Abstract
Purpose of review
Diabetic patients with heart failure have a poor prognosis. Although it has been demonstrated in animal models that metabolic maladaptation plays a pivotal role in contractile dysfunction of the heart, the understanding of ‘diabetic cardiomyopathy’ and its treatment in humans remains incomplete.
Recent findings
Epidemiological studies show that structural changes in the left ventricle can be demonstrated before onset of clinical diabetes. Diastolic dysfunction is the earliest manifestation that is associated with increasing level of serum-free fatty acids and worsening glycemic control. Spectroscopic and histologic evidence in the human myocardium indicates a maladaptive metabolic response in diabetes, characterized by intramyocellular triglyceride accumulation. Studies also suggest a link between myocardial isoform switching, calcium homeostasis and altered metabolism in the development of heart failure. However, treatment directed at deranged metabolic control in diabetes is effective only in animals, and not in humans.
Summary
Although clinical studies suggest the existence of ‘diabetic cardiomyopathy’, it is still difficult to prove causality. However, animal models and human studies suggest that systemic metabolic derangements may lead to metabolic, functional and structural maladaptation of the heart. The exact mechanisms of heart failure in diabetes remain elusive.
Keywords: adaptation, diabetes mellitus, heart failure, substrate metabolism
Introduction
Nonischemic heart failure in diabetes mellitus has been recognized by clinicians for more than a century. Not too long after Minkowski’s [1,2] classic experiments on the cause of diabetes mellitus, it was already speculated that heart disease in diabetes can be traced to an abnormality in intermediary metabolism. Whereas heart failure is primarily a disease of contractile dysfunction of the heart and diabetes mellitus is primarily a systemic disease of metabolic dysregulation, it is becoming increasingly apparent that both diseases are interrelated [3]. Similar to heart failure, diabetes mellitus is a growing problem with prevalence increasing steadily from 2.9% in 1974 to 4.7% in 1998 and is estimated to reach 5.5% in 2025 [4•]. Epidemiological studies [4•] have shown that patients with diabetes also suffer from cardiovascular diseases, and that the population attributable risk for cardiovascular diseases is increasing. In addition, patients with diabetes mellitus, especially women, are at a high risk for developing heart failure [5]. Nonischemic cardiomyopathy in the form of diabetic cardiomyopathy (DCM) forms a large part of the problem.
Background
The existence of a DCM was first proposed in epidemiological and experimental studies documenting structural and functional changes of the heart. However, the term suffers from poor definition. As established by Koch [6] at the end of the 19th century, a cause and effect relationship must exist between a given factor and the disease. It is difficult to apply the principle of Koch’s postulates for a causative link between diabetes mellitus and a specific cardiomyopathy causing heart failure. This difficulty stems partly from the fact that natural history is interrupted by the treatment, and that most patients are already receiving antidiabetic medications by the time they present with overt heart failure. Also, the effect of excess insulin in the insulin-resistant state leading to accelerated atherosclerosis and endothelial dysfunction contributing to heart failure further complicates the issue [3]. Finally, the presence of myocardial metabolic changes before there are any functional changes and the development of diastolic dysfunction in the heart before the onset of symptoms make DCM difficult to define. It is not clear whether DCM is a cause or a consequence of insulin resistance [7]. We have speculated that metabolic dysregulation in the body as a whole and impaired metabolic flexibility in the heart precede, trigger and sustain functional and structural changes [8].
Here we discuss the newer epidemiological data on heart failure in diabetic patients, new data for suggested mechanisms of DCM in humans, and advances in the early detection as well as in the treatment of DCM. There are several excellent reviews of established concepts in DCM from animal models, and we wish to direct the reader to them for further details on the potential molecular mechanisms that link diabetes mellitus and heart failure [9,10••,11].
Metabolic concepts and hypotheses
The pathogenesis of heart failure in diabetic patients is multifactorial and includes not only the metabolic changes of insulin resistance (high blood levels of glucose, fatty acids, and insulin) but also the increased fibrosis and cardiomyocyte apoptosis driven by increased inflammation and over activity of the renin–angiotensin system [12].
Metabolic dysregulation in diabetes mellitus involves both glucose and fatty acid metabolism [13]. We have earlier proposed a model of adaptation and maladaptation of the heart in response to the altered metabolism [3,14]. A prominent feature is that the cardiac myocyte returns to the fetal gene program leading to an increase in the expression of myosin heavy chain beta (MHCβ) and a decrease in the expression of adult isoform MHCα [15,16]. We have postulated that a decrease in glucose oxidation causes an accumulation of glycolytic intermediates that decreases the sarcoplasmic reticulum calcium ATPase 2a (SERCA2a) expression, an essential enzyme in calcium homeostasis, and leads to a diastolic dysfunction, frequently observed in DCM. Moreover, we have recently identified glucose-6-phosphate, the first intermediate in the glycolytic pathway, to be an intracellular signaling molecule that regulates protein synthesis in cardiomyocytes through mammalian target of rapamycin (mTOR) activation. This adds strength to our hypothesis of metabolic regulation of cardiac hypertrophy in diabetic patients [17].
