Abstract
Background
Premenopausal women undergoing chemotherapy are at risk for amenorrhea and impaired fertility. Our objective was to assess levels of Mullerian Inhibitory Substance (MIS), Estrodiol(E2), Follicle Stimulating Hormone(FSH) and menstrual status, in women undergoing chemotherapy.
Patients and Methods
We conducted a nested prospective cohort study in women aged <40 years with breast cancer (BC) undergoing adjuvant chemotherapy (n=26). Serum MIS, FSH, and E2 were measured before chemotherapy (baseline) and at weeks 6, 12, 36 and 52. Controls were 134 age-matched women with known fertility. Hormone levels were compared between the cases and controls at baseline. Differences between amenorrhea and age subgroups were tested with the non-parametric Wilcoxon two-sample test using a two-sided alpha of 0.05.
Results
Subjects with BC and age-matched controls had similar baseline MIS levels (median 0.94 vs. 0.86 ng/ml, p>0.05). Serum MIS decreased significantly at 6 weeks and remained suppressed for 52 weeks. E2 levels decreased, and FSH levels increased during chemotherapy, however, at 52 weeks, the levels returned to baseline. At 52 weeks, only1 patient had MIS above the lower normal range, 15 had return of menstrual function, 11 had premenopausal levels of FSH, and 13 had follicular phase levels of E2. In women <35, 25% remained amenorrheic, whereas in women over 35, 50% were amenorrheic. Amenorrheic and menstruating women had similar MIS values at baseline and follow-up.
Conclusions
In young women with BC, chemotherapy decreases MIS rapidly and dramatically. Rapid reductions in MIS do not predict subsequent menstrual function. Ovarian reserve and endocrine function may be affected differently by chemotherapy.
INTRODUCTION
Due to improvements in the diagnosis and treatment of early breast cancer (BC) there are now more than 2.4 million breast cancer survivors in the US.1 Premenopausal women diagnosed with BC are often concerned about long-term consequence of chemotherapy, such as early menopause and infertility.2,3 Even for women who remain premenopausal following chemotherapy, there is an increased risk of early onset menopause compared to women with BC who do not receive chemotherapy.4 The risk of chemotherapy-related amenorrhea (CRA), menopause, and infertility are associated with patient age and type of treatment received, with age over 40 being the strongest predictor of ovarian failure.5 For women under 40, identifying patients early who are at greatest risk of CRA may alter treatment decisions, therefore early predictors of CRA risk are needed.
Serum Mullerian Inhibiting Substance (MIS), also known as Anti-Mullerian Hormone (AMH), has emerged as a valuable marker for ovarian reserve in healthy women, and helps to predict oocyte quality and yield in in vitro fertilization (IVF).6 MIS is secreted by ovarian granulosa cells of primordial, preantral and small antral follicles. After puberty and before perimenopause, MIS level remains stable in healthy women. MIS levels are consistent throughout the menstrual cycle and correlate well with other markers such as antral follicle counts (AFC), and measurements of inhibin B.7 MIS is considered to be a direct marker of ovarian reserve, as it is produced by FSH-sensitive early antral follicles. It has been suggested that MIS may be a more sensitive predictor of ovarian reserve than the other markers.
Published studies evaluating fertility preservation methods have used menstrual status and traditional markers of ovarian endocrine function such as estradiol (E2) and follicle stimulating hormone (FSH) to help predict future fertility potential. However, there has been a paucity of data on how menstrual status, ovarian endocrine function, or markers of ovarian reserve are affected by chemotherapy, and if any hormonal parameters helps predict future fertility potential. In a prior study of twenty cancer survivors who maintained menses after receiving adjuvant chemotherapy, MIS and antral follicle count were lower than in age matched controls. The authors concluded that further studies should assess the predictive value of these markers for pregnancy potential in this population.8
The primary objective of this study was to evaluate the effect of adjuvant chemotherapy on markers of ovarian reserve and endocrine function in women under the age of 40 with BC. We evaluated the predictive potential of changes in these biomarkers for determining future menstrual status.
PATIENTS AND METHODS
Subjects were premenopausal women undergoing adjuvant chemotherapy for early stage BC who participated in a randomized, double-blind, multi-center phase III trial comparing zoledronic acid 4 mg intravenously every 3 months versus placebo for one year.9 Patients were enrolled from March 2002 to June 2006 following surgery, but before initiating chemotherapy. Upon enrollment, information on demographics, reproductive and menstrual history, tobacco exposure, alcohol intake, medications taken, and tumor characteristics was collected. The protocol was initially limited to Columbia University Medical Center (CUMC) in New York, NY; and then was open to four additional sites in the Northeast. The Institutional Review Board (IRB) of CUMC and the additional sites approved the protocol.
