions that would either delay or prevent onset of clinical symptoms. In addition to the EOFAD mutations, genetic factors that confer increased risk have been identified, including the most powerful common genetic risk factor, the APOE ε4 allele of apolipoprotein E (APOE), which contributes to approximately 40%–50% of cases of AD (6). The molecular pathogenesis of AD is defined by this and other identified genes; however, no other genetic factor has an effect on AD risk to rival that associated with APOE ε4.
GAMMA-SECRETASE AND THE RETROMER REPRESENT POTENTIAL THERAPEUTIC TARGETS IN DEVELOPING DRUGS TO TREAT OR PREVENT ALZHEIMER’S DISEASE
γ-Secretase is the high-molecular-weight complex of four proteins [presenilin 1 or 2 (PS1, PS2), nicastrin, APH1, PEN2] that releases Aβ from APP and specifies the length of the Aβ peptide as either 40 or 42 amino acids. Aβ42 initiates Aβ deposition in all forms of AD. New data indicate that γ-secretase can also perform an exopeptidase function, trimming the initial ε cleavage product at its carboxyl terminus and generating various Aβ carboxyl termini (7) (Figure 1). In this proposed model, excess long Aβ species occur as a result of inadequate trimming or “processivity,” a concept consistent with data indicating that pathogenic PS1 mutants lead to reduction in γ-secretase catalytic activity (8). In two elegant in vitro γ-secretase reconstitution papers (7, 9), Wolfe and colleagues clarified the relationships between mutant PS1 action and altered processivity and between membrane lipid composition and processivity. Notably, modulation of γ-secretase processivity by cholesterol may explain at least some of the recognized but poorly understood role(s) of cholesterol in the etiology of AD (for a comprehensive review of the role of cholesterol in AD, see References 10, 11).
Figure 1.
Polyacrylamide gel electrophoresis reveals a family of amyloid beta (Aβ) peptides generated by the processivity (trimming) function of γ-secretase following ε cleavage. (a) Effects of presenilin 1 (PS1) mutations (L166P, A246E, L286V, G384A, ΔE9) on γ-secretase processivity. Relative intensities of bands correspond to the differential generation of indicated species. (b) Schematized version of the data in panel a, illustrating the cleavage sites in the transmembrane domain of the amyloid precursor protein that specifies generation of each species. WT, wild type. Reprinted with permission from Reference 7.
Protein trafficking is a key issue in AD pathogenesis, as exemplified by the multiple links between the vacuolar protein sorting (Vps) family of proteins and the risk of AD. Genetic linkage to SORL1 was reported in 2007 (12), and pathogenic dominant mutations in SORL1 were reported recently (12, 13). Lane et al. (14) showed that another Vps protein, SorCS1, was linked functionally to the AD phenotype as well as to the phenotype associated with type 2 diabetes insulin resistance in a pathway that converges on the retromer, the protein complex responsible for retrograde transport of cargos from the endosomal system backward to the trans-Golgi network (15). Willnow and colleagues (16) showed that SorL1 requires another retromer component, Vps26, to control Aβ metabolism, further strengthening the connection between the retromer and AD. These observations suggest that a novel pathway may underlie links between type 2 diabetes and AD and, as proposed by Small & Gandy (17), that the SorL1 and SorCS1 links to AD are mediated by the retromer (Figure 2).
Figure 2.
Schematized pathway showing the sorting of amyloid precursor protein (APP) (red dumbbell ) and BACE (β-secretase) (blue dumbbell ) into post-trans-Golgi network (TGN) compartments. The retromer retrieves endosomal proteins and conveys them to the TGN. Retromer deficiency leads to excess retention in the endosome and, theoretically, excess generation of Aβ because of enhanced cleavage of APP by BACE or (not shown) cleavage of APP carboxyl terminal fragments by γ-secretase. Reprinted with permission from Reference 17.
THE DIAGNOSIS OF ALZHEIMER’S BECOMES BIOMARKER BASED AND BRAIN AMYLOID IMAGING BECOMES COMMERCIALLY AVAILABLE IN 2012
For decades, physicians had often stated that the diagnosis of AD could not be confirmed prior to brain biopsy or autopsy. Yet, in 2004, the first nuclear medicine ligand for the visualization of brain amyloid was revealed (18) (Figure 3). Klunk and colleagues (18), working at the University of Pittsburgh, modified the structure of thioflavine T so that the new compounds—termed Pittsburgh compounds A and B (PiA and PiB)—incorporated the positron emitter 11C, crossed the blood-brain barrier, and selectively bound to brain amyloid fibrils. Thus, the brain could be imaged using standard nuclear medicine positron emission tomography (PET) detection technology to reveal the burden of brain fibrillar amyloid. The performance of PiB was superior to that of PiA, heralding the era of PiB and brain amyloid imaging (BAI).
Figure 3.
[11C] Pittsburgh compound B–based positron emission tomography reveals high fibrillar amyloid burden in the brain of a patient with Alzheimer’s disease (red signal, top row) as compared with the brain of an age-matched control subject (lower row). Reprinted with permission from Reference 18.
