Abstract
Over the past 2 decades, stem cells have created enthusiasm as a regenerative therapy for ischemic heart disease (IHD). Transplantation of bone marrow stem cells, skeletal myoblasts, and endothelial progenitor cells has shown to improve myocardial function after infarction. More recently, attention has focused on the potential use of embryonic stem cells (ESC) and induced pluripotent stem cells (iPS), as they possess the capacity to differentiate into various cell types, including cardiac and endothelial cells. Clinical trials have shown positive effects on the functional recovery of heart after myocardial infarction (MI) and have answered questions on timing, dosage, and cell delivery route of stem cells such as those derived from bone marrow. Despite the current advances in stem cell research, one main hurdle remains the lack of reliable information about the fate of cell engraftment, survival, and proliferation after transplantation. This review will discuss the different cell types used in cardiac cell therapy as well as molecular imaging modalities relevant to survival issues.
Introduction
Despite the significant progress in the field of cardiovascular therapy and prevention, ischemic heart disease (IHD) remains the number one cause of morbidity and mortality in the Western world, with a coronary event related death occurring about every minute in the United States alone (Lloyd-Jones et al. 2009). A wide variety of treatments is available with ever evolving cardiac interventional therapy, medical therapy, and prevention therapy, but many patients still develop refractory symptoms, eventually requiring more aggressive approaches such as left ventricular assist devices or orthotopic heart transplantation (Rose et al. 2001). Although cardiac transplantation is an established treatment for end-stage heart failure, it is limited to approximately 2000 patients per year in the United States due to a chronic shortage of suitable donor hearts. Clearly, alternative approaches to restoring heart function are sought.
Over the past 2 decades, stem cells have created enthusiasm as a regenerative therapy for IHD. Transplantation of bone marrow stem cells (Orlic et al. 2001), skeletal myoblasts (Murry et al. 1996), and endothelial progenitor cells (Assmus et al. 2002) has shown to improve myocardial function after infarction. More recently, attention has focused on the potential use of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSC) (Takahashi and Yamanaka 2006), as they possess the capacity to differentiate into various cell types, including cardiac and endothelial cells (Yamashita 2010)
Several clinical trials have shown benefits of cell transplantation. The BOOST trial studied the use of bone marrow stem cell transplantation to enhance ST-elevation infarct regeneration (Wollert et al. 2004). The REPAIR-AMI trial evaluated the use of bone marrow–derived mononuclear cells in a placebo controlled randomized double blinded study (Schachinger et al. 2006). Skeletal myoblast transplantation has been studied in the MAGIC trial (Menasche et al. 2008). These trials represent only a fraction of the representative human clinical trials of cell-based therapy for myocardial ischemia contributed by research groups worldwide. Using direct injection of stem cells, indirect mobilization of stem cells by granulocyte-colony stimulating factor (G-CSF) or a combination of both, currently over 20 randomized cell therapy clinical trials for either acute or chronic myocardial ischemia are under way. These trials, mostly using bone marrow derived stem cells, aim to answer questions related to their timing, dosage, and cell delivery route. The details of these trials have been summarized in excellent recent reviews (George 2010; Herrmann et al. 2009; Janssens 2010).
Despite these ongoing clinical advances in stem cell research, one of the main hurdles remains cell engraftment, survival, and proliferation after transplantation (Haider and Ashraf 2008). This review will focus on the molecular imaging modalities that are used to address survival issues after stem cell transplantation.
Different stem cells used for cardiac cell therapy
Different stem cells or stem cell derived cells are currently being used in both preclinical research as well as in human clinical trials. Despite the many reports of positive effects of different types of stem cells on functional outcome after cardiac ischemia, only a handful of cell types were utilized for human clinical trials. Most of these clinical trials involved the use of autologous adult stem cell populations mainly due to immunogenicity issues.