Fasting morning serum samples were collected at baseline (prior to chemotherapy), 6, 12, 24, 36 and 52 weeks and stored in aliquots at −80°C until measurement. At each time point, serum MIS, FSH, and E2 levels were measured and menstrual status was determined. Baseline and follow-up blood draws were not timed with menstrual cycles in order to coordinate study visits with routine physician office visits and due to the irregularity of menstrual cycles especially during the chemotherapy. It has been established that MIS levels remain relatively constant throughout the menstrual cycle, but FSH and E2 levels fluctuate depending on different phases of the menstrual cycle.
MIS was measured via ELISA (Diagnostic Systems Laboratories, Inc, Webster, TX) with sensitivity of 0.05 ng/ml. Estradiol was measured via radioimmunoassay (RIA, Siemens Medical Solutions Diagnostics, Los Angeles, CA) with sensitivity of 5 pg/ml. FSH was measured via chemiluminescent enzyme immunoassays (Immulite, Siemens Medical Solutions Diagnostics, Los Angeles, CA) with sensitivity of 20 pg/ml or 0.1 mIU/ml. All serum analyses were performed in batches at Columbia Presbyterian Medical Center research laboratories. Women were classified as menstruating if they had at least 1 menstrual cycle following chemotherapy.
Of 86 patients who completed all endpoints, 26 were under the age of 40 and included in this analysis, all of whom were treated with 6 months of chemotherapy. The ages ranged from 27 to 40 years, with 24 patients aged 30 to 40, and the two youngest patients aged 27 and 29 (Table 1). In addition to chemotherapy, 14 patients were subsequently treated with tamoxifen and 3 patients were treated with an aromatase inhibitor. No patient received gonadotropin-releasing hormone (GnRH) agonists or antagonists.
Table 1.
Baseline Clinical and Demographic Charateristics
| Characteristics | Number of Patients (% of total) |
|---|---|
| Number of Enrolled Patients | 26 |
| Median Age (age range) | 37 years (range 27 to 40) |
| Age <35 | 8 (36%) |
| Age >35 | 14 (64%) |
| Race | |
| Caucasian | 4 (15%) |
| African-American | 13 (50%) |
| Hispanic | 9 (35%) |
| Chemotherapy Regimen* | |
| ACT | 24 (93%) |
| CMF | 1 (4%) |
| CAF | 1 (4%) |
| Adjuvant Hormone Therapy | |
| Tamoxifen | 14 (54%) |
| Aromatase inhibitor | 3 (11%) |
| None | 9 (35%) |
| Menstrual status at 52 weeks | |
| Amenorrhea | 11 (42%) |
| Menstruating | 15 58%) |
A=doxorubicin; C=cyclophosphamide; M=methotrexate; T-=paclitaxel; F=5-FU
Controls were selected from women seeking infertility treatment at the Center for Women’s Reproductive Care of Columbia University. One hundred thirty four women, aged 30 to 40, with only male factor infertility had MIS levels assessed before undergoing IVF, and had proven fertility with documented pregnancies after IVF. These women all had spontaneous regular menstrual cycles, but may have been undergoing stress related to infertility. Their serum samples were collected on days 2 to 5. MIS levels of these healthy fertile women were compared with those of breast cancer patients at baseline and after chemotherapy.
Statistical Analysis
At each time point, medians and standard deviations of serum MIS, FSH, and E2 were calculated for all cancer patients and also in subgroups categorized according to age and menstrual status. Patients were categorized as younger (<35 years) and older (>35 years) women. Chemotherapy-related amenorrhea (CRA) was defined as the absence of menses at 52 weeks. For patients with MIS values of <0.05 ng/ml, the value was imputed to be 0.01 for the purpose of statistical analysis.
Differences between amenorrhea and age subgroups on all time points were tested with the non-parametric Wilcoxon two-sample test using a two-sided alpha of 0.05. The Wilcoxon test was also used to test for differences on change in hormone levels at different time points before, during, and after chemotherapy.