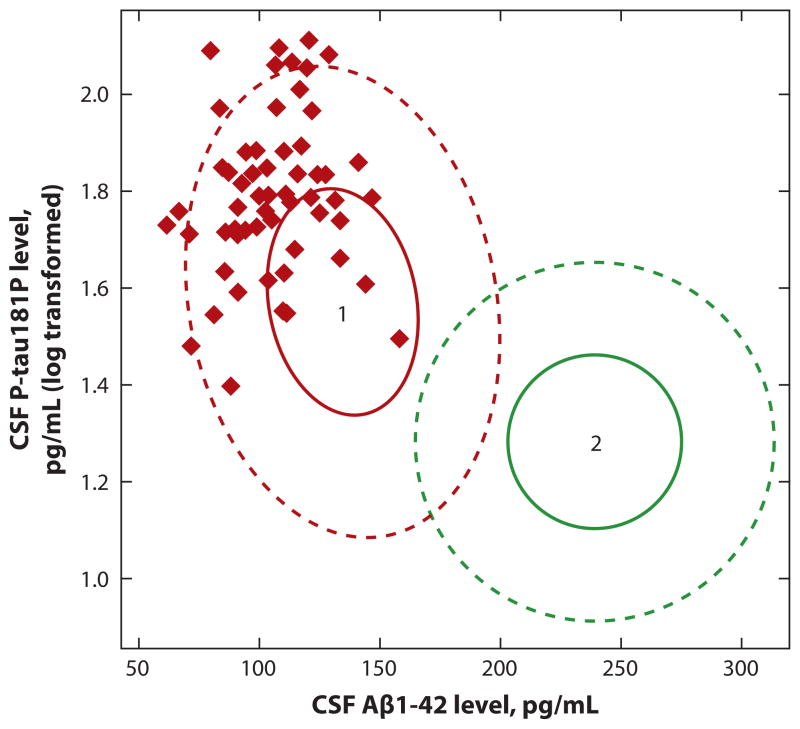
PiB scanning, as amyloid scanning with [11C] PiB became known, was rapidly embraced worldwide. The large multi-institutional natural history study known as the National Institute on Aging Alzheimer’s Disease Neuroimaging Initiative (19) also embraced the new technology. From the beginning, this study had included a biomarker component; the invention of BAI made it possible to confirm that both BAI and cerebrospinal fluid (CSF) Aβ peptide 42 (Aβ42) could identify subjects with cerebral amyloidosis (20). BAI with 11C-PiB reveals elevated 11C signals from involved cortical regions in subjects with amyloidosis, whereas CSF Aβ42 falls as amyloidosis develops, presumably representing depletion of extracellular Aβ42 (20). By 2010, the combination of increased BAI signal, low CSF Aβ42, and high CSF tau in a subject with dementia was recognized as diagnostic of AD, and patients with mild cognitive impairment and the appropriate BAI and CSF profiles could be predicted to progress to frank dementia within a period of ~5 years (21) (Figure 4). Using these biomarker data, clinical trials now can be enriched for subjects who are impaired and have a high probability of progressing from mild cognitive impairment to mild AD within 5 years. Exceptions to the reliability of amyloid imaging do occur, although they are rare (see 22–24). One explanation is that amyloid ligands fail to recognize the toxic Aβ oligomers that cause neuronal dysfunction because these oligomers lack the fibrillar structure required for PiB binding (24–26).
Figure 4.
A plot of cerebrospinal fluid (CSF) levels of phospho-tau (P-tau) ( y axis) versus levels of amyloid beta (Aβ)1–42 reveals that all subjects in the cohort destined to develop Alzheimer’s disease (red diamonds in area 1) within 5 years showed a pattern of high CSF P-tau and low CSF Aβ1–42 and were excluded from areas of the plot defined by low CSF Aβ1–42 levels or low P-tau levels (defined by area 2 inside green circle). Reprinted with permission from Reference 21.
A number of amyloid imaging ligands have now been developed by several pharmaceutical companies. All commercial ligands employ 18F; the short half-life of 11C precludes its use for commercial applications. Only PET scanners with immediate access to a cyclotron can employ binding agents with the very short-lived 11C isotope. Florbetapir, a compound developed by Avid Pharmaceuticals (27) (Figure 5) and subsequently acquired by Eli Lilly and Company, was first to gain approval from the US Food and Drug Administration (FDA) for commercial use. Related compounds [flutemetamol, florbetaben (Bayer), 18F PiB (GE Healthcare)] are in the pipeline and are expected to be approved during 2012 and 2013. Immediately after approval of florbetapir (trade name Amyvid®), the Center for Medicaid and Medicare Services announced that BAI would not be covered by Medicare, so as of this writing, BAI is available on a cash-only basis.
Figure 5.

Amyvid® (18F-florbetapir)-based positron emission tomography images of the brains of (a) a subject with Alzheimer’s disease and (b) an age-matched control. The intense signal from the cerebral cortex indicates the presence of a heavy burden of fibrillar amyloid. Reprinted from http://www.amyvid.com with the permission of Eli Lilly and Company.
Another issue has also arisen: A potential insurer evaluating an applicant for a long-term care insurance policy is unlikely to approve coverage for individuals with neuroimaging or CSF biomarkers indicating cerebral amyloidosis or presymptomatic AD. A question that frequently arises is, “What exactly does a positive brain amyloid scan mean for cognitively intact subjects?” In autopsies of cognitively normal individuals over age 85, at least 25% have sufficient AD pathology (that is, adequate numbers of amyloid plaques and neurofibrillary tangles) to meet diagnostic criteria for AD (28). The “resistance” of functional cognition to the presence of AD pathology is termed cognitive reserve (29). The actual underpinnings of cognitive reserve are believed to include premorbid intelligence, level of education, complexity of vocation, and other factors that are currently impossible to measure. As a result, both health professionals and consumers should keep in mind that—as far as is known to date—a positive brain amyloid scan does not portend the inevitable appearance of clinical dementia.
CSF and neuroimaging biomarkers provide objective molecular indications of AD pathogenesis. The research criteria for diagnosing AD-associated syndromes are currently in evolution (30–33). Investigators have proposed that asymptomatic subjects with low CSF Aβ42 or high amyloid signal on BAI be designated with “asymptomatic cerebral amyloidosis.” Subjects with low CSF Aβ42 or high BAI signal plus high CSF tau, but still with mild or no clinical symptoms, would be designated with “presymptomatic AD,” whereas the clinical picture of what used to be called mild cognitive impairment plus low CSF Aβ42, or high BAI retention, plus high CSF tau would be designated with “incipient or prodromal AD.”