The different stem cell populations used for cardiac repair can be divided into 3 groups: (1) adult stem/progenitor cell populations (including tissue resident stem cells), (2) ESC population, and (3) iPSC population. The developmental potential differs among different cell types. The totipotent stem cell, the most primitive embryonic stem cell, has the potential to develop into a complete embryo. With subsequent divisions, the cells lose their totipotency and become pluripotent embryonic stem cells, capable of differentiating into the 3 embryonic germ layers (ectoderm, mesoderm and endoderm) (Brignier and Gewirtz 2010). Further divisions result in a more restricted developmental potential, called multipotency, with the cells being capable of differentiating only into a limited number of cell types. Adult stem cells are multipotent. Progenitor cells have the least developmental potential since these cells are thought to be mostly unipotent (Seaberg and van der Kooy 2003). Although ESC are considered to be the most promising for myocardial tissue regeneration, to date however, no clinical trial has examined the use of ESC transplantation as a therapeutic option for cardiac damage due to the many remaining hurdles. Recently, the US Food and Drug Administration (FDA) has approved a phase 1 multi-center trial to assess the safety and tolerability of transplantation of hESC-derived oligodendrocyte progenitor cells, called GRNOPC1, in patients with spinal cord injury (www.geron.com 2010). Three main issues continue to hamper clinical translation: 1) teratoma formation (Cao et al. 2007b; Lee et al. 2009), 2) immunogenical rejection (Swijnenburg et al. 2008a; Swijnenburg et al. 2008b), and 3) ethical and political issues in the United States and elsewhere. In vitro differentiation of ESCs into cardiomyocytes or endothelial cells before transplantation could theoretically resolve the teratoma-related issues, but without an existing method of isolation that results in a 100% pure differentiated ES-derived cell population, this still remains a concern (Lee et al. 2009).
Direct reprogramming of adult somatic cells such as fibroblasts to become iPSCs as reported independently by Yamanaka (Takahashi and Yamanaka 2006) and Thomson (Yu et al. 2007), may be able to circumvent the issues of immunogenicity and ethical or political dilemma. Similar to ESCs, cardiomyocytes can be derived from iPSCs and transplantation of iPSC-derived cardiomyocytes has been shown to improve cardiac function after myocardial infarction in rodents (Nelson et al. 2009; Zhang et al. 2009).
Molecular imaging
The promise of stem cell therapy and recent clinical trials showing cardiac improvement after stem cell transplantation (Herrmann et al. 2009) as mentioned above, have opened a growing gap between our lack of basic knowledge of stem cell behaviour and the pressing need to implement stem cell therapy in the clinic. Recent clinical stem cell trials such as the REPAIR-AMI (Schachinger et al. 2009) and the MAGIC (Menasche et al. 2008) focused on secondary endpoints as contractility and perfusion to determine functional outcome. The next step is to demonstrate in vivo longitudinal tracking of transplanted stem cells to investigate their behaviour and fate. Therefore finding safe and reliable in vivo molecular tracking modalities is of utmost importance.
To this end, a variety of diverse approaches have been suggested in the literature, which can be divided in two main approaches, direct (e.g., physical labelling) versus indirect (e.g, reporter gene labelling). These topics have already extensively been reviewed (Lee et al. 2008; Narsinh et al. 2009; Pearl and Wu 2008). A recent review by Lau et al (Lau et al. 2009) addresses the clinical applicability of the different imaging approaches, including MRI-based approaches, radionuclide approaches, reporter gene approaches, and multimodality approaches.
Imaging approaches
Most common direct imaging approaches use radionuclides (Aicher et al. 2003), superparamagnetic iron particles (SPIOs) (Bulte and Kraitchman 2004), or gadolinium chelates (Anderson et al. 2006). The general mechanism physically labels cells ex vivo with a detectable probe to enable the subsequent monitoring of cells by the appropriate imaging technique after transplantation.
Indirect imaging works by transfecting or transducing the cells of interest with reporter genes, allowing cell tracking after administering the reporter substrate. After the reporter gene is integrated into the cell, it will produce a reporter protein that will interact with the administered reporter substrate, thus creating a signal that can be monitored with various imaging techniques, depending on the construct used.
Direct molecular imaging has several disadvantages compared to indirect imaging, principally the limited half-life of its contrast agent (due to degradation or radioactive decay) and dilution (because of cell proliferation). In addition, phagocytosis by other cells (Pawelczyk et al. 2008) may result in reduced cell viability. Li and colleagues compared longitudinal tracking of undifferentiated ESC and ESC-derived endothelial cells in a SCID mouse model using a firefly luciferase based reporter gene (via bioluminescent imaging; BLI) or SPIO based physical labelling (via magnetic resonance imaging; MRI) (Li et al. 2008). They concluded that the long term monitoring of cell survival is more accurate using the reporter gene approach, because the slow tissue clearing of SPIOs can give a false positive representation of cell viability.
Reporter gene imaging
Reporter gene technology offers the best options in cell survival tracking because the reporter protein engages in transcription and synthesis within the cell, which conclusively prove cell viability. Furthermore, as the reporter genes are incorporated in the cell's genome, imaging is not hampered by cell division when the reporter gene is passed on to daughter cells. For these reasons, indirect imaging utilizing reporter genes seems a promising alternative.