RESULTS
Baseline MIS in comparison with Controls
One hundred and thirty four healthy women, aged 30 to 40, with documented fertility had MIS levels measured within 3 months prior to pregnancy. The median MIS value was 0.94 ng/ml and values ranged from 0.2 to 7.7 ng/ml. Compared with these age-matched fertile women, patients with early stage BC had similar baseline MIS levels (median 0.86 ng/ml, range 0.07 – 9.1 ng/ml, p>0.05) (Table 2). However, 29% of cancer patients had baseline MIS levels below 0.2 ng/ml which was the lower limit of healthy controls, and of these, 86% occurred in women older than 35 years of age. There was no significant difference in MIS levels between younger women (≤35) and older women (>35) both in healthy fertile controls (median 1.0 vs 0.88 ng/ml, p>0.05) and cancer patients at baseline (median 1.7 vs 0.5 ng/ml, p> 0.05).
Table 2.
Serum concentrations of MIS, E2, and FSH over time.
| Week 0 median (range) | Week 6 median (range) | Week 12 median (range) | Week 36 median (range) | Week 52 median (range) | |
|---|---|---|---|---|---|
| MIS (ng/ml) | 0.86 (0.07–9.1) | 0.08 a (<0.05 – 0.21) | 0.05 a (<0.05 – 0.07) | 0.05 a (<0.05 – 1.7) | 0.07 a (<0.05 – 1.2) |
| E2 (pg/ml) | 78 (9–304) | 19 a (5–459) | 7 a (5–75) | 24 a (5–723) | 72 (5–654) |
| FSH (IU/L) | 5.2 (0.6–19) | 21.6 a (2–90) | 55 a (6.1– 121) | 43 a (4.2–137) | 15.3 a (2–159) |
significant difference (p<0.05) from pre-chemotherapy level.
Effect of Chemotherapy on MIS Levels
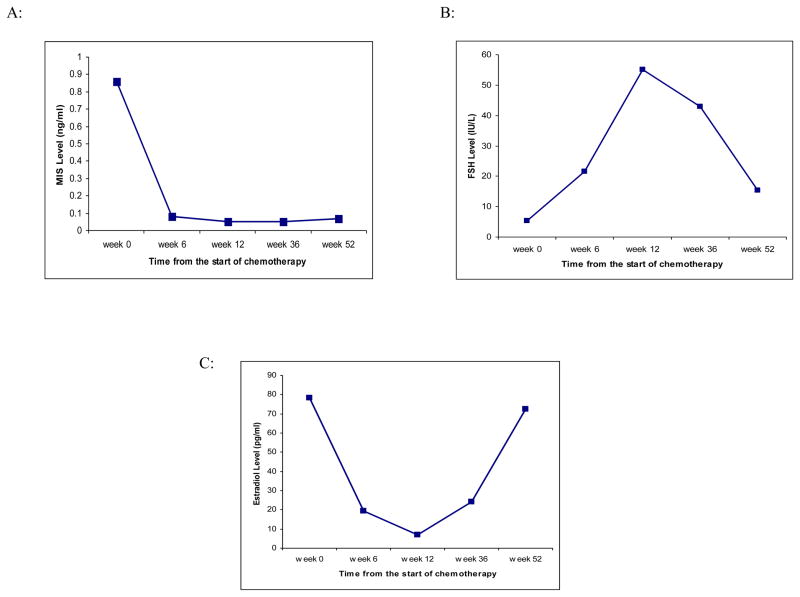
MIS levels decreased sharply and rapidly after the initiation of chemotherapy (median 0.08 ng/ml [range <0.05 to 0.21 ng/ml] after 6 weeks, 0.05 ng/ml [range <0.05 to 0.07 ng/ml] after 12 weeks of chemotherapy, compared to 0.86 ng/ml [range 0.07 to 9.1 ng/ml] before the start of chemotherapy, p<0.05 respectively). At 36 and 52 weeks after the start of chemotherapy, MIS levels remained suppressed (median 0.05 ng/ml [range <0.05 to 1.7 ng/ml] at 36 weeks and 0.07 ng/ml [range <0.05 to 1.16 ng/ml] at 52 weeks). At 52 weeks only one patient had MIS level above the sensitivity limit of 0.05 ng/ml (Figure 1a, Table 2).
Figure 1.
Median serum concentrations of (A) MIS, (B) FSH, and (C) estradiol from the start of chemotherapy.