The metabolic adaptation in the form of activation of fatty acid metabolizing pathways through the nuclear receptor peroxisome proliferator activated receptor-α (PPARα) activation and the inhibition of glucose utilization pathways through the inhibition of the pyruvate dehydrogenase complex (PDC) and phosphofructokinase results in the use of fatty acid as a preferential, albeit relatively inefficient, fuel. In the diabetic heart, increased fatty acid oxidation is suspected to promote mitochondrial uncoupling, a mechanism that may contribute to diminished myocardial high-energy reserves and contractile dysfunction [18].
Several years ago we postulated that the accumulation of glucose and fatty acid metabolites results in complex consequences finally leading to lipotoxicity, glucotoxicity, or glucolipotoxicity [14]. Glucotoxicity and lipotoxicity are associated with advanced glycosylation end-product (AGE) deposition and ceramide formation, which leads to reactive oxygen species (ROS) generation [19,20]. ROS, in turn, affect intracellular homeostasis, Ca2+ cycling, mitochondrial function and programmed cell death [21,22]. Several advances have been made since our earlier reviews of the subject.
Newer epidemiological evidence
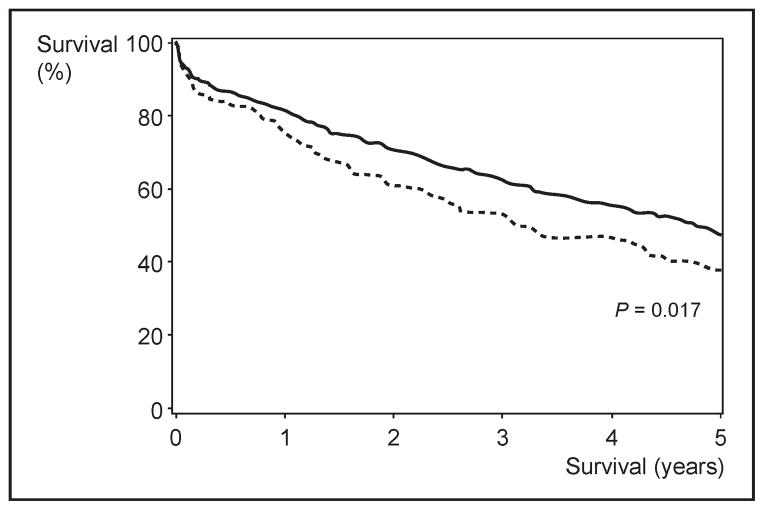
In the Framingham Heart Study population, the diagnosis of diabetes decreased the lifespan by 8.2 years in women and 7.5 years in men [23]. Similar decreasing survival was seen in patients with heart failure. In a retrospective cohort study of 665 heart failure patients from the Mayo Clinic, Rochester, the presence of diabetes decreased the 5-year survival from 46 to 37% (P = 0.017) after adjustment for covariates [24] (Fig. 1). Although none of these studies has identified DCM as a cause of premature death and disability in diabetic patients, it appears that diabetes adversely affects the life expectancy in the general population and also in patients with heart failure. It also raises the question of whether insulin resistance is associated with heart failure.
Figure 1. Kaplan–Meier survival curve of two cohorts of patients with heart failure.
The survival is less in patients with diabetes mellitus. Adapted with permission [24].
Early heart disease and diastolic dysfunction in diabetes
The effects of insulin resistance on the heart are seen even before the onset of clinical diabetes. In an analysis of 2623 patients in the Framingham Study [25], left ventricular mass increased across categories of worsening glucose tolerance even when adjusted for BMI and blood pressure. The effect was more prominent in women (P <0.001) than in men (P = 0.054). Similar gender differences have been reported in the Strong Heart Study [26].
The earliest functional abnormality in DCM is impaired diastolic function. Decrease in the rate of early diastolic filling, decreased early-to-late velocity ratio, and a prolongation of isovolumetric relaxation are the most popular features to diagnose diastolic dysfunction by echocardiography [27]. In nondiabetic obese patients, there is an inverse relation between serum free fatty acid levels and diastolic function as assessed by tissue Doppler imaging [28,29]. In a study of 25 type 1 diabetic patients [30], the severity of diastolic dysfunction correlated well with hemoglobin A1c levels (correlation coefficient r = 0.68; P = 0.0002). Clinical examination or serum markers such as brain natriuretic peptide level are not useful to diagnose diastolic dysfunction in DCM [31]. Recent studies in diabetic patients indicate that early detection of myocardial dysfunction is also possible by using exercise Doppler echocardiography. In contrast to resting mitral annular and inflow velocities, diastolic and systolic velocities during exercise in type 2 diabetic patients are substantially reduced [32]. Hence, early detection of diastolic dysfunction may be possible by the assessment of left ventricular (LV) functional reserve over time.