META-ANALYSES AND GENOME-WIDE ASSOCIATION STUDIES EXPAND THE LIST OF GENES CONFIRMED TO INCREASE THE RISK FOR SPORADIC ALZHEIMER’S DISEASE
For a decade or more, the only genes associated reliably with AD were the APP genes, PS1 and PS2, and the risk allele APOE ε4 (4). The list of confirmed risk genes now includes nine genes besides APOE ε4, although APOE ε4 remains the most important and powerful risk polymorphism (3). The protein products of the APP, PS1, and PS2 genes lie clearly on the pathway to Aβ biogenesis (http://www.alzgene.org) (34), but the roles of APOE ε4 and the other linked genes in specifying increased risk are more difficult to pin down. APOE ε4, present in approximately half of all AD patients, appears to alter Aβ conformation and/or clearance (35–39).
The “top 10” genes in AD (other than APOE ε4) include protein-sorting genes (PICALM, BIN1), the amyloid binding protein apolipoprotein J (CLU), a complement receptor (CR1), and an adaptor gene (CD2AP), among others. The conventional model for the disease begins with amyloidosis and is followed by inflammation and tauopathy. However, each of the molecules identified by genetic searches must be assessed for its role in the disease pathologic cascade. One can envision that initiating events lead to amyloid mismetabolism and, either serially or in parallel, precipitate other cascades that are facilitated or retarded by the allelic variations and subsequent altered protein activity.
Two new strategies hold promise for further elucidation of the molecular pathogenesis of AD, especially sporadic AD. The first involves whole-genome or exome sequencing and network systems analysis of the data to determine the occurrence of spontaneous mutations that perturb relevant network components along a neurodegenerative pathway (40). These analyses take multiple genes and pathways into account and, when analyzed with the proper informatics, predict common potentially pathogenic nodal molecules that may not be obvious otherwise. However logical this strategy may be, its usefulness in AD remains to be determined. Current costs of such analyses are also formidable, but the expense will decline over time.
The other strategy for elucidating novel pathways for AD pathogenesis involves neuronal differentiation of fibroblasts or induced pluripotent stem cells from patients with familial or sporadic AD (41–43). These mature human “AD neurons” can then be characterized at the molecular and cellular levels. The initial template for the eventual study of mature neurons from sporadic human AD subjects has been established using fibroblasts from patients with PS1 mutations. Qiang et al. (42) showed that mature neurons from patients with mutant PS1 generate a typical pathogenic Aβ42/40 profile while also displaying unusually large endosomes (Figure 6). This subcellular phenotype, if confirmed, would provoke a search to determine whether abnormal endosomal size is a consistent feature of all forms of AD. If so, great attention might well turn toward how endosomal size is regulated in order to identify potential sources of insights into pathogenesis as well as novel therapeutic intentions.
Figure 6.

Differentiation of skin fibroblasts (UND, panel a) into neurons with the biochemical and physiological properties of cerebral cortical neurons reveals an apparent anomaly in endosomal morphology in the neurons from a subject with familial Alzheimer’s disease due to a presenilin 1 mutation. Differentiation of neurons (n) from the same subject (FAD, panel b) caused an exaggerated amyloid beta (Aβ)42/40 as compared with either the undifferentiated fibroblasts ( f ) or with Aβ42/40 generated by neurons differentiated from a control subject (not shown). Reprinted with permission from Reference 42. APP, amyloid precursor protein; MPR, mannose-6-phosphate receptor.
THE DIAGNOSIS OF CHRONIC TRAUMATIC ENCEPHALOPATHY IS COINED TO EXPAND THE DEFINITION OF DEMENTIA PUGILISTICA TO INCLUDE SPORTS PLAYERS AND SOLDIERS
The quest to identify environmental influences associated with AD risk has brought heightened awareness to the importance of traumatic brain injury (TBI). Across several populations, a frequently identified acquired risk for AD is a history of a severe head injury with extended loss of consciousness (30 min or more) typically associated with a fall or an automobile crash (44, 45). Evidence indicates that repetitive mild TBI leads to neuropathology that is distinct from that of AD. The classical example has been boxer’s dementia (dementia pugilistica). Several reports have shown clearly that the identical pathology is associated with contact sports such as football (46), professional wrestling (47), and soccer (48) and with exposure to battlefield blasts associated with improvised explosive devices (49) that have been so common in the Middle East wars in Iraq and Afghanistan. Because an indistinguishable tauopathy is associated with all these conditions, the term chronic traumatic encephalopathy (CTE) has been coined to indicate the common neuropathological picture (46). Mild, repetitive TBI—rather than association with any sport in particular—is the most important causative factor.
As suggested above, a type of dichotomy has developed: Whereas a single severe TBI (with loss of consciousness more than 30 min) increases the risk for typical AD, repetitive mild TBI increases the risk for CTE (44). Interestingly, subjects carrying one or two APOE ε4 alleles are at risk for both post-traumatic AD and CTE (50, 51), despite the fact that APOE ε4 alleles in AD are associated with the amyloid pathology and not with the tau pathology (52). Furthermore, APOE ε4 does not increase the risk for frontotemporal dementia (53), progressive supranuclear palsy, or any of a number of other tauopathies. The best model for why APOE ε4 alleles increase the risk for dementia pugilistica/CTE holds that bursts of increased APP synthesis and subsequent acute Aβ deposition accompany each TBI and drive the tau pathology and that Aβ accumulation (or slowed removal) is abetted by the APOE ε4 allele (44). Evidence for this derives from the observation that Aβ deposits are observed in traumatized brain within hours of acute TBI (Figure 7) (54). The “pure tauopathy without amyloidosis” pathological picture in CTE may be attributable, at least in part, to the fact that brains from CTE patients are usually autopsied years following the repetitive traumas and any amyloid produced in response to the trauma may have been removed by normal amyloid clearance mechanisms, leaving only the insoluble tauopathy, albeit in a different distribution than the tauopathy of AD.
Figure 7.

Anti–amyloid beta (Aβ) immunocytochemistry reveals acute deposition in amputated temporal lobes (a) at 2 h or (b) at 16 h following severe traumatic brain injury. Reprinted with permission from Reference 54.