Effect of Chemotherapy on FSH Levels
FSH levels increased to postmenopausal levels (> 20 mIU/ml) 6 weeks after the start of chemotherapy (median 5 mIU/ml at week 0 vs 22 mIU/ml at week 6, p<0.05), and remained in the postmenopausal range at week 36 (median 43 mIU/ml at week 36). However, 52 weeks after the start of chemotherapy, FSH levels decreased significantly (median 15 mIU/ml at week 52, p<0.05 compared with week 36) and 48% of patients had FSH < 20 mIU/ml, although levels remained elevated compared to baseline (median 5 mIU/ml at week 0, p<0.05) (Figure 1b, Table 2).
Effect of Chemotherapy on Estradiol Levels
Estradiol levels rapidly decreased during chemotherapy (median 78 pg/ml at week 0 vs. 19 pg/ml at week 6, p<0.05). However, at 52 weeks from the start of chemotherapy, the levels returned to pre-chemotherapy levels (median 72 pg/ml at week 52 vs. 78 pg/ml at week 0, p>0.05) (Figure 1c, Table 2).
Relationship of Menstrual Status with Hormonal Levels
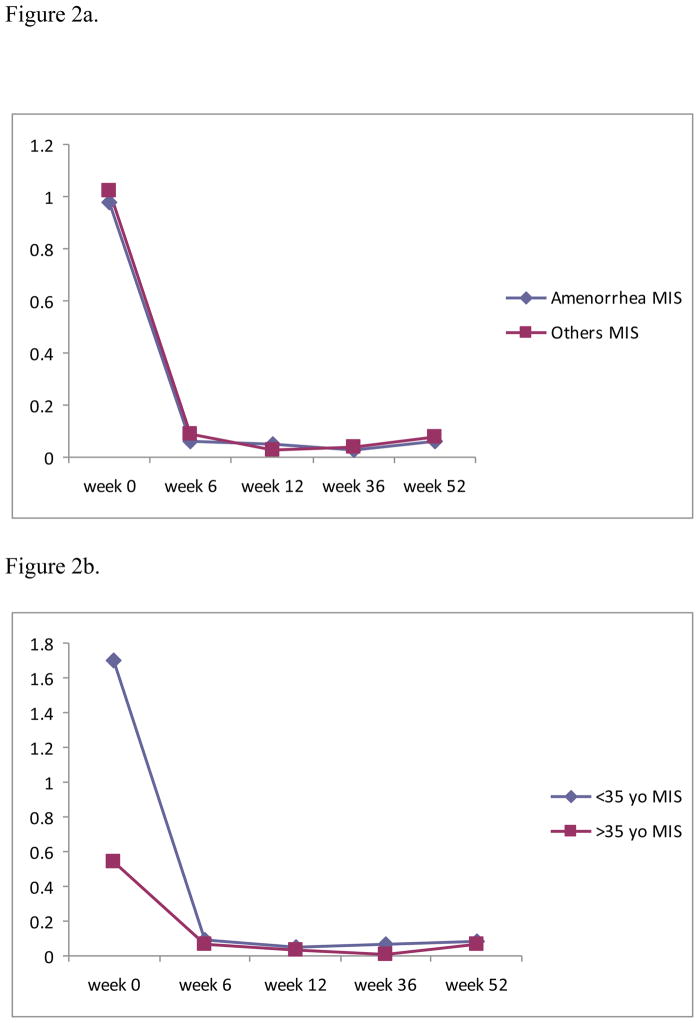
At week 52, 11 out of 26 cancer patients (42%) remained amenorrheic. In the <35 years age group, only 25% of women remained amenorrheic. In women >35 years, 50% of patients stopped menstruating. Compared to other women, the amenorrheic group had similar MIS levels at baseline (median 0.98 vs 0.74 ng/ml, p>0.05) and at 52 weeks (median 0.06 vs 0.08, p>0.05) (Figure 2a). FSH levels at baseline and at 52 weeks were also similar among the amenorrheic and menstruating groups (median 4.5 vs 6.8 mIU/ml at baseline, 11 vs 24 mIU/ml at week 52, p>0.05 respectively). More women in the amenorrheic group had FSH in the postmenopausal range (>20 mIU/ml) at 52 weeks than in the mentruating group (60% in the amenorrheic group; 23% in menstruating group). Each group had similar number of patients with follicular phase levels of E2 (>50 pg/ml) at 52 weeks (50% in amenorrheic group; 57% in menstruating group).
Figure 2.