Links between metabolic dysregulation and diabetic cardiomyopathy in humans
A link between myocardial dysfunction in DCM, lipo-toxicity and glucotoxicity has been proposed in animal models. Recent studies [33,34•] using myocardial biopsy specimens ex vivo as well as advanced imaging techniques with proton MRI spectroscopy in vivo extended these hypotheses to the human heart. Although all the pathways from initial insult to the development of DCM are not fully understood, these studies form the framework of a jigsaw puzzle yet to be completed. New imaging techniques raise the hope that in the future, molecular imaging may help diagnose DCM earlier than it is possible today and to follow the results of pharmacological interventions. The studies reviewed for the purpose of this article are listed in Table 1
Table 1.
Potential mechanisms linking metabolism, energy transfer, calcium homeostasis and contractile function in the heart of patients with DCM
| Topic and reference of the study | Population studied | Investigative technique | Finding |
|---|---|---|---|
| Altered myocardial metabolism | |||
| Herrero et al. [36] | T1DM | PET | Increased glucose and fatty acids oxidation |
| Sharma et al. [33] | T2DM | qPCR | Increased PPARα expression |
| Lipotoxicity | |||
| Sharma et al. [33] | T2DM | Histology | Increased myocardial lipid deposition |
| McGavock et al. [34•] | T2DM | MRS | Increased myocardial triglyceride content |
| Glucotoxicity | |||
| van Heerebeek et al. [54] | T1DM, T2DM | Immunostaining | Increased AGE deposition (mainly in the wall of small heart vessels) |
| Impaired energy transfer | |||
| Diamant et al. [50] | T2DM | MRS/MRI | Decreased PCr/ATP ratio |
| Impaired calcium homeostasis | |||
| Jweied et al. [46] | T2DM | Electro-physiology | Decreased cardiac myofilament Ca2+ responsiveness and maximum Ca2+-saturated force-development |
| Razeghi et al. [45] | T2DM | qPCR | Decreased SERCA2a expression |
| Altered contractile function | |||
| Razeghi et al. [45] | T2DM | qPCR | Decreased MHCα expression |
| Sharma et al. [33] | T2DM | qPCR | Increased MHCβ expression |
AGE, advanced glycation end products; DCM, diabetic cardiomyopathy; MHC, myosin heavy chain; MRS, magnetic resonance spectroscopy; PET, positron emission tomography; PCr, phosphocreatine; PPARα, peroxisome proliferator activated receptor-α; qPCR, real-time polymerase chain reaction; SERCA2a, sarcoplasmic reticulum Ca2+ ATPase; T1DM/T2DM, type 1/type 2 diabetes mellitus.
Altered cardiac metabolism in diabetes
The initial changes of cardiac energy substrate metabolism in diabetes are an increase in fatty acid oxidation and a decrease in glucose oxidation. This metabolic shift was first described by Randle et al. [35] in normal perfused rat hearts. Recently the same metabolic shift has also been demonstrated in the hearts of a cohort of 11 patients with type 1 diabetes using PET [36]. Although the plasma insulin, lactate level and myocardial blood flow were similar to those in the controls, in diabetic patients the heart exhibited higher rates of fatty acid oxidation and utilization. At the same time, the rate of glucose oxidation decreased [36].
Our laboratory reported an upregulation of PPARα-regulated genes in patients with diabetes and high BMI who presented with nonischemic cardiomyopathy [33,37]. PPARα is a key regulator of enzymes of fatty acid oxidation, which suggests that in diabetic patients there is an increase in fatty acid oxidation as a result of metabolic adaptation due to the increased fatty acid supply to the heart [33].
Epicardial adipose tissue
In the heart, epicardial adipose tissue (EAT) covers 80% of its surface and constitutes approximately 20% of the total heart weight [38•]. Adipose tissue is an organ with both endocrine and paracrine properties that are actively involved in crosstalk with muscle tissue [39]. The exchange of hormones and metabolic mediators has been implicated in the induction of insulin resistance in skeletal muscle cells [39]. It is reasonable to speculate that the fat surrounding the heart plays a role in insulin sensitivity and function of the cardiomyocyte [40•]. One indication of its effect on insulin resistance is EAT’s low levels of lipoprotein lipase [41] that has been associated with insulin resistance in the metabolic syndrome [42]. Moreover, given the shared embryonic origins of EAT and visceral adipose tissue and its close proximity to the coronary vessels and access to the myocardium [43], there is a good possibility that excess EAT may contribute to cardiac dysfunction, much in the same way as excess visceral adipose tissue does so in hepatic steatosis. Furthermore, the crosstalk between EAT and the myocardium is facilitated by the lack of a fascia layer that would otherwise impede the diffusion of metabolites [43]. This creates contiguity between the two tissues and may facilitate the development of insulin resistance and cardiac dysfunction in excess EAT. In general, our understanding of EAT is still limited. Given the biochemical and physiological differences between the fat depots in the body, the time is ripe to elucidate what role EAT may play in insulin sensitivity and cardiac function.