The “CTE crisis” has been especially highlighted by its discovery in professional football players and, more recently, in a college student. However, a data-driven estimate of the size of the CTE problem in relation to sports and the military is lacking (55). The decades-long latent period between the TBIs and the discovery/appearance of neuropathology complicates attempts to generate these data. Both the Department of Defense and the National Football League are establishing centers for CTE care and research. There are at least some instances in which a significantly shorter period of sports participation during childhood and/or adolescence can lead to clinically important CTE in young adults. Even if this is a vanishingly rare event, focused research should identify genetic risk factors for CTE that will enable development of screening programs to identify those at highest risk, so that such individuals may be counseled to avoid elective sources of TBI. Because current findings are from selected cases (and are not population derived), it is currently difficult to estimate the size of the problem.
THE SEEDING, OR TEMPLATING, “PRION-LIKE” BEHAVIOR OF ALZHEIMER’S PROTEIN AGGREGATES MAY EXPLAIN THE STEREOTYPED PROGRESSION OF PATHOLOGY THROUGH THE BRAIN
In three independent studies described in a series of reports that were widely covered by the media, Tolnay and colleagues (56), Duff and colleagues (57), and Hyman and colleagues (58) found that aggregated protein pathology can propagate itself from neuron to neuron and from brain region to brain region. This phenomenon was proposed to explain the sequential progression of AD pathology from the perirhinal/entorhinal cortex to the hippocampus and beyond. For decades, neuroscientists have hypothesized that neuronal contact along specific projection pathways underlies the progression of pathological (and clinical) findings in AD, but there was no evidence of any mechanism by which this might occur. This phenomenon, alternatively known as seeding or templating, was described originally for prions, in which the misfolded molecules of prion protein instruct the wild-type molecules to adopt the pathogenic conformation (59). This phenomenon may explain the spread of amyloidosis, tauopathy, and synucleinopathy from brain region to brain region, which likely moves along known neuroanatomical tracts. Although some connected tracts appear vulnerable and conducive, others are not. The key difference between typical prion diseases (e.g., Creutzfeldt-Jakob disease) and these other protein-aggregation diseases is that the former can spread from person to person.
Even though the unexpected nature of this trans-synaptic conveyance of protein aggregates composed the initial “headline,” no conventional subcellular mechanism has been proposed to underlie this “spread” phenomenon. Exosomal and/or autophagic secretion of macromolecular assemblies is one plausible possibility. In addition to this proposition of exciting, unexpected cell biology, elucidation of such a trans-synaptic transmission mechanism may well provide a novel therapeutic opportunity to interrupt the process and arrest disease progression, either in symptomatic subjects or presymptomatic individuals.
TO DATE, ALZHEIMER’S TREATMENT TRIALS HAVE YIELDED NO CONSISTENT ROBUST BENEFITS
The first study to prove target engagement by a disease-modifying drug in living humans was reported by Rinne and colleagues in 2010 (60). In a 78-week trial of infusion of either a control solution or the monoclonal anti-Aβ antibody bapineuzumab, a significant reduction in the burden of fibrillar cerebral amyloid was demonstrated in a subgroup of subjects, although the change was relatively small in magnitude (approximately 15%–25%). This was the first example in any trial in which amyloid burden progressed in the placebo group while minimally regressing in the treatment group. Notably, although this trial showed progressive reduction of brain amyloid burden by bapineuzumab over 18 months, there was no cognitive benefit overall. Further readouts in the fall of 2012 confirmed the absence of clinical benefit.
There are several possible explanations for this failure (61). First, a 25% reduction may not be of sufficient magnitude, or perhaps all the amyloid must be removed to rid the brain of the reserve of amyloid molecules in equilibrium with the extracellular fluid. Also, amyloid imaging fails to visualize Aβ oligomers well (13); thus, their contribution and response to therapy remain undocumentable. The favored (though likely not sole) explanation is that perhaps prevention of amyloidosis (i.e., primary prevention) will be required prior to development of the tau pathology, which correlates more seriously (along with synapse numbers) with level of cognition (62). Mouse model studies in the late 1990s showed that vaccination prior to age at onset of plaque pathology could prevent all amyloidosis, whereas initiation of vaccination following onset of pathology could achieve a maximum of only 50% reduction (63).
The challenges of the financial and time investments associated with prevention trials have given rise to two efforts in which presymptomatic carriers of mutant presenilin alleles will begin receiving passive immunotherapy prior to the expected age of onset based on specific mutation and family histories. One is the Alzheimer Prevention Initiative, which is headed by Eric Reiman and Pierre Tariot at Sun City Research Institute and focuses on a large kindred of mutant PS1 carriers from Colombia. This initiative has announced its intent to use crenezumab for AD prophylaxis in the Colombian PS1 FAD kindred. The other is the Dominantly Inherited Alzheimer Network, which is sponsored by the National Institute on Aging (NIA) and headed by John Morris and Randall Bateman at Washington University in St. Louis. This network focuses on other PS1 mutation carriers in the United States and worldwide, though it has not yet announced any intervention trials.
Individuals carrying the pathogenic mutation show biomarker changes in CSF and neuroimaging that begin as many as 25 years before clinical symptoms appear (64). Thus, any prevention trial may have to run for many years before any separation between the cognitive declines of the reference and treatment groups can be resolved. It is worth recalling that mutant PS1-related AD and typical sporadic AD may arrive at the same pathological phenotype via entirely distinct pathways. For this reason, despite the issues of convenience and economics that attract researchers to use mutant PS1-related familial AD patients or presymptomatic subjects as surrogates for typical sporadic AD, these cannot be considered to be identical diseases. Indeed, EOFAD due to presenilin mutations appears to cause a relative increase in the biosynthetic limb of the Aβ life cycle (4), whereas APOE ε4, which is present in approximately half of all AD patients, appears to alter Aβ conformation and/or clearance (35–39). Furthermore, all the mutant PS1 EOFAD cases appear to fill the basal ganglia with diffuse amyloid plaque prior to deposition in the posterior cingulate and frontal cortices, which are the first regions of deposition in sporadic AD (93).