Figure 2a. Comparison of median MIS levels between amenorrheic patient group (N=11) and menstruating patient group (N=15).
Figure 2b. Comparison of median MIS levels between two age groups ( <35 yo vs >35 yo).
(baseline p<0.05)
Despite the fact that only one patient had MIS level above the lower normal range of 0.05ng/ml at 52 weeks, 15 patients had resumed menstrual function, 11 patients had premenopausal levels of FSH (<20 mIU/ml), and 13 patients had follicular phase levels of E2 (>50 pg/ml).
Pregnancy after Chemotherapy
One 31-year-old patient was pregnant 2 years after completion of chemotherapy. Before the start of chemotherapy, her MIS level was 5.9 ng/ml, FSH level was 3.2 IU/ml, and E2 level was 288 pg/ml. At 52 weeks, her MIS level was 0.09 ng/ml, FSH level was 24 IU/L, and E2 level was 35 pg/ml, despite resuming regular menstrual cycles.
DISCUSSION
Our results indicate that both women who regain their menses following chemotherapy, and those that do not, have rapid declines in MIS to the undetectable range as early as 6 weeks after initiating treatment. MIS levels in all cancer patients remained low despite normalization of FSH and E2 following treatment. Baseline MIS levels in breast cancer patients were similar to age-matched fertile controls. Therefore neither baseline nor change in MIS predicts potential for future return of menses in women undergoing chemotherapy.
Our finding that ovarian function was intact prior to initiation of therapy is consistent with prior studies in breast cancer patients. In the study by Lutchman Singh et al., markers for ovarian reserve were tested in 22 women with breast cancer aged 22 to 42 years before and after chemotherapy, and before and after a transient ovarian stimulation post chemotherapy. The baseline levels and response to ovarian stimulation in breast cancer patients were compared with age-matched controls. They found that ovarian function in cancer patients was not significantly different than controls. However, another study in women with hematologic malignancies found that MIS levels prior to chemotherapy were lower than in controls, even though the control group was older.10 This may indicate that in hematologic malignancies the ovaries may be affected as part of the disease process or that patients may be sicker as a result of the systemic nature of hematologic malignancies.
Chemotherapy disrupts ovarian function by depleting the primordial follicle pool in a drug- and dose-dependent manner.11 Analyses of ovarian function following cancer therapy have mostly described the prevalence of ovarian failure following treatment, although follicular depletion may occur despite maintenance of regular menstrual cycles.12,13 In a small study of 3 patients, Oktay et al. demonstrated that with each cycle of chemotherapy the MIS levels fall significantly, also dropping to undetectable by 6-weeks in women who have disruption of their menses. They, however, found less of a decline in a woman who maintained her periods through treatment.14
We have demonstrated that diminished ovarian reserve, or decreased MIS levels, may be the only persistent change that can be detected after gonadotoxic damage from chemotherapy. Six months after the completion of chemotherapy, although many women had the return of menstrual function and had normal E2 and FSH levels, their MIS levels remained undetectable. This pattern of early MIS decline is similar to the perimenopausal transition changes associated with natural ovarian aging. A recent study on healthy peri-menopausal women showed a linear decline in MIS profiles to values below detection at a time 5 years before the final menstrual period (FMP)15, while mean serum E2 levels were sustained until approximately 2 years prior to the FMP.16 The decrease in MIS that was observed with advancing age may be present before changes in other ovarian aging-related markers, including antral follicle count, FSH, and inhibin B.15
Our study indicates that baseline MIS level is not a useful predictive marker for subsequent menstrual status following chemotherapy. Amenorrheic patients had similar baseline MIS levels compared with menstruating patients. This result is different from two previous studies on women with BC. In the study by Anderson et al.17, among approximately 40 premenopausal BC patients treated with various chemotherapy regimens, pre-chemotherapy MIS levels were lower among women who became amenorrheic at 6 months after chemotherapy compared to those who resumed menses (0.58 versus 1.9 ng/mL, p = 0.0007). Anders et al.18 showed that pre-chemotherapy median MIS levels were lower among women with chemotherapy-related amenorrhea (CRA) compared to those who resumed menses (0.16 vs. 1.09 ng/mL, p = 0.02). The major difference between our study and these two previous studies is the age of the patients. In both the analysis by Anderson17 and Anders18, women up to age 52 were included. The women who became amenorrheic were older (44.4 ± 0.9 versus 36.7 ± 1.2 years, P < 0.0001). All three studies indicate that older women have a higher risk of developing amenorrhea than a younger cohort. The younger age of the patients in the current study may best represent the cohort of patients who have future reproductive needs. Even though chemotherapy causes profound damage to the ovarian reserve as indicated by the undetectable MIS levels months after completion of chemotherapy, pregnancy can still occur. However, the dramatic decrease in MIS levels in patients with normal menstrual cycles and E2 levels suggest a dissociation in hormonal parameters in women having undergone chemotherapy; with more subtle alterations in ovarian reserve occurring as reflected by MIS levels.