Myosin heavy chain isoform switching
Part of adaptation of the myocardium to stressor involves the switching of the MHC isoform from alpha to beta, and this response is augmented by diabetes. We have postulated a role for the hexosamine biosynthetic pathway (N-acetyl-glucosamine) in the regulation of the fetal isoform switch [44]. The hypothesis was based on the analysis of transcripts of myocyte enhancer factor 2C (MEF2C), glucose transporter (GLUT4) and MHCα in failing human hearts of diabetic patients compared with nonfailing hearts of nondiabetic patients [45].
Altered calcium homeostasis
Diastolic dysfunction is the earliest feature of contractile abnormality in diabetic patients. Dysregulation of calcium homeostasis has been implicated in this in animal models [3]. In a study of human cardiac myocytes from patients with T2DM, confirmation of calcium dysregulation was seen. Jweied et al. [46] demonstrated a decreased cardiac myofilament responsiveness to calcium as shown by decreased calcium sensitivity and a trend toward a reduction in maximum Ca2+-saturated force generation.
SERCA2a is a key regulator of intracellular calcium levels. Our laboratory demonstrated that in failing diabetic hearts, SERCA2a transcripts were decreased along with other transcripts that were regulated by glucose. This confirmed the findings in mouse models, which indicate that intermediates of glucose metabolism play a role in calcium homeostasis [45].
Lipotoxicity in heart muscle
Maladaptation in the heart results in lipid accumulation in mouse models of diabetes [33]. In the human heart, cardiac steatosis seems to precede the development of contractile dysfunction [34•]. Using localized 1H magnetic resonance spectroscopy (MRS) and cardiac MRI to measure myocardial triglyceride content and LV function, the investigators compared lean patients, obese patients, patients with impaired glucose tolerance and T2DM. Myocardial triglyceride content increased as the insulin resistance increased despite LV ejection fraction remaining normal and comparable across all groups. This suggests that steatosis precedes the full-blown picture of DCM and plays an important role in its pathogenesis [34•].
Mitochondrial dysfunction
The main pathways of energy substrate metabolism provide the electromotive force that drives the oxidative phosphorylation of ADP. Hence, it is easily deducible that mitochondrial dysfunction would be seen in DCM. Mitochondrial function in humans has been studied using 31P nuclear MRS. The phosphocreatinine (PCr)/ATP ratio has been used as a surrogate marker for mitochondrial function in the human myocardium [47,48]. Using these criteria, a decrease in the PCr/ATP ratio and PCr by half has been observed in the hearts of patients with T2DM [49].
In a study [50] of 12 newly diagnosed, well controlled patients with type 2 diabetes and 12 nondiabetic subjects, diastolic dysfunction, LV mass and high energy phosphate metabolism were studied simultaneously. The investigators found diastolic dysfunction in the absence of increased LV mass or systolic dysfunction independent of age and blood pressure. Diastolic dysfunction is associated with a lower PCr/ATP ratio [50]. Although these studies have demonstrated mitochondrial dysfunction in DCM, it is still not proven whether the dysfunction is a cause or effect of DCM.
Protein glycosylation and advanced glycation end products
The stiffness in the myocardium leading to diastolic dysfunction has also been attributed to the deposition of advanced glycation end (AGE) products. It was observed already some time ago that the serum levels of AGE products correlated with diastolic dysfunction [51]. An increased flux of glucose carbon through the hexosamine biosynthetic pathway in the heart muscle cell may be responsible for UDP-N-acetylglucosamine (O-GlcNAc) acylation [52•]. There is good experimental evidence that an increase of O-GlcNAc on specific proteins may contribute to impaired cardiomyocyte function in the Zucker diabetic fatty rat [53•]. Recently, it was demonstrated that the human myocardium in patients with diabetes had a greater deposition of AGE products, especially in patients with reduced ejection fraction. However, the myocytes from diabetes mellitus patients with intact ejection fraction had decreased contractility [54]. Thus, the role of AGE in the modulation of contractile performance of the heart still needs to be defined.
Therapeutic interventions
On the basis of deranged pathophysiology outlined above, interventions to prevent or treat DCM have been directed toward prevention of further insults of insulin resistance, hyperglycemia and increased plasma fatty acid levels and disruption of the subsequent metabolic pathways that are upregulated in response to the increased load.
The first goal can be achieved by the use of traditional glucose-lowering regimens including insulin and insulin-sensitizing agents. The second goal is harder to achieve, but it is exciting because of newer pharmaceutical targets. In the following sections we present animal and human data regarding clinically relevant interventions. The most important studies are summarized in Table 2.
Table 2.
Metabolic interventions in the treatment of diabetic cardiomyopathy
| Therapy | Mechanism of action | Benefit | Disadvantages/risk |
|---|---|---|---|
| Insulin | Improves glucose uptake and utilization, contributes to normalize plasma lipids levels | Improves LVEF [56] | Undetermined increased mortality in patients with diabetes mellitus and HF [57] |
| Thiazolidinediones | Lower plasma lipids levels, increases fatty acids oxidation, improves glucose uptake | Improves LVEF [73,74] | Increased risk of edema [75] without HF increased mortality [76,77] |
| Metformin | Decreases hepatic glucose production, increases insulin action in muscle and fat, activates AMPK | Insufficient data | Insufficient data |
| GLP-1 inhibitors | Increases secretion of insulin, direct effect on myocardium | Improves LVEF [79] | Short duration of action requires continuous infusion |
AMPK, AMP-activated protein kinase; GLP-1, glucagon-like peptide 1; HF, heart failure; LVEF, left ventricular ejection fraction.