WHICH TRIALS DO PATIENTS WANT TO KNOW ABOUT?
Bapineuzumab, Solaneuzumab, Crenezumab, and Similar Biologics
Bapineuzumab and solanezumab are the anti-Aβ antibodies that are farthest along in clinical trials. As mentioned above, the 18-month outcomes revealed a modest reduction in brain fibrillar amyloid burden but no effect on cognition. In a post hoc analysis (65, 66), stratification by APOE genotype of the cognitive outcomes in the bapineuzumab trial subjects after 18 months of infusions showed that the cognitive outcomes in subjects not harboring APOE ε4 alleles were more promising than were the outcomes in those who were APOE ε4 carriers. However, bapineuzumab recently failed in several late-stage trials, and its future use is uncertain, at least in symptomatic patients. Another monoclonal antibody, solanezumab, was recently shown to be ineffective on all outcome targets, but when two separate solanezumab trials were pooled, a statistically significant benefit was observed in mild but not moderate AD. This result further indicates that earlier intervention may have greater clinical effects. As discussed above, crenezumab is being used in presymptomatic treatment trials of Colombian mutant PS1 kindred.
In addition to straightforward Aβ vaccines and anti-Aβ monoclonals, two related agents are under study. A small, randomized clinical trial (67) reported that intravenous immunoglobulin (IVIG), a blood product derived from thousands of donors, stabilized cognitive function in AD. The experience with using IVIG over the ensuing years led to the initiation of two IVIG trials: a study of 300 subjects in a trial administered collaboratively by the NIA Alzheimer’s Disease Cooperative Study Group and Baxter Pharmaceuticals (the producer of one of the most widely used IVIG preparations, known as Gammagard®) and a second trial of 400 subjects that Baxter is conducting on its own at different sites. Recent preliminary reports indicate that subjects with mild AD receiving IVIG remained clinically stable for at least 3 years (68). The “active ingredient” in IVIG has been proposed to be naturally occurring anti-Aβ oligomer antibodies (69), although other potentially active IVIG ingredients are also receiving attention (70). The second special case focuses on antibodies against non-Aβ antigens that are receiving increased attention as targets for AD therapeutics. The most promising of these is an antibody directed against β-secretase [properly known as BACE (β-APP site cleaving enzyme)], the rate-limiting enzyme in Aβ biogenesis (71). Anti-ankyrin antibodies have been recently reported to be successful in mouse models (72).
Secretase Modulators
Clinical trials in various stages focus on the evaluation of β- and γ-secretase modulators and inhibitors. A recent discovery that an APP mutation that inhibits BACE cleavage may be protective against AD will undoubtedly fuel enthusiasm for BACE inhibitors (73) as well as strengthen the hypothesis that amyloid mis-metabolism is a primary cause of AD. Development of small-molecule BACE inhibitors has been slow, owing to the challenge of engineering an active, nontoxic drug to penetrate the blood-brain barrier, which, in turn, is due in part to the large size of the cavity containing the catalytic site of the enzyme.
The failure of the Lilly γ-secretase inhibitor semagacestat has reoriented researchers interested in γ-secretase metabolism to focus on the development of γ-secretase modulators. Such drugs would shift the cleavage site, leaving total enzyme catalytic efficiency intact. This also avoids the long-feared risk that interfering with the γ-secretase inhibitor in the Notch pathway would ultimately thwart that strategy by causing intolerable side effects. Greengard’s (74) recent discovery of a γ-secretase activating protein and its role as the target for Gleevec modulation of Aβ generation (75) have also provided a new Notch-sparing therapeutic opportunity.
Bexarotene
In 2011, Landreth and colleagues (76) reported that a peroxisome proliferator activated receptor gamma (PPAR-γ) modulator (already FDA-approved for treatment of cutaneous T cell lymphoma) can reduce levels of human Aβ in the brains of APP transgenic mice by 50% in the short span of 72 h. However, as of this writing, the effect of bexarotene in APP transgenic mice has not been confirmed, and human clinical trials are still on the drawing board. Recent editorials have warned against any premature off-label use of bexarotene for AD (94).
Resveratrol and Other Phenols
A review focused on AD cannot do justice to the enormous body of research available on the topic of resveratrol, the proposed active ingredient responsible for the putative salutary benefits of red wine, which has extended life in a number of laboratory species and benefitted behavioral function in APP transgenic mice (77). Currently under way is an NIA AD Cooperative Study Group clinical trial that employs escalating doses never before tested. This high-dose resveratrol trial was designed to achieve brain concentrations of the drug that are predicted to be required to match levels that generate the benefits observed in animals.
Various preparations labeled with the name resveratrol can be purchased at pharmacies and health food stores, making resveratrol a very popular self-prescribed agent for those seeking to treat or to reduce their risk for AD. This situation closely parallels that encountered by one of us (S.T.DeK.) in the assessment of Ginkgo biloba (78). As with Gingko biloba, the contents of these over-the-counter preparations cannot be assured, and the extraction and formulation of high-quality material are required for proper clinical trials. Assessing the components of these natural products for something that would interfere with prescription medications is always recommended. For example, with AD, huperzine, a Chinese tea remedy used to treat memory loss, contains anticholineresterase activity. As such, huperzine should not be taken with prescription cholinesterase inhibitors such as donepezil, galantamine, or rivastigmine that are commonly prescribed in AD. Although huperzine has been studied in formal clinical trials, it has not been found to be successful.
Dimebon (Latrepirdine)
A recent AD trial involving the drug dimebon (trade name latrepirdine) produced dramatic results. Dimebon caused improvement in cognitive function in rats with basal forebrain lesions that deprived the cortex of choline acetyltransferase, mimicking the cholinergic deficit in AD (79). Using the resulting data, a team of US AD researchers oversaw a randomized clinical trial of latrepirdine in Russia that was dramatically successful (80). However, when the trials were repeated in the United States, they all failed.