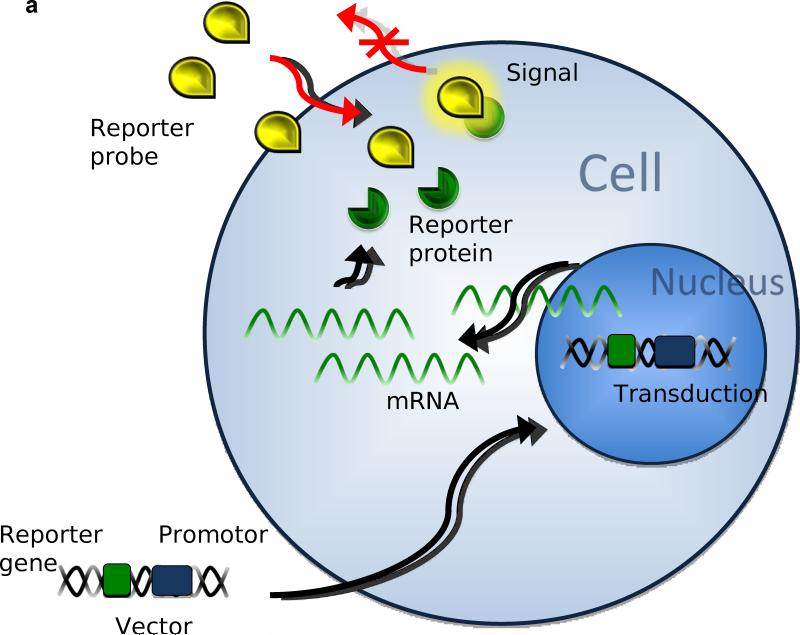
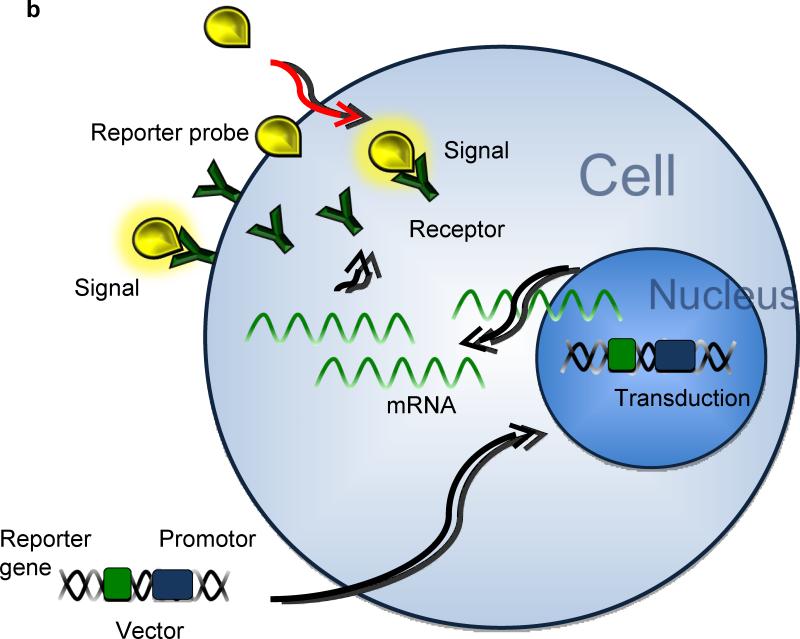
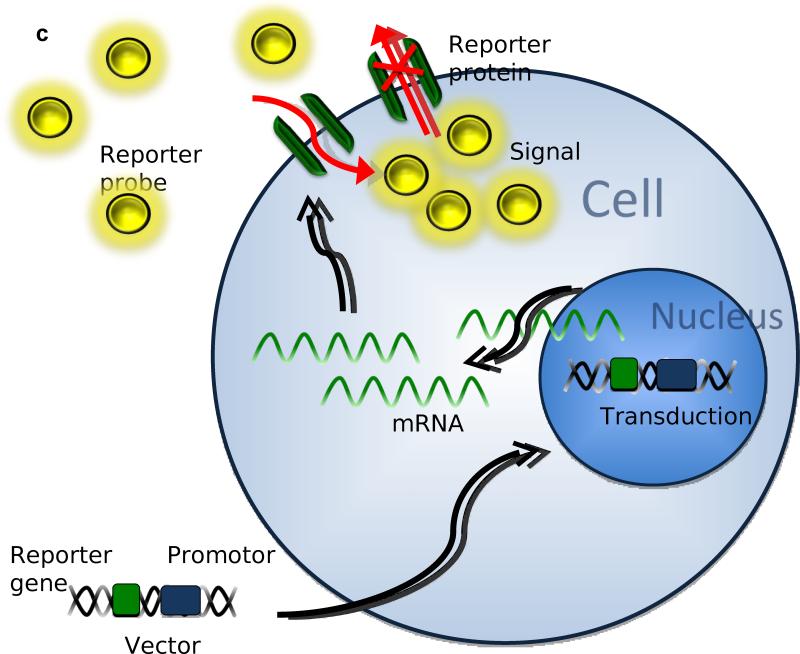
Three major approaches have been used in reporter gene imaging (Zhang et al. 2008). 1) In enzyme based reporter genes (Figure 1A), the inserted reporter gene codes for a reporter enzyme and intracellularly interacts with the reporter substrate, trapping it inside the cell membrane (Green et al. 2004). 2) Similarly, with receptor based reporter genes (Figure 1B), the inserted gene transcribes for a receptor that binds the reporter substrate inside and outside the cell (Gambhir et al. 2000). 3) Less popular than the other approaches, is the transporter based reporter gene method (Figure 1C). The inserted gene transcribes for a transporter protein, which actively accumulates reporter substrate inside the cell (Doubrovin et al. 2007). The last technique has also been applied in MRI based approaches (Genove et al. 2005).
Figure 1.
After transduction in the cell's genome, the reporter gene will transcribe mRNA coding for the following: A) reporter proteins that bind, capture, and activate the reporter probes entering the cell. B) Reporter proteins binding reporter probes on the cell membrane and intracellularly. C) Reporter proteins that actively accumulate and trap active reporter proteins inside the cell.
Disadvantages of reporter gene system
Reporter genes do have certain disadvantages or risks. By incorporating the reporter gene in the cell genome, a number of undesirable effects could occur, depending on the type of viral vector used (Arbab et al. 2009). Risks include alteration of the cells features through unexpected DNA instability (possibly even leading to malignant behavior (Nienhuis et al. 2006)), immune responses from the recipient to the transformed cells (Zhou et al. 2006), gene silencing (Krishnan et al. 2006), and over-expression of reporter protein through uncontrolled insertion of multiple reporter gene copies in the cell (Lau et al. 2009).
Imaging modalities