Insulin
Insulin is an inotropic agent that lowers systemic vascular resistance [55] and increases hemodynamic performance in both normal and diabetic patients. However, the increase in the LV ejection fraction is less pronounced in diabetic than in nondiabetic patients [56]. Also, the increases in LV function may not translate into survival benefit in patients with heart failure. In a systematic review of four trials, the effect of insulin on mortality in diabetic patients with heart failure was conflicting. In the three smaller studies that compared the use of insulin to insulin secretagogues and insulin sensitizers, insulin use was associated with increased unadjusted all-cause mortality. However, in a large study of 8187 patients [57], adjusted all-cause mortality was not increased. Because all these studies were not randomized control trials comparing insulin with other treatment regimens, it is hard to determine the exact effect of insulin on long-term outcomes in diabetic patients with heart failure.
Metformin
Metformin is also an insulin sensitizer in the peripheral tissues. Although the precise mechanism is not known, it is hypothesized that metformin improves metabolism by activating AMP-activated protein kinase (AMPK) in several tissues [58]. Whereas the drug inhibits hepatic gluconeogenesis [59], recent in-vitro studies [60] have suggested that the activation of AMPK may also promote glucose uptake. However, MRS studies in the human myocardium [61] have shown that there was no increase in the myocardial glucose uptake when compared with placebo. Metformin has also been seen to alleviate lipo-toxicity in cultured myocytes by promoting fatty acid oxidation and subsequently decreasing ceramide levels [62]. Although it remains to be shown in humans, metformin may also inhibit cardiac hypertrophy through AMPK-mediated inhibition of protein synthesis [63]. Traditionally, metformin has not been used in the patients with heart failure because of concerns of lactic acidosis. In a recent systematic review [57], use of metformin in type 2 diabetes patients with heart failure was not associated with harm, and these patients did not have increased admissions for heart failure. But the role of metformin in improving function and cardiac performance in DCM is currently unknown.
Thiazolidinediones
Thiazolidinediones (TZDs) improve insulin sensitivity by lowering plasma-free fatty acids levels and modulating transcription of metabolic enzymes through the activation of the PPARγ receptor [64–66]. Experimental studies on myocardial effects of TZDs have shed light on the various pathways regulating insulin responsiveness. In animal studies [67], rosiglitazone was shown to increase the concentrations of glucose transporters 1 and 4 and to improve myocardial glucose uptake. In Zucker diabetic rats, TZDs lower myocardial triglyceride and ceramides content and prevent loss of cardiac function [68]. Similar effects including increased rates of myocardial glucose oxidation were observed with a non-TZD compound [69]. An intriguing finding is that in patients on TZD pioglitazone, decreased myocardial triglyceride levels (P = 0.02) have been observed, when compared with insulin [70]. A recent study [71] of the rat ventricular myocytes also found that rosiglitazone upregulates adiponectin receptors in the myocardium, which in turn increases fatty acid oxidation and glucose uptake in the myocardium. In humans, rosiglitazone improves insulin-stimulated myocardial glucose uptake when assessed by fluorodeoxy-D-glucose (FDG) and PET scanning in patients with T2DM [61]. Similar findings were seen even in the myocardium of type 2 diabetes patients with coronary artery disease [72]. These changes in the myocardial metabolism by rosiglitazone are reflected in the improvement of myocardial function.
Both pioglitazone and troglitazone have been shown to improve LV diastolic function without affecting LV mass in hypertensive patients and in patients with T2DM [73,74]. Although all these studies provide a strong biological basis for TZDs being beneficial in heart failure, the long-term follow-up of patients with diabetes taking TZDs has revealed a higher risk of heart failure. This is primarily related to TZD-related fluid retention [75]. Even though the exact mechanism of fluid retention remains unknown, it is considered to be noncardiac in origin. This led to American Diabetes Association/American Heart Association (ADA/AHA) guidelines restricting the use of TZDs in patients with class III and IV heart failure [75]. In a recent meta-analysis of seven randomized control trials (RCTs), TZDs (either pioglitazone or rosiglitazone) increased the risk of development of heart failure [relative risk 1.72; 95% confidence interval (CI) 1.21–2.42; P = 0.002] in patients with type 2 diabetes and prediabetes when compared with controls. However, it did not increase the risk of cardiovascular mortality suggesting that the heart failure that develops with TZD use may be different from heart failure that develops due to systolic or diastolic dysfunction [76]. Similar results of increased risk for heart failure without an increase in mortality were reported from another meta-analysis of 19 RCTs of pioglitazone use in type 2 diabetes [77].