New research suggests that latrepirdine could arrest progression of a mouse model of very aggressive AD pathology, apparently by activating autophagy (81). In these studies (from the laboratory of one of us, S.G.), latrepirdine also stimulated clearance of synuclein, a known substrate for autophagy (82). The association of latrepirdine to activation of autophagy provides a plausible connection to a growing body of literature on the role of autophagy in neurodegenerative diseases and the therapeutic potential for autophagy activators. Regardless of the fate of latrepirdine, there is substantial enthusiasm for bringing autophagy activators into clinical trials for neurodegenerative diseases, and we predict that this will be an important emerging area in the near future.
Diet and Lifestyle
One of the most common questions from lay audiences focuses on the role of diet and lifestyle in the risk for AD. The best answer is the aphorism “what’s good for the heart is good for the head.” Control of cardiovascular risk factors (obesity, hypertension, diabetes, metabolic syndrome, hypercholesterolemia) will lead to fewer cerebrovascular events, which, in turn, will lead to a reduction in both vascular dementia and the poorly understood contribution of vasculopathy to AD (83). Some evidence suggests that dementia progression rate is reduced when both vascular dementia and AD, a common comorbidity, are present (84). The meaningful benefits of souvenaid, a “medical food” being marketed for AD, remain uncertain (85). However, as with heart disease, much of the risk for AD is set through midlife, as hypercholesterolemia in middle age is associated with increased risk of AD, but hypercholesterolemia in late life is not.
The best-established piece of the diet/lifestyle puzzle in terms of AD benefit is physical exercise. In randomized trials, protocols consisting of 30 min of vigorous exercise or resistance training (i.e., weightlifting) three times per week have shown convincing reduction in the rate of cognitive decline in AD subjects (86, 87). Physical exercise shows consistent benefits in mouse models of AD (88) and has been associated with induction of autophagy and neurogenesis (89, 90). The elucidation of the molecular basis for exercise benefits is an area of interest and dovetails with the autophagy narrative as well as ongoing human trials of neurotrophin-producing brain implants that have shown promise in nonhuman primates (91). Again, good health habits in midlife appear to be protective against AD risk in late life.
THE FOCUS OF ALZHEIMER’S DISEASE RESEARCH SHIFTS TOWARD PREVENTION
In summary, the fields of AD diagnosis, pathology, and genetics have made enormous strides in the 20 years since the first AD gene was discovered. The rapid progress in modeling and preventing disease in mouse models has been slow to translate into therapies because of the combination of the slow progression of the disease and the economic factors associated with AD trials. Disease-modifying trials for AD (i.e., trials seeking the end point of slowed progression of cognitive decline) require large numbers of subjects (hundreds), long trial times (18–24 months minimum), and extraordinary financial resources (~$50 milion per drug for phases I and II). Pharmaceutical companies can be expected to be skeptical until the first successful disease-modifying drug trial is reported.
In addition, prevention trials in sporadic AD will be even longer and more expensive (62, 92), requiring careful and continuous follow up over a decade or more. The interim plan, while the design of sporadic AD trials is being perfected, focuses on affordable trials in highly selected populations (i.e., the presymptomatic carriers of PS1 mutations in the Colombian cohorts discussed above) (88, 124). A challenge of eliminating AD by 2025 has been issued by one of the AD advocacy groups. Such a goal is well meaning and likely essential to mobilizing the federal resources required for future studies. A more likely, yet optimistic, scenario is that we could have a measurable, statistically significant (but perhaps not meaningful) delay in disease onset in mutant PS1 carriers within 5–10 years. A more conservative aim is for intervention before any biochemical change is detectable. The new data indicating that Aβ changes and amyloid plaque deposition precede clinical onset by 25 years would mandate that primary prevention trials must begin 25–30 years before cognitive changes occur. Establishment of the definitive cognitive endpoints would require that neuropsychological assessments continue until subjects had attained an age at least 1.5 standard deviations beyond the age at onset. Thus, 10–20 years may pass before we have a definitive result. At that point, well-informed prevention trials in sporadic AD populations can begin in earnest. These predictions are often interpreted as pessimistic. Both authors of this review have confidence that AD is preventable and that researchers are on the right path. The main questions to contemplate remain: When might we reasonably see the first clinically meaningful benefit of a successful disease-modifying therapy? How much economic and emotional havoc will AD have wrought between now and then?
Acknowledgments
S.G. gratefully acknowledges the support of the Cure Alzheimer’s Fund, National Institute of Neurological Diseases and Stroke grant R01 NS075685, VA MERIT Review grant 5I01BX000348, Amicus Therapeutics, and Baxter Pharmaceuticals. S.G. is also supported by National Institute on Aging grant P50 AG05138 to Mary Sano, PhD. S.T.DeK. gratefully acknowledges consultation for or research support from NIA, Elan, Novartis, Janssen, Pfizer, Baxter, Myriad, Neurochem, and GlaxoSmithKline in the past 5 years. S.T.DeK is also supported by NIA grants AG05133 to Oscar Lopez and AG025204 to William Klunk, both at the University of Pittsburgh.
Footnotes
DISCLOSURE STATEMENT
Within the past five years, S.G. has received personal honoraria for advisory service to the Pfizer/J&J Alzheimer’s Immunotherapy Initiative, Diagenic, Amicus Therapeutics, Balance Therapeutics, and Baxter Pharmaceuticals, and he has received research grants from Amicus Therapeutics and Baxter Pharmaceuticals. Within the past five years, S.T.DeK. has received personal honoraria for advisory service to Pfizer, Merck, Elan/Wyeth, Novartis, Lilly, Janssen, Helicon Therapeutics, Genzyme, Astra Zeneca, Abbott, Baxter, Daichi, Psychogenics, Myriad, Servier, and Wyeth.
Contributor Information
Sam Gandy, Email: samuel.gandy@mssm.edu.
Steven T. DeKosky, Email: dekosky@virginia.edu.