Numerous animal investigations have shown promising results in several different approaches to monitor specific cells. Kraitchman and colleagues monitored MSC migration with MRI after magnetically labelling the cells with SPIO particles in a swine myocardial infarction (MI) model (Kraitchman et al. 2003). Wu and his group transiently transfected and monitored cardiomyoblasts injected in a rat heart with PET using a mutant herpes simplex type 1 (sr39) thymidine kinase (HSV1-sr39TK) reporter gene with 9-[4-[18F]fluoro-3-(hydroxymethyl)butyl]guanine (18F-FHBG) as reporter probe (Wu et al. 2003). The HSV1-TK enzyme can monophosphorylate 18F–FHBG and thus can be used to image cells that express the HSV1-TK PET reporter gene. Bioluminescent imaging using charge coupled device (CCD) cameras and fluorescent imaging have proven to be very suitable for small animal imaging (Sheikh et al. 2007; Wu et al. 2003). Cell survival analyses utilizing bioluminescence imaging is shown in Figure 2. Although researchers are seeking to improve methods (Loening et al. 2007; Rabinovich et al. 2008) thus far fluorescent and bioluminescent imaging of stem cells have limited clinical value due to limited tissue penetration (Zhao et al. 2005). More promising approaches are MRI, positron emission tomography (PET) and single photon emission computed tomography (SPECT), which are not affected by the penetration problem. Although MRI has the advantage of reaching near cellular resolution, as mentioned earlier MRI based on (pre)labeled cells can give a false positive representation of cell viability through dilution of contrast agent by cell division and phagocytosis by macrophages. MRI reporter genes have not yet been as widely applied as PET and SPECT, mostly due to concerns regarding their detection sensitivity threshold (Vande Velde et al.). As such PET and SPECT based imaging modalities seem the most promising and have been extensively applied in various studies (Pomper et al. 2009; Zhang et al. 2008). Other imaging techniques such as ultrasound and X-ray based methods for molecular imaging are dependant on ex vivo cell labeling or contrast agents and have thus far seen little application in molecular imaging of stem cells (Kuliszewski et al. 2009).