In a systematic review of eight studies comparing different antidiabetic agents in patients with heart failure, TZDs were associated with increased admission for heart failure [pooled odds ratio (OR) 1.13; 95% CI 1.04–1.22; P = 0.004]. However, TZDs were associated with a decreased all-cause mortality (pooled OR 0.83; 95% CI 0.71–0.97; P = 0.02) [57]. In the light of the new data that TZDs do not increase mortality despite increasing heart failure, we can speculate that TZDs may unmask a previously existing dysfunction by causing volume overload, and as long as the heart failure is managed with appropriate therapy, it may have survival benefit in type 2 diabetes patients. The use of TZDs in diabetic patients is also associated with an increased risk of myocardial infarction without a significant increase in death from cardiovascular causes according to a recent meta-analysis by Nissen and Wolski [78](OR = 1.43;95%CI = 1.03–1.98; P = 0.03). This may further limit the use of TZDs in diabetic patients and highlights the complex nature of metabolic modulation.
Glucagon-like peptide 1
Glucagon like peptide 1 (GLP-1) is an incretin hormone that promotes insulin secretion in response to glucose. GLP-1 has been shown to have direct protective effect on the myocardium directly acting via the GLP-1 receptor. Infusion of GLP-1 has been shown to improve LV ejection fraction in patients with acute myocardial infarction and heart failure [78,79]. Although no effect on DCM has been studied, GLP-1 appears to be an exciting new drug for DCM as it affects glucose metabolism.
Conclusion
DCM remains a disease without a proper definition. Attempts to characterize the pathogenesis of DCM in humans have been aided by animal models. Mitochondrial dysfunction with related ROS production seems to be one of the major pathways for myocardial injury. Newer imaging techniques, including PET and MRS, have added powerful tools to assess dysregulated myocardial metabolism in vivo, although it is still elusive whether insulin resistance and diabetes are a cause or a consequence of heart failure. It is hoped that ongoing research will lead to the identification of new intracellular targets for identifying patients with diabetes at risk of developing heart failure.
Acknowledgments
Work in the authors’ laboratory is supported in part by the National Heart, Lung and Blood Institute (RO1 HL-073162) of the US Public Health Service. We thank Mohamed F. Algahim for contributions to this paper and Roxy A. Tate for expert editorial assistance.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 288).
- 1.Mayer J. Regarding the connection between diabetes mellitus and heart disease [in German] Zeitschr Klin Med. 1886;14:212–239. [Google Scholar]
- 2.Taegtmeyer H, Passmore JM. Defective energy metabolism of the heart in diabetes. Lancet. 1985;I:139–141. doi: 10.1016/s0140-6736(85)91907-5. [DOI] [PubMed] [Google Scholar]
- 3.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 4•.Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. A cohort study showing an increase in the proportion of cardiovascular diseases which can be attributed to diabetes mellitus over the last 50 years. [DOI] [PubMed] [Google Scholar]
- 5.Kannel W, McGee D. Diabetes and cardiovascular disease: The Framingham Study. J Am Med Assoc. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 6.Koch R. The aetiology of tuberculosis [in German] Mitt Kaiser Gesundh. 1884;2:1–88. [Google Scholar]
- 7.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Taegtmeyer H, Golfman L, Sharma S, et al. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann N Y Acad Sci. 2004;1015:202–213. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 9.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291:H1489–H1506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 10••.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. Excellent review of the pathogenetic mechanisms of diabetic cardiomyopathy in animal models and human studies. [DOI] [PubMed] [Google Scholar]
- 11.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 12.Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 13.Stein DT, Esser V, Stevenson BE, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105:1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 15.Depre C, Young ME, Ying J, et al. Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol. 2000;32:985–996. doi: 10.1006/jmcc.2000.1139. [DOI] [PubMed] [Google Scholar]
- 16.Rajabi M, Kassiotis C, Razeghi P, et al. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Guthrie PH, Chan SS, et al. Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart. Cardiovasc Res. 2007;76:71–80. doi: 10.1016/j.cardiores.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258. doi: 10.1152/physiol.00008.2006. [DOI] [PubMed] [Google Scholar]
- 19.Suematsu N, Tsutsui H, Wen J, et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Kho AL, Anilkumar N, et al. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113:1235–1243. doi: 10.1161/CIRCULATIONAHA.105.581397. [DOI] [PubMed] [Google Scholar]
- 21.Fauconnier J, Andersson DC, Zhang SJ, et al. Effects of palmitate on Ca(2+) handling in adult control and ob/ob cardiomyocytes: impact of mitochondrial reactive oxygen species. Diabetes. 2007;56:1136–1142. doi: 10.2337/db06-0739. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh S, Pulinilkunnil T, Yuen G, et al. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol. 2005;289:H768–H776. doi: 10.1152/ajpheart.00038.2005. [DOI] [PubMed] [Google Scholar]