LITERATURE CITED
- 1.Yaffe K, Middleton LE, Lui LY, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–36. doi: 10.1001/archneurol.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen DM, Smith CB, Davison AN. Molecular changes in senile dementia. Brain. 1973;96:849–56. doi: 10.1093/brain/96.4.849. [DOI] [PubMed] [Google Scholar]
- 3.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–81. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Glenner GG. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts) N Engl J Med. 1980;302:1333–43. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- 5.Masters CL, Simms G, Weinman NA, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–23. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 7.Quintero-Monzon O, Martin MM, Fernandez MA, et al. Dissociation between the processivity and total activity of γ-secretase: implications for the mechanism of Alzheimer’s disease-causing presenilin mutations. Biochemistry. 2011;50:9023–35. doi: 10.1021/bi2007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:141–46. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes O, Paturi S, Ye W, et al. Effects of membrane lipids on the activity and processivity of purified γ-secretase. Biochemistry. 2012;51:3565–75. doi: 10.1021/bi300303g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol. 2011;68:1239–44. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol. 2011;68:1385–92. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pottier C, Hannequin D, Coutant S, et al. High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatry. 2012;17:875–79. doi: 10.1038/mp.2012.15. [DOI] [PubMed] [Google Scholar]
- 14.Lane RF, Raines SM, Steele JW, et al. Diabetes-associated SorCS1 regulates Alzheimer’s amyloid-beta metabolism: evidence for involvement of SorL1 and the retromer complex. J Neurosci. 2010;30:13110–15. doi: 10.1523/JNEUROSCI.3872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clee SM, Yandell BS, Schueler KM, et al. Positional cloning of Sorcs1, a type 2 diabetes quantitative trait locus. Nat Genet. 2006;38:688–93. doi: 10.1038/ng1796. [DOI] [PubMed] [Google Scholar]
- 16.Fjorback AW, Seaman M, Gustafsen C, et al. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J Neurosci. 2012;32:1467–80. doi: 10.1523/JNEUROSCI.2272-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 19.Weiner MW, Veitch DP, Aisen PS, et al. Alzheimer’s Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8 (Suppl 1):S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–19. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 21.DeMeyer G, Shapiro F, Vanderstichele H, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67:949–56. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cairns NJ, Ikonomovic MD, Benzinger T, et al. Absence of Pittsburgh Compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol. 2009;66:1557–62. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikonomovic MD, Abrahamson EE, Price JC, et al. Early AD pathology in a [C-11]PiB-negative case: a PiB-amyloid imaging, biochemical, and immunohistochemical study. Acta Neuropathol. 2012;123:433–47. doi: 10.1007/s00401-012-0943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schöll M, Wall A, Thordardottir S, et al. Low PiB PET retention in presence of pathologic CSF biomarkers in Arctic APP mutation carriers. Neurology. 2012;79:229–36. doi: 10.1212/WNL.0b013e31825fdf18. [DOI] [PubMed] [Google Scholar]
- 25.Gandy S, Simon AJ, Steele JW, et al. Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-beta oligomers. Ann Neurol. 2010;68:220–30. doi: 10.1002/ana.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lublin AL, Gandy S. Amyloid-beta oligomers: possible roles as key neurotoxins in Alzheimer’s disease. Mt Sinai J Med. 2010;77:43–49. doi: 10.1002/msj.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78. doi: 10.1016/S1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- 28.Maarouf CL, Daugs ID, Kokjohn TA, et al. Alzheimer’s disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS ONE. 2011;6:e27291. doi: 10.1371/journal.pone.0027291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern Y, Albert S, Tang MX, et al. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–47. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- 30.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 31.Gabel MJ, Foster NL, Heidebrink JL, et al. Validation of consensus panel diagnosis in dementia. Arch Neurol. 2010;67:1506–12. doi: 10.1001/archneurol.2010.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–69. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeKosky ST, Carrillo MC, Phelps C, et al. Revision of the criteria for Alzheimer’s disease: a symposium. Alzheimers Dement. 2011;7:e1–12. doi: 10.1016/j.jalz.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 35.Yang DS, Small DH, Seydel U, et al. Apolipoprotein E promotes the binding and uptake of beta-amyloid into Chinese hamster ovary cells in an isoform-specific manner. Neuroscience. 1999;90:1217–26. doi: 10.1016/s0306-4522(98)00561-2. [DOI] [PubMed] [Google Scholar]
- 36.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–97. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deane R, Sagare A, Hamm K, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Investig. 2008;118:4002–13. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wexler EM, Rosen E, Lu D, et al. Genome-wide analysis of a Wnt1-regulated transcriptional network implicates neurodegenerative pathways. Sci Signal. 2011;4:ra65. doi: 10.1126/scisignal.2002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi SH, Tanzi RE. iPSCs to the rescue in Alzheimer’s research. Cell Stem Cell. 2012;10:235–36. doi: 10.1016/j.stem.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Qiang L, Fujita R, Yamashita T, et al. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–71. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Sproul AA, Jacob S, Nestor MW, et al. Development of an induced pluripotent stem cell Alzheimer’s disease model using PSEN1 mutant fibroblasts. Alzheimers Dement. 2012;8:310. [Google Scholar]
- 44.DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury: football, warfare, and long-term effects. N Engl J Med. 2010;363:1293–96. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- 45.Costanza A, Weber K, Gandy S, et al. Review: contact sport-related chronic traumatic encephalopathy in the elderly: clinical expression and structural substrates. Neuropathol Appl Neurobiol. 2011;37:570–84. doi: 10.1111/j.1365-2990.2011.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128–34. doi: 10.1227/01.neu.0000163407.92769.ed. discuss. 128–34. [DOI] [PubMed] [Google Scholar]
- 47.Omalu BI, Fitzsimmons RP, Hammers J, et al. Chronic traumatic encephalopathy in a professional American wrestler. J Forensic Nurs. 2010;6:130–36. doi: 10.1111/j.1939-3938.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 48.Kim N, Zimmerman M, Lipton R, et al. Making soccer safer for the brain: DTI-defined exposure thresholds for white matter injury due to soccer heading. Presented at Radiolog. Soc. North Am. Sci. Assem. Annu. Meet., 97th, McCormick Place; 2011. http://rsna2011.rsna.org/search/event_display.cfm?em_id=11001570. [Google Scholar]