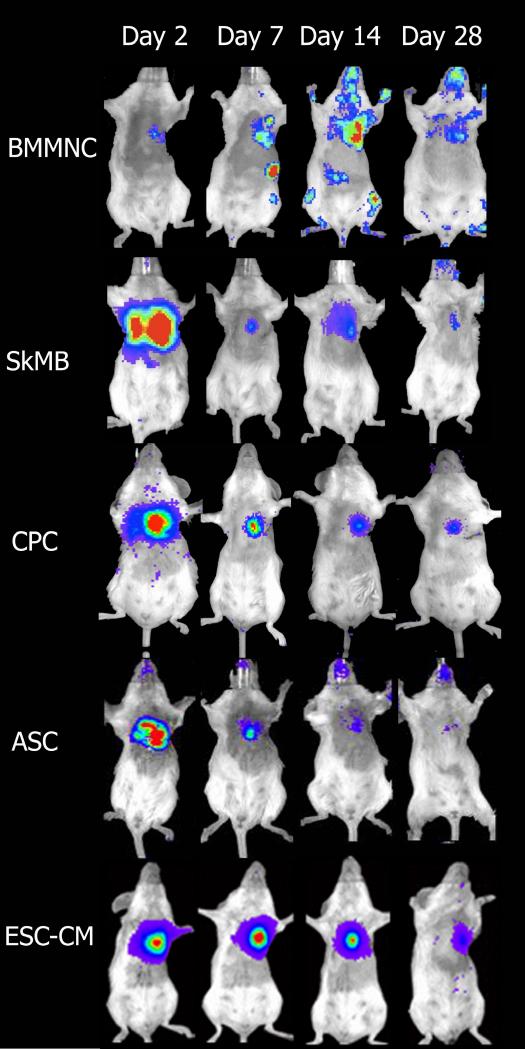
Figure 2.
Bioluminescence imaging of the survival of the different cell types in a mouse transplantation model as used in our laboratory: bone marrow mononuclear cells (BMMNC), skeletal myoblasts (SkMB), cardiac progenitor cells (CPC), adipose tissue derived stromal cells (ASC), and embryonic stem cell derived cardiomyocytes (ESC-CM). Loss of cell survival is seen in all cell types following intramyocardial infarction and injection of cells. Reprinted with permission (Cao et al. 2008; Li et al. 2009; van der Bogt et al. 2009; van der Bogt et al. 2008).
Recent proceedings in molecular imaging
Frangioni and Hajjar (2004) list the traits of an ideal imaging technology for the monitoring of stem cells: (1) The agent should be biocompatible, safe, and nontoxic. (2) No genetic modification or perturbation should occur to the stem cell. (3) Single-cell detection should be possible at any anatomic location. (4) The number of cells should be quantifiable. (5) Cell dilution through division should be minimal to none, and minimal or no transfer of contrast agent to non-stem cells should occur. (6) Noninvasive imaging in the living subject should be possible over months or years, without requiring an injectable contrast agent.
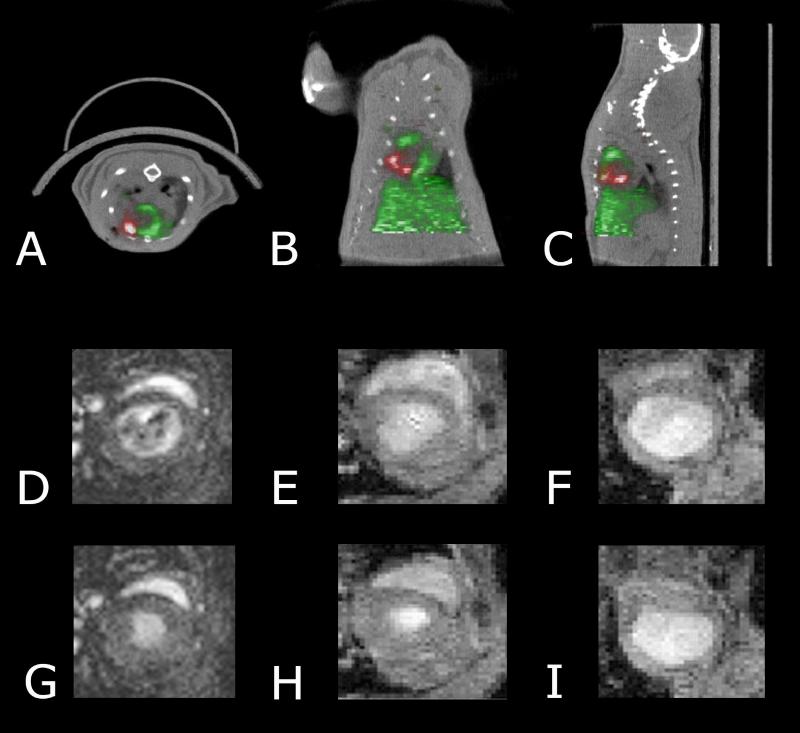
To date, no perfectly ideal imaging modality exists, as none of the most promising techniques meets all these demands. For the time being, a possible solution is the use of multimodality imaging systems that combines several modalities in one construct. Cao and colleagues (Cao et al. 2007a) created a versatile imaging platform using a lentiviral based vector to stably transfect murine embryonic stem cells with a combined PET and BLI imaging reporter gene. Furthermore, by utilizing the PET reporter gene as a suicide gene in the gangciclovir treatment, teratoma formation was diminished in comparison to control animals. The added value of the dual role of the PET reporter gene could prove to be a valuable advantage in the clinical application of this technique. Further developments include dual fusion reporter genes combining MRI and PET, or even triple fusion reporter genes combining fluorescence, bioluminescence, and PET. Kraitchman and colleagues (Kraitchman et al. 2005) combined single-photon emission computed tomography CT (SPECT/CT) and MRI to monitor MSCs in a large-animal model of acute myocardial infarction. Ray and colleagues (Ray et al. 2007) reported the construction and validation of an improved triple fusion reporter gene that is compatible with three different imaging modalities: PET, BLI and fluorescence. Representative images are shown in Figure 3.
Figure 3. PET/CT and MRI imaging of stem cells.