In response to the growing recognition of issues related to fertility and cancer treatment, the American Society of Clinical Oncology (ASCO) has published guidelines recommending that oncologists discuss with patients the possibility of infertility when treated during their reproductive years.19 Furthermore, ASCO guidelines recommend that oncologists be prepared to discuss possible fertility preservation options or to refer to a reproductive specialist.19 However, there is a lack of awareness among cancer patients and their oncologists of fertility preservation options.20 In a survey of 440 breast cancer survivors who registered with Young Survival Coalition, 247 women (56%) recalled that they had desired a future pregnancy at diagnosis, but only 43 women (10%) took steps to preserve fertility.21
For young women looking for fertility preservation methods before the start of chemotherapy, the most reliable method is embryo freezing, although many other options are currently under investigation. Oocyte or ovarian tissue cryopreservation, GnRH agonist or antagonist in conjunction with chemotherapy are all active areas of research.5 Breast cancer patients undergoing controlled ovarian hyperstimulation for embryo or oocyte cryopreservation should be induced by the method that leads to the least increase in E2 levels. Oktay et al. reported using a combined letrozole-FSH protocol for ovulation induction in these patients which produced comparable results to standard in vitro fertilization (IVF), without a significant increase in estradiol levels.22 After following 79 patients who underwent ovarian stimulation with this protocol for a median interval of 23.4 months, they concluded that the letrozole-FSH protocol does not increase cancer recurrence risk nor compromise survival compared to BC patients who underwent no fertility-preserving procedure.23
It is possible that endocrine therapies administered to some of the patients during the second 6-months of the study could have influenced serum hormone levels we measured. Selective estrogen receptor modulators (SERM) such as tamoxifen have been reported to increase circulating estrogen levels and decrease FSH levels, and aromatase inhibitors (AI) have been shown to profoundly decrease estrogen levels and increase FSH levels.24 There has been limited data demonstrating the effect of these adjuvant hormonal therapy on MIS levels, although theoretically MIS levels should not be altered by these therapies. One study on patients taking tamoxifen without previous chemotherapy showed stable MIS levels throughout 12-month follow-up.17
This is one of the first prospective studies investigating the effect of chemotherapy on ovarian reserve, specifically using MIS as a serum marker. The breast cancer patients were younger than in previous studies, which alleviated the concern for natural aging as a confounding factor. We also had a large cohort of healthy women with only male factor infertility as a control group. All women were treated with a 6-month chemotherapy regimen that included alkylating agents, and this ensured that any differences we observed in our study was not caused by the difference in duration of treatment. However, we realize that our study has some limitations, including a small sample size, only one year of follow up, and hormonal levels that were not measured consistently in early follicular phase of menstrual cycles. Due to the short period of follow-up, the menstrual status was not well established in some women. Although we classified women as menstruating if they had at least one menstrual cycle following chemotherapy, we realized that some of these women might not have subsequent regular cycles.
CONCLUSION
Chemotherapy decreases ovarian reserve rapidly and dramatically. Although the secretory function of the ovary may recover to some extent and menses may return following completion of chemotherapy treatment, ovarian reserve remains persistently affected. Neither baseline nor change in MIS predicts return of menstrual function, suggesting that ovarian reserve and endocrine function may be affected differently or may recover differently from chemotherapy. Even in this very young cohort, age remains an important factor for the recovery of endocrine function after chemotherapy, and potentially for fertility as well.
Acknowledgments
Dr. Hershman received research support for this study from a K07 Award from the NCI (CA95597) and an Advanced Clinical Research Award in Breast Cancer from the American Society of Clinical Oncology.