- 49.Omalu B, Hammers JL, Bailes J, et al. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus. 2011;31:E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- 50.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–57. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 51.Jordan BD, Relkin NR, Ravdin LD, et al. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–40. [PubMed] [Google Scholar]
- 52.Rebeck GW, Reiter JS, Strickland DK, et al. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–80. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 53.Geschwind D, Karrim J, Nelson SF, et al. The apolipoprotein E epsilon4 allele is not a significant risk factor for frontotemporal dementia. Ann Neurol. 1998;44:134–38. doi: 10.1002/ana.410440122. [DOI] [PubMed] [Google Scholar]
- 54.Ikonomovic MD, Uryu K, Abrahamson EE, et al. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190(1):192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Gandy S, DeKosky ST. APOE ε4 status and traumatic brain injury on the gridiron or the battlefield. Sci Transl Med. 2012;4:134ed4. doi: 10.1126/scitranslmed.3004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu L, Drouet V, Wu JW, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Calignon A, Polydoro M, Súarez-Calvet M, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–97. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones EM, Surewicz WK. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell. 2005;121:63–72. doi: 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 60.Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–72. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 61.Gandy S. Testing the amyloid hypothesis of Alzheimer’s disease in vivo. Lancet Neurol. 2010;9:333–35. doi: 10.1016/S1474-4422(10)70055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–77. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 64.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimers disease. N Eng J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–70. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaufer D, Gandy S. APOE ε4 and bapineuzumab: infusing pharmacogenomics into Alzheimer disease therapeutics. Neurology. 2009;73:2052–53. doi: 10.1212/WNL.0b013e3181c6784a. [DOI] [PubMed] [Google Scholar]
- 67.Relkin NR, Szabo P, Adamiak B, et al. 18-month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009;30:1728–36. doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 68.Relkin N, Bettger L, Tsakanikas D, et al. Three-year follow up on the IVIG for Alzheimer’s Phase II Study. Presented at Alzheimer’s Assoc. Int. Conf; Vancouver. 2012. [Google Scholar]
- 69.Szabo P, Relkin N, Weksler ME. Natural human antibodies to amyloid beta peptide. Autoimmun Rev. 2008;7:415–20. doi: 10.1016/j.autrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–73. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 71.Atwal JK, Chen Y, Chiu C, et al. A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci Transl Med. 2011;3:84ra43. doi: 10.1126/scitranslmed.3002254. [DOI] [PubMed] [Google Scholar]
- 72.Santuccione AC, Merlini M, Shetty A, et al. Active vaccination with ankyrin G reduces β-amyloid pathology in APP transgenic mice. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.70. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 73.Jonsson KT, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 74.He G, Luo W, Li P, et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Netzer WJ, Dou F, Cai D, et al. Gleevec inhibits beta-amyloid production but not Notch cleavage. Proc Natl Acad Sci USA. 2003;100:12444–49. doi: 10.1073/pnas.1534745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cramer PE, Cirrito JR, Wesson DW, et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–6. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mouchiroud L, Molin L, Dallière N, et al. Life span extension by resveratrol, rapamycin, and metformin: the promise of dietary restriction mimetics for an healthy aging. Biofactors. 2010;36:377–82. doi: 10.1002/biof.127. [DOI] [PubMed] [Google Scholar]
- 78.Snitz BE, O’Meara ES, Carlson MC, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302:2663–70. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bachurin S, Bukatina E, Lermontova N, et al. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann NY Acad Sci. 2001;939:425–35. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 80.Doody RS, Gavrilova SI, Sano M, et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–15. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]
- 81.Steele JW, Lachenmayer ML, Ju S, et al. Latrepirdine improves cognition and arrests progression of neuropathology in an Alzheimer’s mouse model. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.106. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steele JW, Ju S, Lachenmayer ML, et al. Latrepirdine stimulates autophagy and reduces accumulation of α-synuclein in cells and in mouse brain. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.115. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.James BD, Bennett DA, Boyle PA, et al. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA. 2012;307:1798–800. doi: 10.1001/jama.2012.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scheltens P, Twisk JW, Blesa R, et al. Efficacy of souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J Alzheimers Dis. 2012;31(1):225–36. doi: 10.3233/JAD-2012-121189. [DOI] [PubMed] [Google Scholar]
- 85.Rolland Y, Pillard F, Klapouszczak A, et al. Exercise program for nursing home residents with Alzheimer’s disease: a 1-year randomized, controlled trial. J Am Geriatr Soc. 2007;55:158–65. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 86.Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Dement. 2011;26:381–88. doi: 10.1177/1533317511418956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Head D, Bugg JM, Goate AM, et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69:636–43. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.García-Mesa Y, López-Ramos JC, Giménez-Llort L, et al. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis. 2011;24:421–54. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- 89.Kempermann G, van Praag H, Gage FH. Activity-dependent regulation of neuronal plasticity and self repair. Prog Brain Res. 2000;127:35–48. doi: 10.1016/s0079-6123(00)27004-0. [DOI] [PubMed] [Google Scholar]
- 90.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–15. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagahara AH, Merrill DA, Coppola G, et al. Long-term reversal of cholinergic neuronal decline in aged non-human primates by lentiviral NGF gene delivery. Exp Neurol. 2009;215:153–59. doi: 10.1016/j.expneurol.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gandy S. Perspective: prevention is better than cure. Nature. 2011;475:S15. doi: 10.1038/475S15a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klunk WE, Price JC, Mathis CA, et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–84. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lowenthal J, Hull SC, Pearson SD. The ethics of early evidence. N Engl J Med. 2012;367:488–90. doi: 10.1056/NEJMp1203104. [DOI] [PMC free article] [PubMed] [Google Scholar]