Transverse (A), coronal (B), and sagittal (C) image orientations of the detection of intramyocardially injected 18F-FDG–labeled cardiac-derived stem cells (red) in rat heart (green) by small-animal PET/CT after ligation of mid left anterior descending coronary artery. PET was performed immediately after cell transplantation. Representative magnetic resonance images of normal mouse hearts (D&G), mouse embryonic stem cell transplanted hearts (E&H), and normal saline–treated hearts (F&I) shown in end-diastole (D-F) and end-systole (G-I) at 1 week after left anterior descending coronary artery ligation and cell transplantation. Reprinted with permission (Hendry et al. 2008; Wu et al. 2010).
Clinical application
Clinical investigation of both direct and indirect reporter agents has so far been limited, as many approaches have not yet been clinically approved. Hofmann and colleagues (Hofmann et al. 2005) monitored BMSCs labeled with 18F-fluorodeoxyglucose ([18F]-FDG) after transplantation in patients with MI using PET imaging. As mentioned before, the main drawback in the clinical application of indirect labelling is the fact that this technique requires genetic manipulation of the cells. Several studies have investigated whether reporter genes can lead to undesirable changes of the altered cells. Using proteomic and genomic analysis, we have found no significant functional differences between ES cells expressing reporter genes and control ES cells (Wu et al. 2006a; Wu et al. 2006b). Wang and colleagues (Wang et al. 2009) demonstrated that lentiviral transduction of the same triple reporter gene in human MSCs did not affect the basic properties of these cells. Yaghoubi and colleagues (Yaghoubi et al. 2001) proved that the PET reporter probe [18F]-FHBG can be safely applied in human subjects and recently successfully transfected ex vivo expanded human autologous CD8+ T cells and HSV1-tk PET reporter gene, administering them to a patient with glioma (Yaghoubi et al. 2009). So far this is the first documented case report of reporter gene imaging based therapy used in humans.
Conclusion
A wide variety of stem cell types are available for the treatment of myocardial damage, with distinct advantages and disadvantages. Overall, we conclude that despite considerable advances in our knowledge and repertoire of imaging techniques, many issues remain to be resolved in order to accurately explain the mechanisms and survival of stem cells in human tissues. Since no perfectly ideal imaging modality has emerged so far the use of multimodality imaging systems that combines several modalities in one construct seem to be most suitable to overcome the current limitations posed by individual techniques. Nevertheless, the first documented case of reporter gene imaging based therapy in humans is an exciting development that may lead to other useful clinical applications in the near future.
Acknowledgement
This work is supported by NIH R01 EB009689 (JCW), RC1 HL099117 (JCW), U01 HL099776 (RCR) and by the Netherlands organisation for health research and development (MAL).