- 23.Franco OH, Steyerberg EW, Hu FB, et al. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–1151. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- 24.From AM, Leibson CL, Bursi F, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Rutter MK, Parise H, Benjamin EJ, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 26.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 27.Kirkpatrick JN, Vannan MA, Narula J, et al. Echocardiography in heart failure: applications, utility, and new horizons. J Am Coll Cardiol. 2007;50:381–396. doi: 10.1016/j.jacc.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 28.Leichman JG, Aguilar D, King TM, et al. Association of plasma free fatty acids and left ventricular diastolic function in patients with clinically severe obesity. Am J Clin Nutr. 2006;84:336–341. doi: 10.1093/ajcn/84.1.336. [DOI] [PubMed] [Google Scholar]
- 29.Leichman JG, Aguilar D, King TM, et al. Improvements in systemic metabolism, anthropometrics, and left ventricular geometry 3 months after bariatric surgery. Surg Obes Relat Dis. 2006;2:592–599. doi: 10.1016/j.soard.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shishehbor MH, Hoogwerf BJ, Schoenhagen P, et al. Relation of hemoglobin A1c to left ventricular relaxation in patients with type 1 diabetes mellitus and without overt heart disease. Am J Cardiol. 2003;91:1514–1517. doi: 10.1016/s0002-9149(03)00414-4. [DOI] [PubMed] [Google Scholar]
- 31.Fang ZY, Schull-Meade R, Leano R, et al. Screening for heart disease in diabetic subjects. Am Heart J. 2005;149:349–354. doi: 10.1016/j.ahj.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Ha JW, Lee HC, Kang ES, et al. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart. 2007;93:1571–1576. doi: 10.1136/hrt.2006.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 34•.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. Interesting paper demonstrating an increased myocardial triglyceride content in patients with insulin resistance and type 2 diabetes mellitus in the absence of heart failure. [DOI] [PubMed] [Google Scholar]
- 35.Randle PJ, Garland PB, Hales CN, et al. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 36.Herrero P, Peterson LR, McGill JB, et al. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Razeghi P, Young ME, Alcorn JL, et al. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 38•.Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev. 2007;8:253–261. doi: 10.1111/j.1467-789X.2006.00293.x. Timely review of the anatomy and physiological role of epicardial fat and its potential role in the development of coronary atherosclerosis in obese patients. [DOI] [PubMed] [Google Scholar]
- 39.Sell H, Dietze-Schroeder D, Eckel J. The adipocyte-myocyte axis in insulin resistance. Trends Endocrinol Metab. 2006;17:416–422. doi: 10.1016/j.tem.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 40•.Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Des. 2007;13:2180–2184. doi: 10.2174/138161207781039670. A superb review of epicardial adipose tissue structure and function by the pioneers of this field of research. [DOI] [PubMed] [Google Scholar]
- 41.Barber MC, Ward RJ, Richards SE, et al. Ovine adipose tissue mono-unsaturated fat content is correlated to depot-specific expression of the stearoyl-CoA desaturase gene. J Anim Sci. 2000;78:62–68. doi: 10.2527/2000.78162x. [DOI] [PubMed] [Google Scholar]
- 42.Goodarzi MO, Guo X, Taylor KD, et al. Lipoprotein lipase is a gene for insulin resistance in Mexican Americans. Diabetes. 2004;53:214–220. doi: 10.2337/diabetes.53.1.214. [DOI] [PubMed] [Google Scholar]
- 43.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Young ME, Yan Z, Razeghi P, et al. Proposed regulation of gene expression by glucose in rodent heart. Gene Reg Systems Biol. 2007;1:251–262. doi: 10.4137/grsb.s222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razeghi P, Young ME, Cockrill TC, et al. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation. 2002;106:407–411. doi: 10.1161/01.cir.0000026392.80723.dc. [DOI] [PubMed] [Google Scholar]
- 46.Jweied EE, McKinney RD, Walker LA, et al. Depressed cardiac myofilament function in human diabetes mellitus. Am J Physiol Heart Circ Physiol. 2005;289:H2478–H2483. doi: 10.1152/ajpheart.00638.2005. [DOI] [PubMed] [Google Scholar]
- 47.de Roos A, Doornbos J, Luyten PR, et al. Cardiac metabolism in patients with dilated and hypertrophic cardiomyopathy: assessment with proton-decoupled P-31 MR spectroscopy. J Magn Reson Imaging. 1992;2:711–719. doi: 10.1002/jmri.1880020616. [DOI] [PubMed] [Google Scholar]
- 48.Gyulai L, Roth Z, Leigh JS, Jr, et al. Bioenergetic studies of mitochondrial oxidative phosphorylation using 31phosphorus NMR. J Biol Chem. 1985;260:3947–3954. [PubMed] [Google Scholar]
- 49.Scheuermann-Freestone M, Madsen PL, Manners D, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–3046. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 50.Diamant M, Lamb HJ, Groeneveld Y, et al. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42:328–335. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 51.Berg TJ, Snorgaard O, Faber J, et al. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care. 1999;22:1186–1190. doi: 10.2337/diacare.22.7.1186. [DOI] [PubMed] [Google Scholar]