References
- 1.American Cancer Society. Breast Cancer Facts and Figures. Atlanta, Georga: 2008. pp. 1–27. [Google Scholar]
- 2.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–83. doi: 10.1200/JCO.2004.01.159. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15483028. [DOI] [PubMed] [Google Scholar]
- 3.Thewes B, Meiser B, Taylor A, Phillips KA, Pendlebury S, Capp A, et al. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23(22):5155–65. doi: 10.1200/JCO.2005.07.773. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16051957. [DOI] [PubMed] [Google Scholar]
- 4.Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43(11):1646–53. doi: 10.1016/j.ejca.2007.04.006. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17512721. [DOI] [PubMed] [Google Scholar]
- 5.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360(9):902–11. doi: 10.1056/NEJMra0801454. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19246362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakhuda GS. The role of mullerian inhibiting substance in female reproduction. Curr Opin Obstet Gynecol. 2008;20(3):257–64. doi: 10.1097/GCO.0b013e3282fe99f2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18460940. [DOI] [PubMed] [Google Scholar]
- 7.van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–87. doi: 10.1016/j.fertnstert.2004.11.029. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15820810. [DOI] [PubMed] [Google Scholar]
- 8.Partridge AGS, Schapira L, Abusief M, Meyers M, Winer EP, Ginsburg ES. Ovarian reserve in women who remain premenopausal after chemotherapy for early stabe breast cancer. J Clin Oncol. 2008 doi: 10.1016/j.fertnstert.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 9.Hershman DL, McMahon DJ, Crew KD, Cremers S, Irani D, Cucchiara G, et al. Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2008;26(29):4739–45. doi: 10.1200/JCO.2008.16.4707. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18711172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lie Fong S, Lugtenburg PJ, Schipper I, Themmen AP, de Jong FH, Sonneveld P, et al. Anti-mullerian hormone as a marker of ovarian function in women after chemotherapy and radiotherapy for haematological malignancies. Hum Reprod. 2008;23(3):674–8. doi: 10.1093/humrep/dem392. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18216040. [DOI] [PubMed] [Google Scholar]
- 11.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10(3):251–66. doi: 10.1093/humupd/dmh021. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15140872. [DOI] [PubMed] [Google Scholar]
- 12.Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18(11):2368–74. doi: 10.1093/humrep/deg473. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14585889. [DOI] [PubMed] [Google Scholar]
- 13.Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab. 2003;88(11):5307–14. doi: 10.1210/jc.2003-030352. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14602766. [DOI] [PubMed] [Google Scholar]
- 14.Oktay K, Oktem O, Reh A, Vahdat L. Measuring the impact of chemotherapy on fertility in women with breast cancer. J Clin Oncol. 2006;24(24):4044–6. doi: 10.1200/JCO.2006.06.9823. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16921067. [DOI] [PubMed] [Google Scholar]
- 15.Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93(9):3478–83. doi: 10.1210/jc.2008-0567. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18593767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab. 2008;93(10):3847–52. doi: 10.1210/jc.2008-1056. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18647803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21(10):2583–92. doi: 10.1093/humrep/del201. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16820385. [DOI] [PubMed] [Google Scholar]
- 18.Anders C, Marcom PK, Peterson B, Gu L, Unruhe S, Welch R, et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest. 2008;26(3):286–95. doi: 10.1080/07357900701829777. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18317970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–31. doi: 10.1200/JCO.2006.06.5888. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16651642. [DOI] [PubMed] [Google Scholar]
- 20.Partridge AH. Fertility preservation: a vital survivorship issue for young women with breast cancer. J Clin Oncol. 2008;26(16):2612–3. doi: 10.1200/JCO.2008.16.1976. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18509170. [DOI] [PubMed] [Google Scholar]
- 21.Partridge AH, Gelber S, Peppercorn J, Ginsburg E, Sampson E, Rosenberg R, et al. Fertility and menopausal outcomes in young breast cancer survivors. Clin Breast Cancer. 2008;8(1):65–9. doi: 10.3816/CBC.2008.n.004. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18501060. [DOI] [PubMed] [Google Scholar]
- 22.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91(10):3885–90. doi: 10.1210/jc.2006-0962. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16882752. [DOI] [PubMed] [Google Scholar]
- 23.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26(16):2630–5. doi: 10.1200/JCO.2007.14.8700. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18509175. [DOI] [PubMed] [Google Scholar]
- 24.Rossi E, Morabito A, Di Rella F, Esposito G, Gravina A, Labonia V, et al. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: the HOBOE trial. J Clin Oncol. 2009;27(19):3192–7. doi: 10.1200/JCO.2008.18.6213. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19380451. [DOI] [PubMed] [Google Scholar]