References
- Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–9. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Lee KK, Frank JA. Gadolinium-fullerenol as a paramagnetic contrast agent for cellular imaging. Invest Radiol. 2006;41:332–8. doi: 10.1097/01.rli.0000192420.94038.9e. [DOI] [PubMed] [Google Scholar]
- Arbab AS, Janic B, Haller J, et al. In Vivo Cellular Imaging for Translational Medical Research. Curr Med Imaging Rev. 2009;5:19–38. doi: 10.2174/157340509787354697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- Brignier AC, Gewirtz AM. Embryonic and adult stem cell therapy. J Allergy Clin Immunol. 2010;125:S336–44. doi: 10.1016/j.jaci.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Bulte JW, Kraitchman DL. Monitoring cell therapy using iron oxide MR contrast agents. Curr Pharm Biotechnol. 2004;5:567–84. doi: 10.2174/1389201043376526. [DOI] [PubMed] [Google Scholar]
- Cao F, Drukker M, Lin S, et al. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells. 2007a;9:107–17. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- Cao F, van der Bogt KE, Sadrzadeh A, et al. Spatial and temporal kinetics of teratoma formation from murine embryonic stem cell transplantation. Stem Cells Dev. 2007b;16:883–91. doi: 10.1089/scd.2007.0160. [DOI] [PubMed] [Google Scholar]
- Cao F, Wagner RA, Wilson KD, et al. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubrovin MM, Doubrovina ES, Zanzonico P, et al. In vivo imaging and quantitation of adoptively transferred human antigen-specific T cells transduced to express a human norepinephrine transporter gene. Cancer Res. 2007;67:11959–69. doi: 10.1158/0008-5472.CAN-07-1250. [DOI] [PubMed] [Google Scholar]
- Gambhir SS, Herschman HR, Cherry SR, et al. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–38. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. A new transgene reporter for in vivo magnetic resonance imaging. Nat Med. 2005;11:450–4. doi: 10.1038/nm1208. [DOI] [PubMed] [Google Scholar]
- George JC. Stem cell therapy in acute myocardial infarction: a review of clinical trials. Transl Res. 2010;155:10–9. doi: 10.1016/j.trsl.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Green LA, Nguyen K, Berenji B, et al. A tracer kinetic model for 18F-FHBG for quantitating herpes simplex virus type 1 thymidine kinase reporter gene expression in living animals using PET. J Nucl Med. 2004;45:1560–70. [PubMed] [Google Scholar]
- Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–66. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SL, 2nd, van der Bogt KE, Sheikh AY, et al. Multimodal evaluation of in vivo magnetic resonance imaging of myocardial restoration by mouse embryonic stem cells. J Thorac Cardiovasc Surg. 2008;136:1028–1037. e1. doi: 10.1016/j.jtcvs.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Herrmann JL, Abarbanell AM, Weil BR, et al. Cell-based therapy for ischemic heart disease: a clinical update. Ann Thorac Surg. 2009;88:1714–22. doi: 10.1016/j.athoracsur.2009.05.079. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- Janssens S. Stem cells in the treatment of heart disease. Annu Rev Med. 2010;61:287–300. doi: 10.1146/annurev.med.051508.215152. [DOI] [PubMed] [Google Scholar]
- Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–3. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–61. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan M, Park JM, Cao F, et al. Effects of epigenetic modulation on reporter gene expression: implications for stem cell imaging. FASEB J. 2006;20:106–8. doi: 10.1096/fj.05-4551fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliszewski MA, Fujii H, Liao C, et al. Molecular imaging of endothelial progenitor cell engraftment using contrast-enhanced ultrasound and targeted microbubbles. Cardiovasc Res. 2009;83:653–62. doi: 10.1093/cvr/cvp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JF, Anderson SA, Adler E, Frank JA. Imaging approaches for the study of cell-based cardiac therapies. Nat Rev Cardiol. 2009;7:97–105. doi: 10.1038/nrcardio.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Tang C, Cao F, et al. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–12. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Z, Dennis JE, Gerson SL. Imaging stem cell implant for cellular-based therapies. Exp Biol Med (Maywood) 2008;233:930–40. doi: 10.3181/0709-MR-234. [DOI] [PubMed] [Google Scholar]
- Li Z, Lee A, Huang M, et al. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53:1229–40. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Suzuki Y, Huang M, et al. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26:864–73. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Loening AM, Wu AM, Gambhir SS. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods. 2007;4:641–3. doi: 10.1038/nmeth1070. [DOI] [PubMed] [Google Scholar]