- 52•.McNulty PH. Hexosamine biosynthetic pathway flux and cardiomyopathy in type 2 diabetes mellitus. Focus on ‘Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart’. Am J Physiol Cell Physiol. 2007;292:C1243–C1244. doi: 10.1152/ajpcell.00521.2006. A well written editorial that provides perspective to hexosamine biosynthetic pathway flux and reversible O-GlcNa cylation in heart muscle. [DOI] [PubMed] [Google Scholar]
- 53•.Fülöp N, Mason MM, Dutta K, et al. Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol. 2007;292:C1370–C1378. doi: 10.1152/ajpcell.00422.2006. A nice demonstratrion that increase in GlcNAc on specific proteins may contribute to impaired cardiomyocyte function in diabetes. [DOI] [PubMed] [Google Scholar]
- 54.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 55.Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267:E187–E222. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 56.Sasso FC, Carbonara O, Cozzolino D, et al. Effects of insulin-glucose infusion on left ventricular function at rest and during dynamic exercise in healthy subjects and noninsulin dependent diabetic patients: a radionuclide ventriculographic study. J Am Coll Cardiol. 2000;36:219–226. doi: 10.1016/s0735-1097(00)00717-8. [DOI] [PubMed] [Google Scholar]
- 57.Eurich DT, McAlister FA, Blackburn DF, et al. Benefits and harms of anti-diabetic agents in patients with diabetes and heart failure: systematic review. BMJ. 2007;335:497. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stumvoll M, Nurjhan N, Perriello G, et al. Metabolic effects of metformin in noninsulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 60.Bertrand L, Ginion A, Beauloye C, et al. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardio-myocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol. 2006;291:H239–H250. doi: 10.1152/ajpheart.01269.2005. [DOI] [PubMed] [Google Scholar]
- 61.Hallsten K, Virtanen KA, Lonnqvist F, et al. Enhancement of insulin-stimulated myocardial glucose uptake in patients with type 2 diabetes treated with rosiglitazone. Diabet Med. 2004;21:1280–1287. doi: 10.1111/j.1464-5491.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 62.An D, Kewalramani G, Chan JK, et al. Metformin influences cardiomyocyte cell death by pathways that are dependent and independent of caspase-3. Diabetologia. 2006;49:2174–2184. doi: 10.1007/s00125-006-0338-9. [DOI] [PubMed] [Google Scholar]
- 63.Chan AY, Soltys CL, Young ME, et al. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 64.Kramer D, Shapiro R, Adler A, et al. Insulin-sensitizing effect of rosiglitazone (BRL-49653) by regulation of glucose transporters in muscle and fat of Zucker rats. Metabolism. 2001;50:1294–1300. doi: 10.1053/meta.2001.27202. [DOI] [PubMed] [Google Scholar]
- 65.Lebovitz HE, Dole JF, Patwardhan R, et al. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001;86:280–288. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 66.Miyazaki Y, Mahankali A, Matsuda M, et al. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care. 2001;24:710–719. doi: 10.2337/diacare.24.4.710. [DOI] [PubMed] [Google Scholar]
- 67.Oakes ND, Kennedy CJ, Jenkins AB, et al. A new antidiabetic agent, BRL 49653, reduces lipid availability and improves insulin action and glucoregulation in the rat. Diabetes. 1994;43:1203–1210. doi: 10.2337/diab.43.10.1203. [DOI] [PubMed] [Google Scholar]
- 68.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Golfman LS, Wilson CR, Sharma S, et al. Activation of PPARgamma enhances myocardial glucose oxidation and improves contractile function in isolated working hearts of ZDF rats. Am J Physiol Endocrinol Metab. 2005;289:E328–E336. doi: 10.1152/ajpendo.00055.2005. [DOI] [PubMed] [Google Scholar]
- 70.Zib I, Jacob AN, Lingvay I, et al. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med. 2007;55:230–236. doi: 10.2310/6650.2007.00003. [DOI] [PubMed] [Google Scholar]
- 71.Ding G, Qin Q, He N, et al. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor gamma. J Mol Cell Cardiol. 2007;43:73–84. doi: 10.1016/j.yjmcc.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lautamaki R, Airaksinen KE, Seppanen M, et al. Rosiglitazone improves myocardial glucose uptake in patients with type 2 diabetes and coronary artery disease: a 16-week randomized, double-blind, placebo-controlled study. Diabetes. 2005;54:2787–2794. doi: 10.2337/diabetes.54.9.2787. [DOI] [PubMed] [Google Scholar]
- 73.Horio T, Suzuki M, Suzuki K, et al. Pioglitazone improves left ventricular diastolic function in patients with essential hypertension. Am J Hypertens. 2005;18:949–957. doi: 10.1016/j.amjhyper.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Ghazzi MN, Perez JE, Antonucci TK, et al. Cardiac and glycemic benefits of troglitazone treatment in NIDDM. The Troglitazone Study Group. Diabetes. 1997;46:433–439. doi: 10.2337/diab.46.3.433. [DOI] [PubMed] [Google Scholar]
- 75.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27:256–263. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- 76.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 77.Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Jama. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 78.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 79.Sokos GG, Nikolaidis LA, Mankad S, et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]