- Menasche P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–23. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narsinh KH, Cao F, Wu JC. Molecular imaging of human embryonic stem cells. Methods Mol Biol. 2009;515:13–32. doi: 10.1007/978-1-59745-559-6_2. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Martinez-Fernandez A, Yamada S, et al. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–16. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis AW, Dunbar CE, Sorrentino BP. Genotoxicity of retroviral integration in hematopoietic cells. Mol Ther. 2006;13:1031–49. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Pawelczyk E, Arbab AS, Chaudhry A, et al. In vitro model of bromodeoxyuridine or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: implications for cellular therapy. Stem Cells. 2008;26:1366–75. doi: 10.1634/stemcells.2007-0707. [DOI] [PubMed] [Google Scholar]
- Pearl J, Wu JC. Seeing is believing: tracking cells to determine the effects of cell transplantation. Semin Thorac Cardiovasc Surg. 2008;20:102–9. doi: 10.1053/j.semtcvs.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Pomper MG, Hammond H, Yu X, et al. Serial imaging of human embryonic stem-cell engraftment and teratoma formation in live mouse models. Cell Res. 2009;19:370–9. doi: 10.1038/cr.2008.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich BA, Ye Y, Etto T, et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci U S A. 2008;105:14342–6. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P, Tsien R, Gambhir SS. Construction and validation of improved triple fusion reporter gene vectors for molecular imaging of living subjects. Cancer Res. 2007;67:3085–93. doi: 10.1158/0008-5472.CAN-06-2402. [DOI] [PubMed] [Google Scholar]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Erbs S, et al. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail. 2009;11:973–9. doi: 10.1093/eurjhf/hfp113. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–83. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26:125–31. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Sheikh AY, Lin SA, Cao F, et al. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–84. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swijnenburg RJ, Schrepfer S, Cao F, et al. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev. 2008a;17:1023–9. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swijnenburg RJ, Schrepfer S, Govaert JA, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008b;105:12991–6. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- van der Bogt KE, Schrepfer S, Yu J, et al. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–52. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bogt KE, Sheikh AY, Schrepfer S, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–9. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Velde G, Rangarajan JR, Toelen J, et al. Evaluation of the specificity and sensitivity of ferritin as an MRI reporter gene in the mouse brain using lentiviral and adeno-associated viral vectors. Gene Ther. doi: 10.1038/gt.2011.2. [DOI] [PubMed] [Google Scholar]
- Wang F, Dennis JE, Awadallah A, et al. Transcriptional profiling of human mesenchymal stem cells transduced with reporter genes for imaging. Physiol Genomics. 2009;37:23–34. doi: 10.1152/physiolgenomics.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- Wu JC, Abraham MR, Kraitchman DL. Current perspectives on imaging cardiac stem cell therapy. J Nucl Med 51 Suppl. 2010;1:128S–136S. doi: 10.2967/jnumed.109.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Cao F, Dutta S, et al. Proteomic analysis of reporter genes for molecular imaging of transplanted embryonic stem cells. Proteomics. 2006a;6:6234–49. doi: 10.1002/pmic.200600150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–5. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Spin JM, Cao F, et al. Transcriptional profiling of reporter genes used for molecular imaging of embryonic stem cell transplantation. Physiol Genomics. 2006b;25:29–38. doi: 10.1152/physiolgenomics.00254.2005. [DOI] [PubMed] [Google Scholar]
- 2010 www.geron.com. www.geron.com/GRNOPC1Trial/
- Yaghoubi S, Barrio JR, Dahlbom M, et al. Human pharmacokinetic and dosimetry studies of [(18)F]FHBG: a reporter probe for imaging herpes simplex virus type-1 thymidine kinase reporter gene expression. J Nucl Med. 2001;42:1225–34. [PubMed] [Google Scholar]
- Yaghoubi SS, Jensen MC, Satyamurthy N, et al. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–8. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita JK. ES and iPS cell research for cardiovascular regeneration. Exp Cell Res. 2010;316:2555–9. doi: 10.1016/j.yexcr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ruel M, Beanlands RS, et al. Tracking stem cell therapy in the myocardium: applications of positron emission tomography. Curr Pharm Des. 2008;14:3835–53. doi: 10.2174/138161208786898662. [DOI] [PubMed] [Google Scholar]
- Zhao H, Doyle TC, Coquoz O, et al. Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J Biomed Opt. 2005;10:41210. doi: 10.1117/1.2032388. [DOI] [PubMed] [Google Scholar]
- Zhou R, Acton PD, Ferrari VA. Imaging stem cells implanted in infarcted myocardium. J Am Coll Cardiol. 2006;48:2094–106. doi: 10.1016/j.jacc.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]